Abstract
In late mitosis and G1, Mcm2-7 are assembled onto replication origins to “license” them for initiation. At other times licensing is inhibited by cyclin dependent kinases (CDKs) and geminin, thus ensuring that origins fire only once per cell cycle. We show here that, paradoxically, CDKs are also required to inactivate geminin and activate the licensing system. On exit from metaphase in Xenopus egg extracts, CDK-dependent activation of the Anaphase Promoting Complex (APC/C) results in the polyubiquitination of geminin. This ubiquitination triggers geminin inactivation without requiring ubiquitin-dependent proteolysis, and is essential for replication origins to become licensed.
Keywords: DNA replication, replication licensing, Xenopus, geminin, ubiquitination
Introduction
For most eukaryotic cells, chromosomal DNA must be replicated once and only once in each cell cycle. This is ensured by separating DNA replication into two distinct phases. In the first phase, which normally occurs in late mitosis and early G1, replication origins are “licensed” for a single initiation event by loading heterohexamers of the Mcm2-7 proteins, thus forming the pre-replicative complex (pre-RC) (reviewed in 1,2,3). The second phase occurs during S phase, when replication forks are initiated at licensed origins. As each origin initiates Mcm2-7 are displaced from it. So long as the ability to license origins ceases before DNA replication commences, then no replication origin will initiate replication more than once.
The central role of Mcm2-7 regulation in preventing re-replication of DNA was first demonstrated in Xenopus egg extracts4-6. Chong et al4 and Kubota et al5 both exploited the observation that the replication licensing system, as originally defined7, could be specifically inhibited by treating metaphase extracts with certain protein kinase inhibitors such as staurosporine and 6-dimethylaminopurine (6-DMAP). Both these treatments inhibit licensing by ultimately preventing the loading of Mcm2-7 onto chromatin. However, 6-DMAP-treated extracts can efficiently replicate chromatin that has already been licensed. The ability to assay licensing activity using a 6-DMAP-based assay has permitted a systematic chromatographic fractionation of the Xenopus licensing system and the complete reconstitution of the licensing of Xenopus sperm chromatin with purified proteins8.
Despite the effectiveness of this assay, it has remained unclear how 6-DMAP and staurosporine selectively inhibit the licensing system. Inhibition only occurs if 6-DMAP or staurosporine are added to extracts arrested in metaphase; no inhibition of licensing occurs when they are added to extracts already in interphase9,10. Further, 6-DMAP and staurosporine only inhibit licensing at concentrations that are sufficient to cause the extracts to spontaneously exit from metaphase arrest and enter interphase9-11. They are therefore effective at concentrations that inhibit the metaphase-inducing activity of Cdk1-cyclin B, originally defined in Xenopus as MPF (Maturation Promoting Factor)12,13. Olomoucine, an inhibitor that is more specific for CDKs, is able to inhibit licensing in the same way as 6-DMAP and staurosporine14. These results raise the possibility that in Xenopus, the activation of the licensing system that normally occurs on exit from metaphase depends on CDK function.
This suggestion appears at odds with much data showing that CDKs inhibit the licensing system and normally act to prevent inappropriate origin licensing late in the cell cycle1-3. In yeasts, inhibition of CDKs in G2 phase is sufficient to permit re-licensing and hence re-replication of DNA15-18. CDKs block licensing in a number of different ways that appears to vary from organism to organism19-21. In metazoans, inappropriate licensing of replication origins late in the cell cycle is also inhibited by a small protein called geminin. In Xenopus eggs, geminin provides the major activity preventing DNA re-replication22,23. Geminin specifically binds and inhibits the Cdt1/RLF-B component of the licensing system22,24-27. However, no geminin homologue has so far been identified in yeasts.
In this paper, we demonstrate a new link between these two licensing inhibitors in Xenopus egg extracts. We show that CDKs play an essential role in activating the licensing system on exit from metaphase, being required for the non-proteolytic inactivation of geminin mediated by its ubiquitination.
Results
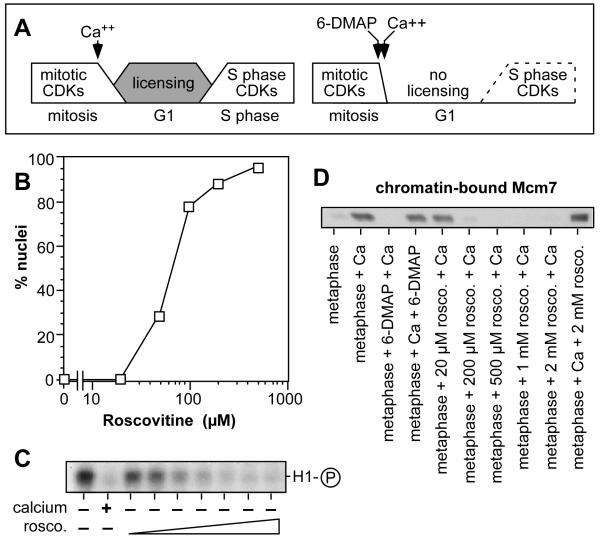
Xenopus eggs are naturally arrested at meiotic metaphase II; on fertilization a transient increase in cytosolic calcium induces them to complete mitosis and enter interphase. Extracts prepared from Xenopus eggs can likewise be released into interphase by addition of calcium. As these extracts exit metaphase, there is a rapid activation of the replication licensing system (Fig 1A, left). If metaphase-arrested egg extracts are treated with CDK inhibitors such as 6-DMAP, staurosporine or olomoucine, MPF is inhibited and the extracts enter an interphase-like state where DNA is assembled into functional nuclei9,10,14. Even if stimulated with calcium, these extracts are unable to support replication licensing, though they can efficiently replicate DNA templates that have already been licensed (Fig 1A, right). To provide further evidence that the failure of licensing in such extracts is due to CDK inhibition we repeated these experiments with roscovitine, an adenine derivative with a high specificity for CDKs28. We first determined the ability of roscovitine to inhibit MPF by titrating roscovitine into metaphase-arrested extracts and monitoring whether they could assemble DNA into interphase nuclei in the absence of calcium stimulation. Figure 1B shows that 50 - 200 μM roscovitine inhibited MPF and caused spontaneous mitotic exit, similar to the concentration of roscovitine required to block entry into mitosis in cycling extracts28. This corresponded to a decline in the total histone H1 kinase activity present in the extracts (Figure 1C). Metaphase extracts treated with ≥200 μM roscovitine prior to calcium addition were severely compromised in their ability to load Mcm7 onto chromatin (Fig 1D). As has been previously shown with 6-DMAP and staurosporine9,10, inhibition of Mcm7 loading was only seen when roscovitine was added to metaphase extract, and did not occur when roscovitine was added to extract after release into interphase (Fig 1D). The close correlation between the ability of roscovitine to inhibit MPF and replication licensing (which is also seen with 6-DMAP, staurosporine and olomoucine9,10,14) provides strong evidence that CDK activity is required for the activation of the licensing system that normally occurs on exit from metaphase.
Figure 1.
Roscovitine inhibits licensing at concentrations where MPF is inhibited.
A, Schematic picture showing events occurring after calcium release of metaphase-arrested Xenopus egg extracts. In the left hand panel, calcium addition causes inactivation of mitotic CDK activity, and activates the licensing system. When S phase CDKs are activated the licensed DNA can replicate. In the right hand panel, 6-DMAP is added to the metaphase extract before calcium. The licensing system fails to become active, so no DNA replication takes place. B, Metaphase extracts were supplemented with Xenopus sperm and the indicated concentrations of roscovitine. The percentage of sperm heads assembled into interphase nuclei possessing an intact nuclear envelope was measured. C, Metaphase extracts were incubated for 15 min plus and minus calcium and roscovitine as indicated, before being used for a histone H1 kinase assay. Roscovitine concentrations were: 10, 20, 50, 100, 200, 500 and 1000 μM. D, Metaphase extracts were supplemented with 3 mM 6-DMAP or various concentrations of roscovitine prior to addition of 0.3 mM calcium (“metaphase + 6-DMAP + Ca” or “metaphase + rosco. + Ca”). Alternatively, 6-DMAP or roscovitine were added after calcium addition (“metaphase + Ca + 6-DMAP” or “metaphase + Ca + rosco.”). Xenopus sperm were then added to the extract, and after incubation for 20 min the chromatin was isolated and immunoblotted for Mcm7.
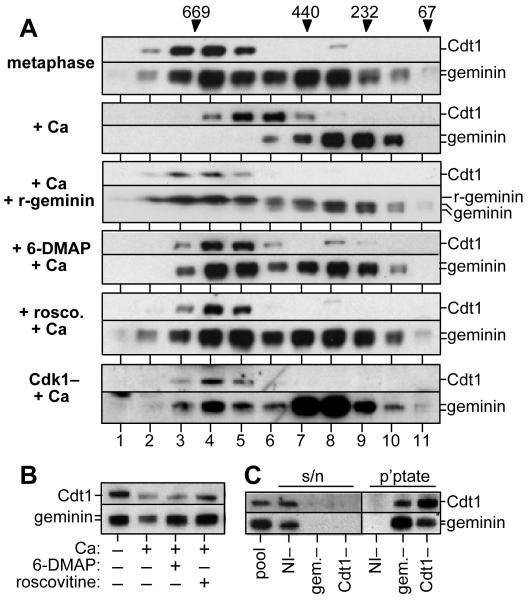
Metaphase extracts treated with 6-DMAP (“6-DMAP-treated extracts”) not only lack licensing activity but also contain an inhibitor of the Cdt1/RLF-B component of the licensing system11. It has recently been shown that the major inhibitor of licensing in Xenopus egg extracts is geminin, a specific inhibitor of Cdt1/RLF-B22-24,27. We therefore asked whether geminin is responsible for the licensing defect seen in metaphase extract treated with CDK inhibitors. Gel filtration of metaphase extracts showed two peaks of geminin (Fig 2A), a high molecular weight peak (fractions 3-5) consisting of complexes between geminin and Cdt1, and a low molecular weight peak (fractions 7-10) consisting of free geminin23. On exit from metaphase, a proportion of the geminin was degraded (Fig 2B), but the remaining geminin no longer formed a complex with Cdt1 (Fig 2A, “+Ca”)23. In order to determine whether the lack of complex formation was due to a change in Cdt1 or a change in geminin, we supplemented extract with excess recombinant geminin (gemininDEL)24 after release into interphase. The recombinant geminin complexed with all of the Cdt1 in the extract (Fig 2A). Previous experiments have shown that in early interphase, endogenous geminin is incapable of interacting with recombinant Cdt123. Together these results strongly suggest that in early interphase, endogenous Cdt1 is fully competent to bind to geminin and that the lack of complex formation is due to a defect in geminin’s Cdt1-binding ability.
Figure 2.
6-DMAP and roscovitine prevent release of geminin from Cdt1.
Metaphase extracts were supplemented with or without 3 mM 6-DMAP or 500 μM roscovitine or were immunodepleted of Cdk1, and were then supplemented with 0.3 mM CaCl2 and incubated for 15 min. Alternatively, metaphase extract was supplemented with 0.3 mM calcium, followed by addition of recombinant gemininDEL and incubation for 15 min. Metaphase extract without addition is also shown. A, Extracts were separated by gel filtration and fractions immunoblotted for Cdt1 and geminin. The migration of molecular weight markers (in kDa) is indicated above. ‘r-geminin’ indicates the migration of gemininDEL. B, Immunoblot of total Cdt1 and geminin in: 1, metaphase extract alone; 2, metaphase extract + calcium; 3, metaphase extract + 6-DMAP + calcium; 4, metaphase extract + roscovitine + calcium. Endogenous Xenopus geminin migrates as a doublet, presumably reflecting the existence of two closely related geminin genes (geminin-H and geminin-L24). C, Gel filtration fractions 3-5 from extract supplemented with 500 μM roscovitine prior to CaCl2 addition were pooled together. They were then immunodepleted with antibodies against geminin (“gem.-”), Cdt1 or non-immune antibodies (“NI-”). Immunodepleted extract was then separated by SDS PAGE and immunoblotted for geminin and Cdt1. The starting pool without any immunodepletion is also shown (“pool”).
We next examined the effect of CDK inhibitors on complex formation. When metaphase extracts were treated with 6-DMAP prior to calcium addition so as to block activation of the licensing system, geminin levels remained high (Fig 2B) and a significant proportion remained bound to Cdt1 (Fig 2A, “+6-DMAP+Ca”). A similar effect was seen when metaphase extracts were treated with roscovitine prior to calcium addition (Figs 2A and B, “+rosco.+Ca”). Immunodepletion of the high molecular weight fractions 3-5 confirmed that the geminin and Cdt1 were complexed together, since all the Cdt1 was co-depleted with anti-geminin antibodies (Fig 2C, “gem-”) and all the geminin was co-depleted with anti-Cdt1 antibodies (Fig 2C, “Cdt1-”). To confirm an essential role for CDKs in inactivating geminin, we immunodepleted metaphase extracts of Cdk1 before addition of calcium. Although some Cdt1 and geminin were co-depleted with the anti-Cdk1 antibodies (data not shown), the remaining Cdt1 was fully complexed to geminin (Fig 2B, “Cdk1- + Ca”). These Cdk1-depleted extracts were devoid of licensing activity, though licensing activity could be restored by addition of Cdt1 (data not shown). This result is consistent with previous observations showing that immunodepletion of Cdk1 (Cdc2) from metaphase-arrested Xenopus extracts blocks subsequent DNA replication29.
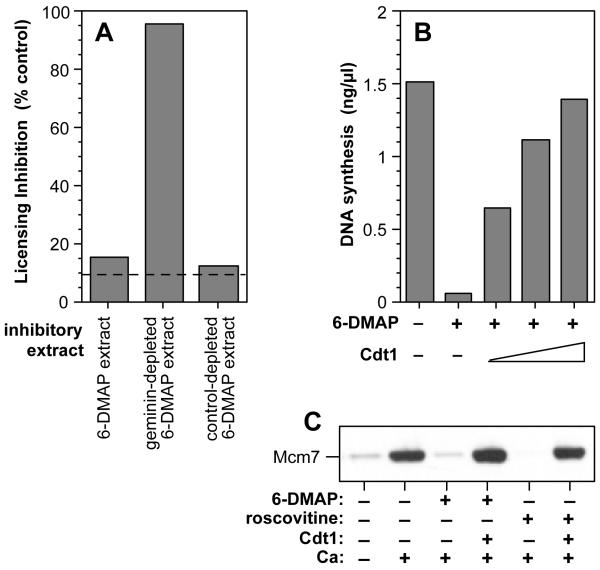
To address whether the presence of active geminin explains the ability of 6-DMAP-treated extracts to inhibit licensing, we performed a functional assay for licensing inhibitors. As expected from previous results11,23, 6-DMAP-treated extract inhibited the licensing activity of normal interphase extract (Fig 3A, ‘6-DMAP extract’). However, immunodepletion of geminin from the 6-DMAP-treated extract almost completely abolished its inhibitory activity, whilst control depletion with non-immune antibodies had no effect. This shows that geminin (or a geminin-associated protein) is the major licensing inhibitor in 6-DMAP-treated extract. Next, we supplemented 6-DMAP-treated extracts with recombinant Cdt1 to titrate out the inhibitory activity of geminin. Fig 3B shows that this completely restored the ability of 6-DMAP-treated extracts to support DNA replication. These results suggest that the only replication defect in 6-DMAP-treated extracts is the presence of active geminin.
Figure 3.
The only inhibitory activity in treated extracts is geminin.
A, Metaphase extract was supplemented with 3 mM 6-DMAP and then 0.3 mM calcium (“6-DMAP extract”). 6-DMAP extracts were immunodepleted with antibodies to geminin, or with non-immune antibodies. The resultant extracts were assayed for their ability to inhibit the licensing of interphase Xenopus extracts. Licensing is expressed as the % of that seen with no inhibitory extract. Dashed lines, background licensing of sperm nuclei incubated in buffer alone. B, Metaphase extract was supplemented with or without 3 mM 6-DMAP and then 0.3 mM calcium. Various concentrations of recombinant Cdt1 (1.8, 5.4 and 16 nM) were then added, and the extracts were assayed for their ability to replicate sperm nuclei. C, Metaphase extract was supplemented plus or minus 3 mM 6-DMAP or 500 μM roscovitine or 300 nM recombinant Cdt1, prior to addition of 0.3 mM calcium. Untreated metaphase extract is also shown. Sperm nuclei were then added to the extract, and after 20 min incubation chromatin was isolated and immunoblotted for Mcm7.
Analogous replication assays could not be performed with roscovitine-treated extracts because roscovitine, as well as inhibiting replication licensing, also inhibits CDK activity required for the initiation of DNA replication (ref 28 and data not shown). Instead, we investigated whether the addition of recombinant Cdt1 to licensing-defective extracts would restore their ability to load Mcm2-7 onto chromatin. Metaphase extracts were treated with 6-DMAP or roscovitine before addition of calcium, and as expected, these extracts did not load Mcm7 onto chromatin (Fig 3C). However, subsequent addition of recombinant Cdt1 to the extract induced efficient Mcm7 loading. Taken together, these results suggest that on exit from metaphase, CDKs have an essential function in inactivating geminin and activating the licensing system. This contrasts with other well-documented roles of CDKs in inhibiting the licensing system at other cell cycle stages1-3.
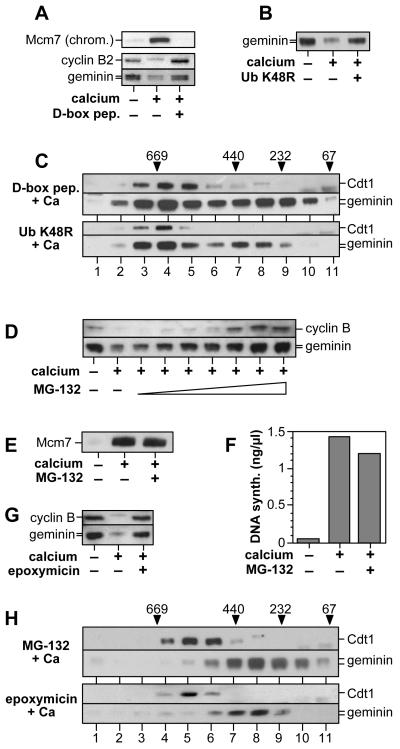
One known function of CDKs on exit from metaphase is the activation of the Anaphase Promoting Complex (APC/C)30,31, and it has been shown that addition of 6-DMAP to metaphase extracts prevents the APC/C-mediated proteolysis of cyclin B11,32. We therefore investigated whether inactivation of geminin is dependent on APC/C function. To do this we used a peptide representing the consensus APC/C-recognition sequence (D-box peptide) that is a competitive inhibitor of the APC/C33. Addition of the D-box peptide to metaphase extracts before addition of calcium blocked the degradation of cyclin B and geminin, and blocked the ability of the extracts to load Mcm7 onto chromatin (Fig 4A). Gel filtration of D-box peptide-treated extract showed Cdt1 and geminin still complexed together (Fig 4C). Thus the D-box peptide prevents the activation of the licensing system and the inactivation of geminin that normally occur on exit from mitosis. Similar results were obtained by supplementing extracts with a mutant form of ubiquitin (ubiquitin K48R) that prevents formation of polyubiquitin chains (Fig 4B, C). These results suggest that the ubiquitin ligase activity of the APC/C (which in turn is dependent on CDK activity) is essential for inactivating geminin on metaphase exit. The effect of ubiquitin K48R suggests that polyubiquitination via lysine 48 is likely to be required for geminin inactivation.
Figure 4.
Inhibition of the APC/C prevents activation of the licensing system.
Metaphase extracts were supplemented with D-box peptide (2 mM; A, C) or ubiquitin K48R (1.2 mM; B, C) then plus or minus 0.3 mM calcium as indicated. A, After incubation for 20 min, extracts were immunoblotted for cyclin B and geminin (bottom panels). Alternatively, sperm nuclei were incubated in the extracts for 20 min, chromatin was then isolated and immunoblotted for Mcm7 (top panel). B, After incubation for 20 min, extracts were immunoblotted for geminin. C, After incubation for 20 min extracts were gel filtered and immunoblotted for Cdt1 and geminin.
Proteins known to be ubiquitinated by the APC/C, such as cyclin B and securin, are subsequently degraded by the 26S proteasome. We therefore used MG-132, a potent inhibitor of the 26S proteasome, to investigate whether proteasome function is required for the inactivation of geminin. Although proteasome mediated degradation of cyclin B and geminin was inhibited by MG-132 (Fig 5A) it had no effect on the activation of the licensing system (Fig 5B) or on the inactivation of geminin (Fig 5E). Similar results were obtained with epoxymicin, another highly-specific proteasome inhibitor (Fig 5D, E). Remarkably, metaphase extracts treated with MG-132 were still able to support efficient DNA replication (Fig 5C); under these conditions Cdk1-cyclin B activity is inhibited by ubiquitination in the absence of proteolysis (ref 34 and data not shown; see also 35). Taken together, the results presented here suggest that whilst activity of the APC/C is essential for geminin inactivation, proteasome-mediated degradation is not.
Figure 5.
Proteasome-mediated proteolysis is not required for activation of licensing.
A, Metaphase extracts were supplemented with various concentrations of MG-132 (0, 0.51, 0.256, 1, 6, 32, 160, 800 μmol) and then plus or minus 0.3 mM calcium. After incubation for 20 min, extracts were immunoblotted for cyclin B and geminin. B, Metaphase extracts were supplemented plus or minus 800 μM MG-132 or 0.3 mM calcium. Sperm nuclei were then added, and after incubation for 20 min, chromatin was isolated and immunoblotted for Mcm7. C, Metaphase extracts were supplemented with or without 800 μM MG-132 or 0.3 mM calcium. Extracts were then assayed for their ability to replicate sperm nuclei. D, Metaphase extracts were supplemented with epoxymicin (500 μmol) and then plus or minus 0.3 mM calcium. After incubation for 20 min, extracts were immunoblotted for cyclin B and geminin. E, Metaphase extracts were supplemented with MG-132 (800 μM) or epoxymicin (500 μM) and then 0.3 mM calcium, after which they were gel filtered and immunoblotted for Cdt1 and geminin.
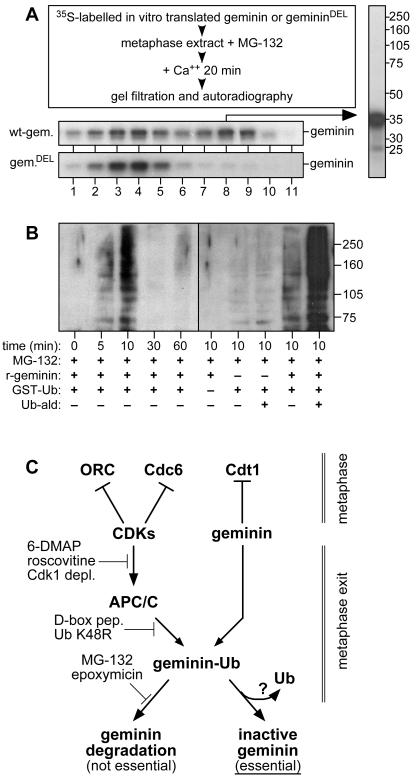
Geminin has previously been reported to be ubiquitinated by the APC/C on exit from metaphase24. Since we have shown that APC/C activity is essential for geminin inactivation, we investigated whether geminin itself is an essential APC/C substrate in this process. We prepared two forms of 35S-labelled recombinant geminin: the first wild-type and the second with a deletion of 9 amino acids in the APC/C recognition motif (gemininDEL) so that it is no longer a substrate for the APC/C24. In order to prevent degradation of the wild-type recombinant protein, proteolysis was inhibited with MG-132. Fig 6A shows that on incubation in Xenopus extract exiting from mitosis, about half of the recombinant wild-type geminin was inactivated so that it did not associate with Cdt1. In contrast, the gemininDEL mutant was completely refractory to inactivation. This suggests that APC/C-mediated ubiquitination of geminin is required for it to be inactivated.
No significant proportion of the inactive geminin seen in interphase extracts is itself ubiquitinated, as ubiquitination would cause an increase in molecular weight of >8 kDa that is clearly not observed (Figs 2, 4 and 5 and data not shown). Since geminin forms octomeric or decameric complexes (ref 23 and S. Shreeram, data not shown) we entertained the possibility that inactive complexes of geminin contain a mixture of both ubiquitinated and non-ubiquitinated forms. However, examination by SDS PAGE of gel filtration fractions containing 35S-labelled wild-type geminin in the inactive complex revealed no significant quantity of high molecular weight ubiquitinated geminin (Fig 6A, right panel).
Xenopus eggs contain high levels of isopeptidases that remove ubiquitin from proteins36, so an alternative explanation for these results is that geminin is only transiently ubiquitinated on exit from metaphase, but de-ubiquitination leaves geminin locked into an inactive conformation. To test this idea, metaphase extracts supplemented with GST-ubiquitin, recombinant geminin and MG-132 were released into interphase by calcium addition and incubated for various times. GST-tagged proteins were then collected on glutathione beads, separated by SDS PAGE and immunoblotted with anti-geminin antibodies. 5-10 minutes after calcium release, a ladder of high molecular weight geminin was detected (Fig 6B, left panel). The lowest band in this ladder was ~70 kDa, the expected size of geminin conjugated to GST-ubiquitin. By 30 min, this ladder had almost completely disappeared, suggesting that the geminin had been de-ubiquitinated (proteolysis having been blocked by MG-132). A similar ladder, though considerably fainter, was seen without addition of recombinant geminin, which suggests that the same reaction is taking place on endogenous geminin (Fig 6B, right panel). The intensity of the ladder was increased by addition of ubiquitin aldehyde, an inhibitor of deubiquitinating enzymes. Taken together, these results suggest that geminin is transiently ubiquitinated on exit from metaphase, but the ubiquitin is subsequently removed leaving the geminin in an inactive form.
Discussion
We have detailed here an unexpected role for CDKs in activating the licensing system in Xenopus extracts. As these extracts exit from metaphase, the licensing system is abruptly activated, and this requires geminin to no longer complex with Cdt1. We show that CDKs are required for this inactivation of geminin by a pathway that depends on the APC/C-mediated ubiquitination of geminin, but does not depend on proteasome-mediated proteolysis. Therefore inhibition of CDK activity in metaphase extracts prevents the activation of the licensing system that normally occurs on exit from metaphase. When the MPF activity of metaphase extracts is inhibited by rather non-specific CDK inhibitors such as 6-DMAP, activation of the licensing system is completely inhibited but the treated extracts can still efficiently replicate DNA templates that have previously been licensed9. The ability of 6-DMAP to inhibit MPF without inhibiting CDK activity required for the initiation of S phase (SPF activity37) may reflect the ability of 6-DMAP to also inhibit other kinases such as MAP kinases14 that play a role in maintaining metaphase arrest38. These results therefore explain how treatment of metaphase extracts with CDK inhibitors can provide a functional assay for licensing system components4,5,8-10. Reconstitution experiments based on this assay have shown that Xenopus sperm nuclei must be incubated with a combination of nucleoplasmin, ORC, Cdc6, Cdt1 and Mcm2-7 before the chromatin becomes competent to replicate when transferred to 6-DMAP-treated extract8,39.
The results presented here provide strong support for the model outlined in Fig 7. In metaphase-arrested Xenopus egg extracts, geminin binds and inhibits Cdt1, whilst active CDKs reduce the affinity of ORC and Cdc6 for chromatin. After calcium addition, the APC/C is activated and this activation requires ongoing CDK activity30-32. The active APC/C then polyubiquitinates geminin24. Some of the ubiquitinated geminin is degraded, but this is not essential for activation of the licensing system. In order for the licensing system to be activated, the remaining geminin must be inactivated. This occurs as a consequence of APC/C-mediated ubiquitination. The polyubiquitination of geminin is only transient, but after de-ubiquitination geminin remains in an inactive state. One possible way this could occur might be by a second covalent modification that is dependent on prior geminin ubiquitination. 2-dimensional gels indeed show many different geminin isoforms, though none seem to correlate precisely with activity (data not shown). Thus geminin joins an expanding collection of proteins whose activity is known to be regulated by ubiquitination independently of proteolysis40,41.
The inhibitory role of CDKs on replication licensing and DNA re-replication has been extensively documented1-3. In contrast, we show here that in Xenopus egg extracts CDKs are required to inactivate geminin on exit from metaphase, thus playing an essential positive role in replication licensing. There are, however, precedents for CDKs playing a positive role in this process. During Drosophila endocycles, Mcm2-7 are re-loaded onto DNA only as cyclin E levels rise at the start of each endocycle42. In cell free extracts prepared from human cells, the loading of Mcm2 onto DNA is stimulated by cyclin E/Cdk243. It is possible that these observations also reflect CDK-dependent ubiquitination and inactivation of geminin, since the function of other SCF-type ubiquitin ligases also depends on CDK activity. The ubiquitination-dependent inactivation of geminin may therefore be a widespread means of regulating the licensing system.
Materials and Methods
Preparation and use of egg extracts
Metaphase-arrested and interphase low speed supernatant Xenopus egg extracts were prepared as described44. All extracts were supplemented with 250 μg/ml cycloheximide, 25 mM phosphocreatine, 10 μg/ml creatine phosphokinase before use, and incubations were performed at 23°C. Xenopus sperm nuclei were demembranated with lysolecithin as described44 and frozen in aliquots in liquid nitrogen. For DNA synthesis experiments, sperm nuclei were incubated at a final concentration of 3 ng DNA/μl extract (~1,000 nuclei/μl), and extracts (typically 10 μl) were supplemented with 50 μCi/ml [α32P]dATP. DNA synthesis was assessed after 90 min incubation by TCA precipitation as described44. The histone H1 kinase assay was performed essentially as described45. Gel filtration of Xenopus extracts was performed as described23.
Immunodepletion of Cdk1 from extract was performed essentially as described44. Affinity-purified anti-Cdk1 antibody was coupled to protein A agarose beads at 15mg antibody/ml beads and washed in EDB-S buffer (50 mM Hepes, 50 mM KCl, 2 mM DTT, 0.4 mM MgCl2, 0.4 mM EGTA, 10% sucrose, 10 μg/ml each of leupeptin, pepstatin and aprotinin, pH 7.6). Metaphase-arrested extract was mixed with 0.2 volumes of antibody beads for 1 hr at 4°C, after which the beads were removed. The process was repeated two further times. Immunoblotting of the depleted extract showed no detectable Cdk1 or cyclin B. The depleted extract spontaneously assembled sperm DNA into interphase nuclei, consistent with the removal of MPF activity.
Recombinant protein and antibodies
Recombinant 6xHis-tagged Xenopus Cdt125 was produced as described8. 35S-labelled geminin was produced from pET28(a) plasmid DNA containing wild-type geminin or gemininDEL 24 using the TnT Coupled Reticulocyte Lysate System (Promega) according to the manufacturer’s instructions. Antibodies against Xenopus geminin, Cdt1 and Mcm7 were as previously described22,46. Antibody against Xenopus cyclin B2 was a kind gift of Tim Hunt. The D-box peptide (RRTALGDVTNKVSE) as described33 was a kind gift of Jim Hutchins and Paul Clarke. Antibody against Cdk1 was raised against a C-terminal peptide (CFDDLDKSSLPANQIRN) and was a kind gift of Johannes Walter and Tanya Prokhorova. Immunoblots were developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford IL) and pre-flashed film.
Chromatin isolation
For immunoblotting experiments, extracts were treated in various ways, then 20 μl aliquots were supplemented with demembranated sperm nuclei at a final concentration of 10 ng DNA/μl extract (~3,000 nuclei/μl). After 20 min incubation, each reaction was diluted in 500 μl NIBA (50 mM KCl, 50 mM Hepes KOH pH 7.6, 5 mM MgCl2, 2 mM DTT, 0.5 mM spermidine, 0.15 mM spermine, 2.5 mM Mg-ATP, 1 μg/ml each of leupeptin, pepstatin and aprotinin) supplemented with 0.1% Triton X-100 and underlayered with 100 μl of the same buffer containing 15% sucrose. The chromatin was pelleted at 6,000g in a swinging bucket rotor at 4°C followed by SDS PAGE and immunoblotting.
Licensing inhibition assay
Licensing inhibition assays were performed essentially as described23. Briefly, 1.5 μl fractions of interest were incubated with 0.5 μl interphase extract and 0.3 μl of sperm nuclei (80 ng DNA/μl) for 30 min. The degree of licensing was then assessed by addition of 6 μl interphase extract containing 1.5 ng/μl gemininDEL and [α32P]dATP and incubation for a further 90 min; total DNA synthesis was measured by TCA precipitation as described above.
Ubiquitination assay
20 μl metaphase arrested extract was supplemented with 800 μM MG-132 and 25 μg/ml GST-ubiquitin, 50 μg/ml ubiquitin-aldehyde, or 5 μg/ml recombinant wild-type geminin as appropriate. Extracts were released into interphase with 0.3 mM CaCl2 and incubated at 23°C. Reactions were stopped by dilution in 30 μl of ice cold Bead Binding Buffer (50 mM K phosphate, pH8, 0.05 % Tween 20) supplemented with 150 mM NaCl, 10 mM N-ethyl maleimide and 10 μl glutathione beads. After 30 min incubation on rotating wheel at 4° C the beads were washed three times in 200 μl Bead Binding Buffer containing 500 mM NaCl and once in 200 μl Bead Binding Buffer without NaCl. Beads were then resuspended in SDS gel loading buffer followed by SDS PAGE and immunoblotting for geminin.
Figure 6.
Geminin is only transiently ubiquitinated on exit from metaphase.
A, Recombinant 35S-labelled wild-type geminin or gemininDEL was incubated for 15 min in metaphase extract supplemented with 800 μM MG-132 before being released into interphase with 0.3 mM calcium; after a further 15 min incubation, extracts were gel filtered and fractions examined by SDS PAGE and fluorography (bottom panels). The entire lane containing fraction 8 of inactive 35S-labelled wild-type geminin is also shown, with the migration of molecular weight markers (kDa) to the right. B, Metaphase extract was supplemented with 800 μM MG-132 and then the indicated combinations of recombinant wild-type geminin, GST-ubiquitin and ubiquitin-aldehyde. At the indicated time, GST-conjugated proteins were isolated on glutathione beads, run on SDS PAGE and immunoblotted with anti-geminin antibodies. Molecular weight markers (kDa) are shown on the right.
Figure 7.
Positive and negative effects of CDKs on the licensing system.
In metaphase (top part of figure), CDKs inhibit the licensing system by reducing the affinity of ORC and Cdc6 for chromatin, whilst geminin inhibits Cdt1. On exit from metaphase (lower part of figure) CDKs activate the licensing system via APC/C-dependent ubiquitination of geminin. See text for further details.
Acknowledgments
Thanks to Conrad Nieduszynski, S. Shreeram and Anna Woodward for comments on the manuscript and to Johannes Walter and Tanya Prokhorova for anti-Cdk21 antibody. This work was supported by Cancer Research UK grants SP2385/0101 and C303/A3135.
References
- 1.Diffley JFX. Building the perfect switch. Current Biol. 2001;11:R367–370. doi: 10.1016/s0960-9822(01)00196-8. [DOI] [PubMed] [Google Scholar]
- 2.Blow JJ, Hodgson B. Replication licensing - defining the proliferative state? Trends Cell Biol. 2002;12:72–78. doi: 10.1016/s0962-8924(01)02203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishitani H, Lygerou Z. Control of DNA replication licensing in a cell cycle. Genes Cells. 2002;7:523–534. doi: 10.1046/j.1365-2443.2002.00544.x. [DOI] [PubMed] [Google Scholar]
- 4.Chong JP, Mahbubani HM, Khoo CY, Blow JJ. Purification of an MCM-containing complex as a component of the DNA replication licensing system. Nature. 1995;375:418–421. doi: 10.1038/375418a0. [DOI] [PubMed] [Google Scholar]
- 5.Kubota Y, Mimura S, Nishimoto S, Takisawa H, Nojima H. Identification of the yeast MCM3-related protein as a component of Xenopus DNA replication licensing factor. Cell. 1995;81:601–609. doi: 10.1016/0092-8674(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 6.Madine MA, Khoo C-Y, Mills AD, Laskey RA. MCM3 complex required for cell cycle regulation of DNA replication in vertebrate cells. Nature. 1995;375:421–424. doi: 10.1038/375421a0. [DOI] [PubMed] [Google Scholar]
- 7.Blow JJ, Laskey RA. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- 8.Gillespie PJ, Li A, Blow JJ. Reconstitution of licensed replication origins on Xenopus sperm nuclei using purified proteins. BioMed Central Biochem. 2001;2:15. doi: 10.1186/1471-2091-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blow JJ. Preventing re-replication of DNA in a single cell cycle: evidence for a replication licensing factor. J. Cell Biol. 1993;122:993–1002. doi: 10.1083/jcb.122.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubota Y, Takisawa H. Determination of initiation of DNA replication before and after nuclear formation in Xenopus egg cell free extracts. J. Cell Biol. 1993;123:1321–1331. doi: 10.1083/jcb.123.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahbubani HM, Chong JP, Chevalier S, Thömmes P, Blow JJ. Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J. Cell Biol. 1997;136:125–135. doi: 10.1083/jcb.136.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 13.Doree M, Hunt T. From Cdc2 to Cdk1: when did the cell cycle kinase join its cyclin partner? J. Cell Sci. 2002;115:2461–2464. doi: 10.1242/jcs.115.12.2461. [DOI] [PubMed] [Google Scholar]
- 14.Vesely J, et al. Inhibition of Cyclin-Dependent Kinases by purine analogs. Eur. J. Biochem. 1994;224:771–786. doi: 10.1111/j.1432-1033.1994.00771.x. [DOI] [PubMed] [Google Scholar]
- 15.Broek D, Bartlett R, Crawford K, Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991;349:388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- 16.Hayles J, Fisher D, Woollard A, Nurse P. Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2-mitotic B cyclin complex. Cell. 1994;78:813–822. doi: 10.1016/s0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 17.Dahmann C, Diffley J, Nasmyth K. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Current Biol. 1995;5:1257–1269. doi: 10.1016/s0960-9822(95)00252-1. [DOI] [PubMed] [Google Scholar]
- 18.Noton E, Diffley JFX. CDK inactivation is the only essential function of the APC/C and the mitotic exit network proteins for origin resetting during mitosis. Mol. Cell. 2000;5:85–95. doi: 10.1016/s1097-2765(00)80405-0. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen VQ, Co C, Li JJ. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature. 2001;411:1068–1073. doi: 10.1038/35082600. [DOI] [PubMed] [Google Scholar]
- 20.Saha P, et al. Human CDC6/Cdc18 associates with Orc1 and cyclin-cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol. Cell. Biol. 1998;18:2758–2767. doi: 10.1128/mcb.18.5.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen BO, Lukas J, Sorensen CS, Bartek J, Helin K. Phosphorylation of mammalian CDC6 by Cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999;18:396–410. doi: 10.1093/emboj/18.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tada S, Li A, Maiorano D, Mechali M, Blow JJ. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat. Cell Biol. 2001;3:107–113. doi: 10.1038/35055000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgson B, Li A, Tada S, Blow JJ. Geminin becomes activated as an inhibitor of Cdt1/RLF-B following nuclear import. Current Biol. 2002;12:678–683. doi: 10.1016/s0960-9822(02)00778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 25.Maiorano D, Moreau J, Mechali M. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature. 2000;404:622–625. doi: 10.1038/35007104. [DOI] [PubMed] [Google Scholar]
- 26.Nishitani H, Lygerou Z, Nishimoto T, Nurse P. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature. 2000;404:625–628. doi: 10.1038/35007110. [DOI] [PubMed] [Google Scholar]
- 27.Wohlschlegel JA, et al. Inhibition of eukaryotic replication by geminin binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- 28.Meijer L, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 29.Blow JJ, Nurse P. A cdc2-like protein is involved in the initiation of DNA replication in Xenopus egg extracts. Cell. 1990;62:855–862. doi: 10.1016/0092-8674(90)90261-c. [DOI] [PubMed] [Google Scholar]
- 30.Zachariae W, Nasmyth K. Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- 31.Peters JM. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- 32.Luca FC, Ruderman JV. Control of programmed cyclin destruction in a cell-free system. J. Cell Biol. 1989;109:1895–1909. doi: 10.1083/jcb.109.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peter M, et al. The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nat. Cell Biol. 2001;3:83–87. doi: 10.1038/35050607. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama A, et al. A nonproteolytic function of the proteasome is required for the dissociation of Cdc2 and cyclin B at the end of M phase. Genes Dev. 2000;14:2344–2357. doi: 10.1101/gad.823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahaffey DT, Gorbea C, Rechsteiner M. Evidence that DNA replication is not regulated by ubiquitin-dependent proteolysis in Xenopus egg extract. Exp. Cell Res. 2003;288:225–234. doi: 10.1016/s0014-4827(03)00088-0. [DOI] [PubMed] [Google Scholar]
- 36.Mahaffey D, Yoo Y, Rechsteiner M. Ubiquitin metabolism in cycling Xenopus egg extracts. J. Biol. Chem. 1993;268:21205–21211. [PubMed] [Google Scholar]
- 37.Blow JJ. Control of chromosomal DNA replication in the early Xenopus embryo. EMBO J. 2001;20:3293–3297. doi: 10.1093/emboj/20.13.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tunquist BJ, Maller JL. Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev. 2003;17:683–710. doi: 10.1101/gad.1071303. [DOI] [PubMed] [Google Scholar]
- 39.Gillespie PJ, Blow JJ. Nucleoplasmin-mediated chromatin remodelling is required for Xenopus sperm nuclei to become licensed for DNA replication. Nucleic Acids Res. 2000;28:472–480. doi: 10.1093/nar/28.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bach I, Ostendorff HP. Orchestrating nuclear functions: ubiquitin sets the rhythm. Trends Biochem. Sci. 2003;28:189–195. doi: 10.1016/S0968-0004(03)00055-0. [DOI] [PubMed] [Google Scholar]
- 41.Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J. Biol. Chem. 2003;278:35857–35860. doi: 10.1074/jbc.R300018200. [DOI] [PubMed] [Google Scholar]
- 42.Su TT, O’Farrell PH. Chromosome association of minichromosome maintenance proteins in Drosophila endoreplication cycles. J. Cell Biol. 1998;140:451–460. doi: 10.1083/jcb.140.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coverley D, Laman H, Laskey RA. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat. Cell Biol. 2002;4:523–528. doi: 10.1038/ncb813. [DOI] [PubMed] [Google Scholar]
- 44.Chong JP, Thömmes P, Rowles A, Mahbubani HM, Blow JJ. Characterization of the Xenopus replication licensing system. Methods Enzymol. 1997;283:549–564. doi: 10.1016/s0076-6879(97)83043-1. [DOI] [PubMed] [Google Scholar]
- 45.Strausfeld UP, et al. Both cyclin A and cyclin E have S-phase promoting (SPF) activity in Xenopus egg extracts. J. Cell Sci. 1996;109:1555–1563. doi: 10.1242/jcs.109.6.1555. [DOI] [PubMed] [Google Scholar]
- 46.Prokhorova TA, Blow JJ. Sequential MCM/P1 subcomplex assembly is required to form a heterohexamer with replication licensing activity. J. Biol. Chem. 2000;275:2491–2498. doi: 10.1074/jbc.275.4.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]