Abstract
Curcumin exhibits anti-inflammatory and antitumor activity and is being tested in clinical trials as a chemopreventive agent for colon cancer. Curcumin's chemopreventive activity was tested in a transgenic mouse model of lung cancer that expresses the human Ki-rasG12C allele in a doxycycline (DOX) inducible and lung-specific manner. The effects of curcumin were compared with the lung tumor promoter, butylated hydroxytoluene (BHT), and the lung cancer chemopreventive agent, sulindac. Treatment of DOX-induced mice with dietary curcumin increased tumor multiplicity (36.3 ± 0.9 versus 24.3 ± 0.2) and progression to later stage lesions, results which were similar to animals that were co-treated with DOX/BHT. Microscopic examination showed that the percentage of lung lesions that were adenomas and adenocarcinomas increased to 66% in DOX/BHT, 66% in DOX/curcumin and 49% in DOX/BHT/curcumin-treated groups relative to DOX only treated mice (19%). Immunohistochemical analysis also showed increased evidence of inflammation in DOX/BHT, DOX/curcumin and DOX/BHT/curcumin mice relative to DOX only treated mice. In contrast, co-treatment of DOX/BHT mice with 80 p.p.m. of sulindac inhibited the progression of lung lesions and reduced the inflammation. Lung tissue from DOX/curcumin-treated mice demonstrated a significant increase (33%; P = 0.01) in oxidative damage, as assessed by the levels of carbonyl protein formation, relative to DOX-treated control mice after 1 week on the curcumin diet. These results suggest that curcumin may exhibit organ-specific effects to enhance reactive oxygen species formation in the damaged lung epithelium of smokers and ex-smokers. Ongoing clinical trials thus may need to exclude smokers and ex-smokers in chemopreventive trials of curcumin.
Introduction
Lung cancer is the leading cause of cancer-related deaths in the USA (1). Despite decades of research and the recent development of novel therapeutic approaches, the 5 year survival rate of lung cancer patients has remained an abysmal 10–15%. This is mainly due to the fact that patients often present with late stage disease. Thus, the development of chemopreventive strategies that could prevent the progression of lung lesions to malignant cancers would reduce the mortality and morbidity resulting from this deadly disease.
The population of tobacco smokers and ex-smokers constitutes a readily identifiable group of individuals at risk for lung cancer who would benefit from intervention with chemopreventive regimens. While non-steroidal anti-inflammatory agents have received considerable attention as potential chemopreventive agents in a variety of organ systems, recent concerns regarding their gastrointestinal and cardiac toxicities make use of these compounds in the disease-free, aysmptomatic smoking population problematic (2,3). As such, current efforts have focused on identifying less toxic agents that could provide chemopreventive activity without life-threatening toxicities. One such compound is curcumin (diferuloylmethane), a naturally occurring plant polyphenol, that has been shown to exhibit anti-inflammatory, antioxidant and chemopreventive properties with minimal toxic side effects (4–6).
In conjunction with its antioxidant and anti-inflammatory properties, curcumin inhibits a number of transcription factors and pro-oxidant enzymes implicated in lung tumor progression. Curcumin has been shown to inhibit activation of the nuclear factor κβ (NF-κβ) pathway and cycloxygenase-2 (COX-2) enzyme activity in a variety of tumor cell lines, including ovarian (7), lymphoma and thymoma (8), myeloid (9), prostate (10), breast (11) and colorectal (12,13) cells. A number of in vivo studies conducted in rodent models have shown that curcumin exhibits potent chemopreventive activity in different organ systems, including colon (14–16), breast (17,18) and skin (19,20). In addition, epidemiological studies (21,22) have provided evidence that the low incidence of colorectal cancers observed in India was associated with diets high in curcumin.
As a result of these preclinical studies, curcumin has entered clinical trials with the goal to test this compound's efficacy in the prevention of colon cancer in high-risk patients (23,24). Because of its relatively low absorption (25–27), it has been assumed that curcumin's effects would be limited primarily to the gastrointestinal tract. Indeed, one study conducted with A/J mice co-treated with benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone suggested that curcumin had no effect on lung tumor multiplicity in vivo (28), although its effects on tumor histology were not reported in this study. However, a recent study demonstrated that pretreatment with dietary curcumin prevented the initiation of lung tumors by benzo[a]pyrene as a result of curcumin's effects on phase I and phase II enzyme levels in liver and lung tissue (29). These results suggest that curcumin can exert effects on lung tissue despite its poor absorption.
As curcumin has been shown to prevent breast tumor progression, we initiated studies to determine the potential chemopreventive activity of curcumin in a transgenic lung tumor mouse model developed in our laboratory. These mice utilize the inducible tetracycline-on system to express the mutant human Ki-rasG12C gene in a lung-specific manner (30). Transgenic mice were developed that contain the cDNA of Ki-rasG12C cloned downstream of a tetracycline-inducible promoter. These mice are then crossed to a second strain of transgenic mice containing a reverse tetracycline trans-activator (rtTA) protein linked to the Clara cell secretory protein (CCSP) promoter, resulting in constitutive expression of the rtTA gene product specifically in the lungs (31). Treatment of these double transgenic, or bitransgenic, mice (designated CCSP-rtTA/Ki-rasG12C mice) with doxycycline (DOX) induces transcription of the Ki-rasG12C gene specifically in the lung. Previous work by this laboratory has shown that administration of DOX for 9–12 months resulted predominantly in the development of benign hyperplastic foci and adenomas (ADs) (32). Interestingly, these lesions remained small (the majority were ≤1 mm), benign and well circumscribed, indicating that they failed to progress to more advanced stage lesions. The benign lung lesions exhibited increased levels of both proliferative and antimitotic signaling, which likely accounted for the benign phenotype observed (32,33).
In the current study, we demonstrate that curcumin unexpectedly promoted the progression of lung lesions from benign hyperplasias to ADs and carcinomas, similar to the known lung tumor promoter butylated hydroxytoluene (BHT) (34–36). Furthermore, we provide evidence that after 1 week of curcumin administration in the diet, lung tissue demonstrated enhanced levels of oxidative damage. This early pro-oxidant effect may account for the tumor-promoting effects of curcumin in lung tissue.
Materials and methods
Animal treatment and histological analysis of lungs
All mice used in this study were on a FVB/N background. CCSP-rtTA/Ki-rasG12C bitransgenic and Ki-rasG12C monotransgenic control mice were treated with or without DOX and either vehicle (olive oil) or BHT. DOX was given in the drinking water (500 μg/ml) beginning 8 weeks after birth. BHT was administered 1 week after initiation of DOX treatment and consisted of six weekly intraperitoneal injections of 150 mg/kg of BHT in olive oil. Control mice received olive oil vehicle at a dose of 0.5 ml/25 gm. Separate groups of mice were fed either the chemopreventive agent sulindac at a dose of 80 p.p.m. or 4000 p.p.m. of curcumin starting 2 days after the initiation of DOX. The dose of curcumin that we employed is twice that utilized in a previous lung chemoprevention study (28) and well within the range of doses employed in other in vivo models and human clinical trials as reviewed recently in Goel et al. (37); the dose for sulindac has been shown to be effective in a previous lung chemoprevention study (38). Curcumin or sulindac containing AIN-76A diets were formulated by the Wake Forest University Diet Core Laboratory.
For the long-term experiments to assess the effects of curcumin on lung tumorigenesis, mice were euthanized 45 weeks (9 months) following the initiation of DOX treatment by CO2 asphyxiation and cervical dislocation. The lungs were removed, carefully examined for pulmonary masses and all macroscopic pulmonary lesions were recorded. The lung was processed for histopathology and immunohistochemistry (IHC) by fixation for 24 h in 4% chilled paraformaldehyde fixative and stored in 70% ethanol until embedding in paraffin. Tissue slices cut 4 μ thick were stained with hematoxylin and eosin and proliferative lesions classified by standard murine pulmonary tumor characteristics (39). One representative slide was cut from 12 individual mice in each group to assess tumor pathology. The study included the following treatment groups: (i) bitransgenic mice receiving no treatment (n = 27; 10♂, 17♀); (ii) bitransgenic mice treated with DOX (n = 19; 15♂, 4♀); (iii) bitransgenic mice treated with BHT (n = 14; 9♂, 5♀); (iv) bitransgenic mice treated with DOX and BHT (n = 20; 9♂, 11♀); (v) bitransgenic mice treated with DOX, BHT and curcumin (n = 20; 11♂, 9♀); (vi) bitransgenic mice treated with DOX and curcumin (n = 15; 11♂, 4♀); (viii) bitransgenic mice treated with DOX, BHT and sulindac (n = 26; 20♂, 6♀); (xi) bitransgenic mice treated with DOX and sulindac (n = 16; 10♂, 6♀); (x) monotransgenic Ki-rasG12C mice receiving no treatment (n = 16; 10♂, 6♀); (xi) monotransgenic mice treated with DOX (n = 14; 8♂, 6♀) and (xii) FVB/N mice that were not treated (n = 17; 7♂, 10♀).
Determination of oxidative damage by protein carbonylation
Eight CCSP-rtTA/Ki-rasG12C bitransgenic mice were treated with DOX as described above. The mice were divided into two groups of four mice each; one group was fed the normal diet and the other fed diet supplemented with 4000 p.p.m. of curcumin. Nine days after the initiation of DOX treatment, mice were euthanized as described above. The lungs were removed and homogenized in a Tissue Terra for 20 s at the highest setting using digestion buffer from the Millipore Protein Extraction Kit (Millipore, Billerica, MA). Samples were processed according to the manufacturer's instructions and stored at −80°C until use. Five microliters (0.13–2.2 mg) of supernatant were denatured and derivatized with 2,4-dinitrophenylhydrazine using the OxyBlot™ Protein Oxidation Detection Kit (Chemicon, Temecula, CA). Proteins were separated on duplicate 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, transferred onto nitrocellulose membranes and incubated with a primary antibody to either the DNP moiety of the protein or β-actin (Abcam, Cambridge, MA) followed by a secondary horseradish peroxidase-conjugated antibody. Proteins were visualized with the enhanced chemiluminescence system (GE Healthcare, Pistacaway, NJ) and quantified using Image J software. Curcumin-mediated increases in protein oxidation were determined by scanning the entire lane. Multiple exposures were used to assure that intensities were measured in the linear range of the film. Changes in protein oxidation were expressed as percentage change in intensity of the DNP signal for DOX/curcumin-treated samples relative to DOX only treated samples. Signal intensities for the DNP antibody were normalized to β-actin by dividing the absorbance obtained with for the DNP signal by the absorbance for β-actin in each individual lane.
IHC
IHC was performed following the standard procedure provided by Cell Signaling Technology (Danvers, MA). Primary antibodies are as follows: rabbit antimouse NF-κβ p65 1:200 (Thermo Scientific, Waltham, MA; cat. # RB-10612-P1) and rabbit antimouse COX-2 1:150 (Cayman Chemical, Ann Arbor, MI; cat. #160126). Samples as well as negative controls (which lacked the primary antibody) and positive control-tissue specific for each antibody were incubated overnight at 4°C and then washed 3× for 5 min with 1× PBS. Tissues were stained with biotinylated goat antirabbit antibody (Vector Laboratories, Burlingame, CA). Slides were then counterstained with hematoxylin. Slides from four to six individual mice from each group were stained with each antibody.
Statistical analysis
Tumor incidence was calculated from the proportion of mice that had one or more tumors from the total number of mice. Chi-square or Fisher's exact tests were used to test for differences in tumor incidence between groups. The distribution of tumor multiplicity was determined graphically and descriptively for skewness and normality. Analysis of variance was used to test for significant differences in tumor multiplicity. Post hoc pairwise comparisons were made using Tukey's honestly significant differences. All analyses were performed using SAS v9.1.3 (SAS Institute, Cary, NC). A two-sided P-value <0.05 was considered statistically significant.
Results
We utilized a transgenic mouse model exhibiting DOX-inducible regulation of the mutant human Ki-rasG12C allele in lung tissue to determine the potential chemopreventive effects of curcumin on lung tumor progression in early stage lesions. Previous studies from our laboratory demonstrated that treatment of CCSP-rtTA/Ki-rasG12C mice with DOX for 9–12 months resulted in the development of hyperplastic foci and small (≤1 mm), benign, well-differentiated ADs that did not progress to more severe tumor phenotypes (32). Analysis of signaling events downstream of RAS revealed that mutant RASCYS12 signaled to a subset of its downstream effectors, as we saw increased levels of extracellular signal-regulated kinases (ERK) and p38 phosphorylation but no activation of the AKT and JUN kinase (JNK) pathways (32,33). This appears to account for the benign phenotype of the lung lesions. We compared the effects of curcumin, which is currently in clinical trials as a chemopreventive agent for colon cancer, to the known lung tumor promoter BHT and the known lung cancer chemopreventive agent sulindac in order to validate the bitransgenic model for use in chemopreventive studies. BHT mediates its tumor-promoting effects by creating an inflammatory environment in the lung (35,36), whereas sulindac inhibits several mediators of oxidative stress (40,41).
Co-treatment of bitransgenic mice with DOX and BHT resulted in a statistically significant (P = 0.01) increase in tumor multiplicity, as assessed by counting the number of visible surface tumors, in the DOX/BHT animals compared with the DOX only treated group (Figure 1). Introduction of curcumin into the diets of DOX and DOX/BHT-treated mice caused similar increases in lung tumor multiplicity as observed in mice treated with DOX/BHT (Figure 1). Indeed, much to our surprise, there were no statistically significant differences between the DOX/BHT treated and DOX/BHT/curcumin and DOX/curcumin-treated bitransgenic mice (analysis of variance; P = 0.6), indicating that curcumin exhibited tumor promoter activity comparable with that seen with BHT. Only five lung lesions were detected among the control groups—one lesion was detected in two individual untreated bitransgenic mice, one lesion was detected in two different DOX-treated monotransgenic Ki-rasG12C mice and one lesion was detected in a BHT-treated bitransgenic mouse that did not receive DOX. The only significant difference in body weights between the various treatment groups was a 1.3-fold increase in body weight of DOX/curcumin male-treated mice relative to the other treatment groups at euthanasia.
Fig. 1.
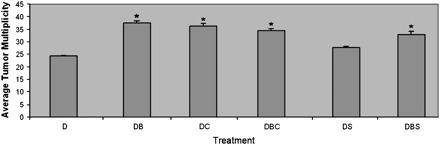
Average tumor multiplicity of mice at 45 weeks after the initiation of DOX treatment. The average tumor multiplicity for each treatment group is expressed as number of tumors per mouse/total number of mice with tumors ±SE. DOX only treated group (D), DOX/BHT co-treated group (DB), DOX/BHT/sulindac co-treated group (DBS), DOX/sulindac co-treated group (DS), DOX/BHT/curcumin co-treated group (DBC) and DOX/curcumin co-treated group (DC). *Denotes statistical significance from DOX-treated group, where P-value is <0.05.
Microscopic examination of lung sections from 12 individual mice for each treatment group revealed that there was an increase in adenoma (62 ± 7%, 61 ± 8% and 47 ± 10%) and large atypical adenoma, defined as ADs with a small focus of more anaplastic looking cells, and adenocarcinoma development (4, 5 and 2%) in the DOX/BHT, DOX/curcumin and DOX/BHT/curcumin-treated groups, respectively, compared with the DOX only treated group, where only 19 ± 12.4% of lesions were classified as ADs (Figure 2). In contrast, while co-treatment of DOX/BHT mice with 80 p.p.m. of the non-steroidal anti-inflammatory agent sulindac in the diet had little or no effect on the average lung tumor multiplicity of the DOX/BHT bitransgenic mice after 9 months, sulindac prevented tumor progression. As shown in Figure 2, distribution of hyperplastic foci and ADs in DOX/BHT/sulindac and DOX/sulindac-treated mice resembled that seen in DOX only treated mice. Sulindac has been shown to inhibit lung tumor progression in a variety of murine models (40,41). Thus, the CCSP-rtTA/Ki-rasG12C mice respond appropriately to known lung tumor-promoting and chemopreventive agents.
Fig. 2.

Percent microscopic lesions grouped by tumor stage and treatment group. Percent proliferative lesions are expressed as the total number of lesions for each histological type within each treatment group/total number of proliferative lesions for each treatment group multiplied by 100 ± SE. The percentage for each microscopic lesion type is shown for hyperplasias (H), AD and large AD with atypical features and/or carcinomas (C) for the DOX (D), DOX/sulindac (DS), DOX/BHT (DB), DOX/BHT/sulindac (DBS), DOX/curcumin (DC) and DOX/BHT/curcumin (DBC) treatment groups. *Denotes statistical significance between hyperplasias (H), ** AD and *** carcinomas (C) when compared with the DOX-treated group.
Photomicrographs of representative lung sections are shown in Figure 3. Figure 3A shows lung tissue from normal lung and demonstrates the lack of proliferative lesions or atypia in control lung tissue. Upon treatment of the CCSP-rtTA/Ki-rasG12C bitransgenic mice with DOX, we noted the development of hyperplastic lesions within the lungs of treated animals (Figure 3B), consistent with our previous observations (32). Figures 3C–E demonstrate the severe proliferative lesions seen in DOX/BHT, DOX/curcumin and DOX/BHT/curcumin bitransgenic animals.
Fig. 3.
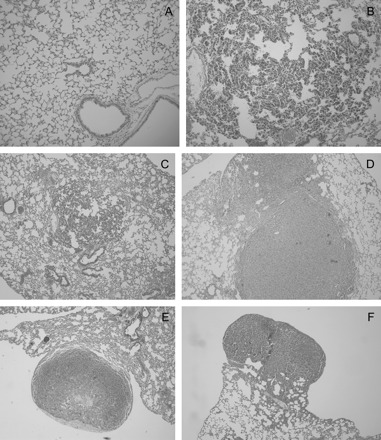
Photomicrographs of mouse lung tissue. Hematoxylin and eosin stains of tissue from control and treated mice. (A) Normal lung with typically thin alveolar walls. (B) Diffuse pneumocyte hyperplasia in a mouse treated with DOX. Alveolar walls are thickened by plump, closely spaced pneumocytes. (C) Central focus of pneumocyte hyperplasia in a mouse treated with DOX. Alveolar walls are thickened about twice normal by plump, closely spaced pneumocytes. More normal lung is present adjacent. (D) Two solid coalescing AD in a mouse treated with DOX/BHT. Cells form dense sheets which expand the adjacent parenchyma. (E) Subpleural adenoma in a mouse treated with DOX/curcumin. The central area of pallor reflects necrosis. (F) Subpleural adenoma with papillary morphology in a mouse treated with DOX/BHT/curcumin. Panels A and B are ×10 and panels C–F are ×4 magnification.
Four to six representative slides from individual mice in each group were examined for evidence of leukocyte infiltration by hematoxylin and eosin staining and expression of COX-2 and NF-κβ by IHC. As shown in Figure 4, lung tissue isolated from DOX-treated mice exhibited mild hyperplasia with little evidence of inflammation (panels A and B). There was minimal staining for either COX-2 or NF-κβ. In contrast, tumors isolated from DOX/BHT, DOX/curcumin and DOX/BHT/curcumin-treated mice (Figures 5, 6 and 7, respectively) exhibited inflammation with a high density of macrophages (cells with black dots visible in Figure 6, panel B for example) which also appeared to be positive for staining for NF-κβ (Figures 5D, 6D and 7D), whereas elevated COX-2 expression was seen in the endothelial cells in the tumor compartment (Figures 5C, 6C and 7D). The inflammation appeared to be somewhat more severe in DOX/curcumin versus DOX/BHT-treated mice (Figure 6A and B and Figure 5A and B, respectively) and was the most severe in mice treated with DOX/BHT/curcumin. Mice treated with DOX/BHT/sulindac exhibited milder inflammation, being similar to that seen in DOX/BHT mice (data not shown).
Fig. 4.

Hematoxylin and eosin and immunohistochemical staining of lung tissue from DOX-treated mice. Panels (A) and (B) are ×10 and ×40 magnifications, respectively, of hematoxylin and eosin-stained slides showing relatively normal-appearing tissue with evidence of some hyperplasia. Panels (C) and (D) are immunohistochemical staining of lung tissue slides with antibodies to COX-2 and NF-κβ (Ser276) showing little staining for either protein in the tissues; magnification for IHC ×40.
Fig. 5.
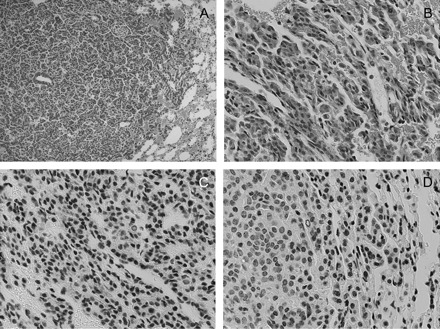
Hematoxylin and eosin and immunohistochemical staining of lung tissue from DOX/BHT-treated mice. Panels (A) and (B) are ×10 and ×40 magnifications, respectively, of hematoxylin and eosin-stained slides showing mild edema around the tumor; panels (C) and (D) are immunohistochemical staining of lung tissue slides with antibodies to COX-2 and NF-κβ (Ser276) showing COX-2 and NF-κβ positivity in blood vessels and stromal cells, respectively; magnification for IHC ×40.
Fig. 6.
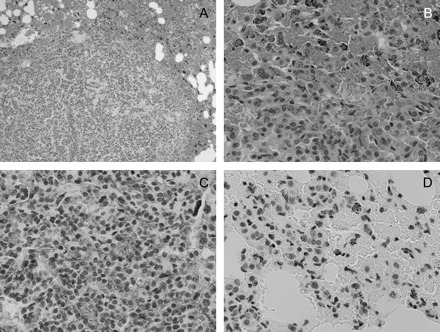
Hematoxylin and eosin and immunohistochemical staining of lung tissue from DOX/curcumin-treated mice. Panels (A) and (B) are ×10 and ×40 magnifications, respectively, of hematoxylin and eosin-stained slides showing marked inflammation around the tumors and a high density of red blood cells suggestive of edema (panel A) with infiltration of macrophages (panel B, cells with black dots in panel D). (C) Immunohistochemical staining of lung tissue slides with an antibody to COX-2. The tumor has a high-density vasculature that contains endothelial cells staining positively for COX-2. (D) Immunohistochemical staining of lung tissue slides with an antibody to NF-κβ (Ser276) demonstrating NF-κβ positive macrophages infiltrating the tumor tissue; magnification for IHC ×40.
Fig. 7.
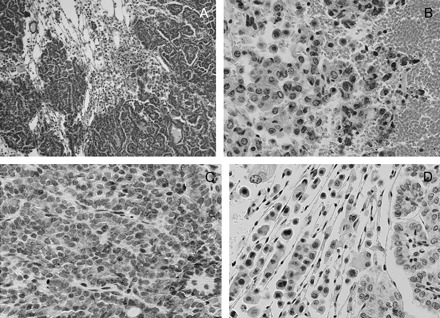
Hematoxylin and eosin and immunohistochemical staining of lung tissue from DOX/BHT/curcumin-treated mice. Panels (A) and (B) are ×10 and ×40 magnifications, respectively, of hematoxylin and eosin-stained slides showing severe inflammation and leukocyte infiltration of the tumor. Panels (C) and (D) are immunohistochemical staining of lung tissue slides with antibodies to COX-2 and NF-κβ (Ser276) showing COX-2 and NF-κβ positivity in blood vessels, stromal and immune cells in the tumor microenvironment; magnification for IHC ×40.
Based on the marked inflammation we observed in the tumor tissues with curcumin treatment and results obtained in the β-carotene chemoprevention trials, where patients in the trial exhibited higher incidences of lung cancer (42,43) that have been attributed to the pro-oxidant effects of β-carotene in the lungs of smokers (44), we hypothesized that curcumin may similarly exhibit pro-oxidant effects in the highly oxygenated environment of the lungs. To this end, we assessed the pro-oxidant effects of dietary curcumin exposure using a protein carbonylation assay as a biomarker for oxidative damage to proteins in the lungs of Ki-ras-expressing mice. We examined the effects of curcumin after 1 week of dietary exposure as any observed effects could not be attributed to transient effects of curcumin but at the same time the exposure would be very early in the tumorigenic process. In addition, the levels of mutant Ki-ras expression would be near maximal levels by 1 week of DOX treatment (32). After 9 days of DOX treatment and 1 week of dietary curcumin exposure, we found that DOX/curcumin-treated mice exhibited a 33% increase (P = 0.010) in the levels of modified protein relative to DOX-treated mice on the control diet (Figure 8). These results suggest that, in the lungs of smokers, curcumin may increase the formation of reactive oxygen species to enhance tumor progression as a result of the curcumin-mediated oxidative damage to additional cellular macromolecules that act in concert with the genetic damage already prevalent in the lungs of smokers and ex-smokers.
Fig. 8.

Curcumin-mediated increase in oxidative damage in lung tissue. CCSP-rtTA/Ki-rasG12C bitransgenic mice were treated with 500 μg/ml of DOX in the drinking water. Two days after the initiation of DOX treatment, mice were kept on the normal diet or were fed diet supplemented with 4000 p.p.m. of curcumin. Nine days after the initiation of DOX treatment, the mice were euthanized and the lungs removed and homogenized in digestion buffer. Five microliters (0.13–2.2 mg) of supernatant were denatured and derivatized with 2,4-dinitrophenylhydrazine (DNPH), the derivatized proteins separated on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels, transferred onto a nitrocellulose membrane and incubated with a primary antibody to either the DNP moiety of the protein or β-actin followed by a secondary horseradish peroxidase-conjugated antibody. The upper bands in the blot are non-specific binding that occurs when 2,4-dinitrophenylhydrazine-treated lysates are probed with the antibody to β-actin.
Discussion
Because of its potent anti-inflammatory and antioxidant activities, curcumin has entered clinical trials as a potential chemopreventive agent for colon cancer (23,24). In this study, we compared the effects of curcumin to the known tumor promoter, BHT, and known lung tumor chemopreventive agent, sulindac, to determine its effects on lung tumor progression in a conditionally expressed, RAS-driven transgenic mouse model for the early stages of lung cancer. These mice, when treated with DOX, develop small, benign lung lesions that do not appear to progress, at least over the 12 month observation period, and thus are representative of the asymptomatic smoker or ex-smoker who harbors genetic damage to their lung epithelial cells but have not progressed to frank carcinoma (32,33,45). As expected, treatment with BHT resulted in increased tumor multiplicity (as determined by counting visible surface tumors) and enhanced tumor progression (as determined by histological analysis of sectioned slides) whereas co-treatment of DOX/BHT mice with sulindac reversed the histological alterations induced by BHT, demonstrating that this transgenic model accurately can determine the promoter and chemoprevention activities of chemicals identified in other mouse models (34–36,40,41).
Results of this study suggest that, in the lung, curcumin exerts tumor-promoting activity. Curcumin caused a statistically significant increase in tumor multiplicity and a clear shift from the predominantly hyperplasia development typically seen in the DOX only treated animals to larger atypical ADs and carcinomas in the DOX/curcumin and DOX/BHT/curcumin-treated animals, similar to the results obtained when DOX was administered to the bitransgenic mice in conjunction with BHT. In addition, histopathological examination of the tumors indicated that, relative to DOX-treated mice, co-treatment with DOX/BHT, DOX/curcumin or DOX/BHT/curcumin resulted in the attraction of leukocytes to the tumor and an increase in inflammation, probably resulting in an increase in oxidative stress in the tumor microenvironment.
Interestingly, co-administration of the two agents did not cause additive or synergistic effects in terms of increase in either tumor multiplicity or tumor progression, suggesting that the two agents may act via similar mechanisms. However, co-treatment of mice with DOX/BHT/curcumin clearly caused a more severe inflammatory response. Given these findings and the fact that BHT mediates its lung tumor-promoting effects through an inflammatory process, resulting in a pro-oxidant environment and the generation of reactive oxygen species, we examined the early effects of curcumin on lung tissue using a protein carbonylation assay to measure the levels of oxidative damage. We used protein carbonylation as a marker for oxidative stress in the lung environment. Our results demonstrated that after 1 week of curcumin exposure in the diet, mice exhibited an increased level of proteins modified by oxidative damage. Thus, the curcumin-mediated increase in reactive oxygen species probably predisposes lung tissue to increased genomic instability and tumor progression. One could envision several oncogenic and tumor suppressor pathways that could be affected by the increase in reactive oxygen species, including signal transduction pathways related to mitogen-activated protein kinase kinases, NF-κβ and p53 (which are all redox sensitive) in addition to the potential mutations and genetic damage to genes in this pathway as a result of the increased oxidative stress in the tumor microenvironment. Indeed, our IHC analyses indicated elevated levels of NF-κβ in tumor stromal tissue and COX-2 in surrounding endothelial cells. In this regard, curcumin has been shown to interfere with the activity of the p53 tumor suppressor pathway (46). While most carcinogenicity studies conducted in mice and rats have failed to establish a relationship between tumorigenesis and administration of curcumin (47,48), in one study (49) curcumin was shown to cause hyperplasia of the mucosal epithelium in the cecum and colon of male and female rats, an increased incidence of hepatocellular adenoma in male and female mice, a significant increase in thyroid gland follicular cell hyperplasia in female mice and a small but significant increase in sister chromatid exchanges and chromosomal aberrations in cultured Chinese hamster ovary cells.
A number of studies have shown that the antioxidant effects of curcumin are preceded by an oxidative stimulus, which is time and dose dependent (48,50–52). These findings support the premise that curcumin, under some conditions, could have adverse effects on cellular macromolecules. A shortcoming of the clinical development of many cancer chemopreventive agents has been the lack of knowledge of the relationship between markers of pharmacologic activity and levels required for efficacy. For example, the lack of pharmacokinetic information for β-carotene led to a randomized trial using β-carotene and vitamin A for prevention of lung cancer which had to be terminated early due to the unexpected increase in the incidence of lung cancer in patients who smoked or had previous exposure to asbestos (42,43). Subsequent studies have shown that β-carotene can exert pro-oxidant effects in the damaged lung epithelial cells of smokers and ex-smokers that may result in further damage to key cellular macromolecules involved in lung tumor progression (44). Thus, we proposed that in the highly oxygenated environment of the lung, a similar phenomenon may occur whereby curcumin initially acts as a pro-oxidant, causing an increase in reactive oxygen species and potential damage to critical oncogenic or tumor suppressor pathways (27,53). Indeed, recent studies have shown that treatment of mice with pharmacological doses of ascorbate, another putative chemopreventive agent with known antioxidant effects, resulted in increased levels of ascorbate radical and H2O2 in tumor xenografts (54), thereby producing a pro-oxidant environment in vivo.
Future studies will need to focus on the potential mechanisms by which curcumin mediates its apparent pro-oxidant effects and examine the effects of curcumin at early time points utilizing toxicogenomic approaches to identify the global effects of this agent on key regulatory pathways involved in cell proliferation, survival, inflammation and oxidative stress. Our results suggest that, in ongoing clinical trials assessing the potential chemopreventive activity of curcumin for colon cancer, it may be necessary to screen patients for smoking status and exclude those patients who have a history of smoking.
Funding
National Cancer Institute (CA91909 to M.S.M. and CA91909-S1 to S.T.D.-B); American Foundation for Aging Research (GTA #31424).
Acknowledgments
This work was performed in partial fulfillment for the requirements for a PhD degree in the Department of Cancer Biology at the Wake Forest University Graduate School of Arts and Sciences. A preliminary report of this work was presented at the 47th annual meeting of the Society of Toxicology.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AC
adenocarcinomas
- AD
adenoma
- BHT
butylated hydroxytoluene
- CCSP
Clara cell secretory protein
- COX-2
cycloxygenase-2
- DOX
doxycycline
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IHC
immunohistochemistry
- NF-κβ
nuclear factor κβ
- RT–PCR
reverse transcription-polymerase chain reaction
- rtTA
reverse tetracycline trans-activator
References
- 1.Jemal A, et al. Cancer statistics, 2007. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Sandler AB, et al. COX-2 inhibition and lung cancer. Semin. Oncol. 2004;31:45–52. doi: 10.1053/j.seminoncol.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Psaty BM, et al. Risks and benefits of celecoxib to prevent recurrent adenomas. N. Engl. J. Med. 2006;355:950–952. doi: 10.1056/NEJMe068158. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB, et al. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 5.Duvoix A, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–190. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 6.Thangapazham RL, et al. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8:E443–E449. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin YG, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin. Cancer Res. 2007;13:3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 8.Brennan P, et al. Inhibition of nuclear factor kappaB by direct modification in whole cells—mechanism of action of nordihydroguaiaritic acid, curcumin and thiol modifiers. Biochem. Pharmacol. 1998;55:965–973. doi: 10.1016/s0006-2952(97)00535-2. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, et al. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J. Biol. Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 10.Hour TC, et al. Curcumin enhances cytotoxicity of chemotherapeutic agents in prostate cancer cells by inducing p21(WAF1/CIP1) and C/EBPbeta expressions and suppressing NF-kappaB activation. Prostate. 2002;51:211–218. doi: 10.1002/pros.10089. [DOI] [PubMed] [Google Scholar]
- 11.Lambert JD, et al. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am. J. Clin. Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 12.Plummer SM, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- 13.Lev-Ari S, et al. Celecobix and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin. Cancer Res. 2005;11:6738–6744. doi: 10.1158/1078-0432.CCR-05-0171. [DOI] [PubMed] [Google Scholar]
- 14.Perkins S, et al. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol. Biomarkers Prev. 2002;11:535–540. [PubMed] [Google Scholar]
- 15.Huang MT, et al. Effect of dietary curcumin and ascorbyl palmitate on azoxymethanol-induced colonic epithelial cell proliferation and focal areas of dysplasia. Cancer Lett. 1992;64:117–121. doi: 10.1016/0304-3835(92)90071-3. [DOI] [PubMed] [Google Scholar]
- 16.Kawamori T, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- 17.Inano H, et al. Chemoprevention by curcumin during the promotion stage of tumorigenesis of mammary gland in rats irradiated with gamma-rays. Carcinogenesis. 1999;20:1011–1018. doi: 10.1093/carcin/20.6.1011. [DOI] [PubMed] [Google Scholar]
- 18.Singletary K, et al. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumorigenesis and DMBA-DNA adduct formation by curcumin. Cancer Lett. 1996;103:137–141. doi: 10.1016/0304-3835(96)04224-3. [DOI] [PubMed] [Google Scholar]
- 19.Huang MT, et al. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48:5941–5946. [PubMed] [Google Scholar]
- 20.Huang MT, et al. Inhibitory effects of curcumin on tumor initiation by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1992;13:2183–2186. doi: 10.1093/carcin/13.11.2183. [DOI] [PubMed] [Google Scholar]
- 21.Sinha R, et al. Cancer risk and diet in India. J. Postgrad. Med. 2003;49:222–228. [PubMed] [Google Scholar]
- 22.Mohandas KM, et al. Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J. Gastroenterol. 1999;18:118–121. [PubMed] [Google Scholar]
- 23.Cheng AL, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 24.Sharma RA, et al. Curcumin: the story so far. Eur. J. Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Ravindranath V, et al. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–265. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 26.Wahlstrom B, et al. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenh) 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 27.Ireson CR, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 28.Hecht SS, et al. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett. 1999;137:123–130. doi: 10.1016/s0304-3835(98)00326-7. [DOI] [PubMed] [Google Scholar]
- 29.Garg R, et al. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: mechanism of its anti-initiating action. Carcinogenesis. 2008;29:1022–1032. doi: 10.1093/carcin/bgn064. [DOI] [PubMed] [Google Scholar]
- 30.Tichelaar JW, et al. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 2000;275:11858–11864. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 31.Hayashida S, et al. Regulation and function of CCSP during pulmonary Pseudomonas aeruginosa infection in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L452–L459. doi: 10.1152/ajplung.2000.279.3.L452. [DOI] [PubMed] [Google Scholar]
- 32.Floyd HS, et al. Conditional expression of the mutant Ki-rasG12C allele results in formation of benign lung adenomas: development of a novel mouse lung tumor model. Carcinogenesis. 2005;26:2196–2206. doi: 10.1093/carcin/bgi190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Floyd HS, et al. Genetic and epigenetic alterations in lung tumors from bitransgenic Ki-rasG12C expressing mice. Mol. Carcinog. 2006;45:506–517. doi: 10.1002/mc.20181. [DOI] [PubMed] [Google Scholar]
- 34.Witschi H, et al. Enhancement of urethan tumorigenesis in mouse lung by butylated hydroxytoluene. J. Natl. Cancer Inst. 1977;58:301–305. doi: 10.1093/jnci/58.2.301. [DOI] [PubMed] [Google Scholar]
- 35.Bauer AK, et al. Butylated hydroxytoluene (BHT) induction of pulmonary inflammation: a role in tumor promotion. Exp. Lung Res. 2001;27:197–216. doi: 10.1080/019021401300053948. [DOI] [PubMed] [Google Scholar]
- 36.Bauer AK, et al. The lung tumor promoter, butylated hydroxytoluene (BHT), causes chronic inflammation in promotion-sensitive BALB/cByJ mice but not in promotion-resistant CXB4 mice. Toxicology. 2001;169:1–15. doi: 10.1016/s0300-483x(01)00475-9. [DOI] [PubMed] [Google Scholar]
- 37.Goel A, et al. Curcumin as “Curecumin”: from kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Castonguay A, et al. Inhibition of lung tumourigenesis by sulindac: comparison of two experimental protocols. Carcinogenesis. 1997;18:491–496. doi: 10.1093/carcin/18.3.491. [DOI] [PubMed] [Google Scholar]
- 39.Nikitin AY, et al. Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res. 2004;64:2307–2316. doi: 10.1158/0008-5472.can-03-3376. [DOI] [PubMed] [Google Scholar]
- 40.Duggan DE. Sulindac: therapeutic implications of the prodrug/pharmacophore equilibrium. Drug Metab. Rev. 1981;12:325–337. doi: 10.3109/03602538108994035. [DOI] [PubMed] [Google Scholar]
- 41.Duperron C, et al. Chemopreventive efficacies of aspirin and sulindac against lung tumorigenesis in A/J mice. Carcinogenesis. 1997;18:1001–1006. doi: 10.1093/carcin/18.5.1001. [DOI] [PubMed] [Google Scholar]
- 42.Omenn GS, et al. CARET, the beta-carotene and retinol efficacy trial to prevent lung cancer in asbestos-exposed workers and in smokers. Anticancer Drugs. 1991;2:79–86. doi: 10.1097/00001813-199102000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Omenn GS, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N. Engl. J. Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 44.Alija AJ, et al. Beta-carotene breakdown products enhance genotoxic effects of oxidative stress in primary rat hepatocytes. Carcinogenesis. 2006;27:1128–1133. doi: 10.1093/carcin/bgi342. [DOI] [PubMed] [Google Scholar]
- 45.Dance-Barnes ST, et al. Effects of mutant human Ki-ras(G12C) gene dosage on murine lung tumorigenesis and signaling to its downstream effectors. Toxicol. Appl. Pharmacol. 2008;231:77–84. doi: 10.1016/j.taap.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moos PJ, et al. Curcumin impairs tumor suppressor p53 function in colon cancer cells. Carcinogenesis. 2004;25:1611–1617. doi: 10.1093/carcin/bgh163. [DOI] [PubMed] [Google Scholar]
- 47.Ammon HP, et al. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 48.Kawanishi S, et al. Evaluation for safety of antioxidant chemopreventive agents. Antioxid. Redox Signal. 2005;7:1728–1739. doi: 10.1089/ars.2005.7.1728. [DOI] [PubMed] [Google Scholar]
- 49.National Institute of Environmental Health Sciences. Toxicology and Carcinogenesis Studies of Turmeric Oleoresin (CAS No. 8024-37-1) (Major Component 79-85% Curcumin, CAS No. 458-37-7) in F344/N Rats and B6C3F1 Mice (Feed Studies) 1993. [PubMed] [Google Scholar]
- 50.Dutta S, et al. Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorg. Med. Chem. Lett. 2005;15:2738–2744. doi: 10.1016/j.bmcl.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Farombi EO, et al. Curcumin attenuates gentamicin-induced renal oxidative damage in rats. Food Chem. Toxicol. 2006;44:1443–1448. doi: 10.1016/j.fct.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Galati G, et al. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology. 2002;177:91–104. doi: 10.1016/s0300-483x(02)00198-1. [DOI] [PubMed] [Google Scholar]
- 53.Sakano K, et al. Metal-mediated DNA damage induced by curcumin in the presence of human cytochrome P450 isozymes. Arch. Biochem. Biophys. 2002;405:223–230. doi: 10.1016/s0003-9861(02)00302-8. [DOI] [PubMed] [Google Scholar]
- 54.Chen Q, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl Acad. Sci. USA. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]


