Abstract
The cyclin-dependent kinase (Cdk) inhibitor p27Kip1 (p27) is a marker of prognosis in many cancers, including breast cancer. Low p27 expression correlates with poor prognosis, especially in hormone receptor positive breast tumors. This association suggests a role for p27 in hormone-dependent cancer. We used the Wnt-1 transgenic mouse model to further explore the role of p27 in hormone-driven breast cancer. We found that p27 deficiency did not alter breast cancer rate in either male or female Wnt-1 mice. However, we did find p27−/− females had reduced levels of serum progesterone (P) and increased variability in estradiol (E), which could have affected their cancer susceptibility. To equalize hormone levels, an additional cohort of Wnt-1 female mice was ovariectomized and implanted with slow release pellets of E and P. Although this treatment did not alter the breast cancer rate, it did accelerate the development of pituitary and gastric tumors in p27−/− mice. This study shows that while not a significant inhibitor of Wnt-1-driven breast cancer, p27 inhibits gastric tumors, whose latency is modulated by sex steroids.
Introduction
The cyclin-dependent kinase (Cdk) inhibitor p27Kip1 (p27) regulates the G0 to S cell cycle transition by binding to and inhibiting the activity of Cdks (1). Many extracellular signals that increase or decrease cell proliferation act by decreasing or increasing p27 levels, respectively. Reduced expression of p27 is frequently observed in human cancers and this correlates with poor prognosis (2). Notable examples include cancers of the lung, colon, prostate and breast. In breast cancer, where p27 has been extensively studied, 11 of 17 studies show reduced p27 correlates significantly with poor prognosis in multivariate analysis (2). Reduced p27 increased the risk of relapse or death 1.3- to 4-fold over 5–17 years of follow up.
Porter et al. (3) recently reported results from a large study of >3000 breast cancer patients, all of whom received similar adjuvant chemotherapy, confirming that p27 levels were prognostic, when controlled for treatment. This association was strongest in steroid receptor positive, tamoxifen-treated patients. In addition, high levels of p27 were strongly associated with positive estrogen receptor (ER) and progesterone receptor status and were an independent predictor of response to hormonal therapy (4). Ovarian hormones, notably estradiol (E) and progesterone (P) play a major role in the development of breast cancer (5). Over 70% of human breast cancers are ER positive and show estrogen-dependent growth. One proposed mechanism of estrogen-stimulated breast cancer growth is through the regulation of p27. Estrogen-treated MCF-7 breast cancer cells show a decrease in p27 and increased cyclinE/Cdk2 activity and G1–S transition (6). Conversely, levels of p27 increase in tamoxifen-treated ER-positive breast cancer cells and p27 was required for subsequent cell cycle arrest (7). Together, these studies suggest p27 may provide a causal link between steroid hormones and breast cancer.
Despite the accepted role of p27 as a cell cycle inhibitor and the correlation of reduced expression with tumor aggressiveness, mutations in the CDKN1B gene encoding p27 are rarely observed in human tumors (8–10). A causal role for p27 in tumor suppression was demonstrated by the tumor predisposition of p27-deficient mice (11). In susceptible genetic backgrounds or after exposure to carcinogens, both p27-null and p27 heterozygous knockout mice exhibit increased susceptibility to tumor development in multiple tissues, including pituitary (12–14), lung, small intestine, colon (11), lymphoma (15–18), prostate (19) and bladder (20). Recently, germline mutations in the CDKN1B gene were found in patients with multiple endocrine neoplasia syndrome, confirming a tumor suppressor role for p27 in human neoplasia (21).
In general, mouse models have demonstrated tumor suppression by p27 increases with gene dosage, the more p27 the more tumor suppression. However, several exceptions have been reported. For example, tumor development in ErbB2 transgenic mice (22) or Nkx3.1 PTEN-deficient mice (23) was paradoxically delayed in a p27-null background but accelerated in p27 heterozygous mice compared with p27 intact mice. In another example, p27 inhibits intestinal tumor development in mice carrying a mutant Apc allele, but not SMAD3-deficient mice (24). Thus, tumor suppression by p27 is context dependent and varies depending on the primary oncogenic lesion driving tumor development.
Wnt-1 is a secreted glycoprotein that binds and activates the frizzled receptor, triggering a signaling cascade through Apc and glycogen synthase kinase 3-beta (25). In the canonical pathway, this leads to stabilization and nuclear accumulation of β-catenin and Tcf-4-dependent gene expression. Transgenic Wnt-1 female mice develop mammary tumors with myoepithelial, acinar, or glandular phenotypes (26,27) with a median latency of ∼6 months. Wnt-1 breast tumors express both estrogen and P receptor (28) and tumor development is delayed in ovariectomized or ER-α-deficient Wnt-1 mice (29), indicating a role for endocrine hormones in this model. The Apc tumor suppressor is a key component of the Wnt-signaling pathway and we showed previously that p27 is a potent inhibitor of intestinal neoplasia in ApcMin mice (24). Given this background, we tested the hypothesis that p27 suppresses Wnt-1-driven breast cancer.
Materials and methods
Mice
B6SJL-Tg(Wnt1)1Hev/J mice were obtained from Jackson Laboratories (Bar Harbor, ME). The Wnt-1 transgene was backcrossed at least five times to the C57BL/6 strain. These mice were then intercrossed to 129 p27+/− mice (14) to generate C57BL/6 129 (B6129) Wnt-1 p27-experimental mice. Genotyping protocols are available on request. A second cohort of B6129 Wnt-1 mice underwent ovariectomy (OVX) at 5 weeks of age and implanted subcutaneous between the scapula with a 90 day release pellet of E (0.1 mg) and P (10 mg) (Innovative Research of America, Sarasota, FL). Pellets were replaced every 90 days. Both male and female mice were observed regularly and killed when tumors reached 1.5 cm in diameter or when they showed symptoms of tumor development at other sites. Mice were necropsied and tumors were removed and sections were frozen in liquid nitrogen or fixed in 10% neutral-buffered formalin and then processed and embedded in paraffin. Hematoxylin and eosin-stained slides from tumors were analyzed for morphologic features as described (30).
Immunohistochemistry and immunoblotting
Immunohistochemical staining for p27 and β-catenin and western blotting for p27 and cyclin D1 from tissue lysates was performed as described previously (24).
Serum hormone levels
Blood was collected by cardiac puncture from female mice of all three p27 genotypes between 15 and 25 weeks of age. Serum was separated from the whole blood clot and frozen at −20°C. Enzyme-linked immunosorbent assay kits (Diagnostics Systems Laboratories, Webster, TX) were used to measure serum E and P levels. Each sample was run in duplicate from 12 mice of each p27 genotype. A logarithmic curve of absorbance versus concentration was established using the kit standards and GraphPad Prism 3.0; from this curve, the sample concentrations were calculated.
Statistical analysis
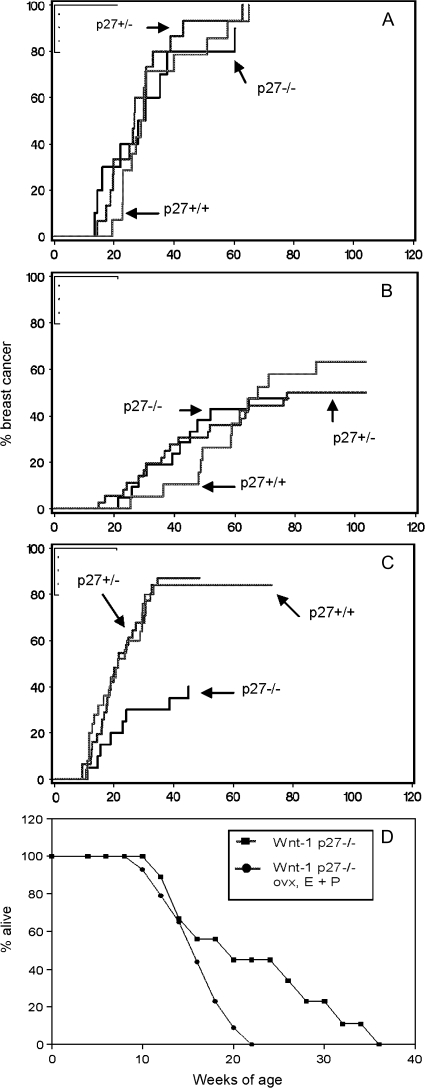
Comparisons of breast tumor incidence rates were performed using Cox regression. The development of other tumor types was treated as a competing risk in these analyses. Because of this competing risk, the incidence curves for breast cancer (Figure 1) may not necessarily reflect the underlying incidence rates (Table I). This is particularly notable in Figure 1C, where the apparent incidence of breast tumors in p27−/− mice is reduced because of the high incidence of other tumor types, not because of intrinsically lower incidence among mice at risk.
Fig. 1.
Effect of p27 genotype on breast tumor latency. The percentage of mice that developed breast cancer is plotted versus age in weeks. (A) Intact B6129 Wnt-1 female mice of the indicated p27 genotypes. (B) Intact B6129 Wnt-1 male mice of the indicated p27 genotypes. (C) Ovariectomized, estrogen and P-supplemented B6129 Wnt-1 female mice of the indicated p27 genotypes. (D) Kaplan–Meier survival analysis of Wnt-1 p27−/− mice versus Wnt-1 p27−/− ovariectomized and implanted with E + P.
Table I.
Statistical analysis of tumor development in B6129 Wnt-1 mice
| Sex | Genotype | n | Breast cancer ratea | P-valuebc | Other cause ratea | P-valueb |
| Intact female | p27−/− | 10 | 3.3 | 0.68 | 0.4 | |
| p27+/− | 15 | 3.5 | 0.97 | 0.0 | ||
| p27+/+ | 14 | 2.9 | 0.48, 0.44 | 0.0 | 0.20 | |
| Male | p27−/− | 21 | 1.0 | 0.82 | 1.1 | |
| p27+/− | 36 | 0.9 | 0.56 | 0.9 | ||
| p27+/+ | 19 | 0.9 | 0.58, 0.98 | 0.6 | 0.0005 | |
| OVX female | p27−/− | 20 | 2.0 | 0.44 | 3.0 | |
| p27+/− | 31 | 3.8 | 0.27 | 0.6 | ||
| p27+/+ | 25 | 3.4 | 0.25, 0.93 | 0.6 | 0.0001 |
OVX: ovariectomized and implanted with pellets of estrogen and P.
Events per 100 weeks of observation.
From Cox regression.
The first line P-value is the overall test of equality. The second line is the test of p27+/− versus p27−/−. The third line lists the tests of p27+/+ versus p27−/− and p27+/+ versus p27+/−.
Results
Breast cancer rate in Wnt-1-transgenic mice is dependent on gender but not p27 status
We crossed p27-deficient mice to Wnt-1-transgenic mice and examined tumor latency in Wnt-1 mice of all three p27 genotypes (+/+, +/− and −/−). We examined both males and females to determine if tumor suppression by p27 interacted with gender. The rate of breast cancer development, expressed as events per 100 weeks, in p27 intact Wnt-1 females (2.9) was significantly greater than Wnt-1 males (0.9) (Figure 1 and Table I). In total, 100% of female mice developed breast cancer by 70 weeks compared with only 60% of male mice by 100 weeks. This shows the prominent influence of sex hormonal environment on Wnt-1-driven breast cancer, but also that breast tumors do develop in male mice at fairly high incidence in the absence of high levels of E and P. Loss of either one or both alleles of p27 had negligible affect on breast tumor development in both male and female mice. The rate of breast cancer in Wnt-1 female p27+/+, p27+/− and p27−/− mice was similar (2.9, 3.5 and 3.3 events per 100 weeks, respectively). Likewise, the rate of breast cancer in Wnt-1 male p27+/+, p27+/− and p27−/− mice was similar (0.9, 0.9 and 1.0 events per 100 weeks, respectively) (Figure 1 and Table I). In contrast, p27-null mice of both genders developed a greater number of pituitary tumors (six tumors/43 mice) compared with p27 wild-type mice (zero tumors/41 mice), confirming previous reports of increased susceptibility of p27-deficient mice to these tumors (12–14). We conclude that the difference in breast tumor development that exists between male and female mice is largely independent of p27 status.
Tumor morphology and grade
To determine if p27 status affected tumor grade, breast tumors from each genotype were evaluated histologically and categorized by morphological subtypes (acinar, papillary, cribiform, comedo or solid), and for tumor grade, which encompasses degree of tubularity, nuclear pleomorphism, mitotic and apoptotic activity (30). Morphology of Wnt-1 breast tumors was consistent with published studies (27) and no differences were observed between p27 genotypes with respect to morphological subtype or tumor grade (data not shown).
β-Catenin staining in Wnt-1 breast tumors
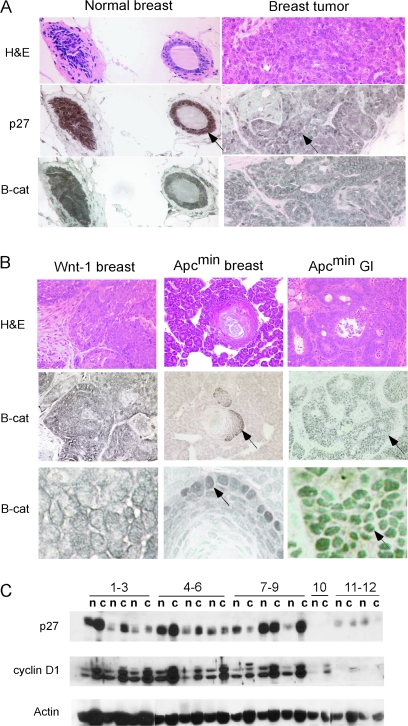
The lack of cooperation between p27 deficiency and Wnt-1 in breast tumor development differed from the previously reported strong cooperation between p27 loss and Apc mutation in gastrointestinal neoplasia (24). Both Wnt-1 and mutant Apc are thought to act through a similar signaling pathway, culminating in stabilization of nuclear β-catenin and Tcf-dependent gene expression (25). However, direct comparison of nuclear β-catenin revealed significant differences. Prominent nuclear β-catenin was clearly evident in the majority of cells from ApcMin intestinal adenomas, whereas breast tumors from Wnt-1 mice were uniformly negative for nuclear β-catenin staining (Figure 2B). To determine if this differential activation of β-catenin was due to tumor type or to differential signaling from Wnt-1 versus mutant Apc, we took advantage of the fact that ApcMin mice from our colony (24) occasionally developed breast tumors. Breast tumors from ApcMin mice displayed small, focal areas of nuclear β-catenin staining, surrounded by a majority of unstained tumor cells, as compared with uniformly positive nuclear staining in ApcMin intestinal tumors (Figure 2B). This shows that breast tumors driven by mutant Apc exhibit reduced activation of β-catenin compared with intestinal tumors driven by the same mutation, indicating tumor-specific regulation of β-catenin. Thus, differential activation of β-catenin between breast and intestinal tumors provides at least one explanation for the dissimilar tumor suppressing effects of p27 in the two models.
Fig. 2.
p27 and β-catenin (B-cat) expression in Wnt-1 tumors. (A) p27 and B-cat staining of normal breast and breast tumor from Wnt-1 mice. Arrows point to positive cells. Left column photographs taken at ×100 and right column at ×600 magnification. (B) Comparison of B-cat staining in breast and intestinal (gastrointestinal) tumors from ApcMin mice and Wnt-1 transgenic mice. Middle row photographs taken at ×100 and bottom row at ×600 magnification. (C) Immunoblot analysis of p27 and cyclin D1 in Wnt-1 breast tumors from the following mice. Lanes 1–3: intact female; lanes 4–6: E- and P-treated female; lanes 7–9: male; lane 10: p27 null; lane 11 and12: normal breast. n, nuclear fraction and c, cytoplasmic fraction.
p27 expression in Wnt-1 breast tumors
p27 protein levels are frequently reduced in tumors due to increased p27 degradation, reduced transcription or other pathways (2). We examined p27 expression in Wnt-1 breast tumors with both immunohistochemistry and western blot analysis. Prominent nuclear staining for p27 was seen in normal breast ductal epithelia (Figure 2A). Wnt-1 breast tumors showed more variable p27 expression, but prominent nuclear staining was clearly detected in many cells throughout the tumors. Immunoblotting of nuclear/cytoplasmic fractions from tissue lysates showed variable p27 expression in breast tumors, but an overall increase compared with normal breast tissue (Figure 2C). No major difference was seen in p27 levels in tumors from male or female mice. p27 was markedly reduced in ApcMin intestinal adenomas relative to normal intestinal tissue (24), further indication of differential signaling pathways through p27 in Wnt-1 breast tumors compared with mutant Apc intestinal tumors. Cyclin D1 levels were markedly increased in Wnt-1 breast tumors consistent with increased proliferation compared with normal breast tissue (31).
p27 deficiency affects circulating levels of E and P
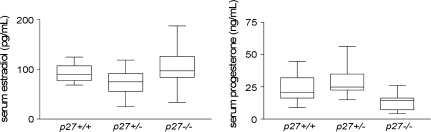
Previous studies revealed that Wnt-1 breast tumors express ER-α (28) and that breast tumor development is delayed in ER-α-null or -ovariectomized mice (29,32), indicating a supporting role for ovarian-derived hormones such as E and P in Wnt-1-driven tumor development. p27-null female mice are infertile, exhibiting a disordered estrus cycle and impaired formation of the corpus luteum (12–14). As E and P are produced by the corpus luteum and both play important roles in the natural history of breast cancer, we asked if E and P levels were altered in p27-null mice. The mean serum P concentration in p27−/− mice (13.9 ng/ml) was significantly lower than wild-type (24.3 ng/ml) (P < 0.02) or p27+/− (29.3 ng/ml) (P < 0.01) mice (Figure 3). Serum P concentrations did not differ significantly between p27 wild-type and p27+/− mice (P > 0.28). The mean serum E concentration did not differ appreciably between wild-type (92.4 pg/ml) and p27−/− (104.6 pg/ml) female mice; however, there was greater intermouse variation in p27−/− mice, with values ranging from 34.0 to 188.0 pg/ml (Figure 3). E levels in p27+/− mice (73.6 pg/ml) were significantly reduced compared with p27−/− mice (P < 0.04 using a two-sided t-test). This suggests E levels are less tightly regulated in p27-deficient mice, consistent with previous findings of perturbed estrous cycling (13).
Fig. 3.
Serum concentrations of E and P in p27-deficient mice. Median concentrations are plotted with 25–75 percentiles (boxes) and ranges (vertical bars).
Circulating levels of E and P affect tumor predisposition of p27-null mice
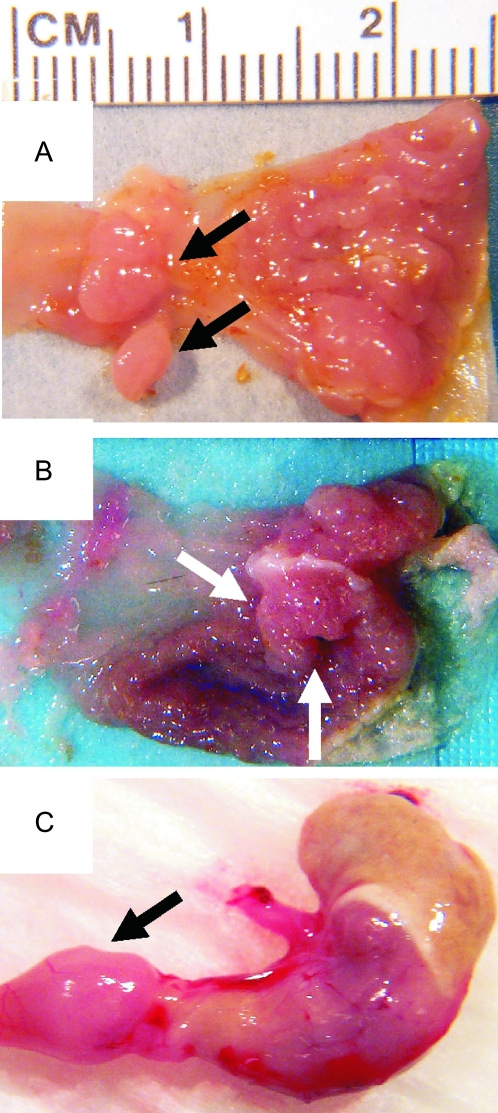
Having shown that p27-deficient mice have altered the serum levels of E and P, we asked if this affected their susceptibility to breast or other hormonally responsive cancers. To address this, we equalized E and P levels between p27 genotypes and measured tumor susceptibility. A cohort of Wnt-1 female mice of all three p27 genotypes was subjected to OVX and implanted with slow release pellets of E and P (see Materials and Methods). Compared with intact mice, the rate of breast tumor development in E- and P-implanted mice was modestly increased in p27 wild-type and p27 heterozygous mice (from 2.9 to 3.4 and 3.5 to 3.8 events per 100 weeks, respectively) but appeared to be decreased in p27-null mice (from 3.3 to 2.0 events per 100 weeks) although this did reach statistical significance (Figure 1, Table I). Measurement of breast cancer rate in p27 nulls was complicated by early lethality from other tumor types, notably pituitary, stomach and duodenal tumors (Figure 1D). The rate of development of these non-breast tumors in treated p27−/− mice was independent of Wnt-1 (data not shown), was significantly greater than in treated p27+/− or p27+/+ mice (3.0, 0.6 and 0.6 events per 100 weeks, respectively, P = 0.0001) and was increased compared with intact untreated p27−/− mice (0.4 events per 100 weeks, P = 0.001) (Table I). Thus, OVX followed by E and P pellets only affected tumor development in p27−/− mice, indicating that the hormonal dysfunction of p27-null mice influences their tumor predisposition. Ninety-three percent (13/14) of E- and P-treated p27-null mice developed pituitary tumors of both the distal and intermediate lobes, compared with only 12% (2/16) in E- and P-treated wild-type and 15% (2/13) in intact p27-null females over a similar time frame (P < 0.001, Chi squared test). In addition, 71% of E- and P-treated p27-null mice developed stomach lesions, located within the cardiac region of the glandular stomach or at the duodenal junction, with an average latency of 4.0 ± 0.8 months. These lesions were often large and in several cases perforated through the stomach wall or obstructed the duodenal passage (Figure 4). Histologically, these were classified as a mixture of hyperplasias, adenomatous polyps and cystic adenocarcinomas of the gastric stomach as well as adenomas of the duodenum. Sixty percentage of intact p27−/− mice also developed stomach tumors, but with a longer latency of 5.5 ± 1.7 months, whereas none of the p27+/− or p27+/+ mice developed such tumors. E- and P-treated mice of all genotypes also presented with uterine endometrial hyperplasia, consistent with known effects of sustained exposure to E and P. Together, these results show that (i) p27 does not significantly alter Wnt-1-driven breast cancer in either male or female hormonal milieus. (ii) p27-null mice are susceptible to gastric cancer. (iii) Tumor spectrum and especially tumor kinetics of p27-null mice is influenced by endocrine dysfunction and when rendered hormonally equivalent, p27-null mice exhibit an even greater susceptibility to tumor development.
Fig. 4.
Gastric tumors from p27-null mice. Arrows point to lesions at gastric region of the stomach or at the duodenal junction.
Discussion
Hormones play a major role in breast cancer in humans (5). In Wnt-1 mice, breast cancer was markedly delayed in males relative to females, indicating the strong hormonal dependence of this tumor model. Germline p27 deficiency did not significantly alter the latency of breast tumors in either male or female mice, indicating that the effect of the sex hormonal environment on breast cancer development is largely p27 independent. Further, the histological subtypes and aggressiveness of breast tumors from p27-null mice were indistinguishable from wild-types. These results, together with the retention of p27 expression in Wnt-1 breast tumors, indicate that the initiation and growth of these tumors is not inhibited by p27. Jackson et al. (33) showed that germline p27 deficiency did not modify breast tumor latency in a Ras transgenic model. These results showing little or no affect of p27 are different to that seen in an ErbB2 transgenic model, where p27 heterozygosity accelerated and nullizygosity delayed breast cancer (22). The delayed tumorigenesis in the absence of p27 was attributed to a requirement for p27 to assemble functional cyclin D–Cdk complex and drive cell proliferation. Consistent with this idea, breast cancer in ErbB2-transgenic mice was completely eliminated in cyclin D1-null mice, indicating a strict requirement for cyclin D1 (34). Together, these findings reinforce the idea that tumor suppression by p27 is pathway specific (24) and suggest that the role of p27 in human cancer is likely strongly influenced by the primary underlying genetic lesion within each tumor type.
The lack of cooperation between p27 loss and Wnt-1 is also in contrast with potent tumor suppression by p27 seen in mutant Apc-driven intestinal tumors (24). This differential effect of p27 between the two models could be attributed to differential signaling between transgenic Wnt-1 in breast tumors and mutant ApcMin in intestinal tumors. Wnt-1 was shown to expand the mammary stem cell pool and this is a proposed explanation for heterogenous cell types seen in Wnt-1 tumors (35,36). Further, nuclear β-catenin staining was much more prominent in ApcMin intestinal tumors compared with breast tumors from either Wnt-1 or ApcMin mice, indicating differential activation of β-catenin in breast compared with intestinal tumors. The increase in cyclin D1 seen in Wnt-1 breast tumors could be due to non-canonical Wnt signaling. Interestingly, Yu et al. (34) showed that cyclin D1 deletion had only a modest affect on breast tumor latency in Wnt-1 transgenic mice, which together with our results indicate that Wnt-1-driven tumor formation is largely independent of the cyclin D1/p27 axis.
In addition to the known function of p27 as a cell autonomous tumor suppressor (37), these studies identify a non-cell autonomous endocrine-mediated pathway that modifies the tumor predisposition of p27 deficient mice. p27-null female mice exhibit altered endocrine function, as shown by reduced fertility, aberrant corpus luteum formation and altered estrus cycling (13). Tong et al. (38) reported that p27 is required for the proper coupling of differentiation and growth arrest during the granulosa to luteal transition. As the corpus luteum produces E and P, this provides an explanation for the aberrant levels of serum P and E seen in p27 deficient mice. When rendered hormonally equivalent to wild-type mice by OVX and E and P implants, p27-null mice developed adenomas and adenocarcinomas of the stomach and duodenum, as well as accelerated distal and intermediate lobe pituitary tumors. Ikeda et al. (39) previously reported accelerated pituitary tumorigenesis in E-treated p27-deficient mice. These results indicate a component of the susceptibility of p27-deficient mice is non-cell autonomous and attributable to an altered hormonal environment. The predisposition of p27-null mice to gastric tumors was also seen in p27-null mice after infection with Helicobacter pylori (40). In human gastric cancer, reduced p27 protein expression correlates with advanced stage, invasiveness and prognosis (41–45) and a protective effect of estrogen in stomach cancer has been reported (46). These findings demonstrate a causal role for p27 in suppression of gastric cancer. Further studies will be required to address the mechanisms of tumor suppression by p27 in hormonally dependent cancers.
Funding
Life Possibilities Fund and National Institutes of Health (R01).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- Cdk
cyclin-dependent kinase
- E
estradiol
- ER
estrogen receptor
- OVX
ovariectomy
- p27
p27Kip1
- P
progesterone
References
- 1.Sherr CJ, et al. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 2.Chu IM, et al. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 3.Porter PL, et al. p27(Kip1) and cyclin E expression and breast cancer survival after treatment with adjuvant chemotherapy. J. Natl Cancer Inst. 2006;98:1723–1731. doi: 10.1093/jnci/djj467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pohl G, et al. High p27Kip1 expression predicts superior relapse-free and overall survival for premenopausal women with early-stage breast cancer receiving adjuvant treatment with tamoxifen plus goserelin. J. Clin. Oncol. 2003;21:3594–3600. doi: 10.1200/JCO.2003.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Pike MC, et al. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol. Rev. 1993;15:17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 6.Foster JS, et al. Estrogens down-regulate p27Kip1 in breast cancer cells through Skp2 and through nuclear export mediated by the ERK pathway. J. Biol. Chem. 2003;278:41355–41366. doi: 10.1074/jbc.M302830200. [DOI] [PubMed] [Google Scholar]
- 7.Cariou S, et al. Down-regulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells. Proc. Natl Acad. Sci. USA. 2000;97:9042–9046. doi: 10.1073/pnas.160016897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietenpol JA, et al. Assignment of the human p27Kip1 gene to 12p13 and its analysis in leukemias. Cancer Res. 1995;55:1206–1210. [PubMed] [Google Scholar]
- 9.Kawamata N, et al. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res. 1995;55:2266–2269. [PubMed] [Google Scholar]
- 10.Spirin KS, et al. p27 mutation found in breast cancer. Cancer Res. 1996;56:2400–2404. [PubMed] [Google Scholar]
- 11.Fero ML, et al. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama K, et al. Mice lacking p27 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 13.Kiyokawa H, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 14.Fero ML, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 15.Philipp-Staheli J, et al. Distinct roles for p53, p27Kip1, and p21Cip1 during tumor development. Oncogene. 2004;23:905–913. doi: 10.1038/sj.onc.1207220. [DOI] [PubMed] [Google Scholar]
- 16.Hwang HC, et al. Identification of oncogenes collaborating with p27Kip1 loss by insertional mutagenesis and high-throughput insertion site analysis. Proc. Natl Acad. Sci. USA. 2002;99:11293–11298. doi: 10.1073/pnas.162356099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins CP, et al. Loss of p27(Kip1) but not p21(Cip1) decreases survival and synergizes with MYC in murine lymphomagenesis. EMBO J. 2002;21:3739–3748. doi: 10.1093/emboj/cdf364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geisen C, et al. Loss of p27(Kip1) cooperates with cyclin E in T-cell lymphomagenesis. Oncogene. 2003;22:1724–1729. doi: 10.1038/sj.onc.1206340. [DOI] [PubMed] [Google Scholar]
- 19.Di Cristofano A, et al. Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat. Genet. 2001;27:222–224. doi: 10.1038/84879. [DOI] [PubMed] [Google Scholar]
- 20.Hikosaka A, et al. Susceptibility of p27 kip1 knockout mice to urinary bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl)nitrosamine may not simply be due to enhanced proliferation. Int. J. Cancer. 2008;122:1222–1228. doi: 10.1002/ijc.23249. [DOI] [PubMed] [Google Scholar]
- 21.Pellegata NS, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc. Natl Acad. Sci. USA. 2006;103:15558–15563. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muraoka RS, et al. ErbB2/Neu-induced cyclin D1-dependent transformation is accelerated in p27-haploinsufficient mammary epithelial cells but impaired in p27-null cells. Mol. Cell. Biol. 2002;22:2204–2219. doi: 10.1128/MCB.22.7.2204-2219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao H, et al. A critical role for p27kip1 gene dosage in a mouse model of prostate carcinogenesis. Proc. Natl Acad. Sci. USA. 2004;101:17204–17209. doi: 10.1073/pnas.0407693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philipp-Staheli J, et al. Pathway-specific tumor suppression. Reduction of p27 accelerates gastrointestinal tumorigenesis in Apc mutant mice, but not in Smad3 mutant mice. Cancer Cell. 2002;1:355–368. doi: 10.1016/s1535-6108(02)00054-5. [DOI] [PubMed] [Google Scholar]
- 25.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 26.Tsukamoto AS, et al. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 27.Rosner A, et al. Pathway pathology: histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. Am. J. Pathol. 2002;161:1087–1097. doi: 10.1016/S0002-9440(10)64269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, et al. Estrogen receptor positivity in mammary tumors of Wnt-1 transgenic mice is influenced by collaborating oncogenic mutations. Oncogene. 2005;24:4220–4231. doi: 10.1038/sj.onc.1208597. [DOI] [PubMed] [Google Scholar]
- 29.Bocchinfuso WP, et al. A mouse mammary tumor virus-Wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-alpha. Cancer Res. 1999;59:1869–1876. [PubMed] [Google Scholar]
- 30.Cardiff RD, et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, et al. Changes in gene expression during the development of mammary tumors in MMTV-Wnt-1 transgenic mice. Genome Biol. 2005;6:R84. doi: 10.1186/gb-2005-6-10-r84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, et al. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19:1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- 33.Jackson RJ, et al. Loss of the cell cycle inhibitors p21(Cip1) and p27(Kip1) enhances tumorigenesis in knockout mouse models. Oncogene. 2002;21:8486–8497. doi: 10.1038/sj.onc.1205946. [DOI] [PubMed] [Google Scholar]
- 34.Yu Q, et al. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 35.Vaillant F, et al. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 36.Liu BY, et al. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc. Natl Acad. Sci. USA. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chien WM, et al. Genetic mosaics reveal both cell-autonomous and cell-nonautonomous function of murine p27Kip1. Proc. Natl Acad. Sci. USA. 2006;103:4122–4127. doi: 10.1073/pnas.0509514103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong W, et al. The absence of p27Kip1, an inhibitor of G1 cyclin-dependent kinases, uncouples differentiation and growth arrest during the granulosa->luteal transition. Cell Growth Differ. 1998;9:787–794. [PubMed] [Google Scholar]
- 39.Ikeda H, et al. Morphologic and molecular analysis of estrogen-induced pituitary tumorigenesis in targeted disruption of transforming growth factor-beta receptor type II and/or p27 mice. Endocrine. 2001;16:55–65. doi: 10.1385/ENDO:16:1:55. [DOI] [PubMed] [Google Scholar]
- 40.Kuzushita N, et al. p27kip1 deficiency confers susceptibility to gastric carcinogenesis in Helicobacter pylori-infected mice. Gastroenterology. 2005;129:1544–1556. doi: 10.1053/j.gastro.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 41.Yasui W, et al. Reduced expression of cyclin-dependent kinase inhibitor p27Kip1 is associated with advanced stage and invasiveness of gastric carcinomas. Jpn. J. Cancer Res. 1997;88:625–629. doi: 10.1111/j.1349-7006.1997.tb00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori M, et al. p27 expression and gastric carcinoma. Nat. Med. 1997;3:593. doi: 10.1038/nm0697-593. [DOI] [PubMed] [Google Scholar]
- 43.Kim DH, et al. Reduced expression of the cell-cycle inhibitor p27Kip1 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology. 2000;36:245–251. doi: 10.1046/j.1365-2559.2000.00842.x. [DOI] [PubMed] [Google Scholar]
- 44.Myung N, et al. Loss of p16 and p27 is associated with progression of human gastric cancer. Cancer Lett. 2000;153:129–136. doi: 10.1016/s0304-3835(00)00359-1. [DOI] [PubMed] [Google Scholar]
- 45.Min YH, et al. Cytoplasmic mislocalization of p27Kip1 protein is associated with constitutive phosphorylation of Akt or protein kinase B and poor prognosis in acute myelogenous leukemia. Cancer Res. 2004;64:5225–5231. doi: 10.1158/0008-5472.CAN-04-0174. [DOI] [PubMed] [Google Scholar]
- 46.Lindblad M, et al. Estrogen and risk of gastric cancer: a protective effect in a nationwide cohort study of patients with prostate cancer in Sweden. Cancer Epidemiol. Biomarkers Prev. 2004;13:2203–2207. [PubMed] [Google Scholar]