Abstract
Breast cancer is the most common cause of cancer death among women worldwide. In order to improve the treatment of this disease, a more complete understanding of its biological basis is necessary. Since the Hedgehog (Hh) pathway was recently found to be required for growth and propagation of a number of different cancers, we discuss here the possible involvement of this pathway in the normal biology and development of cancer in the mammary gland. The use of mouse mammary cancer models has assisted the process of dissecting the mechanisms behind Hh-driven mammary tumour formation and growth. Based on recent studies, we conclude that the inhibition of Hh signalling in breast tumours may interfere with the maintenance of a putative cancer stem cell compartment and the abnormal stimulation of tumour stroma. Therefore, the components of the Hh signalling cascade may provide a set of drug targets, which could be implemented into novel combinatorial strategies for the treatment of breast cancer.
Introduction
Breast cancer is the deadliest form of cancer affecting women worldwide. Although there are effective therapies against some forms of this tumour, such as those with abnormal activation of the HER2/Neu oncogene, the majority of breast cancers remain incurable, evident from the high mortality among affected women.
The hedgehog (Hh) signalling pathway plays a crucial role in vertebrate embryogenesis by controlling cell fate, patterning, proliferation, survival and differentiation. In the adult organism, Hh signalling remains active and is involved in the regulation of tissue homeostasis, regeneration and stem cell maintenance (reviewed in ref. 1). Its importance in development and homeostasis is underlined by the fact that inappropriate activation is implicated in the development of several types of cancer, particularly of the skin, brain, lung, prostate and pancreas (reviewed in refs 2,3). Recent studies underline the importance of tightly controlled Hh pathway activation in mammary gland to ensure proper development and avert tumour formation. Nevertheless, the role of this pathway in breast carcinogenesis is far from understood, leaving room for conjecture (4). Thus far, no pathway has been found to play a definite role in breast cancer induction (5), suggesting that a network of pathways interact during the development of this disease. In this review, we discuss the different possibilities of how the Hh pathway may be involved as part of this network, in causing or contributing to the development of mammary cancer.
The mammalian Hh signalling pathway
Three Hh homologues have been identified in vertebrates, contrasting with the single Hh gene found in Drosophila. These gene homologues are called Sonic hedgehog (Shh), Desert hedgehog (Dhh) and Indian hedgehog (Ihh), which are expressed at different stages of ontogeny in different tissues and may have distinct biological functions [for detailed review see ref. 6].
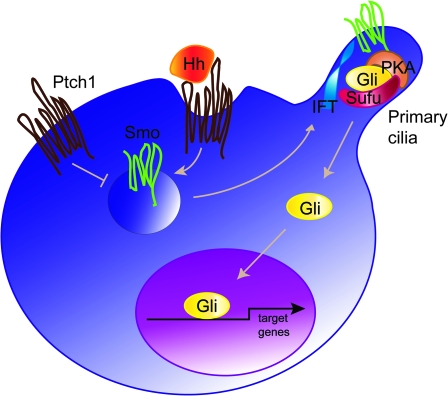
Hh signal transduction is initiated by the binding of the processed and lipid modified Hh ligand to its receptor Patched (Ptch1), a 12-pass transmembrane protein. In the absence of the Hh protein, Ptch1 represses signal transduction by inhibiting the seven transmembrane protein, Smoothened (Smo). Upon Hh binding, the inhibitory function of Ptch1 on Smo is abolished, resulting in Smo activation (reviewed in ref. 7) (Figure 1). The ultimate step in the pathway is mediated by the zinc finger transcription factors Gli1, Gli2 and Gli3, where Gli1 and Gli2 represent the main activators of Hh target genes and Gli3 acts mostly as a repressor (8,9).
Fig. 1.
Hh signalling—a simplified model. In the absence of Hh ligand (Hh), the pathway is inactive. Ptch1 inhibits the activity of Smo, thus the pathway activating Gli transcription factors is prevented from entering the nucleus and the Hh target genes are repressed. Activation of the pathway is initiated upon Hh binding to Ptch1, which leads to de-repression of Smo. As a consequence, a signalling cascade involving a multi-protein complex and the primary cilium as the processing platform leads to the translocation of the active form of Gli transcriptional activators to the nucleus. The Hh/Gli target genes are activated including Ptch1 and Gli1 itself. IFT, intraflagellar proteins; PKA, protein kinase A; Sufu, suppressor of fused.
Gli2 and Gli3 are considered latent transcription factors that can exist both in activator and repressor forms. Upon stimulation of the Hh pathway, Gli2 and Gli3 are activated causing increased transcription of the direct target gene Gli1 (10,11). It has therefore been proposed that the final outcome of the Hh pathway depends on the balance between the Gli activator and repressor forms (reviewed in ref. 12).
The exact mechanism of signal transduction within the cascade from Smo to the Gli proteins is not yet clear, although increasing data suggest that the primary cilium provides a platform for relaying the signal from the cell membrane to the nucleus (reviewed in ref. 13). The primary cilia have been proposed to serve as the processing sites for Gli transcription factors, involving a multi-protein complex consisting of a subset of intraflagellar transport proteins, protein kinase A, glycogen synthase kinase 3, casein kinase and others [(14,15) and for detailed review see ref. 16]. It is known that suppressor of fused (Sufu) plays a key role in negatively regulating Hh/Gli signalling (17), since targeted disruption of the murine Sufu gene leads to neural tube defects, lethality at mid-gestation and altered dorsoventral patterning of the neural tube. This resembles the phenotype caused by an excess of Hh signalling (18).
Hh signalling in mammary tumours
Alterations in hedgehog pathway genes
The possible role for the Hh pathway in development and maintenance of mammary cancer has been proposed only recently, though the data describing the genetic alteration and the modulation of the expression pattern of Hh pathway components in mammary gland are still limited. A contributing factor to the oversight in identifying a role for Hh signalling in breast cancer is the fact that patients with Gorlin's syndrome are not predisposed to breast cancer. A likely interpretation is that the de-regulation of the Hh pathway at the level of PTCH1 in mammary gland may not be the initiating factor for this disease (19). However, earlier studies on small numbers of breast cancer samples did identify putative activating mutations in SHH and missense mutations in the PTCH1 gene (20,21). Yet, this could not be confirmed in later studies with larger sample sets looking for pathway activating mutations in the PTCH1, SHH and SMO (22,23). However, a large screen for genomic mutations revealed that 3 out of 11 breast cancer samples and breast cancer cell lines bear a mutation in GLI1, but the significance of these results is still unclear (24).
Evidence from a single study, where a biallelic Pro1315Leu (C3944T) polymorphism in PTCH1 significantly lowered the risk of breast cancer in premenopausal women taking oral contraceptives gave rise to an interesting new hypothesis: modulating Hh pathway activity could regulate the response of mammary epithelial cells to hormones, which could in turn influence mammary carcinogenesis. Indeed, Chang-Claude et al. (25) located the C3944T polymorphism to the regulatory C-terminus of the PTCH1 protein, opening up the possibility that hormone-driven Hh expression, in combination with the modulated properties of the PTCH1 receptor, might promote mammary cancer formation. Data linking the Hh pathway to mammary cancer were the occurrence of hyperplasia in mammary glands of virgin Ptch1+/− mice, which are reminiscent of human ductal carcinoma in situ. These proliferations disappear during pregnancy and reappear during involution, suggesting a direct link between Ptch1-regulated cellular signalling and hormonal status of the organism (26). This is corroborated by the fact that progesterone and oestrogen induce expression of Ihh in the uterus during pregnancy, arguing in favour of such association (27).
More evidence that Hh signalling is involved in breast cancer comes from a high-resolution comparative genomic hybridization analysis performed on breast cancer samples and breast cancer cell lines. This revealed a frequent loss of the PTCH1 (9q22.1–q31) chromosomal region and amplification of the GLI1 (12q13.2–q13.3) chromosomal region (28,29). Epigenetic mechanisms may provide an alternative mechanism for modulating Hh signalling, hence affecting breast cancer initiation and progression as shown in the MCF7 breast cancer cell line and in a subset of breast cancer samples, where the PTCH1 gene is silenced through promoter methylation (30). Whether the genetic alterations so far described can cause misexpression of Hh pathway components and de-regulate the pathway activity needs to be further investigated.
The expression of Hh pathway members in breast cancer
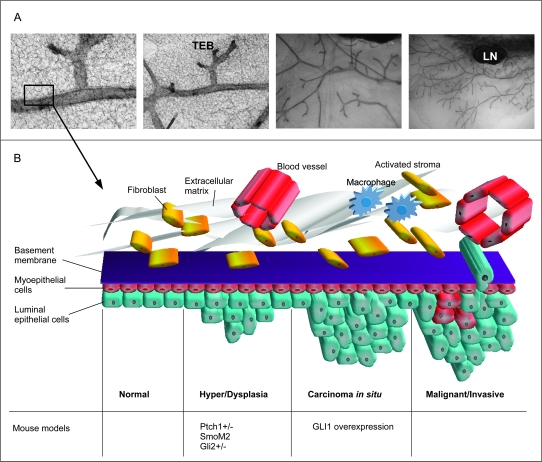
Mammary cancers arise from the epithelial component of the mammary gland. Normal mammary epithelium is composed of (i) a basal layer of myoepithelial cells (also termed basal cells), which is contractile and required for milk ejection and (ii) an inner layer of luminal cells, which produce the milk during lactation (31) (Figure 2). In the normal mammary gland, the basal layer of epithelial cells is situated adjacent to the basement membrane and expresses the cytokeratins K5, K14 and K17, a common characteristic to basal layers of stratified epithelia (32). Additionally, these cells express CD10 and smooth muscle actin (Sma), also found in contractile smooth muscle cells, hence the name ‘myoepithelium’ (33,34). Although the expression of these markers generally coincide in normal mammary glands, there is a small population of cells in which SMA and K5 expression do not overlap (35).
Fig. 2.
The Hh pathway and mammary gland tumour formation. (A) The structure of the normal mouse virgin mammary gland, whole mounts depicting the ductal tree and the terminal end buds are shown in increasing resolution from right to left. (B) A schematic illustration of cancer progression in the mammary gland in relation to mouse models with a de-regulated Hh pathway. Early lesions appear in the form of hyper and/or dysplasias in the mammary epithelium. These changes are seen in mice haploinsufficient for Ptch1 and in mice with targeted expression of a constitutively active form of Smo in the mammary gland (26,40). Hyper and dysplasias are also formed when normal mammary stem cells over-expressing Gli2 are transplanted into immunodeficient hosts (39). In the next step called carcinoma in situ (CIS), transformed cells appear in the dysplastic areas, but no invasive growth or metastases are observed. Such tumours are induced by targeted over-expression of GLI1 in the mammary gland (M.Fiaschi, B.Rozell, Å.Bergström, R.Toftgård, submitted). Development of fully malignant tumours induced by activated Hh signalling has not yet been reported. TEB, terminal end bud; LN, lymph node.
Human breast cancers can be classified as luminal or basal-like tumours, depending on their expression pattern of differentiation markers.
The luminal tumours generally express oestrogen receptor (ER), progesterone receptor and HER2. The basal-like tumours, however, lack ER, progesterone receptor and HER2 and are characterized by K5, K14 and K17 expression. A further subclassification of luminal tumours into luminal A, luminal B (basoluminal) and an intermediary subtype characterized by high HER2 and androgen expression levels is possible, as a result of gene expression profile characterization from large sets of human breast tumours (reviewed in ref. 36). The basal tumours have a poor clinical prognosis, partly due to a high metastatic rate.
The expression data of Hh pathway components in tumours are ambiguous. This may be explained by the heterogeneity in genetic characteristics of tumours and the degree of misregulation of other genes and/or proteins. As a reference, normal breast tissue expression levels for the PTCH1 protein are high, whereas other pathway components have low expression; in invasive ductal carcinoma and ductal carcinoma in situ, however, PTCH1 is expressed at lower levels than in the normal breast tissue (30). In contrast, a different study found positive correlation between increased expression of IHH, PTCH1 and GLI1/2/3 in invasive ductal carcinoma and proliferative index of the cancer, its invasiveness and metastasis (37). Yet, other data have shown that SHH, DHH and GLI1 are expressed at higher levels in certain breast cancer cell lines and in 30% of cancer samples when compared with normal mammary epithelial cells or epithelial tissue (30,38). It should be noted, however, that results obtained using immunohistochemistry are dependent on the quality of antibodies and should ultimately be confirmed at the messenger RNA level.
Histopathology of mammary tumours initiated by a de-regulated Hh pathway
Transgenic mouse models and an in vitro RNAi screen have been used to address the physiological consequences of modulated Hh signalling in the mammary gland.
An indication that Hh components are involved in breast carcinogenesis comes from transgenic expression studies of Smo and Gli2. Constitutively, active human SMO (SmoM2) in the mammary epithelium as well as over-expression of Gli2 in mammosphere (MS) initiating cells transplanted into immunosuppressed female mice both resulted in ductal hyperplasia (39,40). Further evidence was provided by a genome-wide RNAi screen, which demonstrated that GLI2 is necessary for the growth of breast cancer cell lines (41). Breast cancer cell lines, which also show elevated SHH, DHH and GLI1 protein expression undergo apoptosis upon cyclopamine treatment. (30,38). The mechanism of action of cyclopamine and CUR0199691 (Smo inhibitor), however, remains questionable, since these compounds can also inhibit growth of breast cancer cell lines with undetectable SMO expression (42).
A mouse model over-expressing human GLI1 in mouse mammary epithelial cells was the first Hh pathway mouse model to develop tumours (43,44) (M.Fiaschi, B.Rozell, Å.Bergström, R.Toftgård, in press in Cancer Research). The phenotype of these mice included an inability to lactate, accompanied by a reduction in the size and complexity of the lobuloalveolar network and incomplete differentiation of the secretory epithelium. In parallel with these changes, the tumours exhibited a basal or luminal/basal epithelial phenotype. Morphologically, these tumours are classified as ductal adenocarcinomas, solid tumours and squamous carcinomas. In terms of expression patterns, these tumours predominantly exhibited a basal keratin expression profile, are positive for vimentin, but show no Sma expression. Of note, the early GLI1-induced hyperplastic changes show Sma expression confirming their basal nature. In the later stages, loss of Sma coincides with the destruction of the basal membrane and loss of E-cadherin, a hallmark of the epithelial–mesenchymal transition (EMT) (M.Fiaschi, B.Rozell, Å.Bergström, R.Toftgård, submitted).
EMT is a change in the cellular morphology and physiology, which is characterized by the down-regulation of epithelial specific gene expression like cytokeratins and up-regulation of mesenchymal markers like vimentin. Additionally, the expression of several cell–cell adhesion molecules, such as E-cadherin, is down-regulated, enabling cells to escape the structural constraints of the tissue. EMT is considered an important step in the transformation process from normal cells into malignant tumour cells (reviewed in ref. 45). Nevertheless, although the GLI1-induced proliferations exhibited the molecular markers of EMT, no morphological signs of this process were noted. This suggests that GLI1 over-expression prepares cells for EMT but is not sufficient to complete the process (M.Fiaschi, B.Rozell, Å.Bergström, R.Toftgård, submitted).
Potential mechanisms of Hh pathway contribution in breast cancer development
The experimental data described above show that de-regulation of the Hh pathway can act as an initiating event for mammary tumour development or occur as a secondary event following other tumour-initiating genetic alterations. Below, we will discuss two possible scenarios of how Hh signalling could contribute to breast cancer, through: (i) de-regulating the stem cell compartment and (ii) modulating the epithelial–stromal interaction of normal and transformed epithelial cells. Further, we will discuss and summarize potential mechanisms on the molecular level, which could lead to the Hh pathway de-regulation and therefore breast cancer promotion.
Regulation of the epithelial compartment and mammary stem cells
The mammary epithelium is a heterogeneous population of cells, which form a complex tubuloalveolar structure. During each pregnancy–lactation cycle, the mammary epithelium undergoes proliferation and differentiation, followed by apoptosis during the involution process (46). The proliferative capabilities of the mammary epithelium, required for every pregnancy and lactation cycle, are fulfilled by a mammary stem cell population. Adult stem cells are long-lived, relatively undifferentiated cells, which maintain a tissue throughout life and are therefore vulnerable to accumulating mutations over time. These multi-potent and self-renewing cells thus serve as a likely target for cancer formation (47). While not fully characterized, electron microscopy studies have revealed a rare population of small, undifferentiated electro-lucent cells in basal and suprabasal layers, which are thought to represent mammary stem cells (48,49). The use of defined cell surface and biochemical markers and ex vivo culturing methods has enabled the sorting and functional characterization of different mammary epithelial cell populations, with different lineage reconstitution capabilities in human and mouse mammary glands (50–56). The ability to form clonal colonies—so-called mammospheres (‘MSs’)—under anchorage independent growth conditions has also been used to functionally isolate mammary stem cells (57). MSs are typically enriched in the cells able to renew and reconstitute mammary gland epithelial components. This assay can only be used as an indirect readout of changes in stem cell activity and not as a direct measurement for stem cell frequency, since MS can also be formed by lineage restricted luminal or myoepithelial progenitors (58).
Hh signalling is known to regulate the self-renewal of stem cells in the nervous system and human embryonic skin (59,60). It is therefore not surprising to find that PTCH1, GLI1 and GLI2 are expressed in MSs derived from normal human breast tissue. In concordance, activation of the Hh pathway, by adding SHH protein or over-expressing GLI1, GLI2 or SmoM2, results in increased MS formation (40). Conversely, inhibiting the Hh pathway with cyclopamine was reported to decrease MS formation efficiency of human mammary cells (39). GANT61—a potent small molecule GLI inhibitor (61)—also has a negative effect on MS formation in mouse epithelial cells (M.Kasper and R.Toftgård, unpublished data).
Unlike GLI2-transduced human MSs, the mammary reconstitution ability of cells derived from SmoM2-expressing mouse MSs was reduced compared with wild-type cells (39). From this, we can postulate that ectopic Hh signalling might increase the proliferation capabilities of mouse mammary progenitors rather than enlarge the multi-potent stem cell pool, thereby contributing to mammary cancer formation (40). In line with this data, Ptch1+/− mice show an expansion of mammary gland progenitor cells (CD29lowCD24+lin−), whereas the stem cell fraction (CD29highCD24+lin−) was reduced, resulting in an overall enhancement of proliferation. These results indicate that activation of Hh signalling causes a quiescence defect in the mammary stem cell-enriched fraction, altering the stem and progenitor cell balance. This proliferative effect is mediated by Ihh—expressed in progenitor cells—in an autocrine and paracrine manner. Moreover, pregnant Ptch1+/− dams show an increased progenitor population, whereas the stem cell fraction is constant, suggesting that Hh signalling plays a particular role in pregnancy-associated mammary gland expansion (62).
Like normal tissues, malignant tumours are thought to contain stem cell-like or, so-called cancer stem cells (CSCs), which maintain the cancer tissue and are able to reinitiate the tumour if transplanted to a receptive host. CSCs can be isolated from tumours of various organs including breast (63,64). The identification and characterization of breast CSCs may significantly improve breast cancer diagnosis and provide better patient-specific treatment (65). A case in point are the instances of human breast cancer where elevated numbers of cells expressing the stem cell marker ALDH1 correlate with poor prognosis (56). Surface marked CD44+CD24−/lowlin− cells, isolated from primary human breast cancer, represent CSCs, which when analysed, are capable of self-renewing and at the same time giving rise to ‘differentiated’ non-tumourigenic bulk tumour cells (Figure 3). The Hh pathway likely plays a role in CSCs, such as the CD44+CD24−/lowlin− breast cells having increased messenger RNA expression levels of PTCH1, GLI1 and GLI2 compared with the bulk of tumour cells (39,66).
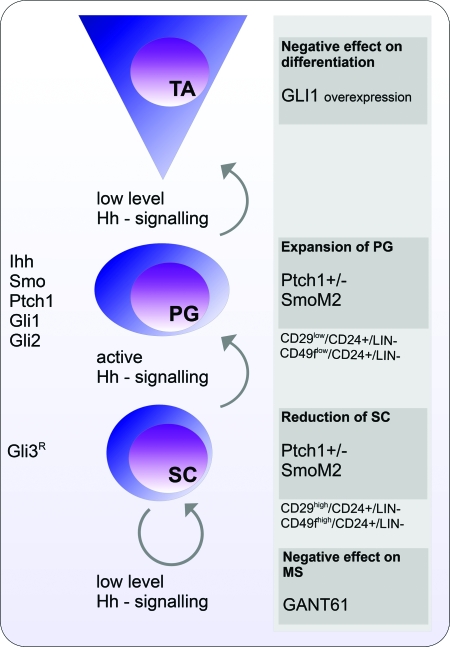
Fig. 3.
A proposed model illustrating the role of Hh signalling in stem cell maintenance and differentiation in the mammary gland. Stem cell (SC) maintenance is dependent on the presence of only a low level of Hh signalling achieved by expression of the Gli3 repressor form (Gli3R). At the same time, effective inhibition of Hh signalling by GANT61, a small molecule Gli inhibitor has a negative effect on MS formation (M.Kasper, unpublished data). When the Hh pathway is activated, progenitor cell (PG) expansion is promoted as shown in the Ptch1+/− and SmoM2 mouse models (26,40). PGs actively express Ihh, Smo, Ptch1, Gli1 and Gli2 (62). Differentiation requires subsequent down-regulation of the Hh pathway activity, since over-expression of GLI1 counteracts mammary gland epithelial cell differentiation. The cell surface markers characterizing the SC and PG compartments are indicated. TA, transit-amplifying cells.
Further evidence that Hh signalling may target the SC compartment comes from mice ectopically expressing GLI1 in the mammary epithelium, giving rise to three different types of tumours in the same mammary gland. This indicates that the oncogenic effect of GLI1 targets early progenitors or stem cells, which later develop into multiple tumour types (M.Fiaschi, B.Rozell, Å.Bergström, R.Toftgård, submitted). The amount of cells expressing cytokeratin 6 (K6), a marker of primitive progenitor cells in the mammary gland (67,68), is also significantly increased in GLI1-induced early basaloid lesions (M.Fiaschi, B.Rozell, Å.Bergström, R.Toftgård, submitted). These cells are negative for differentiation markers, supporting their progenitor cell nature. Interestingly, ectopic Wnt-1 expression in mouse mammary glands (MMTV/Wnt-1) results in the expansion of K6-positive cells and the formation of morphologically diverse tumours. Since this phenotype is similar to the GLI1 mouse model, this suggests that there is a common target cell population for both the Wnt and the Hh signalling pathway (69).
Modulation of the epithelial–mesenchymal interaction
Breast cancers do not only consist of epithelial tumour cells but also endothelial cells, forming intratumoural blood vessels, fibroblasts, infiltrating lymphocytes and macrophages (70). Together, these cells are referred to as the tumour stroma (Figure 2). It is clear that the tumour stroma plays an active role in mammary cancer progression and maintenance and can modulate the growth properties and response of mammary tumour cells to chemotherapy. It has also been shown that during the transformation process of normal mammary epithelial cells into tumour cells, stromal cells must be recruited, underlining the active participation of mammary gland stroma in breast cancer formation (71–74).
Hh signalling plays an important part in the communication between epithelial and stromal compartments of different tissues. For example, during human prostate development, SHH expression and consequently its target genes, PTCH1 and GLI1, are localized to the tip of the elongating tubules and in the surrounding stroma (75). The same expression pattern is seen in the growing hair follicles, where Shh is expressed by the keratinocytes, and Ptch1 and Gli1 can be detected in keratinocytes and in the surrounding stroma (76,77).
During pre-pregnancy mouse mammary gland development, Ihh is expressed in the terminal end buds and in the ductal epithelium. While Ptch1 is expressed in both the epithelium and the stroma, Gli2 is expressed only in the fat pad and the periductal stroma. During pregnancy, however, Gli2 is also expressed in the lobuloalveolar epithelium and in the epithelium of the smaller ducts. Mouse mammary glands, deficient in Gli2, thus exhibit various abnormalities including abnormal branching and ductal growth. The Gli2-deficient mammary explants also have a severely reduced periductal stroma, suggesting that the defects observed can be at least partly attributed to a de-regulated interplay between the mammary epithelium and periductal mesenchyme. Indeed, the abnormalities in mammary structure formation were largely rescued upon transplantation of Gli2-deficient epithelium into wild-type stroma, reinforcing the need for an intact Hh pathway, particularly functioning in the mesenchyme for coordinated mammary gland development (78). Moreover, when GLI1 is over-expressed in the mammary epithelium, an unusually dense layer of fibroblastic stroma is detected around the mammary ducts (44). This shows clearly that Hh signalling is necessary for coordinating epithelial–mesenchymal interactions. Interestingly, the primary role of Gli3 is to repress Hh signalling in the stroma during mammary gland formation, since loss of Gli3 expression induces Gli1 expression in the mammary mesenchymal compartment (79).
There are several indications that, similarly to normal tissues, also in tumours Hh ligands originate mainly from the epithelial compartment while both epithelium and stroma respond to the signal (80). In cells and tissue samples derived from benign and malignant human mammary tumours, the expression of GLI1 and GLI2 is considerably higher in fibroblasts than in epithelial cells. The epithelial cells express SHH and DHH at higher levels than fibroblasts, suggesting that the Hh ligands are produced by epithelial cells and activate the Hh pathway not only in the neighbouring epithelium but also in the stroma. Upon activation, the stroma provides feedback in the form of yet unidentified factors (38). Consistent with this view, observations in gastric adenocarcinomas and in prostate cancers show that SHH is expressed in the tumour tissue but PTCH1 and GLI1 are expressed both in the tumour tissue and the stroma (81,82). Until now, it has been believed that the main role of Hh signalling in cancer progression is cell-autonomous stimulation of the pathway in the Hh-expressing epithelial cells. In contrast, a recent study suggests that rather the Hh signal response in the stroma is the main determinant of tumour growth underlining the importance of stromal response in tumour progression (83).
Potential molecular mechanisms of Hh signalling in breast cancer
We have thus far touched upon the fact that Hh signalling modulates the stem cell compartment and the epithelial–mesenchymal interactions. Here, we will discuss in greater molecular detail how this may occur (Figure 4). The Hh pathway could play a major role in tumour initiation and formation via regulating cell proliferation and survival, regulating the expression of stem cell-related genes and preparing the tumour cells for the EMT. In parallel, Hh signalling can contribute to breast cancer formation by interaction with other main signalling pathways and pro-oncogenic factors. In addition, genetic events within pathways upstream of Gli transcription factors and changes in the local microenvironment can contribute to the activation of the Hh pathway.
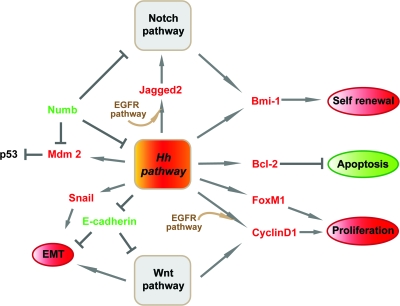
Fig. 4.
Potential molecular mechanisms of Hh signalling in breast cancer. Hh signalling contributes to breast tumourigenesis via its positive effects on proliferation, survival, self-renewal and EMT. These processes involve the activation of tumour promoting genes (red) and the down-regulation of genes with tumour suppressor functions (green). Some factors selectively enhance the expression of Hh target genes (brown).
Hh target genes.
Certain target genes of the Hh pathway, including Ccnd1, Bcl-2 and members of the Myc gene family (9), affect cell proliferation and survival. Consistently, the early proliferative lesions in mammary glands induced by GLI1 contain cells expressing high levels of Ccnd1 (M.Fiaschi, B.Rozell, Å.Bergström, R.Toftgård, submitted). Also the expression of the pro-survival protein BCL-2 is commonly up-regulated in a variety of tumours including human breast cancers (84,85). Moreover, elevated expression or activity of FOXM1—a hedgehog target gene, which is itself a transcription factor—is associated with the development and progression of breast cancer (86,87). FOXM1 regulates the expression of cell cycle-related genes essential for coordinated DNA synthesis and mitosis. Its inhibition causes disruption of mitosis and cell death in breast cancer cells making it a promising new potential target for breast cancer treatment (88,89).
Interaction with other pathways.
Hh signalling may modulate the tumourigenic behaviour of mammary cells in co-operation with other signalling pathways like epidermal growth factor receptor (EGFR) or Notch. For example, transcription of CCND1 and JAG2 (a Notch ligand) is up-regulated as a response to Hh pathway activation in human epithelial cells, which is potentiated due to synergism with active EGFR signalling directed through mitogen-activated protein kinase kinase/extracellular regulated kinase (MEK/ERK) (90). In concordance, a substantial number of breast cancers show increased EGFR expression; nevertheless, a correlation to the clinical outcome cannot yet be made (91). In addition, two recent studies show a correlation of basal-like breast cancer subtypes with EGFR positivity. Comparative genomic hybridization of human breast cancer samples revealed a significant gain in DNA copy number of EGFR in triple negative tumours (92) and protein array analysis of 30 breast cancer cell lines showed that cell lines defined as basal like have high levels of EGFR protein and phosphorylated ERK1/2 (93). There is no evidence so far connecting the HER2 expression, which is a prognostic marker for non-favourable clinical outcome of breast cancer, with the Hh pathway. Of note, basal-like tumours are mostly negative for HER2 (94).
Analysis of the Notch pathway provides another perspective on basal-like and luminal tumours and cell fate. Bouras et al. (95) recently found the Notch pathway to be important for the expansion of luminal progenitor cells (CD29lowCD24+CD61+lin−). As expected, over-expression of human NICD1—a cleaved (active) form of the Notch1 receptor—in luminal progenitor cells leads to formation of hyperplastic nodules and tumours in the mouse mammary gland. The expression pattern of the NCID1-derived luminal tumours is the opposite when compared with the GLI1 or Wnt-1 basal-like induced tumours. Intriguingly, over-expression of NICD1 in the mammary stem cell-enriched population (CD29hiCD24+lin−), which contains the basal epithelial cells, also results in the development of luminal tumours. At this point, one can speculate that inappropriate activation of Notch signalling in the basal epithelial compartment directs the fate of the tumour into the luminal subtype. Whether a developing tumour finally acquires a basal or luminal fate is likely dependent on the balance between the different oncogenic signals and pathways directing the fate from initially basal like into luminal tumours. Consequently, the frequent activation of the Notch pathway in breast tumours is consistent with high prevalence of the luminal subtype of breast cancers. Moreover, the ability of active Notch signalling to sustain progenitor self-renewal and expansion could explain the presence of luminal breast tumours with an unfavourable prognosis.
Both, activated Wnt and Hh signalling in mouse mammary epithelium, lead to tumours with similar basal-like characteristics as discussed previously, and thus, both pathways are believed to target mammary stem cells or early progenitors (69). Hence, one would expect these pathways to synergize in breast cancer formation. Surprisingly, no direct molecular link has been found yet connecting these pathways during mammary cancer development. On the contrary, a gene activated as a response to Hh signalling, SFRP1—a secreted frizzled-related protein counteracting Wnt pathway activation (96,97)—is associated with poor prognosis in human breast carcinomas (98). Interestingly, the expression of SFRP1 and the related SFRP2 and SFRP5 is silenced by promoter methylation in breast cancer and breast cancer cell lines (99–104). It can be speculated that overall the genes regulated by Hh signalling support cancer growth, but simultaneous epigenetic silencing of selected target genes is crucial to allow cancer progression.
EMT.
Ectopic expression of GLI1 in the mouse mammary gland causes elevated expression of the transcription factor Snail and concomitant loss of E-cadherin—processes characteristic for EMT (44). Corroborating evidence includes the up-regulation of Snail in basal cell carcinomas and the induction of Snail expression by Gli1 (105,106). Snail expression is in fact necessary for the repression of E-cadherin, thus promoting EMT (107). It has been suggested that the expression of FOXC2—involved in EMT and the metastatic phenotype of the basal-like breast cancers—can be modulated by Hh signalling in an indirect way presumably via Snail (108,109). Moreover, the majority of invasive ductal and lobular breast carcinomas have lost both, E-cadherin and membranous β-catenin expression (110,111). Possibly, the down-regulation of E-cadherin by Hh signalling promotes the release of β-catenin—bound to a membranous complex involving E-cadherin—and thus activates Wnt signalling.
Stem cells.
Hh signalling leads to an increased expression of Bmi-1 in isolated mammary epithelial stem cells and CSCs (39). Bmi-1 is a transcriptional repressor belonging to the polycomb gene family and its suppressor functions are involved in maintaining neuronal, haematopoietic and mammary gland stem cells (39,112,113). In concordance, the GLI1-induced mammary tumours also show increased Bmi-1 expression (M.Fiaschi, B.Rozell, Å.Bergström, R.Toftgård, submitted). Over-expression of BMI-1 induces telomerase activity and can immortalize normal human mammary cells (114). It should be noted that Bmi-1 is also up-regulated in the CD133+ glioma initiating cells, underlining its universal role in supporting both normal and CSC maintenance (115,116).
Indirect activation of Hh signalling.
In addition to genetic events directly affecting Hh pathway components, the activation of Hh signalling in breast tumours can occur also as a secondary event due to alterations in other pathways or as a response to changes in the metabolic state of cells. For example, loss of the adaptor protein Numb is a common event in breast carcinomas (117). Numb is involved in cell fate determination, though the exact mechanism of action is not clear. The Numb protein interacts with various ubiquitin ligases including Itch, which is able to polyubiquitinate Gli1 and directs the latter to degradation and thus helps to control the activity status of the Hh pathway (118). Loss of Numb also results in the activation of the Notch pathway and the degradation of the tumour suppressor protein p53. Numb normally inhibits the activity of the main p53 E3 ubiquitin ligase Mdm2 and thereby stabilizes p53 (119). The loss of wild-type p53 activity resulting from Numb deletion is thus even more dramatic since the concomitant activation of Hh signalling can increase the expression of Mdm2, resulting in complete loss of p53 function (120). Since it has been suggested that transcription of Bmi-1 is also positively regulated by Notch signalling, the simultaneous activation of both Hh and Notch pathways can result in hyperactivation of Bmi-1 (121). Furthermore, it has been suggested that the Hh and Notch pathways might form a positive feedback loop both in normal mammary development and tumour formation (122). Of note, also Wnt's oncogenic action during transformation of normal human mammary epithelial cells is dependent on an active Notch pathway (69,123).
Hypoxia, often occurring in the malignant tumour environment, induces EMT and neovasculogenesis via hypoxia-inducible factors HIF1 or HIF2. This is accompanied by Twist up-regulation, which is a major determinant in the down-regulation of E-cadherin in breast cancer (124,125). Finally, Twist can activate GLI1 transcription integrating environmental hypoxia with Hh signalling (126,127).
Clinical implications of Hh signalling in breast cancer
Despite lack of conclusive data regarding the biological significance of Hh signalling in human breast cancer maintenance, progression and metastasis, it is relevant to consider possible effects of Hh pathway inhibitors in breast cancer treatment. Hh signalling inhibitors are of potential interest as part of a combinatorial cancer therapy by (i) counteracting multi-drug resistance and CSC maintenance, (ii) inhibiting other tumour-promoting and maintenance signals and/or pathways and (iii) disrupting the growth-supporting interaction with the tumour stroma.
The conventional non-targeted chemotherapies such as anthracycline and taxane-derived drugs mainly target rapidly dividing cells due to their cytotoxic effect (for a detailed review see ref. 128). The resistance of a subset of cancer cells, presumably CSCs, is attributed to their slow-cycling nature and/or multi-drug resistance by modulation of the drug transport activity (reviewed in ref. 129). Increased drug efflux is commonly mediated by members of the adenosine triphosphate-binding cassette transporter family, some of which are known to be induced by SHH. Conversely, the inhibition of Hh signalling increases the response of cancer cell lines to classical chemotherapeutic treatments (130). Today's breast cancer therapies are when possible a combination of cytostatic and targeted drugs. For example, luminal tumours can be treated on the basis of their frequent ER or HER2 positivity. In contrast, the basal-like breast carcinomas, having similarities to those induced by ectopic Hh signalling in animal experiments, have a poor prognosis due to a high metastasis rate and lack of effective therapeutic agents (131).
The main current targeted breast cancer therapies inhibit HER2 (Trastuzumab) or ER (Tamoxifen). Unfortunately, the majority of basal-like tumours are negative for HER2 and ER, though recently reported often EGFR positive (93). Therefore, an interesting future strategy could be treatment with a combination of EGFR and Hh/Gli inhibitors.
Experiments in mice and early phase clinical trials have already shown that the inhibition of Hh signalling at the receptor level can counteract skin cancer and metastasis formation (132,133). There are several small molecule compounds with therapeutic potential under development, which can inhibit the Hh pathway at different levels [for a detailed review see ref. 134]. For instance, inhibition of Hh signalling using HhAntag targeting Smo in medulloblastomas developing in Ptch+/−p53−/− mice decreased cell proliferation, increased cell death and resulted in eradication of the tumour (135). Experiments using a human glioma xenograft model show that Hh signalling can regulate the self-renewal capabilities of the CSC population as indicated by the response to cyclopamine and SMO-small hairpin RNA (shRNA). Moreover, in this study the inhibition of Hh signalling potentiated the anti-proliferative effect of conventional chemotherapy (116). Importantly, the inhibition of Hh signalling (by cyclopamine or SMO-shRNA) in human melanoma xenografts not only reduced proliferation and prevented recurrence but also prevented melanoma cells injected into the tail vein from metastasizing to the lung (136). Similar tumour growth prevention was obtained in xenografts of a human prostate carcinoma cell line using GANT-61, which blocks GLI-mediated transcription of Hh target genes (61). Hh pathway inhibitors possibly potentiate the effect of cytostatic drugs by down-regulation of pro-survival genes such as CCND1 and BCL-2. In addition, Hh inhibitors may reduce formation of metastases, since metastasizing human prostate cancers show higher GLI1 and PTCH1 messenger RNA levels compared with non-metastatic tumours (137).
The initial concept of cell-autonomous Hh signalling in epithelial cancer cells as the driving force for tumour formation has recently been challenged. Yauch et al. (83) showed that Hh proteins are secreted to activate the pathway in non-malignant stroma cells. Intriguingly, specific inhibition of Hh signalling using HhAntag or genetic deletion of Smo in the mouse stroma resulted in growth inhibition in xenograft tumour models. Moreover, elevated expression of GLI1 and PTCH1 has been detected in the surrounding stroma of human esophageal and prostate tumours (81,138) and ectopic SHH expression in prostate tumour cells in xenografts induced Ptch1 and Gli1 expression in the surrounding stroma associated with increased tumour proliferation (81). Hence, Hh inhibitors are likely to disrupt the communication between tumour cells and their microenvironment, a process reminiscent of the normal developmental role of Hh signalling in the mammary gland. However, Nolan-Stevaux et al. (139) showed that although the presence of Smo in epithelial cells in a mouse model of pancreatic cancer was dispensable for tumour development, Gli1 was nonetheless expressed at elevated levels in tumour cells and contributed to tumour cell proliferation. The potential involvement of both autocrine and paracrine Hh signalling in tumour formation argues in favour for evaluation of inhibition of this pathway in the development of new breast cancer therapies.
Concluding remarks
The data linking Hh signalling with mammary cancer are increasing but still limited. We can, however, conclude that up-regulation of Hh signalling is able to cause mammary cancer in a mouse model and many human tumour samples show de-regulated Hh signalling. Although, postulating the involvement of this pathway in human breast cancer initiation is still preliminary, the prior established roles in stem cell maintenance and coordination of epithelial–mesenchymal interactions imply that the Hh pathway is potentially a useful target in a complex multi-target breast cancer therapy. Modulating its activity may affect key aspects of cancer progression such as stem cell maintenance, signalling between epithelium and stroma, and EMT.
Funding
Swedish Cancer Society, Swedish Research Council and NIH (AR47898 and U01CA105491) to R.T.; Wenner-Gren Foundation and Lars Hierta's and Syskonen Svenssons' foundations to M.K.; Marie-Curie Intra-European Reintegration Grant, European Comission's project ECOGENE and Lars Hierta's foundation to V.J.
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CSC
cancer stem cell
- Dhh
Desert hedgehog
- EGFR
epidermal growth factor receptor
- EMT
epithelial–mesenchymal transition
- ER
oestrogen receptor
- Hh
hedgehog
- Ihh
Indian hedgehog
- MS
mammosphere
- Ptch1
Patched
- SFRP
secreted frizzled-related protein
- Shh
Sonic hedgehog
- SMA
smooth muscle actin
- Smo
Smoothened
Note: Human genes and proteins are in capital letters
References
- 1.Hooper JE, et al. Communicating with Hedgehogs. Nat. Rev. Mol. Cell Biol. 2005;6:306–317. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 2.Beachy PA, et al. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 3.Rubin LL, et al. Targeting the Hedgehog pathway in cancer. Nat. Rev. Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 4.Hatsell S, et al. Hedgehog signaling in mammary gland development and breast cancer. J. Mammary Gland Biol. Neoplasia. 2007;12:163–173. doi: 10.1007/s10911-007-9048-2. [DOI] [PubMed] [Google Scholar]
- 5.Stingl J, et al. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat. Rev. Cancer. 2007;7:791–799. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- 6.Ingham PW, et al. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, et al. Hedgehog signaling in development and cancer. Dev. Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai CB, et al. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev. Cell. 2004;6:103–115. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 9.Kasper M, et al. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur. J. Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Dai P, et al. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J. Biol. Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 11.Ikram MS, et al. GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J. Invest. Dermatol. 2004;122:1503–1509. doi: 10.1111/j.0022-202X.2004.22612.x. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz i Altaba A, et al. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oro AE. The primary cilia, a ‘Rab-id’ transit system for hedgehog signaling. Curr. Opin. Cell Biol. 2007;19:691–696. doi: 10.1016/j.ceb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 15.Huangfu D, et al. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl Acad. Sci. USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varjosalo M, et al. Hedgehog signaling. J. Cell Sci. 2007;120:3–6. doi: 10.1242/jcs.03309. [DOI] [PubMed] [Google Scholar]
- 17.Kogerman P, et al. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat. Cell Biol. 1999;1:312–319. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- 18.Svard J, et al. Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev. Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 19.High A, et al. Basal cell nevus syndrome. Curr. Opin. Oncol. 2005;17:160–166. doi: 10.1097/01.cco.0000154108.99236.ed. [DOI] [PubMed] [Google Scholar]
- 20.Oro AE, et al. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 21.Xie J, et al. Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res. 1997;57:2369–2372. [PubMed] [Google Scholar]
- 22.Wicking C, et al. No evidence for the H133Y mutation in SONIC HEDGEHOG in a collection of common tumour types. Oncogene. 1998;16:1091–1093. doi: 10.1038/sj.onc.1201644. [DOI] [PubMed] [Google Scholar]
- 23.Vorechovsky I, et al. The patched/hedgehog/smoothened signalling pathway in human breast cancer: no evidence for H133Y SHH, PTCH and SMO mutations. Eur. J. Cancer. 1999;35:711–713. doi: 10.1016/s0959-8049(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 24.Sjoblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 25.Chang-Claude J, et al. The patched polymorphism Pro1315Leu (C3944T) may modulate the association between use of oral contraceptives and breast cancer risk. Int. J. Cancer. 2003;103:779–783. doi: 10.1002/ijc.10889. [DOI] [PubMed] [Google Scholar]
- 26.Lewis MT, et al. Defects in mouse mammary gland development caused by conditional haploinsufficiency of Patched-1. Development. 1999;126:5181–5193. doi: 10.1242/dev.126.22.5181. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto H, et al. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev. Biol. 2002;245:280–290. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- 28.Naylor TL, et al. High resolution genomic analysis of sporadic breast cancer using array-based comparative genomic hybridization. Breast Cancer Res. 2005;7:R1186–R98. doi: 10.1186/bcr1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nessling M, et al. Candidate genes in breast cancer revealed by microarray-based comparative genomic hybridization of archived tissue. Cancer Res. 2005;65:439–447. [PubMed] [Google Scholar]
- 30.Wolf I, et al. Unmasking of epigenetically silenced genes reveals DNA promoter methylation and reduced expression of PTCH in breast cancer. Breast Cancer Res. Treat. 2007;105:139–155. doi: 10.1007/s10549-006-9440-4. [DOI] [PubMed] [Google Scholar]
- 31.Daniel CW, et al. The mammary gland: a model for development. J. Mammary Gland Biol. Neoplasia. 1999;4:3–8. doi: 10.1023/a:1018796301609. [DOI] [PubMed] [Google Scholar]
- 32.Gusterson BA, et al. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res. 2005;7:143–148. doi: 10.1186/bcr1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gusterson BA, et al. Identification of myoepithelial cells in human and rat breasts by anti-common acute lymphoblastic leukemia antigen antibody A12. J. Natl Cancer Inst. 1986;77:343–349. [PubMed] [Google Scholar]
- 34.Gugliotta P, et al. Specific demonstration of myoepithelial cells by anti-alpha smooth muscle actin antibody. J. Histochem. Cytochem. 1988;36:659–663. doi: 10.1177/36.6.3367051. [DOI] [PubMed] [Google Scholar]
- 35.Zhang RR, et al. A subset of morphologically distinct mammary myoepithelial cells lacks corresponding immunophenotypic markers. Breast Cancer Res. 2003;5:R151–R156. doi: 10.1186/bcr635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sims AH, et al. Origins of breast cancer subtypes and therapeutic implications. Nat. Clin. Pract. Oncol. 2007;4:516–525. doi: 10.1038/ncponc0908. [DOI] [PubMed] [Google Scholar]
- 37.Xuan Y, et al. Expression of Indian Hedgehog signaling molecules in breast cancer. J. Cancer Res. Clin. Oncol. 2009;135:235–240. doi: 10.1007/s00432-008-0451-x. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee S, et al. Hedgehog signaling and response to cyclopamine differ in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol. Ther. 2006;5:674–683. doi: 10.4161/cbt.5.6.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moraes RC, et al. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development. 2007;134:1231–1242. doi: 10.1242/dev.02797. [DOI] [PubMed] [Google Scholar]
- 41.Iorns E, et al. Identification of CDK10 as an important determinant of resistance to endocrine therapy for breast cancer. Cancer Cell. 2008;13:91–104. doi: 10.1016/j.ccr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, et al. Cyclopamine inhibition of human breast cancer cell growth independent of Smoothened (Smo) Breast Cancer Res. Treat. 2008 doi: 10.1007/s10549-008-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunther EJ, et al. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 2002;16:283–292. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- 44.Fiaschi M, et al. Targeted expression of GLI1 in the mammary gland disrupts pregnancy-induced maturation and causes lactation failure. J. Biol. Chem. 2007;282:36090–36101. doi: 10.1074/jbc.M704280200. [DOI] [PubMed] [Google Scholar]
- 45.Hugo H, et al. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J. Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 46.Hennighausen L, et al. Signaling pathways in mammary gland development. Dev. Cell. 2001;1:467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- 47.Dontu G, et al. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(suppl. 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chepko G, et al. Ultrastructure of the putative stem cell niche in rat mammary epithelium. Tissue Cell. 2003;35:83–93. doi: 10.1016/s0040-8166(02)00107-6. [DOI] [PubMed] [Google Scholar]
- 49.Chepko G, et al. Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell. 1997;29:239–253. doi: 10.1016/s0040-8166(97)80024-9. [DOI] [PubMed] [Google Scholar]
- 50.Stingl J, et al. Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation. 1998;63:201–213. doi: 10.1111/j.1432-0436.1998.00201.x. [DOI] [PubMed] [Google Scholar]
- 51.Stingl J, et al. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res. Treat. 2001;67:93–109. doi: 10.1023/a:1010615124301. [DOI] [PubMed] [Google Scholar]
- 52.Stingl J, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 53.Villadsen R, et al. Evidence for a stem cell hierarchy in the adult human breast. J. Cell Biol. 2007;177:87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 55.Smalley MJ, et al. Clonal characterization of mouse mammary luminal epithelial and myoepithelial cells separated by fluorescence-activated cell sorting. In Vitro Cell. Dev. Biol. Anim. 1998;34:711–721. doi: 10.1007/s11626-998-0067-0. [DOI] [PubMed] [Google Scholar]
- 56.Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dontu G, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao MJ, et al. Enrichment of a population of mammary gland cells that form mammospheres and have in vivo repopulating activity. Cancer Res. 2007;67:8131–8138. doi: 10.1158/0008-5472.CAN-06-4493. [DOI] [PubMed] [Google Scholar]
- 59.Zhou JX, et al. Role of sonic hedgehog in maintaining a pool of proliferating stem cells in the human fetal epidermis. Hum. Reprod. 2006;21:1698–1704. doi: 10.1093/humrep/del086. [DOI] [PubMed] [Google Scholar]
- 60.Palma V, et al. Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex. Development. 2004;131:337–345. doi: 10.1242/dev.00930. [DOI] [PubMed] [Google Scholar]
- 61.Lauth M, et al. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc. Natl Acad. Sci. USA. 2007;104:8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li N, et al. Reciprocal intraepithelial interactions between TP63 and hedgehog signaling regulate quiescence and activation of progenitor elaboration by mammary stem cells. Stem Cells. 2008;26:1253–1264. doi: 10.1634/stemcells.2007-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Hajj M. Cancer stem cells and oncology therapeutics. Curr. Opin. Oncol. 2007;19:61–64. doi: 10.1097/CCO.0b013e328011a8d6. [DOI] [PubMed] [Google Scholar]
- 64.Al-Hajj M, et al. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kakarala M, et al. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J. Clin. Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shipitsin M, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Smith GH, et al. Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth Differ. 1990;1:161–170. [PubMed] [Google Scholar]
- 68.Grimm SL, et al. Keratin 6 is not essential for mammary gland development. Breast Cancer Res. 2006;8:R29. doi: 10.1186/bcr1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc. Natl Acad. Sci. USA. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gregoire M, et al. The role of fibroblasts in tumor behavior. Cancer Metastasis Rev. 1995;14:339–350. doi: 10.1007/BF00690602. [DOI] [PubMed] [Google Scholar]
- 71.Orimo A, et al. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 72.Sadlonova A, et al. Breast fibroblasts modulate epithelial cell proliferation in three-dimensional in vitro co-culture. Breast Cancer Res. 2005;7:R46–R59. doi: 10.1186/bcr949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shekhar MP, et al. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- 74.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 75.Lamm ML, et al. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev. Biol. 2002;249:349–366. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- 76.Chiang C, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev. Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- 77.St-Jacques B, et al. Sonic hedgehog signaling is essential for hair development. Curr. Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 78.Lewis MT, et al. The Gli2 transcription factor is required for normal mouse mammary gland development. Dev. Biol. 2001;238:133–144. doi: 10.1006/dbio.2001.0410. [DOI] [PubMed] [Google Scholar]
- 79.Hatsell SJ, et al. Gli3-mediated repression of Hedgehog targets is required for normal mammary development. Development. 2006;133:3661–3670. doi: 10.1242/dev.02542. [DOI] [PubMed] [Google Scholar]
- 80.Ruiz i Altaba A. Therapeutic inhibition of Hedgehog-GLI signaling in cancer: epithelial, stromal, or stem cell targets? Cancer Cell. 2008;14:281–283. doi: 10.1016/j.ccr.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 81.Fan L, et al. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145:3961–3970. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 82.Ma X, et al. Frequent activation of the hedgehog pathway in advanced gastric adenocarcinomas. Carcinogenesis. 2005;26:1698–1705. doi: 10.1093/carcin/bgi130. [DOI] [PubMed] [Google Scholar]
- 83.Yauch RL, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 84.Regl G, et al. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- 85.Callagy GM, et al. Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer. 2008;8:153. doi: 10.1186/1471-2407-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Teh MT, et al. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res. 2002;62:4773–80. [PubMed] [Google Scholar]
- 87.Schuller U, et al. Forkhead transcription factor FoxM1 regulates mitotic entry and prevents spindle defects in cerebellar granule neuron precursors. Mol. Cell Biol. 2007;27:8259–8270. doi: 10.1128/MCB.00707-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kwok JM, et al. Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression. Mol. Cancer Ther. 2008;7:2022–2032. doi: 10.1158/1535-7163.MCT-08-0188. [DOI] [PubMed] [Google Scholar]
- 89.Bektas N, et al. Tight correlation between expression of the Forkhead transcription factor FOXM1 and HER2 in human breast cancer. BMC Cancer. 2008;8:42. doi: 10.1186/1471-2407-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kasper M, et al. Selective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Mol. Cell Biol. 2006;26:6283–6298. doi: 10.1128/MCB.02317-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klijn JG, et al. The prognostic value of epidermal growth factor receptor (EGF-R) in primary breast cancer: results of a 10 year follow-up study. Breast Cancer Res. Treat. 1994;29:73–83. doi: 10.1007/BF00666183. [DOI] [PubMed] [Google Scholar]
- 92.Andre F, et al. Molecular characterization of breast cancer with high-resolution oligonucleotide comparative genomic hybridization array. Clin. Cancer Res. 2009;15:441–451. doi: 10.1158/1078-0432.CCR-08-1791. [DOI] [PubMed] [Google Scholar]
- 93.Boyd ZS, et al. Proteomic analysis of breast cancer molecular subtypes and biomarkers of response to targeted kinase inhibitors using reverse-phase protein microarrays. Mol. Cancer Ther. 2008;7:3695–3706. doi: 10.1158/1535-7163.MCT-08-0810. [DOI] [PubMed] [Google Scholar]
- 94.Rakha EA, et al. Triple-negative/basal-like breast cancer: review. Pathology. 2009;41:40–47. doi: 10.1080/00313020802563510. [DOI] [PubMed] [Google Scholar]
- 95.Bouras T, et al. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 96.He J, et al. Suppressing Wnt signaling by the hedgehog pathway through sFRP-1. J. Biol. Chem. 2006;281:35598–35602. doi: 10.1074/jbc.C600200200. [DOI] [PubMed] [Google Scholar]
- 97.Eichberger T, et al. Overlapping and distinct transcriptional regulator properties of the GLI1 and GLI2 oncogenes. Genomics. 2006;87:616–632. doi: 10.1016/j.ygeno.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Reedijk M, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 2005;65:8530–8537. doi: 10.1158/0008-5472.CAN-05-1069. [DOI] [PubMed] [Google Scholar]
- 99.Lo PK, et al. Epigenetic suppression of secreted frizzled related protein 1 (SFRP1) expression in human breast cancer. Cancer Biol. Ther. 2006;5:281–286. doi: 10.4161/cbt.5.3.2384. [DOI] [PubMed] [Google Scholar]
- 100.Klopocki E, et al. Loss of SFRP1 is associated with breast cancer progression and poor prognosis in early stage tumors. Int. J. Oncol. 2004;25:641–649. [PubMed] [Google Scholar]
- 101.Veeck J, et al. Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene. 2006;25:3479–3488. doi: 10.1038/sj.onc.1209386. [DOI] [PubMed] [Google Scholar]
- 102.Veeck J, et al. Promoter hypermethylation of the SFRP2 gene is a high-frequent alteration and tumor-specific epigenetic marker in human breast cancer. Mol. Cancer. 2008;7:83. doi: 10.1186/1476-4598-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suzuki H, et al. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br. J. Cancer. 2008;98:1147–56. doi: 10.1038/sj.bjc.6604259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Veeck J, et al. Epigenetic inactivation of the secreted frizzled-related protein-5 (SFRP5) gene in human breast cancer is associated with unfavorable prognosis. Carcinogenesis. 2008;29:991–998. doi: 10.1093/carcin/bgn076. [DOI] [PubMed] [Google Scholar]
- 105.Louro ID, et al. Comparative gene expression profile analysis of GLI and c-MYC in an epithelial model of malignant transformation. Cancer Res. 2002;62:5867–5873. [PubMed] [Google Scholar]
- 106.Li X, et al. Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene. 2006;25:609–621. doi: 10.1038/sj.onc.1209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li X, et al. Gli1 acts through Snail and E-cadherin to promote nuclear signaling by beta-catenin. Oncogene. 2007;26:4489–4498. doi: 10.1038/sj.onc.1210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamagishi H, et al. Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer. Genes Dev. 2003;17:269–281. doi: 10.1101/gad.1048903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mani SA, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc. Natl Acad. Sci. USA. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bankfalvi A, et al. Immunophenotypic and prognostic analysis of E-cadherin and beta-catenin expression during breast carcinogenesis and tumour progression: a comparative study with CD44. Histopathology. 1999;34:25–34. doi: 10.1046/j.1365-2559.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- 111.Karayiannakis AJ, et al. Expression patterns of beta-catenin in in situ and invasive breast cancer. Eur. J. Surg. Oncol. 2001;27:31–36. doi: 10.1053/ejso.1999.1017. [DOI] [PubMed] [Google Scholar]
- 112.Park IK, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 113.Molofsky AV, et al. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dimri GP, et al. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 2002;62:4736–4745. [PubMed] [Google Scholar]
- 115.Leung C, et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004;428:337–341. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- 116.Clement V, et al. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pece S, et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J. Cell Biol. 2004;167:215–221. doi: 10.1083/jcb.200406140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Marcotullio L, et al. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nat. Cell Biol. 2006;8:1415–1423. doi: 10.1038/ncb1510. [DOI] [PubMed] [Google Scholar]
- 119.Colaluca IN, et al. NUMB controls p53 tumour suppressor activity. Nature. 2008;451:76–80. doi: 10.1038/nature06412. [DOI] [PubMed] [Google Scholar]
- 120.Abe Y, et al. Hedgehog signaling overrides p53-mediated tumor suppression by activating Mdm2. Proc. Natl Acad. Sci. USA. 2008;105:4838–4843. doi: 10.1073/pnas.0712216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang H, Sr, et al. The study of the relationship between Notch signal transduction pathway and polycomb protein Bmi1 in colorectal cancer cells. J. Clin. Oncol. (Meeting Abstracts) 2007;25:14501. [Google Scholar]
- 122.Liu S, et al. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ayyanan A, et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc. Natl Acad. Sci. USA. 2006;103:3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang L, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J. Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gort EH, et al. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2alpha. Oncogene. 2008;27:1501–1510. doi: 10.1038/sj.onc.1210795. [DOI] [PubMed] [Google Scholar]
- 126.Vesuna F, et al. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem. Biophys. Res. Commun. 2008;367:235–241. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Villavicencio EH, et al. Cooperative E-box regulation of human GLI1 by TWIST and USF. Genesis. 2002;32:247–258. doi: 10.1002/gene.10078. [DOI] [PubMed] [Google Scholar]
- 128.Chabner BA, et al. Timeline: chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 129.Lu C, et al. Chemoresistance in gliomas. Mol. Cell Biochem. 2008;312:71–80. doi: 10.1007/s11010-008-9722-8. [DOI] [PubMed] [Google Scholar]
- 130.Sims-Mourtada J, et al. Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene. 2007;26:5674–5679. doi: 10.1038/sj.onc.1210356. [DOI] [PubMed] [Google Scholar]
- 131.Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tabs S, et al. Induction of the differentiation and apoptosis of tumor cells in vivo with efficiency and selectivity. Eur. J. Dermatol. 2004;14:96–102. [PubMed] [Google Scholar]
- 133.Von Hoff D, et al. Efficacy data of GDC-0449, a systemic Hedgehog pathway antagonist, in a first-in-human, first-in-class Phase I study with locally advanced, multifocal or metastatic basal cell carcinoma patients. 2008 AACR Meeting Abstracts. LB-138. [Google Scholar]
- 134.Kolterud Å, et al. Strategies for Hedgehog inhibition and its potential role in cancer treatment. Drug Discov. Today Ther. Strat. 2007;4:229–235. [Google Scholar]
- 135.Romer JT, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 136.Stecca B, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc. Natl Acad. Sci. USA. 2007;104:5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karhadkar SS, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 138.Ma X, et al. Hedgehog signaling is activated in subsets of esophageal cancers. Int. J. Cancer. 2006;118:139–148. doi: 10.1002/ijc.21295. [DOI] [PubMed] [Google Scholar]
- 139.Nolan-Stevaux O, et al. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]