Abstract
Background and Purpose
Activated protein C (APC), a protease with anticoagulant and cytoprotective activities, protects neurons and endothelium from ischemic injury. Drotrecogin-alfa activated, a hyperanticoagulant form of human recombinant APC, is currently being studied in patients with ischemic stroke. How changes in APC anticoagulant activity influence APC’s neuroprotection and risk for bleeding is not clear.
Methods
We used neuronal and brain endothelial cell injury models and middle cerebral artery occlusion in mice to compare efficacy and safety of drotrecogin-alfa activated and human 3K3A-APC, an APC nonanticoagulant mutant.
Results
Drotrecogin-alfa activated and 3K3A-APC exhibited 148% and 10% of plasma-derived APC’s anticoagulant activity and differ in the carbohydrate content. 3K3A-APC protected mouse neurons from N-methyl-d-aspartate-induced apoptosis and human brain endothelial cell from oxygen-glucose deprivation with 1.8- and 3.1-fold greater efficacy than drotrecogin-alfa activated. Given 5 minutes before transient middle cerebral artery occlusion, 3K3A-APC and drotrecogin-alfa activated (0.5 and 2 mg/kg intravenously) reduced comparably and dose-dependently the infarction lesion up to 85%. 3K3A-APC, but not drotrecogin-alfa activated, improved neurological score dose-dependently (P<0.05). 3K3A-APC did not cause bleeding. In contrast, drotrecogin-alfa activated dose-dependently increased hemoglobin content in postischemic brain. After permanent middle cerebral artery occlusion, 3K3A-APC multidose therapy (1 mg/kg intravenously at 12 hours and 1, 3, 5, and 7 days) improved functional recovery and reduced infarction by 60% with no risk for bleeding, whereas drotrecogin-alfa activated increased hemoglobin deposition in the postischemic brain and showed relatively modest neuroprotection.
Conclusions
Nonanticoagulant 3K3A-APC exhibits greater neuroprotective efficacy with no risk for bleeding compared with drotrecogin-alfa activated, a hyperanticoagulant form of APC.
Keywords: anticoagulant activity, brain ischemia, intracerebral microhemorrhage, neuroprotection, proteases
Activated protein C (APC) is a serine protease with systemic anticoagulant, anti-inflammatory, and antiapoptotic activities.1 Its anticoagulant activity is independent of its cellular effects. APC is generated from its zymogen protein C that is activated by thrombin on the surface of the endothelial cells, which requires 2 membrane receptors, thrombomodulin and endothelial protein C receptor. APC’s anticoagulant activity is mediated by irreversible proteolytic degradation of factors Va and VIIIa with contributions by various cofactors, whereas its cytoprotective activities are mediated by proteolytic activation of protease activated receptor 1.1 APC’s cellular signaling results in cytoprotective alterations of gene expression profiles, anti-inflammatory activity, and antiapoptotic activity.2–6
APC protects neurons and brain endothelial cells from ischemic injury and divergent inducers of apoptosis by inhibiting the mitochondria-mediated and death receptor-mediated apoptotic pathways.4,7–9 Early postischemic administration of APC within 4 hours of an ischemic insult is neuroprotective in rodent models of transient ischemia4,10 and embolic stroke.11 APC also protects against diabetic endothelial and glomerular injury12 and in animal models of sepsis, ischemia–reperfusion injury of kidney and lung, and thrombosis models.13 Regarding central nervous system disorders, APC is protective in rodent models of spinal cord injury,14 amyotrophic lateral sclerosis,15 and multiple sclerosis.16
A form of human recombinant APC with increased anticoagulant activity, ie, xigris (drotrecogin-alfa activated), has been approved by the US Food and Drug Administration for use in patients with severe sepsis.17 Drotrecognin-alfa activated is currently being studied in patients with ischemic stroke (The Activated Protein C in Acute Stroke Trial [APCAST]; http://clinicaltrials.gov/ct2/show/NCT00533546?term=apc&rank=25). The Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial for APC infusion in patients with severe sepsis suggested low but significant risk for bleeding, including increased risk for intracerebral hemorrhage.17 Moreover, at higher pharmacological doses, APC is toxic to nonhuman primates and produces disseminated bleeding (see www.emea.europa.eu/humandocs/PDFs/EPAR/xigris/247102en6.pdf).
How changes in APC anticoagulant activity influence APC’s neuroprotection and risk for intracerebral bleeding is not clear. To address this question, we compared drotrecogin-alfa activated with an APC nonanticoagulant mutant containing 3 alanine substitutions for 3 protease domain positively charged lysines (KKK191–193AAA) designated as 3K3A-APC. 18 This mutation alters factor Va binding exosite in APC resulting in greatly reduced anticoagulant activity, ie, >90%,18 but does not affect exosites that recognize endothelial protein C receptor or protease activated receptor 1, which are important for APC’s cellular activity, resulting in normal antiapoptotic activity in a model of staurosporine-induced apoptosis in human umbilical vein cells.19 Here, we show that nonanticoagulant 3K3A-APC mutant exerts enhanced neuroprotective activity and eliminates risk for intracerebral bleeding that has been seen with drotrecogin-alfa activated, a hyperanticoagulant form of APC.
Methods
Reagents
Human 3K3A-protein C (3K3A-PC) stable cell line was generated in Chinese hamster ovary (CHO) cells. The cells were grown in suspension in CD OptiCHO medium (Invitrogen) containing 2 mmol/L CaCl2, 10 µg/mL vitamin K, and 2 mmol/L GlutaMAX (Invitrogen) in a 2-L Biowave bioreactor for production. A 4-step purification procedure was used: capturing PC using FFQ resin (GE Health), purification of PC using Uno Q column (BioRad), activating with recombinant human thrombin (Zymogenetics), and removal of thrombin using Uno Q column. The purity of 3K3A-APC was determined by reduced SDS-PAGE/silver staining, the enzymatic activity by amidolytic assay, and the concentration by enzyme-linked immunosorbent assay and A280 spectral analysis. There was no detectable thrombin in the purified APC based on thrombin time clotting assays using purified fibrinogen. Human plasma-derived APC was purchased from Enzymes Research Laboratories (South Band, Ind). Xigris (drotrecogin-alpha activated) was from Eli Lilly and Co (Indianapolis, Ind). N-methyl-d-aspartate (NMDA) was purchased from Sigma (St Louis, Mo).
Activated Partial Thromboplastin Time Assay
This assay was accomplished as previously described.18 Briefly, 50 µL of protein C-deficient plasma (American Diagnostica) was mixed with 50 µL Automated APTT Reagent (Biomerieux) and incubated at 37°C for 1 minute. Human plasma-derived APC, drotrecogin-alpha activated, and 3K3A-APC were diluted into buffer (30 mmol/L HEPES, 150 mmol/L NaCl, and 0.3% BSA) and added to the mixture at 30 and 50 nM in a volume of 25 µL and incubated for 3 minutes at 37°C. The clotting was then initiated with the addition of 50 µL of 50 mmol/L CaCl2. Clotting time was measured by the Start4 Coagulometer (Diagnostica Stago). All samples were tested in duplicate.
Carbohydrate Analysis
Sialic Acid Analysis
Sialic acids (glycolyl and acetyl) were hydrolyzed from 3K3A-APC and drotrecogin-alpha activated, labeled with a fluorescent dye orthophenylenediamine, and analyzed using reversed-phase high-pressure liquid chromatography (high-performance liquid chromatography) coupled with fluorescence detection.
N-linked Glycan Profiling
To obtain oligosaccharide profiles for 3K3A-APC and drotrecogin-alfa activated, sugar moieties were removed from protein backbone with N-glycanase and labeled with 2-aminobenzamide and analyzed by high-performance liquid chromatography coupled with fluorescence detection.
Mouse Neuronal Cell Cultures
Primary neuronal cultures were established as described previously. 9,20 In brief, cerebral cortex was dissected from fetal C57BL6 mice (Charles River Laboratories, Inc; Wilmington, Mass) at 16 days of gestation, treated with trypsin for 10 minutes at 37°C, and dissociated by trituration. Dissociated cell suspensions were plated at 5 × 105 cells per well on 12-well tissue culture plates or at 4 × 106 cells per dish on 60-mm tissue culture dishes coated with poly-l-lysine, in serum-free Neurobasal medium plus B27 supplement (Gibco; Rockville, Mass). The medium suppresses glial growth to <2% of the total cell population. Cultures were maintained in a humidified 5% co2 incubator at 37°C for 7 days before treatment. Medium was replaced every 3 days.
N-methyl-d-aspartate-Induced Apoptosis in Neuronal Culture
For induction of neuronal apoptosis, cultures were exposed for 10 minutes to 300 µmol/L NMDA/5 µmol/L glycine in Mg2+-free Earle’s balanced salt solution.20 Control cultures were exposed to Earle’s balanced salt solution alone. After the exposure, cultures were rinsed with Earle’s balanced salt solution, returned to the original culture medium, and incubated with or without different concentrations of drotrecogin-alfa activated (1 to 50 nM) and human 3K3A-APC (1 to 50 nM). All experiments involving treatment with APC included hirudin (Sigma) to block any thrombin signaling.3,4,21
Human Brain Endothelial Cell Cultures
Primary brain endothelial cells (BECs) were isolated from specimens (<3 hours) from neurologically healthy, young individuals after surgery for epilepsy. BECs were characterized and cultured as we previously described.4
Oxygen–Glucose Deprivation in Human Brain Endothelial Cells
Cells were maintained in serum-free Dulbeco’s modified Eagle medium and exposed for 8 hours to oxygen–glucose deprivation (OGD; <2% oxygen, no glucose). Hypoxia was induced using an anaerobic chamber (Forma Scientific; Holbrook, NY).4 The levels of O2 were monitored by O2 Fyrite (Forma Scientific). Different concentrations of drotrecogin-alpha activated (3 to 100 nM) and human 3K3A-APC (3 to 100 nM) were added at the time of OGD treatment. Hirudin was included to block any thrombin signaling.3,4,21
Detection of Cell Survival and Cell Injury
Cell survival in mouse neuronal cultures was detected by water-soluble tetrazolium WST-8 assay (Dojindo Molecular Technologies; Gaithersburg, Md). Cell injury in BEC was detected by the release of lactic acid dehydrogenase (LDH assay, Sigma).
Transient Middle Cerebral Artery Occlusion
All procedures were done according to the National Institutes of Health guidelines for animal care and approved by the Institutional Animal Care and Use Committee at the University of Rochester. Male C57BL6 mice 6 to 8 weeks old (Charles River Laboratories; Wilmington, Mass) were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. We used a 1-hour transient middle cerebral artery occlusion (MCAO) suture model as previously reported4,9,10 followed by 24-hour reperfusion. Physiological parameters (ie, blood gasses, pH, hematocrit, arterial blood pressure) were monitored as described.22 Rectal temperature was kept within normal limits during the MCAO surgical procedure. Mice were randomly assigned to receive 5 minutes before ischemia either vehicle or human recombinant 3K3A-APC (0.4 or 2 mg/kg through the femoral vein) or drotrecogin-alfa activated (0.5 or 2 mg/kg intravenously). Motor neurological deficit was scored on a 5-point scale 24 hours after ischemic insult: no neurological deficit=0; failure to extend left forelimb fully=1; circling to the left=2; falling to the left side=3; unable to walk=4; and stroke-related death=5. After 24 hours, mice were euthanized and brains isolated and prepared for analysis. Coronal brain sections (1-mm thick) were cut and incubated with 2% 2,3,5-triphenyltetrazolium chloride solution in a phosphate-buffered saline for 5 minutes at 37°C as reported.4,10 The injury volume (cubed millimeters) was calculated by multiplying the surfaces of all injured areas in square millimeters by the thickness of brain sections. The infarction volume was obtained by subtracting the edema volume from the injury volume. Edema volume (tissue swelling) was calculated by subtracting the volume of the contralateral nonischemic hemisphere from the volume of the ipsilateral ischemic hemisphere as described.22
Permanent Distal Middle Cerebral Artery Occlusion
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Rochester. Male C57BL6 mice, 6 to 8 weeks old, were anesthetized with ketamine (100 mg/kg intraperitoneally) and xylazine (10 mg/kg intraperitoneally). Animals were allowed to breathe spontaneously. Rectal temperature was maintained at 37°C using a feedback-controlled heating system. The right femoral artery was cannulated for monitoring of blood pressure and blood analysis. A modified permanent distal MCAO technique was used. Under the surgical microscope, the left common carotid artery was isolated through a neck incision and ligated using a 5–0 silk. A skin incision was made between the right orbit and tragus. The zygomatic arch was removed and temporal muscle retracted laterally. The mandible was retracted downward. The middle cerebral artery was visible through the temporal semitranslucent surface of the skull. Craniectomy was performed by drilling with a 0.9-mm round burr. The inner layer of the skull was removed with fine forceps. The dura was carefully opened and the M1 branch of the middle cerebral artery exposed and coagulated using a cauterizer, producing permanent distal MCAO. The wound was sutured, and rectal temperature was controlled until mice regained full consciousness.
Mice were randomly assigned to the vehicle-treated group, the 3K3A-APC-treated group and drotrecogin-alfa activated-treated group. 3K3A-APC and drotrecogin-alfa activated were administered by tail vein at 1 mg/kg at 12 hours and 1, 3, 5, and 7 days poststroke. Vehicle-treated group received saline. At days 1, 3, and 7 postischemia, the forelimb asymmetry test for sensorimotor activity23 and foot-fault test for locomotor assessment24 were applied. Mice were euthanized after 7 days and the brains removed and rapidly frozen in co2-snow. Brains were cut into serial 20-µm cryostat sections. Every tenth section was stained with cresyl-violet and the lesion area determined using an image analysis system (Image J). The infarct volume was calculated as described previously.
Hemoglobin Assay
We used a spectrophotometric hemoglobin assay as reported.9,10 Briefly after 2,3,5-triphenyltetrazolium chloride or cresyl-violet staining, the hemisections of the ischemic hemisphere were homogenized and treated with the Drabkin’s reagent (Sigma) to determine hemoglobin content. Bovine erythrocyte hemoglobin or mouse blood added to brain homogenates was used for standard curves.
Statistical Analysis
Data were presented as mean ± SEM. Student t test and analysis of variance was used to determine statistically significant differences. P<0.05 was considered statistically significant.
Results
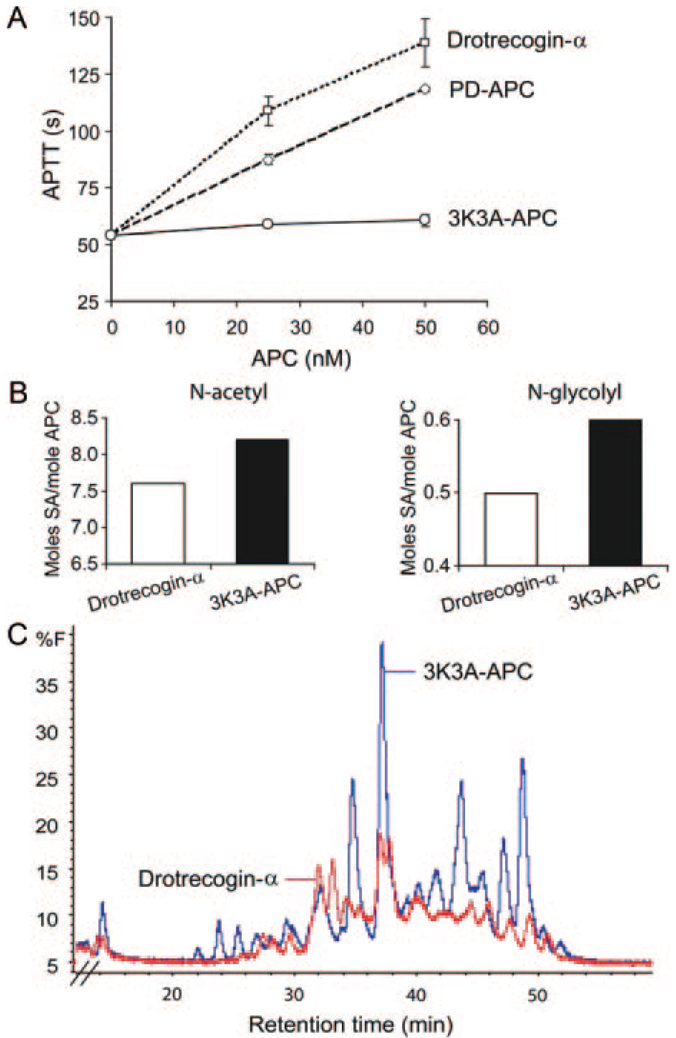
Activated partial thromboplastin time values indicated that drotrecogin-alfa activated compared with plasma-derived APC has by 48% increased anticoagulant activity, whereas 3K3A-APC has approximately 10% of plasma-derived APC anticoagulant activity (Figure 1A). The sialic acid (SA) analysis revealed that 3K3A-APC has 8.8 moles of SA per mole of APC, ie, 8.2 moles of acetyl forms and 0.6 moles of glycolyl forms, whereas drotrecogin-alfa activated had 8.1 moles of SA per mole of protein, or 7.6 moles of acetyl forms and 0.5 moles of glycolyl forms. The comparison of the released oligosaccharides showed that 3K3A-APC and drotrecogin-alfa activated have different N-linked glycan profiles and the content of N-linked glycans was significantly higher in 3K3A-APC (Figure 1B).
Figure 1.
Anticoagulant activity and carbohydrate profiles of drotrecogin-alfa activated and 3K3A-APC mutant. Drotrecogin-alfa activated (drotrecogin-α) derived from human embryonic kidney HEK293 cell line. 3K3A-APC derived from stable Chinese hamster ovary (CHO) cell line. A, Anticoagulant activity was measured by activated partial thromboplastin time (aPTT) and expressed as clotting time in seconds. Drotrecogin-α (□), plasma-derived human APC (PD-APC; ◇), and 3K3A-APC (○) were studied at 25 and 50 nM. Values are mean ± SEM, n=3 independent experiments. B, SA content in drotrecogin-α and 3K3A-APC preparations used in the present study. SA analysis was performed with high-performance liquid chromatography-fluorescence detection as described in “Methods.” N-acetyl-SA and N-glycolyl-SA are expressed in moles SA per mole APC. C, N-linked oligosaccharide profiles of drotrecogin-α and 3K3A-APC preparations used in the present study. The N-linked glycan maps were obtained using high-performance liquid chromatography-fluorescence detection as described in “Methods.” The overlay of the 2 chromatograms is shown. The relative peak quantification was determined by percent fluorescence (%F) for each APC preparation.
3K3A-APC was more potent than drotrecogin-alfa activated in protecting mouse cortical neurons from NMDA-mediated injury as indicated by a shift to the left of its respective dose–response curve (Figure 2A). The IC50 values, ie, the inhibitory concentrations corresponding to the half maximal protection effect, for drotrecogin-alfa activated and 3K3A-APC were 7.66 and 12.8 nM, respectively (Figure 2B), indicating 1.8-fold greater efficacy of 3K3A-APC. In the OGD model in human BEC, the 3K3A-APC protection curve similarly shifted to the left compared with the drotrecogin-alfa activated curve (Figure 3A). The respective IC50 values for drotrecogin-alfa activated and 3K3A-APC were 24.1 and 7.5 nM (Figure 3B), indicating 3.2-fold greater efficacy of 3K3A-APC.
Figure 2.
Protection of mouse cortical neurons from NMDA-induced injury by drotrecogin-alfa activated and 3K3A-APC mutant. A, Dose-dependent neuroprotection by drotrecogin-α and 3K3A-APC at 24 hours of NMDA. Cell survival was quantified with WST-8 assay. B, IC50 values for drotrecogin-α and 3K3A-APC from dose–response curves shown in A. Values are mean ± SEM, n=3 to 5 independent experiments in triplicate.
Figure 3.
Protection of human BECs from OGD by drotrecogin-alfa activated and 3K3A-APC mutant. A, Dose-dependent cyto-protection of BEC by drotrecogin-α and 3K3A-APC within 8 hours of exposure to OGD. Cell injury was quantified by release of lactic acid dehydrogenase into the culture medium. B, IC50 values for drotrecogin-α and 3K3A-APC were calculated from dose–response curves shown in 3A. Values are mean ± SEM, n=3 to 5 independent experiments in triplicate.
During the MCAO surgical procedure, physiological variables (ie, PaO2, Paco2, pH, hematocrit, arterial blood pressure, rectal temperature) remained within normal limits compared with basal values (data not shown) as reported.4,10 Transient MCAO resulted in approximately 80% reduction in the cerebral blood flow compared with the preocclusion baseline values. During reperfusion, the cerebral blood flow recovered to 85% to 87% of the baseline values (not shown) as reported.10
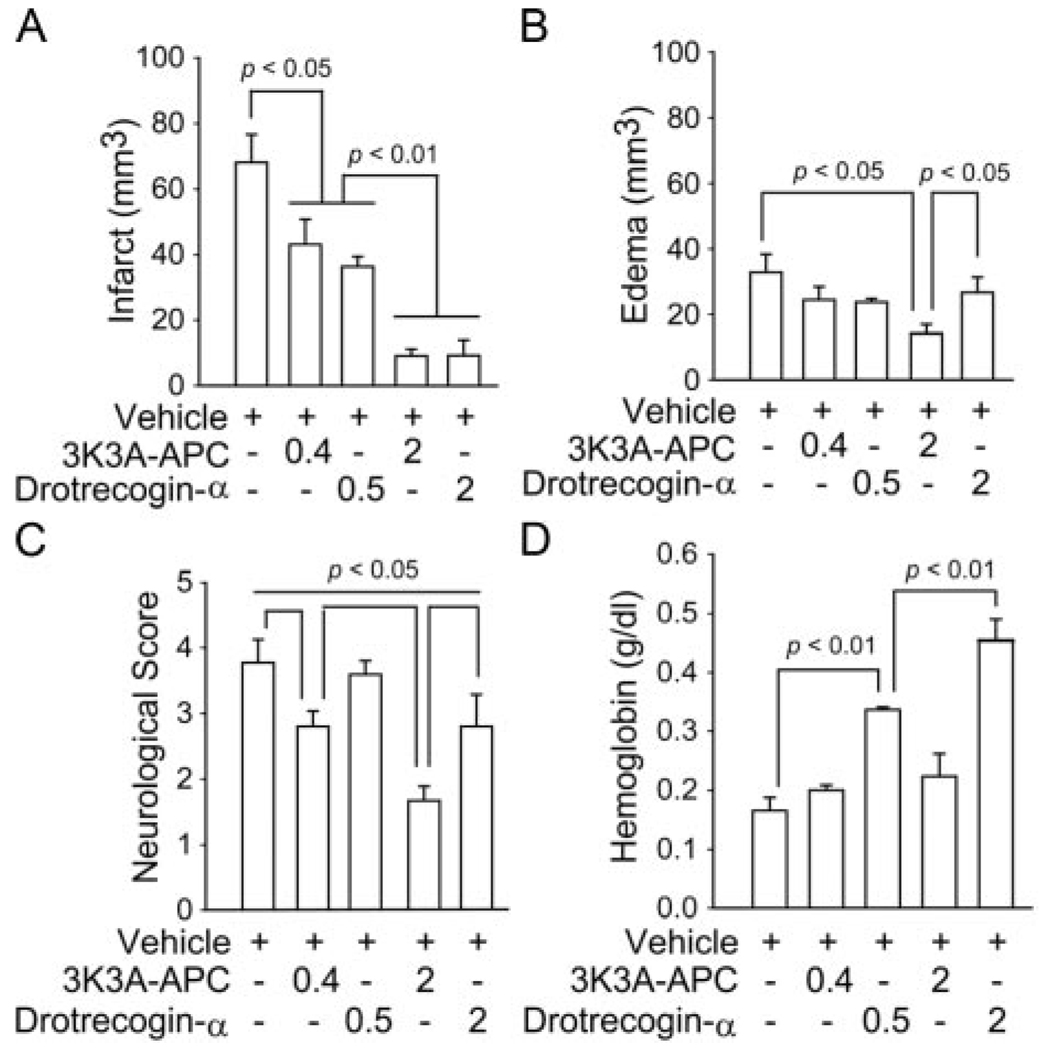
Applied 5 minutes before 1-hour transient MCAO, 3K3A-APC and drotrecogin-alfa activated exhibited comparable dose–response reductions in the infarction volume at 24 hours (Figure 4A). At a higher dose, 3K3A-APC, but not drotrecogin-alfa activated, reduced significantly tissue swelling (edema; Figure 4B). Moreover, 3K3A-APC exhibited a dose-dependent reduction in motor neurological scores, whereas drotrecogin-alfa reduced neurological score only at a higher dose, but still significantly below 3K3A-APC (Figure 4C). The greatest difference between the 2 APC preparations was seen in hemoglobin accumulation within the ischemic hemisphere. Although 3K3A-APC did not increase hemoglobin levels at either concentration, there was a dose-dependent increase in hemoglobin content in the ischemic hemisphere with drotrecogin-alfa activated (Figure 4D), suggesting microbleeding. Microhemorrhages were also confirmed by counting clusters of red blood cells outside the vessels (data not shown) as reported.11
Figure 4.
Reduced neuroprotection and risk for bleeding with drotrecogin-alfa activated compared with 3K3A-APC mutant after transient MCAO. Vehicle, drotrecogin-α (0.5 and 2 mg/kg) and 3K3A-APC (0.4 and 2 mg/kg) were administered through the femoral vein 5 minutes before 1 hour MCAO. Neuropathological analysis, neurological motor score, and hemoglobin levels were determined at 24 hours of reperfusion. A, Infarct volume. B, Brain swelling (edema). C, Motor neurological score. D, Hemoglobin levels in ischemic hemisphere. Mean ± SEM, n=6 mice per group.
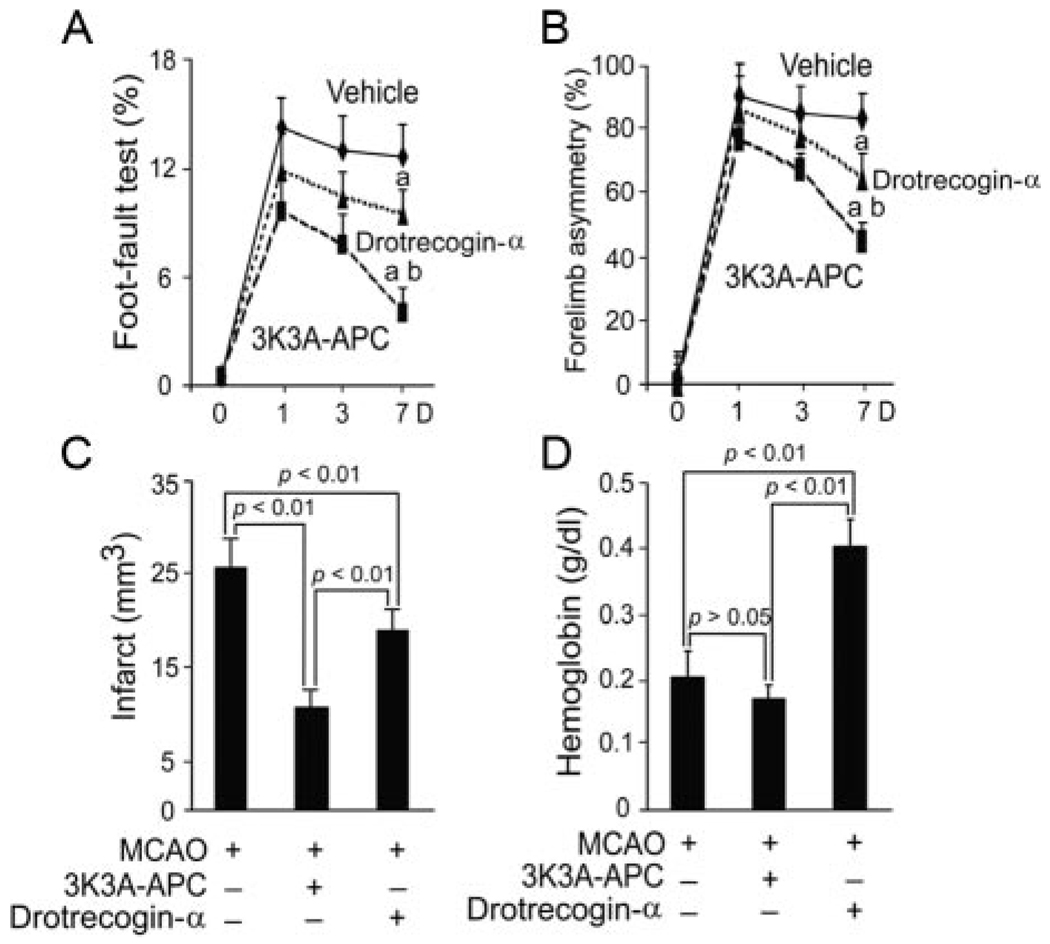
Finally, 2 APC preparations were administered as a multidose therapy (1 mg/kg intravenously) at 12 hours and 1, 3, 5, and 7 days after permanent distal MCAO. 3K3A-APC had superior effects both in terms of functional recovery, as shown by significantly better performance on the foot-fault test and forelimb asymmetry test (Figure 5A–B), as well as in significantly greater reduction in the infarction volume, ie, 60% compared with 25% of control (vehicle-treated) mice for 3K3A-APC and drotrecogin-alfa activated, respectively (Figure 5C). 3K3A-APC did not increase the risk for bleeding as shown by no increase in hemoglobin content in the ischemic hemisphere (Figure 5D) as well as by the absence of red blood cell extravasation (data not shown). In contrast, drotrecogin-alfa activated doubled hemoglobin accumulation in the ischemic brain (Figure 5D) suggesting significant postischemic microhemorrhages.
Figure 5.
Reduced neuroprotection and risk for bleeding with drotrecogin-alfa activated multidose therapy compared with 3K3A-APC mutant after permanent distal MCAO. Vehicle, drotrecogin-α (1 mg/kg) and 3K3A-APC (1 mg/kg) were administered through the tail vein 12 hours and 1, 3, 5, and 7 days after permanent distal MCAO. Behavioral tests, neuropathological analysis, and hemoglobin levels were determined within 7 days. A, Foot-fault test. B, Forelimb asymmetry test. C, Infarct volume. D, Hemoglobin levels in ischemic hemisphere. Mean±SEM, n=5 mice per group. aP<0.05, vehicle versus drotrecogin-α and vehicle versus 3K3A-APC; bP<0.05, drotrecogin-α versus 3K3A-APC.
Discussion
The present study shows superior neuroprotective effects of human nonanticoagulant 3K3A-APC mutant compared with drotrecogin-alfa activated, a hyperanticoagulant form of human recombinant APC. 3K3A-APC was more efficacious in all models studied, including in vitro cultured mouse cortical neurons challenged by NMDA and human BEC subjected to OGD and in vivo models of transient MCAO and permanent distal MCAO in mice. 3K3A-APC’s neuroprotection was not associated with a risk of intracerebral bleeding in vivo. In contrast, treatment with drotrecogin-alfa activated resulted in a dose-dependent postischemic accumulation of hemoglobin in the lesioned hemisphere. Drotrecogin-alfa activated reduced the infarct lesion after transient MCAO to the degree comparable to that seen with 3K3A-APC, but its effect on motor neurological score was very modest and likely due to accumulations of hemoglobin-derived neurotoxic products in ischemic tissue that cause damage through oxidant stress as reported.25–28
The anticoagulant action of APC mainly depends on its binding to factor Va, which is a prerequisite for APC-mediated inactivation of factor Va by proteolytic cleavage at Arg506.1 Factor Va binding to APC depends on positively charged residues in the surface loops on APC’s protease domains, including loop 37 (residues 190 to 193), the Ca2+-binding loop (residues 225 to 235), and the autolysis loop (residues 301 to 316). Substitution of positively charged lysine residues with 3 alanine residues in loop 37 (KKK191-193AAA) decreases APC’s anticoagulant activity by >90% and reduces the risk for bleeding through reductions in both APC’s anticoagulant and APC-dependent profibrinolytic activities.29 Reduced risk for bleeding has been confirmed in vivo in different models of sepsis30 and now in murine models of stroke. It is of note, the KKK191-193AAA mutation in 3K3A-APC leaves intact the N-terminal Gla domain of APC, which is important for its binding to endothelial protein C receptor on the endothelial cells, and does not alter the protease catalytic site that is responsible for activation of protease activated receptor 1.29
The anticoagulant activity of APC is also influenced by its glycosylation profile, which primarily depends on the content of negatively charged sialic acid forms and distribution and branching of the N-linked glycan species. HEK 293-derived drotrecogin-alfa activated (human protein C; xigris) has intact factor Va and VIIIa binding sites, but compared with plasma-derived APC contains 50% less sialic acid forms and the N-linked oligosaccharides are less branched.31 It has been suggested that the reduced glycosylation pattern in HEK 293-derived drotrecogin-alfa activated may influence APC binding to factors Va and VIIIa and/or other cofactors resulting in its higher anticoagulant activity.32
3K3A-APC was more potent than drotrecogin-alfa activated in protecting neurons and brain endothelial cells from injury in vitro, which must be attributed to structural differences between the 3K3A-APC mutant and drotrecogin-alfa activated, including the KKK191-193AAA mutation in loop 37, and significantly higher content of N-linked glycan species and sialic acid forms. Although the exact molecular mechanism for the enhanced efficacy of the mutant compared with drotrecogin-alfa activated remains to be explored, one might speculate that an enriched glycosylation profile in 3K3A-APC would either facilitate proteolytic activation of protease activated receptor 1 and/or 3K3A-APC binding to endothelial protein C receptor on endothelium, resulting in enhanced antiapoptotic signaling in injured neurons and vascular endothelium as reported.4,8
In summary, our data suggest that nonanticoagulant 3K3A-APC mutant has important advantages over hyperanticoagulant drotrecogin-alfa activated as a neuroprotective agent in mouse models of stroke, including significantly greater efficacy and no risk for bleeding.
Acknowledgments
We thank Colleen Mullen for her guidance in 3K3A-APC recombinant protein production in suspended CHO cells, Dayle Schweibert for producing recombinant human 3K3A-APC in bioreactor, Michael Sperber for purifying the protein, and Sarah Lawrence for the initial characterization of 3K3A-APC.
Sources of Funding
This work was supported by the National Institutes of Health grants HL63290 and HL81528 (B.V.Z.) and NS39592 (T.P.D.). ZZ Biotech LLC provided human 3K3A-APC mutant.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
B.V.Z. is the scientific founder of Socratech LLC and its subsidiary ZZ Biotech, startup biotechnology companies with a focus to develop therapeutics for Alzheimer disease and stroke, including therapies based on APC and its functional mutants. B.V.Z. serves as a Chief Scientific Officer on these 2 companies and owns shares in both Socratech LLC and ZZ Biotech, but does not receive salary support. T.P.D. is CEO of ZZ Biotech and receives financial compensation.
References
- 1.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 2.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 3.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 4.Cheng T, Liu D, Griffin JH, Fernández JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 5.Domotor E, Benzakour O, Griffin JH, Yule D, Fukudome K, Zlokovic BV. Activated protein C alters cytosolic calcium flux in human brain endothelium via binding to endothelial protein C receptor and activation of protease activated receptor-1. Blood. 2003;101:4797–4801. doi: 10.1182/blood-2002-12-3680. [DOI] [PubMed] [Google Scholar]
- 6.Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem J. 2003;373:65–70. doi: 10.1042/BJ20030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu D, Cheng T, Guo H, Fernández JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 8.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernández JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 9.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernández JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 10.Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, Zlokovic BV. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–1805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- 11.Zlokovic BV, Zhang CL, Liu D, Fernandez J, Griffin JH, Chopp M. Functional recovery after embolic stroke in rodents by activated protein C. Ann Neurol. 2005;58:474–477. doi: 10.1002/ana.20602. [DOI] [PubMed] [Google Scholar]
- 12.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 13.Griffin JH, Zlokovic B, Fernandez JA. Activated protein C: potential therapy for severe sepsis, thrombosis, and stroke. Semin Hematol. 2002;39:197–205. doi: 10.1053/shem.2002.34093. [DOI] [PubMed] [Google Scholar]
- 14.Taoka Y, Schlag MG, Hopf R, Redl H. The long-term effects of pretreatment with activated protein C in a rat model of compression-induced spinal cord injury. Spinal Cord. 2000;38:754–761. doi: 10.1038/sj.sc.3101096. [DOI] [PubMed] [Google Scholar]
- 15.Zhong Z, Hallagan L, Chow N, Zlokovic BV. Activated protein C inhibits motor neuron degeneration in a transgenic mouse model of amyotrophic lateral sclerosis. Soc Neurosci. 2006 Abstract Viewer/Planner Online (708.8) [Google Scholar]
- 16.Han MH, Hwang SI, Roy DB, Lundgren DH, Price JV, Ousman SS, Fernald GH, Gerlitz B, Robinson WH, Baranzini SE, Grinnell BW, Raine CS, Sobel RA, Han DK, Steinman L. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
- 17.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ, Jr Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 18.Gale AJ, Tsavaler A, Griffin JH. Molecular characterization of an extended binding site for coagulation factor Va in the positive exosite of activated protein C. J Biol Chem. 2002;277:28836–28840. doi: 10.1074/jbc.M204363200. [DOI] [PubMed] [Google Scholar]
- 19.Mosnier LO, Gale AJ, Yegneswaran S, Griffin JH. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 2004;104:1740–1744. doi: 10.1182/blood-2004-01-0110. [DOI] [PubMed] [Google Scholar]
- 20.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riewald M, Ruf W. Protease-activated receptor-1 signaling by activated protein C in cytokine perturbed endothelial cells is distinct from thrombin signaling. J Biol Chem. 2005;280:19808–19814. doi: 10.1074/jbc.M500747200. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Kittaka M, Sun N, Schreiber SS, Zlokovic BV. Chronic nicotine treatment enhances focal ischemic brain injury and depletes free pool of brain microvascular tissue plasminogen activator in rats. J Cereb Blood Flow Metab. 1997;17:136–146. doi: 10.1097/00004647-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Schallert T, Whishaw IQ. Bilateral cutaneous stimulation of the somato-sensory system in hemidecorticate rats. Behav Neurosci. 1984;98:518–540. doi: 10.1037//0735-7044.98.3.518. [DOI] [PubMed] [Google Scholar]
- 24.Gong Y, Hua Y, Keep RF, Hoff JT, Xi G. Intracerebral hemorrhage: effects of aging on brain edema and neurological deficits. Stroke. 2004;35:2571–2575. doi: 10.1161/01.STR.0000145485.67827.d0. [DOI] [PubMed] [Google Scholar]
- 25.Regan RF, Panter SS. Neurotoxicity of hemoglobin in cortical cell culture. Neurosci Lett. 1993;153:219–222. doi: 10.1016/0304-3940(93)90326-g. [DOI] [PubMed] [Google Scholar]
- 26.Regan RF, Guo Y. Toxic effect of hemoglobin on spinal cord neurons in culture. J Neurotrauma. 1998;15:645–653. doi: 10.1089/neu.1998.15.645. [DOI] [PubMed] [Google Scholar]
- 27.Qu Y, Chen J, Benvenisti-Zarom L, Ma X, Regan RF. Effect of targeted deletion of the heme oxygenase-2 gene on hemoglobin toxicity in the striatum. J Cereb Blood Flow Metab. 2005;25:1466–1475. doi: 10.1038/sj.jcbfm.9600143. [DOI] [PubMed] [Google Scholar]
- 28.Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J Biol Chem. 2007;282:33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 30.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan SC, Razzano P, Chao YB, Walls JD, Berg DT, McClure DB, Grinnell BW. Characterization and novel purification of recombinant human protein C from three mammalian cell lines. Biotechnology (N Y) 1990l;8:655–661. doi: 10.1038/nbt0790-655. [DOI] [PubMed] [Google Scholar]
- 32.Grinnell BW, Walls JD, Gerlitz B. Glycosylation of human protein C affects its secretion, processing, functional activities, and activation by thrombin. J Biol Chem. 1991;266:9778–9785. [PubMed] [Google Scholar]