Abstract
Background
Low education has an impact on life expectancy and level of cognition, but little is known on its effect on life expectancy with cognitive impairment.
Methods
The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) collected population-based longitudinal data on people aged 65 years and older including measures of education and cognitive impairment, using the Mini-Mental State Examination (MMSE), for five geographically diverse areas around England and Wales interviewed between 1991 and 2003. Transitions between health states were calculated using Markov chain methods. Life expectancy in different states of cognitive function as measured by MMSE were further explored for different education groups. The effect of fixed and educationally based cut points for cognitive impairment are investigated.
Results
Life expectancy spent with cognitive impairment is fairly constant with increasing age at around 1.4 years in men and 2.5 years in women, though this reflects a large increase in the proportion of life spent with cognitive impairment. The differences seen between education groups for the proportion of total life with cognitive impairment (men 13% and women 22% of life lived for low education vs men 7% and women 12% in high education group) disappear when education-adjusted cut points are used (10% in men and 17% in women at age 65 for all education groups).
Conclusions
The results show that there is a substantial amount of life expectancy with cognitive impairment in both men and women. The impairment burden is just as great for those with high education as the lowest educated group.
Keywords: Life expectancy, Deprivation, cognitive impairment, social class, education
HEALTHY life expectancy is used by policy makers and researchers to monitor the overall health of a population (1). The techniques bring morbidity and mortality into a combined measure and provide a better indication of population health than total life expectancy (TLE) alone, particularly when the population has low mortality but high morbidity rates (2). Cognitive impairment has been shown to have a marked impact on the survival of individuals (3,4); it also is associated with a marked deficiency in an individuals quality of life and that of their carer (5). Life expectancy is known to vary between men and women and by different demographic characteristics (6–8).
Life expectancy with cognitive impairment based on longitudinal data, that is, using mortality data on individuals has been rarely examined. Studies from the United Kingdom, the Netherlands, United States, and Canada, of which two were longitudinal, show that 5%–10% of TLE at age 65 years is spent with impairment and that this impairment burden is larger in women than men, but they do not investigate differences between education groups within the studies (9–13).
Socioeconomic differences at an individual level are usually defined by education, occupation, income, and material circumstances, or some combination of these markers (14). Income and wealth (including dynamic changes in these) have been linked to health in old age (15–17) and Robert and House (18) have provided evidence of an increasing relative impact of income over education on some measures of health with increasing age. In the United Kingdom, occupation has been used as a marker of social status, and household tenure or car ownership have served as markers of material circumstances (19,20). However, especially for older people, contemporaneous measures of social position can be misleading, failing to reflect changing status from middle age or earlier (21). Full-time education, as a marker, has the advantage of generally being completed early in adulthood and therefore less likely to suffer from reverse causation. It is also a good measure of long-term economic position, at least in the United States (17).
Some studies analyzing cognitive impairment adjust for education with a standardized cut point (22,23), others use education-adjusted levels for cognitive impairment measured using Mini-Mental State Examination (MMSE) (24). Education is known to affect both the level of cognition and its measurement, though it is not clear whether the measure is better with or without adjustment (25). In cognitive health, the measurement itself may be educationally biased and may not reflect real cognitive differences, though this is contentious (26).
The aim of this article is to investigate the potential impact of education on life expectancy and cognitively impaired life expectancy by the use of longitudinal data from the Medical Research Council Cognitive Function and Ageing study (MRC CFAS).
METHODS
Data
MRC CFAS (http://www.cfas.ac.uk) is a population-based longitudinal study in which 13,004 individuals were originally interviewed. Details of the study can be found elsewhere (27), but briefly, approximately 2,500 individuals were recruited from each of five centers in England and Wales (Cambridgeshire, Gwynedd, Newcastle, Nottingham, and Oxford), using an age-stratified design that oversampled the participants aged 75 and older from primary care registers where the entire population, including individuals in residential homes are enumerated. All individuals have been followed since first sampled for mortality information at the Office for National Statistics. The 13,004 individuals aged 65 and older at baseline interview have each contributed to a maximum of nine interviews, between 1991 and 2003, with more than 34,000 interviews in total. All individuals have been registered for death notification, and to date (end December 2005) there have been 8,545 deaths (version 8.1 of the data). All interviews contained the MMSE (28), and any individuals with an interview, but incomplete MMSE, have been excluded for that interview only (Table 1 shows numbers missing). All individuals with at least one known state contributed to the analysis (n = 12,880 [99%]).
Table 1.
Numbers and Percentage (in Brackets) of Individuals and the States Used in the Analysis
| Interview and Time in Years From Baseline Screen | Severe/Moderate Impairment | Mild Impairment | No Impairment | Total With MMSE Measured | Death (Cumulative)* | Missing MMSE | Not Interviewed | |
| Baseline screen | 0 | 1,742 (14) | 3,087 (24) | 7,978 (62) | 12,807 | 0 | 73 | 0 |
| Baseline assessment | 0.25 | 1,053 (40) | 719 (28) | 834 (32) | 2,606 | 147 (1) | 29 | 10,098 |
| First assessment | 1 | 365 (40) | 221 (24) | 322 (35) | 908 | 655 (5) | 12 | 11,305 |
| Second follow-up | 2 | 1,233 (14) | 1,975 (23) | 5,550 (63) | 8,758 | 1,497 (12) | 63 | 2,562 |
| Second assessment | 2.25 | 430 (31) | 408 (29) | 570 (40) | 1,408 | 1,582 (12) | 54 | 9,836 |
| Third assessment | 3 | 164 (28) | 172 (29) | 248 (42) | 584 | 2,105 (16) | 5 | 10,186 |
| Fourth assessment | 6 | 560 (23) | 618 (25) | 1255 (52) | 2,433 | 4,092 (32) | 27 | 6,328 |
| Fifth assessment | 8 | 99 (26) | 101 (26) | 185 (48) | 385 | 5,565 (43) | 5 | 6,925 |
| Sixth assessment | 10 | 497 (16) | 682 (21) | 2,014 (63) | 3,193 | 6,796 (53) | 18 | 2,873 |
| Status at December 31, 2005 | Dead | 8,432 (65) | Censored (alive) | 4,448 (35) | ||||
Notes: MMSE = Mini-Mental State Examination.
In the table, deaths are coded approximately to the interview stage, in the analysis exact date of death has been used. A total of 124 individuals with no known cognitive state are excluded from the entire table.
Definitions of Variables
Participants were classified into three groups based on the number of years of full-time education undertaken (0–9 years [n = 7,934], 10/11 years [n = 2,700], and 12+ years [n = 1927]), reflecting basic/higher education as 9 years was the statutory time for this generation. Individuals who were missing education data were excluded (n = 319). The MMSE (28) has proved popular as a measure of cognitive impairment in population studies as it is easy and brief to administer in a standardized manner. We used three cognitive impairment groups: moderate/severe (≤21 MMSE), mild (22–25 MMSE), and no impairment (26–30 MMSE) (29, 30). For analysis with covariates, just the two groups of moderate/severe impairment or mild/none have been used. We also investigated the effect of using cut points for cognitive impairment defined by education groups. The cut points for moderate/severe impairment was defined within each of the three education groups by the position of the 10th percentile of the distribution from the baseline data. Cut points used were MMSE ≤20 for 9 or less years of education, MMSE ≤22 for 10–11 years, and MMSE ≤23.5 for 12 or more years. These values, which have previously been found to be similar to other studies (31), were then used as the cut points throughout the longitudinal phase of analysis.
Statistical Methods
A multistate Markov model in discrete time is used in these analyses to model the transitions between health states where states refer to health, ill-health, and death. Cognitive impaired life expectancies were calculated with software developed specifically for this type of analysis, IMaCh (Interpolation of Markov chains) (32), using principles of discrete-time multistate modeling to calculate incidence rates from longitudinal studies, which then permits calculation of health expectancies and their standard errors and hence confidence intervals (CIs). This technique partitions the time intervals between successive interviews into shorter steps and models the resulting transition probabilities between states by multinomial logistic regression on age. Estimated transition probabilities then act as inputs to a multistate life table. Version 0.98i of IMaCh has been used in this analysis. We used education as a covariate but analyzed men and women separately. The process was repeated for the fixed and education-based impairment states.
Poor cognition is associated with dropout as well as death, so this will cause longitudinal estimates to underestimate cognitive impairment (33,34). Sensitivity analyses were undertaken assuming a missing MMSE score from a successful interview indicated impairment and also multiple imputation of 10 data sets for all missing interviews (due to refusal or migration from the area) (35). Ten imputation data sets have been suggested as a suitable number to enable the full variability to be described (35, 36). The imputation model was based on information from the previous interview, including length of interview, residence, amount of missing data in interview, cognition, and demographic factors.
RESULTS
Of the 13,004 (5,157 men [m] and 7,847 women [w]) participants in the study 12,880 (99%) (5,126 m and 7,754 w) have been included in these analyses. A total of 124 (1%) were excluded because they had no MMSE measured. Complete interview waves were undertaken at baseline, year 2 and year 10, with the proportion with no cognitive impairment being consistent at just more than 60% (Table 1). Sampling for all other interviews was weighted toward the cognitively impaired, and this is reflected in the proportions seen within the impairment states.
Overall Impairment Burden Fixed Cut Points
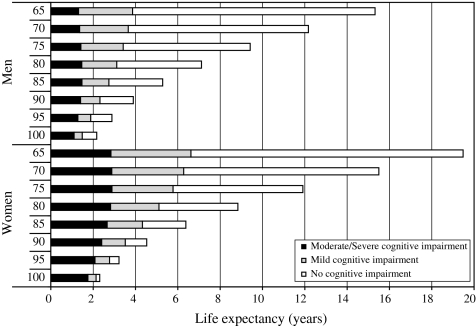
In both men and women, TLE decreases steadily with age: in men from 15.3 years (95% CI, 15.0–15.7) at age 65 to 5.3 years (95% CI, 5.1–5.5) at age 85 and in women from 19.5 years (95% CI, 19.2–19.8) to 6.4 years (95% CI, 6.2–6.5) (Table 2). The life expectancy without cognitive impairment mirrors the decrease seen in the TLE with age; life expectancy with mild impairment decreases more rapidly with increasing age (Figure 1). In men, at age 65 life expectancy with mild impairment is 2.6 years (17% of TLE; 95% CI, 2.4–2.7 years) and moderate/severe cognitive impairment is 1.3 years (9%; 95% CI, 1.2–1.4 years), which reflects a total impaired burden of 3.9 years. In women, the life expectancies are 3.8 years (19%; 95% CI, 3.6–4.0 years) for mild and 2.8 years (15%; 95% CI, 2.7–3.0 years) for moderate/severe, giving 6.6 years combined. Severe cognitive impairment is much less common and therefore has lower life expectancies at just 0.7 years of life expectancy with severe cognitive impairment in men and 1.5 years in women at age 65. In both men and women, cognitively impaired life expectancy increases as a proportion of TLE (Figure 2).
Table 2.
Life Expectancy and Impaired Life Expectancy (for Fixed and Education-Specific Cut Points) for Individuals at Age 70 Years and 80 Years by Education Groups
| TLE | IFLE | ILE | ||
| Men | ||||
| Age 70: overall | 12.0 | 10.7 (89%) | 1.3 (11%) | |
| Fixed cut points ≤21 MMSE | 9 years or less | 11.8 | 10.3 (87%) | 1.5 (13%) |
| 10/11 years | 12.7 | 11.6 (92%) | 1.0 (8%) | |
| 12+ years | 12.9 | 12.0 (93%) | 0.9 (7%) | |
| Education-specific cut points (10%) | 9 years or less | 11.8 | 10.6 (90%) | 1.2 (10%) |
| 10/11 years | 12.7 | 11.4 (90%) | 1.3 (10%) | |
| 12+ years | 13.0 | 11.5 (88%) | 1.5 (12%) | |
| Age 80: overall | 7.0 | 5.6 (80%) | 1.4 (20%) | |
| Fixed cut points ≤21 MMSE | 9 years or less | 6.9 | 5.2 (76%) | 1.7 (24%) |
| 10/11 years | 7.6 | 6.4 (85%) | 1.2 (15%) | |
| 12+ years | 7.5 | 6.5 (87%) | 1.0 (13%) | |
| Education-specific cut points (10%) | 9 years or less | 6.9 | 5.5 (80%) | 1.3 (20%) |
| 10/11 years | 7.5 | 6.1 (81%) | 1.5 (19%) | |
| 12+ years | 7.5 | 5.9 (79%) | 1.6 (21%) | |
| Women | ||||
| Age 70: overall | 15.3 | 12.5 (81%) | 2.9 (19%) | |
| Fixed cut points ≤21 MMSE | 9 years or less | 15.0 | 11.7 (78%) | 3.3 (22%) |
| 10/11 years | 15.9 | 13.4 (84%) | 2.5 (16%) | |
| 12+ years | 16.5 | 14.6 (88%) | 2.0 (12%) | |
| Education-specific cut points (10%) | 9 years or less | 15.0 | 12.3 (82%) | 2.7 (18%) |
| 10/11 years | 15.9 | 13.1 (82%) | 2.9 (18%) | |
| 12+ years | 16.7 | 13.8 (83%) | 2.9 (17%) | |
| Age 80: overall | 8.8 | 6.0 (68%) | 2.8 (32%) | |
| Fixed cut points ≤21 MMSE | 9 years or less | 8.6 | 5.4 (63%) | 3.2 (37%) |
| 10/11 years | 9.1 | 6.7 (74%) | 2.4 (26%) | |
| 12+ years | 9.8 | 7.9 (80%) | 2.0 (20%) | |
| Education-specific cut points (10%) | 9 years or less | 8.6 | 5.9 (69%) | 2.7 (31%) |
| 10/11 years | 9.1 | 6.3 (70%) | 2.8 (30%) | |
| 12+ years | 9.9 | 7.0 (71%) | 2.9 (29%) |
Note: TLE = total life expectancy; IFLE = impairment free life expectancy; ILE = life expectancy with moderate/severe cognitive impairment; MMSE = Mini-Mental State Examination.
Figure 1.
Total life expectancy by impairment level.
Figure 2.
Proportion of total life expectancy spent with none, mild, moderate/severe cognitive impairment.
Education Effects on Fixed Cut Point Cognitive Impairment
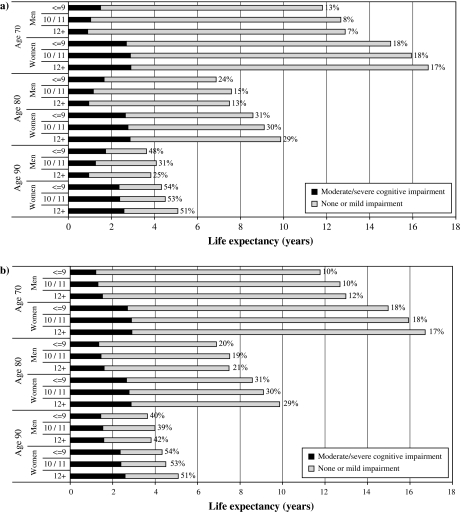
Table 2 and Figure 3a show the cognitive impaired life expectancy and TLE for men and women by educational group. In women, there are differences in the TLEs by educational group with individuals who had 12 years or more of education having 1.6 years (95% CI, 0.8–2.5) more TLE at age 65 than those with 9 years or less of full-time education. This effect appears to endure with age, where at age 85 there is 1.0 years (95% CI, 0.5–1.5) more TLE for those with higher education than those with low education, and these differences relate to approximately 8%–14% increase in TLE for the higher education group across all ages. There is a large reduction in the number of years spent with impairment with increasing education, with 1.3 fewer years (95% CI, 0.9–1.8) for the high education group compared with the lowest educated at age 65. Similar effects are found in men, though the absolute level of impairment is less and the trend between groups slightly weaker.
Figure 3.
(a) Education differences in life expectancy and impaired life expectancy by education groups using fixed cut points, with proportion of total life spent with impairment. (b) Education differences in life expectancy and impaired life expectancy by education groups using education-specific cut points, with proportion of total life spent with impairment.
Education-Based Cut Points
Table 2 and Figure 3b show the cognitive impaired life expectancy by education-specific cut points. The overall life expectancy is the same as before, though there are much smaller differences between the education groups. The proportion of time spent with impairment is almost constant at 10% for men aged 65 years and 17% for women in each of the education groups. These differences contrast with the substantial changes seen without the adjustment for education.
Effect of Cut Point on Transitions (Data Not Shown)
For those without cognitive impairment, both education-based and fixed cut points, the higher education group has lower mortality and a higher proportion remain free of impairment than the lower education group. Comparison of the two analyses showed differences in the transitions from and to cognitive impairment. In the fixed cut points analysis, higher educated individuals are less likely to become cognitively impaired, to “recover” from cognitive impairment, and to remain cognitively impaired, though they have a higher mortality when cognitive impaired. In contrast, for the education-based cut points, incidence of cognitive impairment is similar for all education groups, though recovery from cognitive impairment is greater in higher educated groups, whereas remaining cognitively impaired and mortality are lower in the higher educated groups.
Sensitivity Analyses
The sensitivity analyses indicated that the results were robust to the assumptions of the modeling process and missing data mechanism. Imputation showed little variation within the 10 imputed data sets for either TLE (at age 65: men all 15.3, women 19.3–19.4) or for impaired life expectancy (at age 65: men 1.2–1.3, women 3.0–3.2). No differences became apparent at older ages.
DISCUSSION
The results presented here come from longitudinal analysis of a large U.K. population–based study using survival and transition times with known dates of death. The results show that there is a substantial burden of life expectancy with cognitive impairment for both mild and moderate/severe impairment in both men and women. These burdens are larger for groups with low education, though when education-adjusted cut points are used most of the effect is removed.
The TLE of 15.3 for men and 19.5 for women compares well with national estimates for England and Wales for 1991 (16.0 for men and 19.2 for women from www.ons.gov.uk). Minor differences are seen in the estimates of TLE for different impairment groupings. The life expectancy with severe cognitive impairment of 0.7 years (4.4%) for men and 1.5 years (8.0%) for women found with the longitudinal methods are slightly higher than the 0.5 years (3.7%) for men and 1.2 years (6.8%) for women seen in a previous cross-sectional analysis (37). However, with both methods the life expectancy with cognitive impairment was found to be almost constant with increasing age; this effect was seen not only in the overall amount of impaired life expectancy but also in each education group.
These results demonstrate that differences in TLE by educational groups are large in the elderly population. The effect suggests that measurement of cognitive impairment is educationally dependent, but that this is focused potentially on the misclassification of the impaired state. Despite large differences in TLE, cognitive impairment free life expectancy remains a constant proportion of life expectancy for all education groups when the threshold for impairment is adjusted for education. Adjusting the cut points for education may underestimate the true problem of cognitive impairment. If there exists a cut point that indicates a level of impairment that makes independent living unlikely (a disabling state), that cut point may be reached more easily in those with low education. However, if the cut point does indicate a disabled state, then the cut point is correct. To adjust the cut point lower due to the preexisting educational deficit does not necessarily make the new cut point better at estimating the disabling state of cognitive impairment. It is essential therefore to consider the reason for the analysis before deciding about whether to adjust for education.
The results shown here compare well with other studies, the impairment burden being longer in women than men (9–12). This study has the advantage of being able to investigate not only moderate/severe life expectancy but also the life expectancy with mild cognitive difficulties. This analysis has shown that in addition to the 5%–15% of life spent at age 65 with moderate/severe cognitive impairment there is another 15%–20% of life with mild cognitive difficulties (Figure 2). Individuals spend the majority of their lives with mild-severe cognitive difficulties by age 84 for men and 76 for women.
Critique of Methods
The results come from a large population-based study, including individuals living in institutions, with a long period of follow-up. The study consisted of five centers in England and Wales and give reasonable representation of the diversity of the country (38). The values obtained for the TLE compare well with the national data. The fact that there was a consistent 60% without impairment at each wave where all individuals were interviewed indicates the importance of accounting for attrition in standard longitudinal analysis as this proportion should decrease with age. Information on transitions between states for individuals who refused to take part and who moved out of the areas is unknown. This information is more likely to be missing for individuals with cognitive impairment and with less education (33,34). The result of this bias would be to underestimate transitions into the impaired state where the death rate is higher (33), so in combination, this will underestimate the life expectancy with impairment. Checking whether this influences our results using multiple imputation techniques to estimate these missing states reveals little bias in the impaired life expectancy estimates. TLE is not affected by the missing data as death enumeration is almost 100% complete, and hence, the survival status of all individuals is known independent of the impairment state measurement.
The use of education-adjusted cut points for cognitive impairment reflects a different burden of cognitive change, though it does not always provide a better mechanism for screening than using a fixed cut point (39). Here we have investigated both aspects and have shown that the burden of cognitive impairment is a similar proportion of total life when education has been taken into account. This indicates that each education group has the same population burden of cognitive impairment. Whether cognitive impairment should or should not be adjusted for education is contentious and no one answer is correct (26). The measure of education as years of full-time education is not optimal as the level of formal qualifications (A levels, first degrees, and masters degrees) is unknown. The number of years of education of 9 years was the statutory time for this generation so the majority of the cohort had this level of education.
CONCLUSIONS
The methods used in this article take a complete approach of not only investigating differences in life but also in healthy life. There are large differences in TLE between education groups, though impaired life expectancy does not change if the cut points are made educationally dependent. The duration of remaining life spent with cognitive impairment is a problem, therefore, for the whole population, not just for those with low education.
CONFLICT OF INTEREST
None declared.
FUNDING
F.M. is currently funded by the Medical Research Council (grant numbers MRC/G9901400 and MRC/U.1052.00.013).
Acknowledgments
MRC CFAS is indebted to the respondents for their continuing support. MRC CFAS has received funding from the Department of Health and Medical Research Council (grant number MRC/G9901400). F.M. undertook the analysis, wrote the paper, and is guarantor of the analysis. C.J. revised the paper. L.M. undertook preliminary analysis and revised the paper. C.B. is lead principal investigator on the study and revised the paper. In addition to individual authors, group authorship recognized as MRC CFAS is included. This group meets full authorship criteria and requirements as it is responsible for study design, questionnaire development and implementation, and manuscript reviewing. All named authors have seen and approved the final draft. The Medical Research Council and Department of Health have had no role in the study design, collection of data, analysis, or decision to publish. MRC CFAS has had ethical approval from Eastern Anglia Multicentre research ethics committee and all local ethical committees for the duration of the study (1990 to date). All individuals gave written informed consent.
References
- 1.Robine JM, Jagger C, Mathers CD, Crimmins EM, Suzman RM. Determining Health Expectancies. Chichester: Wiley and Sons; 2003. [Google Scholar]
- 2.Barendregt JJ, Bonneux L, van der Maas PJ. Health expectancy: an indicator for change? Technology Assessment Methods Project Team. J Epidemiol Community Health. 1994;48:482–487. doi: 10.1136/jech.48.5.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: a systematic review of the literature. Int J Geriatr Psychiatry. 2001;16:751–761. doi: 10.1002/gps.397. [DOI] [PubMed] [Google Scholar]
- 4.Arauz A, Alonso E, Rodriguez-Saldana J, et al. Cognitive impairment and mortality in older healthy Mexican subjects: a population-based 10-year follow-up study. Neurol Res. 2005;27:882–886. doi: 10.1179/016164105X49427. [DOI] [PubMed] [Google Scholar]
- 5.Kurz X, Scuvee-Moreau J, Vernooij-Dassen M, Dresse A. Cognitive impairment, dementia and quality of life in patients and caregivers. Acta Neurol Belg. 2003;103:24–34. [PubMed] [Google Scholar]
- 6.Spoerri A, Zwahlen M, Egger M, Gutzwiller F, Minder C, Bopp M. Educational inequalities in life expectancy in German speaking part of Switzerland 1990–1997: Swiss National Cohort. Swiss Med Wkly. 2006;136:145–148. doi: 10.4414/smw.2006.11328. [DOI] [PubMed] [Google Scholar]
- 7.Crimmins EM, Saito Y. Trends in healthy life expectancy in the United States, 1970–1990: gender, racial, and educational differences. Soc Sci Med. 2001;52:1629–1641. doi: 10.1016/s0277-9536(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 8.Rogot E, Sorlie PD, Johnson NJ. Life expectancy by employment status, income, and education in the National Longitudinal Mortality Study. Public Health Rep. 1992;107:457–461. [PMC free article] [PubMed] [Google Scholar]
- 9.Sauvaget C, Jagger C, Arthur AJ. Active and cognitive impairment-free life expectancies: results from the Melton Mowbray 75+ health checks. Age Ageing. 2001;30:509–515. doi: 10.1093/ageing/30.6.509. [DOI] [PubMed] [Google Scholar]
- 10.Gallo JJ, Schoen R, Jones R. Cognitive impairment and syndromal depression in estimates of active life expectancy: the 13-year follow-up of the Baltimore Epidemiologic Catchment Area sample. Acta Psychiatr Scand. 2000;101:265–273. [PubMed] [Google Scholar]
- 11.Suthers K, Kim JK, Crimmins E. Life expectancy with cognitive impairment in the older population of the United States. J Gerontol B Psychol Sci Soc Sci. 2003;58:S179–S186. doi: 10.1093/geronb/58.3.s179. [DOI] [PubMed] [Google Scholar]
- 12.Deeg DJ, Portrait F, Lindeboom M. Health profiles and profile-specific health expectancies of older women and men: The Netherlands. J Women Aging. 2002;14:27–46. doi: 10.1300/J074v14n01_03. [DOI] [PubMed] [Google Scholar]
- 13.Dubois MF, Hebert R. Cognitive-impairment-free life expectancy for Canadian seniors. Dement Geriatr Cogn Disord. 2006;22:327–333. doi: 10.1159/000095593. [DOI] [PubMed] [Google Scholar]
- 14.Lynch J, Kaplan G. Socio-economic position. In: Berkman LF, Kawachi I, editors. Social Epidemiology. New York: Oxford University Press; 1999. pp. 13–35. 1999. [Google Scholar]
- 15.Maddox GL, Clark DO, Steinhauser K. Dynamics of functional impairment in late adulthood. Soc Sci Med. 1994;38:925–936. doi: 10.1016/0277-9536(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 16.Smith JP, Kington R. Demographic and economic correlates of health in old age. Demography. 1997;34:159–170. [PubMed] [Google Scholar]
- 17.Smith JP. Wealth inequality among older Americans. J Gerontol B Psychol Sci Soc Sci. 1997;52:74–81. doi: 10.1093/geronb/52b.special_issue.74. [DOI] [PubMed] [Google Scholar]
- 18.Robert S, House JS. SES differentials in health by age and alternative indicators of SES. J Aging Health. 1996;8:359–388. doi: 10.1177/089826439600800304. [DOI] [PubMed] [Google Scholar]
- 19.Matthews RJ, Jagger C, Hancock RM. Does socio-economic advantage lead to a longer, healthier old age? Soc Sci Med. 2006;62:2489–2499. doi: 10.1016/j.socscimed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Matthews RJ, Smith LK, Hancock RM, Jagger C, Spiers NA. Socioeconomic factors associated with the onset of disability in older age: a longitudinal study of people aged 75 years and over. Soc Sci Med. 2005;61:1567–1575. doi: 10.1016/j.socscimed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 21.House JS, Lepkowski JM, Kinney AM, Mero RP, Kessler RC, Herzog AR. The social stratification of aging and health. J Health Soc Behav. 1994;35:213–234. [PubMed] [Google Scholar]
- 22.Stewart R, Prince M, Mann A. Age, vascular risk, and cognitive decline in an older, British, African-Caribbean population. J Am Geriatr Soc. 2003;51:1547–1553. doi: 10.1046/j.1532-5415.2003.51504.x. [DOI] [PubMed] [Google Scholar]
- 23.Kalmijn S, Launer LJ, Lindemans J, Bots ML, Hofman A, Breteler MM. Total homocysteine and cognitive decline in a community-based sample of elderly subjects: the Rotterdam Study. Am J Epidemiol. 1999;150:283–289. doi: 10.1093/oxfordjournals.aje.a010000. [DOI] [PubMed] [Google Scholar]
- 24.Bo M, Massaia M, Speme S, et al. Risk of cognitive decline in older patients after carotid endarterectomy: an observational study. J Am Geriatr Soc. 2006;54:932–936. doi: 10.1111/j.1532-5415.2006.00787.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones RN, Gallo JJ. Education bias in the mini-mental state examination. Int Psychogeriatr. 2001;13:299–310. doi: 10.1017/s1041610201007694. [DOI] [PubMed] [Google Scholar]
- 26.Jorm AF, Scott R, Henderson AS, Kay DW. Educational level differences on the Mini-Mental State: the role of test bias. Psychol Med. 1988;18:727–731. doi: 10.1017/s0033291700008424. [DOI] [PubMed] [Google Scholar]
- 27.Brayne C, McCracken C, Matthews FE. Cohort profile: the Medical Research Council Cognitive Function and Ageing Study (CFAS) Int J Epidemiol. 2006;35:1140–1145. doi: 10.1093/ije/dyl199. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Brayne C, Calloway P. The case identification of dementia in the community: a comparison of methods. Int J Geriatr Psychiatry. 1990;5:309–316. [Google Scholar]
- 30.Clarke M, Jagger C, Anderson J, Battcock T, Kelly F, Stern MC. The prevalence of dementia in a total population: a comparison of two screening instruments. Age Ageing. 1991;20:396–403. doi: 10.1093/ageing/20.6.396. [DOI] [PubMed] [Google Scholar]
- 31.Chatfield M, Matthews FE, Brayne C. Using the Mini-Mental State Examination for tracking cognition in the older population based on longitudinal data. J Am Geriatr Soc. 2007;55:1066–1071. doi: 10.1111/j.1532-5415.2007.01216.x. [DOI] [PubMed] [Google Scholar]
- 32.Lievre A, Brouard N, Heathcote C. The estimation of health expectancies from cross-longitudinal surveys. Math Popul Stud. 2003;10:211–218. [Google Scholar]
- 33.Matthews FE, Chatfield M, Freeman C, et al. Attrition and bias in the MRC cognitive function and ageing study: an epidemiological investigation. BMC Public Health. 2004;4:12. doi: 10.1186/1471-2458-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews FE, Chatfield M, Brayne C MRC CFAS. An investigation of whether factors associated with short-term attrition change or persist over ten years: data from the Medical Research Council Cognitive Function and Ageing study (MRC CFAS) BMC Public Health. 2006;6:185. doi: 10.1186/1471-2458-6-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 36.Royston P. United Kingdom Stata Users’ Group Meetings. London: Stata Users Group; 2004. Multiple Imputation of Missing Data: An Implementation of van Buuren's MICE. [Google Scholar]
- 37.Brayne C, Matthews FE, McGee MA, Jagger C MRC CFAS. Health and ill-health in the older population in England and Wales. Age and Ageing. 2001;30:53–62. doi: 10.1093/ageing/30.1.53. [DOI] [PubMed] [Google Scholar]
- 38.Matthews FE, Miller LL, Brayne C, Jagger C. Regional differences in multidimensional aspects of health: findings from the MRC Cognitive Function and Ageing study. BMC Public Health. 2006;6:90. doi: 10.1186/1471-2458-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cullen B, Fahy S, Cunningham CJ, et al. Screening for dementia in an Irish community sample using MMSE: a comparison of norm-adjusted versus fixed cut-points. Int J Geriatr Psychiatry. 2005;20:371–376. doi: 10.1002/gps.1291. [DOI] [PubMed] [Google Scholar]