Abstract
Background
The association between body adiposity at older ages and the development of cognitive impairment is unclear.
Methods
The association of body mass index (BMI) and waist circumference in late life with incidence of cognitive impairment was prospectively examined in a cohort study of 1,351 Latinos, aged 60–101 and residents of the Sacramento, CA, area at study baseline. The status of dementia and “cognitive impairment but not demented” (CIND) was determined at baseline and at each of five follow-up examinations by a multistage assessment protocol. Incident cases of dementia and CIND were combined (dementia/CIND) for more than 8 years of follow-up. BMI was categorized as less than 25.0, 25.0–29.9 (overweight), and 30 kg/m2 or greater (obese). Waist circumference was categorized into sex-specific tertiles.
Results
Dementia/CIND was diagnosed in 110 (8.2%) participants. Compared with the lowest BMI category, overweight participants had a 48% decreased rate of dementia/CIND (adjusted hazard ratio [HR] = 0.52, 95% confidence interval [CI]: 0.30–0.91) and obese participants had a 61% decreased rate of dementia/CIND (HR = 0.39, 95% CI: 0.20–0.78). Rates of dementia/CIND for the middle and high tertile of waist circumference, compared with the low tertile, were 80% and 90% higher, respectively (adjusted HR = 1.8, 95% CI: 1.1–3.1, and adjusted HR = 1.9, 95% CI: 0.91–3.8).
Conclusions
Abdominal fat in late life appears to confer an increased risk for dementia/CIND, whereas overall obesity appears to be protective. This may reflect age-related changes in body composition and the association of visceral fat with metabolic dysregulation.
Keywords: Adiposity, Dementia, Cognitive impairment, Body mass index, Waist circumference
IT is well known that aging is associated with changes in body composition, including an increase of fat mass and a decline in lean mass. Abdominal fat, largely caused by the accumulation of visceral fat, increases proportionally more with age than peripheral fat (1–4). Many epidemiological and clinical studies have shown that excess abdominal visceral fat correlates with increased risk for hypertension, dyslipidemia, and hyperglycemia (5–7).
Measurement of body mass index (BMI) in older individuals may not adequately reflect abdominal fat accumulation because of the concurrent decrease in muscle mass. In older individuals particularly, waist circumference is a more accurate indicator of abdominal visceral fat level than BMI, percent body fat, or waist-to-hip ratio (8) and provides good correlation with the gold standard, computed tomography or magnetic resonance imaging (MRI) of the abdomen (9).
Because overweight and obesity increase the risk for vascular and metabolic disorders, body fat, particularly central body adiposity, may be a risk factor for age-related cognitive impairment or dementia. Several reports demonstrate an association between high BMI at midlife and increased risk for dementia in older ages (10–12); however, the association between adiposity at older ages and the development of dementia or cognitive impairment is less clear (13–16). The prevalence of central and general obesity is rapidly increasing in the United States, and prevalence of these conditions in Mexican Americans is disproportionately higher than in non-Hispanic whites and other Hispanic groups (17). We prospectively evaluated the associations of waist circumference and BMI with incident dementia or cognitive impairment in a cohort of older Mexican Americans.
METHODS
Study Population
Data are from the Sacramento Area Latino Study on Aging (SALSA), a cohort of community-dwelling Latinos, aged 60 or older at baseline in 1998–1999. Participants were residents in the Sacramento Metropolitan Statistical Area in California. Details of the sampling frame and recruitment have been previously published (18). At baseline, 1,789 individuals were enrolled in the study. Five follow-up cognitive evaluations were completed between the baseline evaluation (in 1998–1999) and 2006. More than 80% percent of participants had at least three follow-up visits, and the median years of follow-up were 4.5 in this analysis. The study population comprised 1,351 SALSA participants who were free of dementia or cognitive impairment but not demented (CIND) at baseline, had baseline anthropometric measurements, and had at least one follow-up cognitive evaluation during the follow-up period.
All field staff were bilingual in Spanish and English, and all interviews and clinical evaluations of participants (with the exception of MRI) were done at their homes in their language of choice. The study was approved by the University of California at Davis and the University of Michigan institutional review boards. Written informed consent was obtained from all participants.
Outcomes
The status dementia and CIND was determined at baseline and at each follow-up evaluation. Classifications of cognitive status were based on a multistage assessment protocol. At each evaluation, the Modified Mini-Mental State Examination (3MSE) and a verbal delayed word list recall test (DelRec) from the Spanish and English Verbal Learning Test (19) were administered to participants. The 3MSE is a widely used screening tool for dementia that tests a broad variety of cognitive functions including memory, orientation, language, and abstract reasoning (20). The 3MSE score ranges from 0 to 100, with higher scores indicating better global cognitive performance. The DelRec has been shown to be particularly useful in the early detection of abnormal short-term memory decline in older persons (21). The DelRec scores range from 0 to 15, with higher scores indicating better performance. At baseline, a participant was referred for neuropsychological evaluation if age- and education-adjusted screening scores fell below the 20th percentile on either test (3MSE < 84 or DelRec < 7). At each follow-up evaluation, a participant was referred for neuropsychological evaluation if there was a decline from the baseline 3MSE score by more than 8 points and the current 3MSE score was less than 20th percentile or there was a decline from the baseline DelRec score by more than 3 points and the current DelRec score was less than 20th percentile. The neuropsychological test battery (Spanish English Neuropsychological Assessment Scales) was constructed with five scales to provide psychometrically matched English- and Spanish-language measures of cognitive functioning in older persons (22). These scales included measures of semantic memory, verbal attention span, verbal abstraction, and visual perceptual ability. The neuropsychological evaluation also consisted of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (23). Participants were referred for a neuroclinical examination if they scored below the 10th percentile on one or more of the neuropsychological tests and 3.40 or greater on the IQCODE, scored below the 10th percentile on four or more neuropsychological tests regardless of IQCODE, or scored greater than 4.0 on the IQCODE. Dementia was diagnosed using Diagnostic and Statistical Manual of Mental Disorders 3rd edition and National Institute of Neurologic Disorders and Stroke-AD and Related Disorders Association criteria (24). CIND was diagnosed if the participant did not meet diagnostic criteria for dementia but had clinically significant impairment in one or more cognitive domains. Diagnoses of dementia, were adjudicated by a neurologist, a geriatrician, and a neuropsychologist. Seven dementia cases that were not diagnosed by the study were identified from death certificates and were assigned a dementia diagnosis after case review; for these, age at death was assigned as the age at diagnosis. For the present study, prevalent cases of dementia and CIND at baseline were excluded and incident cases of all-cause dementia and CIND were combined (dementia/CIND).
Anthropometric Measurements
Baseline body weight in kilograms, height in meters, and waist circumference (cm) were measured using standard tapes, stadiometers, and scales. Weight and height were measured without shoes. Waist circumference was taken at the level of the umbilicus at mid-respiration with the participant standing erect. BMI was calculated as weight in kilograms divided by height in meters squared.
Covariates
Age in years, education in years, and self-report of physician's diagnosis of stroke were assessed by interview at the baseline home visit. Baseline type 2 diabetes status was determined by a fasting glucose greater than 125 mg/dL, self-report of a physician's diagnosis, or use of a diabetes medication ascertained by a medicine chest inventory. Baseline low-density lipoprotein (LDL)-cholesterol was measured from a fasting blood sample.
Statistical Analysis
Waist circumference was categorized into sex-specific tertiles, and tertile values for men and women were combined. Waist circumference tertiles for women were 43.3–88.9, 91.44–101.6, and 104.14–142.24 cm, and for men, 40–88.9, 91.4–101.6, and 102–147.32 cm.
BMI was categorized as less than 25.0, 25.0–29.9 (overweight), and 30 kg/m2 or greater (obese). BMI categories for adults traditionally include an underweight category of less than 18.5, but because only four study participants had a BMI less than 18.5, the lowest BMI category for this analysis was categorized as less than 25.0.
Multicollinearity among waist circumference, BMI, and height was evaluated using standard diagnostic statistics. Spearman correlation coefficients ranged from −.15 to .65 and the tolerances were between .52 and .91, suggesting the absence of multicollinearity (25).
Descriptive statistics for participants at baseline were computed by waist circumference tertile and BMI category. Incidence rates were obtained for each waist circumference tertile and BMI category by dividing the number of cases by the number of person-years at risk, which was calculated by summing each participant's follow-up time from the study baseline. Confidence intervals for the incidence rates were calculated assuming a Poisson distribution.
Cox proportional hazards regression was used to examine the association between baseline waist circumference and BMI and rate of dementia/CIND during the follow-up period. Age at diagnosis of dementia/CIND (or censoring) was used in the models as the time scale as it was expected to have a larger effect on the hazard than time in study (26,27). The models were fit with each participant contributing time at risk starting from the participant's age at the baseline visit of the study. Participants who did not return for follow-up were censored at the age of their last cognitive evaluation. Participants who died during the follow-up period were censored at their age of death. Death certificates were sought for every participant and obtained for 90% who died during the study period.
Hazard ratio (HRs) with 95% confidence intervals were obtained for waist circumference tertiles and BMI categories with the lowest tertile as the reference group for waist circumference and less than 25.9 kg/m2 category as the reference group for BMI. Regression models were adjusted for age (as the time scale), sex, and education as these variables are risk factors for dementia/CIND and are also associated with body fat. To explore the effect of body fat distribution on the association between BMI and dementia/CIND, the HR was further adjusted for waist circumference. Height, as a measure of body stature and a possible marker of early life exposures, has been shown to have an independent and inverse association with late-life cognitive impairment (28,29); thus, models were additionally adjusted for height to control for the potential effect of body stature on cognitive impairment that is not accounted for by BMI (30). Analysis was done with and without adjustment for baseline diabetes status, stroke, and LDL-cholesterol level as these may be on the causal pathway between body fat and cognitive impairment. Tests for trend were evaluated fitting waist circumference tertiles and BMI categories as continuous variables. Interactions between sex and waist circumference, sex and BMI, age and waist circumference, and age and BMI were tested. Stratified analyses by sex and age at baseline (≤75 vs >75 years) were also performed to evaluate heterogeneity of the associations by sex and age. Statistical analyses were performed using PC-SAS (version 9).
Because weight loss is common in dementia and often occurs before the onset of clinical symptoms (31,32), all dementia/CIND cases that were diagnosed at the first two follow-up visits of the study were excluded in a separate analysis. This was done to exclude any participants who might have had clinically undetectable dementia/CIND early in the study and were experiencing weight loss as a result of the cognitive impairment.
RESULTS
Participants were followed for an average of 5.6 years (range 1–8 years). During this period, 110 (8.2%) of the participants eligible for this analysis were diagnosed with either incident dementia (n = 57) or CIND (n = 53). The average BMI at baseline was 29.8 (standard deviation [SD] = 5.9). The mean waist circumference among women was 94.8 cm (SD = 13.6) and among men 100.1 cm (SD = 12.3).
The Spearman correlation coefficient (r) was .65 (p < .0001) for waist circumference tertiles and BMI categories, r = .02 (p = .42) for waist circumference tertiles and height, and r = −.15 (p < .0001) for BMI categories and height.
Baseline demographic, anthropometric, and health-related characteristics and cognitive screening test scores by waist circumference tertiles and BMI categories are shown in Table 1. With increasing tertile of waist circumference, education and LDL-cholesterol decreased and the prevalence of diabetes increased. With increasing BMI, there was a decrease in mean age, education, and height. The prevalence of diabetes increased with increasing BMI. There was little difference in average baseline 3MSE or DelRec scores by waist circumference tertile or BMI category. More than 40% of men and 60% of women fell above the National Heart, Lung, and Blood Institute cut points of 101.6 cm for men and 88.9 cm for women.
Table 1.
Baseline Characteristics by Waist Circumference Tertile and Body Mass Index Category for 1,351 Study Participants
| Characteristic | Waist circumference (cm), tertiles |
||
| Low Tertile (women: 43.2–88.9; men: 40–88.9) | Middle Tertile (women: 91.44–101.6; men: 91.4–101.6) | High Tertile (women: 104.14–142.24; men: 104.14–147.32) | |
| N (% of total) | 436 (32.3) | 512 (37.9) | 403 (29.8) |
| Age, y | 70.0 (6.6) | 70.0 (6.9) | 69.8 (6.1) |
| Education, y | 8.0 (5.3) | 7.7 (5.4) | 7.5 (5.5) |
| Height, cm | 159.1 (9.6) | 159.6 (10.4) | 160.3 (10.7) |
| LDL-cholesterol, mg/dL | 216 (38) | 214 (40) | 208 (39) |
| Diabetes, % | 19.7 | 32.0 | 42.7 |
| Stroke, % | 5.3 | 9.0 | 8.2 |
| 3MSE score | 87.4 (9.7) | 87.0 (10.5) | 87.0 (10.9) |
| DelRec score | 8.9 (2.9) | 8.7 (2.9) | 8.9 (2.7) |
| Characteristic | Body mass index, kg/m2 | ||
| <25.0 (n = 249) | 25.0–29.9 (n = 521) | ≥30.0 (n = 581) | |
| Age, y | 71.3 (6.7) | 70.1 (6.7) | 69.2 (6.3) |
| Education, y | 8.0 (5.2) | 7.8 (5.4) | 7.6 (5.4) |
| Height, cm | 161.4 (10.9) | 161.1 (10.0) | 157.6 (10.0) |
| LDL-cholesterol, mg/dL | 211 (40) | 217 (39) | 210 (39) |
| Diabetes, % | 16.5 | 32.4 | 36.5 |
| Stroke, % | 7.2 | 6.3 | 8.8 |
| 3MSE score | 87.4 (9.5) | 86.7 (10.4) | 87.3 (10.8) |
| DelRec score | 8.7 (2.8) | 8.7 (2.9) | 9.0 (2.8) |
Note: Data are means (standard deviation) unless otherwise indicated. LDL = low-density lipoprotein; 3MSE = Modified Mini-Mental State Examination; DelRec, delayed word list recall test.
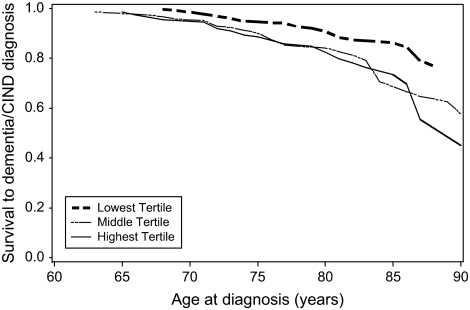
Table 2 shows the number of dementia/CIND cases, person-years at risk, and incidence rates for participants by waist circumference tertile and BMI category. The overall crude incidence rate was 14.6 per 1,000/y. Among BMI categories, the lowest rate was in the obese category. Among waist circumference tertiles, the lowest rate was in the low waist circumference tertile. Table 3 shows the HRs relating waist circumference to the rate of incident dementia/CIND in sequentially adjusted models. In Model 1, with no adjustment for BMI, there was little association of waist circumference with dementia/CIND rate. Model 2 shows an increase in the rate for participants in the middle and high tertiles of waist circumference compared with the participants in the low tertile after adjustment for BMI. Further adjustment for height (Model 3) strengthened the positive association between waist circumference and dementia/CIND. Exclusion of cases that were identified early in the study (Model 4) resulted in a 29% increase in the HR relating the highest waist circumference tertile to dementia/CIND. Figure 1 shows survival to dementia/CIND by waist circumference tertile adjusted for BMI category, sex, education, and height.
Table 2.
Dementia and Cognitive Impairment but not Demented Incidence Rates (per 1,000/y) by Waist Circumference Tertile and Body Mass Index (BMI) Category
| Number of cases | Person—years at risk | Incidence rate (95% confidence interval) | |
| Waist tertile | |||
| Low | 30 | 2,439.1 | 12.3 (7.9–16.7) |
| Middle | 50 | 2,864.2 | 17.5 (12.6–22.3) |
| High | 30 | 2,234.4 | 13.4 (8.6–18.2) |
| BMI category (kg/m2) | |||
| <25.0 | 27 | 1,381.5 | 19.5 (12.2–26.9) |
| 25.0–29.9 | 42 | 2,967.8 | 14.2 (9.9–18.4) |
| ≥30 | 41 | 3,188.4 | 12.9 (8.9–16.8) |
Table 3.
Hazard Ratios Relating Waist Circumference Tertiles to Incident Dementia and Cognitive Impairment but not Demented (Dementia/CIND) During 8 Years of Follow-up From Cox Proportional Hazards Regression Models
| Measure | Hazard Ratio (95% Confidence Interval) |
|||
| Model 1a | Model 2b | Model 3c | Model 4d | |
| Waist circumference | ||||
| Low tertile (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| Middle tertile | 1.3 (0.83–2.1) | 1.7 (1.0–3.0) | 2.1 (1.2–3.5) | 1.9 (1.0–3.4) |
| High tertile | 1.0 (0.62–1.7) | 1.6 (0.79–3.2) | 2.1 (1.0–4.4) | 2.7 (1.2–6.0) |
| p for trend | .86 | .17 | .03 | .02 |
Notes:
Adjusted for age, sex, and education.
Adjusted for age, sex, education, and body mass index category.
Adjusted for age, sex, education, body mass index category, and height.
After exclusion of dementia/CIND cases identified during first two follow-up evaluations and adjusted for age, sex, education, body mass index category, and height.
Figure 1.
Survival to dementia and cognitive impairment but not demented by tertile of waist circumference at baseline, adjusted for body mass index category, sex, education, and height.
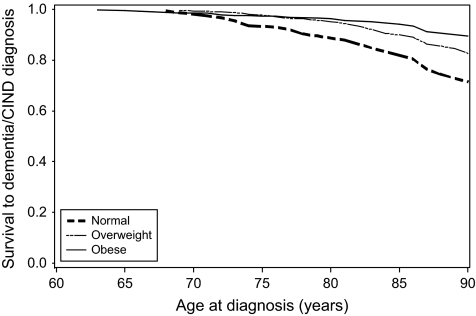
Table 4 shows the HRs relating BMI to the rate of incident dementia/CIND in sequentially adjusted models. There was an inverse effect of BMI on dementia/CIND rate without adjustment for waist circumference (Model 1). Model 2 shows a stronger inverse association between BMI and dementia/CIND after adjustment for waist circumference. Further adjustment for height (Model 3) strengthened the inverse association between BMI and dementia/CIND. After excluding dementia/CIND cases that were identified early in the study (Model 4), the adjusted HRs for BMI and dementia/CIND remained essentially unchanged. Among those in the highest BMI category, dementia/CIND rates were 76% lower compared with the lowest BMI category in Model 4. Figure 2 shows survival to dementia/CIND by BMI category adjusted for waist circumference tertile, sex, education, and height.
Table 4.
Hazard Ratios Relating Body Mass Index (BMI) to Incident Dementia and Cognitive Impairment but not Demented (Dementia/CIND) During 8 Years of Follow-up From Cox Proportional Hazards Regression Models
| Measure | Hazard Ratio (95% Confidence Interval) |
|||
| Model 1a | Model 2b | Model 3c | Model 4d | |
| BMI, kg/m2 | ||||
| <25.0 (reference) | 1.0 | 1.0 | 1.0 | 1.0 |
| 25.0–29.9 | 0.71 (0.43–1.2) | 0.55 (0.32–0.95) | 0.47 (0.27–0.82) | 0.46 (0.25–0.86) |
| >30.0 | 0.69 (0.42–1.1) | 0.49 (0.25–0.95) | 0.34 (0.17–0.70) | 0.24 (0.10–0.54) |
| p for trend | .18 | .05 | .004 | .001 |
Notes:
Adjusted for age, sex, and education.
Adjusted for age, sex, education, and waist circumference tertile.
Adjusted for age, sex, education, waist circumference tertile, and height.
After exclusion of dementia/CIND cases identified during first two follow-up evaluations and adjusted for age, sex, education, waist circumference tertile, and height.
Figure 2.
Survival to dementia and cognitive impairment but not demented by body mass index category, adjusted for waist circumference tertile, sex, education, and height.
Although there was little evidence of interactions of sex or age with BMI or waist circumference (p > .10 for all interaction tests), stratified analysis by sex and age revealed moderately stronger associations of body adiposity and CIND/dementia rate among women and among older participants. Among women, the adjusted HRs were 2.1 and 3.6 for the middle and highest waist circumference tertile, respectively, compared with the lowest tertile (p for trend = .009), and among men, the adjusted HRs were 2.2 and 1.0, respectively (p for trend = .82). The adjusted HR relating the overweight and obese categories to the rate of CIND/dementia among women were 0.41 and 0.22 compared with the lowest BMI category (p for trend = .001). Among men, these adjusted HRs were 0.52 and 0.54, respectively (p for trend = .39).
In stratified analysis by baseline age (≤75 vs >75 years), adjusted HRs in the older age group for middle and high tertiles of waist circumference were 3.3 and 4.3 (p for trend = .01), and were 1.5 and 1.4 (p for trend = .50), respectively, compared with the lowest waist circumference tertile in the younger group. Among older participants, the adjusted HRs were 0.57 and 0.30 for the overweight and obese BMI categories, respectively, compared with the lowest category (p for trend = .03); among younger participants, the adjusted HRs were 0.43 and 0.40 for the overweight and obese categories, respectively, compared with the lowest BMI category, p for trend = .06.
The addition of diabetes, stroke, and LDL-cholesterol to the fully adjusted models attenuated the association of waist circumference with dementia/CIND (HR for middle tertile compared with low tertile = 1.5; HR for high tertile compared with low tertile = 1.3; p for trend = .47). The effect of BMI category did not diminish after the addition of these variables (HR for the overweight category compared with the reference category = 0.55 and HR for the obese category compared with the lowest category = 0.44; p for trend = .01).
DISCUSSION
In this cohort study of older persons, BMI at baseline was inversely associated with rate of dementia/CIND during the follow-up period. In contrast, large waist circumference at baseline was associated with an increased rate of dementia/CIND. Adjustment for the presence of the vascular and metabolic risk factors of diabetes, stroke, and LDL-cholesterol attenuated the relationship between waist circumference and risk of dementia/CIND but had no effect on the association of BMI with risk of dementia/CIND.
The association of BMI with dementia/CIND rate was sequentially strengthened after adjustment for waist circumference and height. Similarly, the association of waist circumference with dementia/CIND rate was strengthened after sequential adjustment for BMI and height. These results suggest that the effects of generalized obesity and central obesity in late life on rate of cognitive impairment may be masked without complete adjustment for body size and stature.
Cross-sectional studies show that older individuals with dementia have a lower BMI than those without dementia (33–35) and longitudinal findings suggest that weight loss may be a precursor to dementia (31,32). To reduce the possibility of low BMI related to the weight loss that can accompany preclinical cognitive impairment, we performed an additional analysis after excluding all incident CIND and dementia cases that occurred during the first 2 years in the follow-up period. Results from this analysis revealed no change in the inverse relationship between BMI and rate of dementia/CIND. Exclusion of these early cases strengthened the association between large waist circumference and dementia/CIND.
Considerable evidence exists to support a relationship between central obesity and vascular and metabolic abnormalities that are risk factors for dementia/CIND. In this study, inclusion of baseline diabetes status, stroke, and LDL-cholesterol levels attenuated the association between waist circumference and dementia/CIND suggesting that metabolic and vascular abnormalities may mediate the association.
Reports from other longitudinal studies relating BMI in older persons to cognitive function are conflicting (36). Mechanisms relating BMI, independent from central obesity, to cognitive impairment or dementia are unclear. A higher BMI can be the result of more lean body mass as well as more fat mass. It is possible that higher lean body mass may be involved in reducing the risk of dementia/CIND in an older population. In a large cross-sectional study on older women, participants in the highest quartile of fat-free soft tissue mass had decreased risk for cognitive impairment compared with the lowest quartile group (37). Increased BMI may also result from increased accumulation of fat in regions other than the abdominal area. Larger leg fat mass in older individuals has been associated with improved glucose metabolism (38), which could potentially have implications for reducing risk of development of cognitive impairment. A recent publication (39) reported increased risk of dementia associated with sagittal fat. It may also be possible that loss in muscle and gain in central fat may act synergistically to increase risk for dementia/CIND in older populations.
The present study has several strengths including a longitudinal assessment of dementia/CIND. Dementia or CIND was diagnosed with a standard battery of cognitive tests and clinical evaluation. Baseline weight, height, and waist circumference were measured by the investigators, and the potentially confounding variables of age, sex, education, and height were controlled for in the analyses.
A limitation of this study is the relatively low frequency of dementia or CIND that prohibited the examination of the effect of body adiposity on risk of specific classifications of dementia or cognitive impairment such as Alzheimer's diease or vascular dementia. Also, we were unable to investigate the effect of weight history of study participants onrisk of dementia/CIND. Additionally, we were unable to explicitly differentiate lean and fat components of the anthropometric measurements.
Accumulation of abdominal fat, but not total body fat, may confer an increased risk for age-related cognitive impairment in late life. The possible inverse effect of BMI on rate of dementia/CIND has relevance to ongoing discussions regarding guidelines for ideal body weight in older populations.
Acknowledgments
This study was supported by grants AG12975 and DK60753 from the National Institutes of Health and used the Chemistry Laboratory of the Michigan Diabetes Research and Training Center funded by NIH5P60 DK20572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Horber FF, Gruber B, Thomi F, Jensen EX, Jaeger P. Effect of sex and age on bone mass, body composition and fuel metabolism in humans. Nutrition. 1997;13:524–534. doi: 10.1016/s0899-9007(97)00031-2. [DOI] [PubMed] [Google Scholar]
- 2.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Hassager C, Ravn P, Wang S, Christiansen C. Total and regional body-composition changes in early postmenopausal women: age-related or menopause-related? Am J Clin Nutr. 1994;60:843–848. doi: 10.1093/ajcn/60.6.843. [DOI] [PubMed] [Google Scholar]
- 4.Zamboni M, Armellini F, Harris T, et al. Effects of age on body fat distribution and cardiovascular risk factors in women. Am J Clin Nutr. 1997;66:111–115. doi: 10.1093/ajcn/66.1.111. [DOI] [PubMed] [Google Scholar]
- 5.Ohlson LO, Larsson B, Svardsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus. 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–1058. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 6.Okosun IS, Cooper RS, Rotimi CN, Osotimehin B, Forrester T. Association of waist circumference with risk of hypertension and type 2 diabetes in Nigerians, Jamaicans, and African-Americans. Diabetes Care. 1998;21:1836–1842. doi: 10.2337/diacare.21.11.1836. [DOI] [PubMed] [Google Scholar]
- 7.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 8.Rankinen T, Kim SY, Perusse L, Despres JP, Bouchard C. The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord. 1999;23:801–809. doi: 10.1038/sj.ijo.0800929. [DOI] [PubMed] [Google Scholar]
- 9.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 10.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 11.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–326. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 14.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 16.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 17.Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004;89:2590–2594. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 18.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English- and Spanish-speaking older people. J Int Neuropsychol Soc. 2001;7:544–555. doi: 10.1017/s1355617701755026. [DOI] [PubMed] [Google Scholar]
- 20.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 21.Welsh K, Butters N, Hughes J, Mohs R, Heyman A. Detection of abnormal memory decline in mild cases of Alzheimer's disease using CERAD neuropsychological measures. Arch Neurol. 1991;48:278–281. doi: 10.1001/archneur.1991.00530150046016. [DOI] [PubMed] [Google Scholar]
- 22.Mungas D, Reed BR, Marshall SC, Gonzalez HM. Development of psychometrically matched English and Spanish language neuropsychological tests for older persons. Neuropsychology. 2000;14:209–223. doi: 10.1037//0894-4105.14.2.209. [DOI] [PubMed] [Google Scholar]
- 23.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Allison PD. Logistic Regression Using SAS®: Theory and Application. Cary, NC: SAS Institute and John Wiley & Sons; 1999. [Google Scholar]
- 26.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 27.Lamarca R, Alonso J, Gomez G, Munoz A. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53:M337–M343. doi: 10.1093/gerona/53a.5.m337. [DOI] [PubMed] [Google Scholar]
- 28.Beeri MS, Davidson M, Silverman JM, Noy S, Schmeidler J, Goldbourt U. Relationship between body height and dementia. Am J Geriatr Psychiatry. 2005;13:116–123. doi: 10.1176/appi.ajgp.13.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petot GJ, Vega U, Traore F, et al. Height and Alzheimer's disease: findings from a case-control study. J Alzheimers Dis. 2007;11:337–341. doi: 10.3233/jad-2007-11310. [DOI] [PubMed] [Google Scholar]
- 30.Michels KB, Greenland S, Rosner BA. Does body mass index adequately capture the relation of body composition and body size to health outcomes? Am J Epidemiol. 1998;147:167–172. doi: 10.1093/oxfordjournals.aje.a009430. [DOI] [PubMed] [Google Scholar]
- 31.Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996;44:1147–1152. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 32.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 33.Berlinger WG, Potter JF. Low body mass index in demented outpatients. J Am Geriatr Soc. 1991;39:973–978. doi: 10.1111/j.1532-5415.1991.tb04043.x. [DOI] [PubMed] [Google Scholar]
- 34.Burns A, Marsh A, Bender DA. Dietary intake and clinical, anthropometric and biochemical indices of malnutrition in elderly demented patients and non-demented subjects. Psychol Med. 1989;19:383–391. doi: 10.1017/s0033291700012423. [DOI] [PubMed] [Google Scholar]
- 35.White H, Pieper C, Schmader K, Fillenbaum G. Weight change in Alzheimer's disease. J Am Geriatr Soc. 1996;44:265–272. doi: 10.1111/j.1532-5415.1996.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 36.Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age Ageing. 2007;36:23–29. doi: 10.1093/ageing/afl123. [DOI] [PubMed] [Google Scholar]
- 37.Nourhashemi F, Andrieu S, Gillette-Guyonnet S, et al. Is there a relationship between fat-free soft tissue mass and low cognitive function? Results from a study of 7,105 women. J Am Geriatr Soc. 2002;50:1796–1801. doi: 10.1046/j.1532-5415.2002.50507.x. [DOI] [PubMed] [Google Scholar]
- 38.Snijder MB, Dekker JM, Visser M, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:327–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 39.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008 doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]