Abstract
Background
Understanding preclinical transitions to impairment in cognitive abilities associated with risks for functional difficulty and dementia. This study characterized in the Women's Health and Aging Study (WHAS) II 9-year declines and transitions to impairment across domains of cognition.
Methods
The WHAS II is an observational study of initially high-functioning, community-dwelling women aged 70–80 years at baseline. Random-effects models jointly compared rates of decline, and discrete-time Cox models estimated hierarchies of incident clinical impairment on measures of psychomotor speed and executive function (EF) using the Trail Making Test and in immediate and delayed verbal recall using the Hopkins Verbal Learning Test. Patterns of transition were related to incidence of global cognitive impairment on the Mini-Mental State Exam (MMSE).
Results
Mean decline and impairment occurred first in EF and preceded declines in memory by about 3 years. Thereafter, memory decline was equivalent to that for EF. Over 9 years, 49% developed domain-specific impairments. Risk of incident EF impairment occurred in 37% of the sample and was often the first impairment observed (23.7%), at triple the rate for psychomotor speed (p < .01). Risk of immediate and delayed recall impairments was nearly double that for psychomotor speed (p values <.01). Incident impairment in EF and delayed recall was associated with greater risk for MMSE impairment.
Conclusions
Executive dysfunction developed first among nearly one quarter of older women and was associated with elevated risk for global cognitive impairment. Because EF declines preceded memory declines and are important to efficient storage and retrieval EF represents an important target for interventions to prevent declines in memory and MMSE both of which are associated with progression to dementia.
Keywords: Cognitive decline, Executive dysfunction, Memory, Cognitive impairment, Mild cognitive impairment
MOST cognitive abilities remain relatively stable during the life course, with declines beginning in one's sixth or seventh decades (1). Declines in memory become more precipitous in the seventh decade (2), and accelerate thereafter (3,4). This period corresponds to that in which population-based risk for dementia begins to rise dramatically (5). The clinical and public health import of characterizing the preclinical cognitive changes that progress to cognitive impairment and dementias, such as Alzheimer's disease (AD), has taken on increased significance given the findings that risk modifiers exert little benefit by the time one reaches diagnosis. One method by which to characterize incipient cognitive changes that precede dementia is via long-term longitudinal observation of age-related declines in an initially high-functioning sample in the age range of increased risk (6–8). Describing and comparing rates of decline across cognitive abilities, such as memory and executive function (EF), further allows us to identify possible hierarchies of decline and incident impairment that could help identify clinically relevant precursor states amenable to intervention.
Some cognitive abilities appear more vulnerable to the effects of aging than other abilities. Speed of information processing and psychomotor speed begin to decline in one's late 30s and decline at a modest linear rate (9). By comparison, one's verbal lexicon and general knowledge continue to improve well into one's sixth decade (1) and remain stable through one's 80s (10). What remains less well understood are the onsets and rates of decline in cognitive abilities associated with functional difficulty and dementia, such as executive attention (11,12), and its onset relative to memory. To date, clinical and epidemiological studies of dementia have generally restricted focus to global cognition and memory, thus limiting understanding about attentional changes.
Clinical wisdom holds that changes in episodic memory mark the early stage of AD among initially nondemented individuals (13,14). Studies of preclinical AD show that deficits often emerge in multiple domains, including memory (15), EF (16,17), and speed of processing (18). Along these lines, studies of the precursor stage to AD, mild cognitive impairment (MCI) (19), now suggest that impairments are not restricted to, and may not necessarily include, memory (20,21).
In this study, we examined in a cohort of well-characterized and initially high-functioning older women entering the age of greatest risk for cognitive impairment. Our first objective was to assess whether cognitive declines were uniform across domains of ability for a 9-year interval, and, if not, which domains of ability declined first and most rapidly. Second, we explored whether these declines corresponded to the order in which individuals made clinical transitions to impairment across domains of cognition associated with risk for global cognitive impairment. Characterizing cognitive changes that may precede clinical transitions to dementia in this population-based sample will provide vital information in the early and accurate identification of at-risk individuals in whom targeted treatments may be applied.
METHODS
Participants
The Women's Health and Aging Study II (WHAS II) is a prospective study of physical functioning among the least disabled two thirds of 70- to 80-year-old, community-dwelling women in eastern Baltimore, MD. Sampling and recruitment of this cohort is described in detail elsewhere (11,22) and complements WHAS I, a study of the one-third most disabled, community-dwelling older women. The sampling frame for WHAS II was drawn from female Medicare beneficiaries on Health Care Financing Administration Medicare eligibility lists. Trained interviewers determined eligibility at sampling according to whether individuals were (a) aged 70–79 years; (b) had sufficient hearing and proficiency in English to be interviewed; (c) could be contacted by telephone; (d) had a Mini-Mental State Exam (MMSE) (23) score >23; and (e) reported difficulty in no more than one of four functional domains: mobility and exercise tolerance, upper extremity, higher functioning (eg, shopping), and basic self-care (22). Of 880 eligible individuals, 436 agreed to participate in the baseline examination. Five follow-up exams were conducted at approximately 1.5-year intervals, with the exception of a 3-year interval between Exams 3 and 4, yielding a 9-year study interval. This study was approved by the Johns Hopkins Institutional Review Board, and each participant gave informed, written consent before completing a standardized interview at each exam. Exams included an adjudicated medical history based on patient record review to ascertain 14 physician-diagnosed chronic conditions or diseases (22), including coronary artery disease, chronic heart failure, chronic obstructive pulmonary disease, cancer, disc disease, diabetes, hip fracture, osteoarthritis, osteoporosis, rheumatoid arthritis, peripheral artery disease, Parkinson's disease, stenosis, and stroke, as well as sensory vision and hearing difficulties. At each exam, participants also completed a cognitive exam, as part of a 1-day evaluation in the Functional Status Laboratory at the Johns Hopkins Outpatient General Clinical Research Center, or in the home, as needed. Education was measured by years of schooling. Race/ethnicity was dichotomized into white versus black (22). Over 9 years, 90 participants died. Additionally, 103 participants were lost to follow-up. Of 436 participants at baseline, 395 had complete cognitive test data for at least one of five follow-up exams, and thus served as the sample for comparative analyses of trajectories of cognitive decline. The 41 not included (due to missing data) had less education (p = .01), lower baseline MMSE scores (p < .001) (23), and were more often nonwhite, (p = .002), but were otherwise comparable in age and most comorbidities (p values >.10).
Study Outcomes
Standardized cognitive testing by a trained technician included a global cognitive screen, the MMSE. The Trail Making Test (TMT) (24,25) was used to evaluate psychomotor speed via Part A and EF via Part B. Participants were allotted a maximum time of 240 seconds on Part A and 360 seconds on Part B. Verbal immediate and delayed recall memory of 12 common objects were assessed using the Hopkins Verbal Learning Test (HVLT-R) (26,27) and its six alternate forms. Participants heard and recalled words during three successive learning trials (maximum = 36) and again after a filled, 20-minute interval. Times on the TMT were transformed into speed (1/time in minutes) so that higher scores across tests were uniformly associated with better performance.
Statistical Analyses
Modeling declines in cognition.—
The primary objective was to compare rates of decline across four cognitive domains. We analyzed each outcome separately using conventional random-effects growth curve models (REGCM) to account for within-subject correlation, unequal follow-up intervals, and data missing at random (28–30). Random effects model individuals with baseline abilities (intercept) and rates of decline (ie, slopes) that differ from the mean. To check for nonlinear changes in rates of domain-specific decline for a period of 9 years, scatterplots were used (31). Performance on the TMT, Part A, declined at a steady, linear rate for a period of 9 years. However, slopes differed before and after Exam (year) 3 on the TMT, Part B, and both HVLT memory measures requiring that a spline term be used at Exam 3 to separately model these two slopes. We used an unstructured variance-covariance matrix for random effects. Goodness of fit criteria were used for all model comparisons. We also identified potential outliers (32).
Assessing hierarchy of declines across cognitive outcomes.—
To compare rates of decline across cognitive outcomes, we performed three complementary analyses focusing on both mean and individual-level order of onset of declines. We first analyzed the four cognitive outcomes jointly in a multivariate REGCM, with the TMT, Part A, serving as the reference test of normative age-related decline on which to compare declines in other, more complex domains. This joint modeling approach allowed us to directly compare and test mean differences in slopes. To compare differences between cognitive domains in the multivariate model, we standardized the mean effects estimates of rates of decline by using the standard deviation (SD) of that domain at baseline. All regression models were adjusted for age (years), race (white vs nonwhite), education (years), and number of chronic diseases.
The second approach applied survival models to investigate population-mean order of incidence of impairment across cognitive domains. Impairment on each of four tests was conservatively defined as a terminal event, according to its first onset, based on performance at or below 10th percentile cutoffs using published age- and education-matched norms: TMT, Part A ≥81 seconds; TMT, Part B ≥225 seconds (33); HVLT immediate recall ≤16; and HVLT delayed recall ≤4 (26). These cut-points matched well with internal norms, corresponding to 1.4–1.8 SDs below internal norms at baseline and between the 5th and 12th percentiles. Cases coded on a given test as incomplete due to cognitive impairment were defined as impaired at that exam. Those 73 participants with prevalent impairment on one or more of the four tests at baseline were removed from incident analyses, yielding a final sample of 322.
Multivariate discrete-time Cox proportional hazards (DTPH) models (34) were selected to compare the hazard of incident impairment in each of three TMT, Part B, and the HVLT immediate and delayed recall outcomes relative to incident impairment on the TMT, Part A, the test which declined least in slope over time and which is more strongly associated with normative aging. The TMT, Part A, again served as the reference test (baseline hazard function) with which to compare elevated hazard, or risk, of incident impairment on other tests. To account for within-subject correlations for multiple impairments, we included person-specific intercepts as random effects in the model. Those missing follow-up visits were defined as missing and conservatively right censored as nonevents. We used PROC MIXED in SAS (version 9.0, SAS Institute, Inc., Cary, NC) to fit the REGCMs and MIXOR 2.0 (available at http://www.dsi-software.com/mixedup.html) (35,36) to fit the DTPH model.
We complemented population-mean Cox models by categorizing individuals according to their first domain-specific impairment and examined associated studywide risk of global cognitive impairment on the MMSE. Participants were placed into one of five groups: those developing EF impairment first (or alone) on the TMT, Part B (EF Group); those developing impairment first (or alone) on the HVLT delayed recall, the ability most often predicting AD (DR Group); those with impairment first on HVLT immediate recall (DI Group); those in whom EF and recall deficits co-occurred (EF+ DR Group); and the Healthy reference Group, who remained cognitively intact during the study interval. Because only 7 cases of incident impairment on the TMT, Part A, occurred first (or alone), they were considered too small to make stable comparisons and were excluded. We then compared these five groups for studywide rates of clinically significant impairment in global cognition (MMSE <24).
RESULTS
Descriptive Analysis
Baseline characteristics for the 395 participants are presented in Table 1. Their average age was 74 years and mean MMSE score was 28. Seventy-nine percent completed a high school education or higher and 17% were African American. The means, SDs, and ranges for all four cognitive tests for six exams spanning a 9-year interval are presented in Table 2. The average follow-up period was 7.23 years. Attrition was minimal for the first three exams (379 at Exam 2 and 377 at Exam 3) and increased at Exams 4 (N = 301), 5 (N = 273), and 6 (N = 243). Although there was an overall decline in performances over time, selective loss of the oldest and most cognitively impaired participants due to death or study withdrawal likely resulted in a conservative estimate of true decline.
Table 1.
Baseline Characteristics of 395 Women in the Women's Health and Aging Study II, and Stratified by Presence or Absence of Incident Impairment in One or More Domains of Cognition
| Characteristics | Overall Sample (N = 395) | Participants Free of Domain-Specific Cognitive Impairment* (N = 163) | Participants With Any Incident Domain-Specific Cognitive Impairment* (N = 159) | p Value Comparing Stratified Groups |
| Age, mean (SD) | 73.9 (2.8) | 73.2 (2.6) | 74.3 (2.9) | <.001 |
| Caucasian, n (%) | 327 (82.8) | 145 (89.0) | 129 (81.1) | .05 |
| Education, mean (SD) | 12.6 (3.3) | 13.6 (3.0) | 12.4 (3.2) | <.001 |
| MMSE, mean (SD) | 28.2 (1.7) | 28.9 (1.3) | 28.2 (1.6) | <.001 |
| Number of chronic diseases†, mean (SD) | 1.5 (1.0) | 1.5 (1.0) | 1.6 (1.1) | .23 |
| CVD, % (n) | 34.4 (136) | 33.1 (54) | 35.9 (57) | .61 |
| DM, % (n) | 7.6 (30) | 3.7 (6) | 10.1 (16) | .02 |
| Stroke, % (n) | 1.3 (5) | 1.8 (3) | 1.3 (2) | .67 |
| Smoking | ||||
| Never smoker, % (n) | 55.6 (219) | 52.2 (85) | 58.2 (92) | .43 |
| Former smoker, % (n) | 34.8 (137) | 39.2 (64) | 32.3 (51) | |
| Current smoker, % (n) | 9.6 (38) | 8.6 (14) | 9.5 (15) | |
| Drink alcohol, % (n) | 32.2 (127) | 35.6 (58) | 32.9 (52) | .61 |
| Kilocalories expenditure of physical activities per week, mean (SD) | 1214.9 (1344.6) | 1274.9 (1306.4) | 1241.0 (1370.4) | .82 |
| IADL difficulty‡, % (n) | 12.0 (47) | 6.8 (11) | 11.5 (18) | .14 |
| Mobility difficulty, % (n) | 30.4 (120) | 20.9 (34) | 37.7 (60) | <.001 |
| Self-care difficulty, % (n) | 0.8 (3) | 0 (0) | 1.9 (3) | .08 |
Notes: MMSE = Mini-Mental State Examination; CVD = cardiovascular disease, CHF = chronic heart failure; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; SD = standard deviation.
Comparison of two groups restricted to those without prevalent impairment at baseline.
Number of chronic diseases is the sum of 14 chronic diseases.
Instrumental activities of daily living difficulty in one or more of the eight items including light housework, preparing meals, using telephone, shopping, taking medication, managing money, driving, and using stove.
Table 2.
Distributions of Cognitive Test Scores Across Six Exams Spanning a 9-Year Interval for 395 Cognitively Intact Women, Aged 70–80 Years at Baseline in the Women's Health and Aging Study II
| Cognitive Test | N | Mean (SD) | Range | p Value |
| TMT, Part A, (s) | ||||
| Exam 1 | 366 | 46.0 (19.2) | 17.0–153.2 | <.001 |
| Exam 2 | 370 | 48.0 (22.3) | 20.9–240.0 | |
| Exam 3 | 371 | 52.6 (31.9) | 18.8–240.0 | |
| Exam 4 | 300 | 56.1 (32.5) | 19.0–240.0 | |
| Exam 5 | 270 | 58.8 (38.5) | 15.9–240.0 | |
| Exam 6 | 239 | 60.2 (35.4) | 18.1–240.0 | |
| TMT, Part B (s) | ||||
| Exam 1 | 364 | 127.5 (70.4) | 44.0–420.0 | <.001 |
| Exam 2 | 367 | 138.7 (87.6) | 35.5–420.0 | |
| Exam 3 | 371 | 165.5 (104.2) | 33.6–420.0 | |
| Exam 4 | 293 | 177.7 (104.6) | 43.7–420.0 | |
| Exam 5 | 269 | 192.6 (115.0) | 50.6–420.0 | |
| Exam 6 | 238 | 195.8 (112.7) | 52.0–420.0 | |
| HVLT immediate recall | ||||
| Exam 1 | 381 | 22.8 (5.0) | 7–35 | <.001 |
| Exam 2 | 373 | 22.5 (5.2) | 7–34 | |
| Exam 3 | 371 | 23.0 (5.8) | 7–34 | |
| Exam 4 | 301 | 21.5 (5.9) | 2–35 | |
| Exam 5 | 271 | 21.2 (5.6) | 6–36 | |
| Exam 6 | 241 | 21.9 (6.6) | 5–34 | |
| HVLT delayed recall | ||||
| Exam 1 | 371 | 8.2 (2.6) | 0–12 | <.001 |
| Exam 2 | 373 | 8.0 (2.7) | 0–12 | |
| Exam 3 | 372 | 8.1 (2.9) | 0–12 | |
| Exam 4 | 300 | 7.4 (3.0) | 0–12 | |
| Exam 5 | 271 | 7.3 (3.2) | 0–12 | |
| Exam 6 | 239 | 7.1 (3.5) | 0–12 | |
| MMSE | ||||
| Exam 1 | 387 | 28.2 (1.7) | 22.0–30.0 | <.001 |
| Exam 2 | 372 | 28.2 (2.0) | 21.0–30.0 | |
| Exam 3 | 382 | 27.4 (2.6) | 17.0–30.0 | |
| Exam 4 | 319 | 27.7 (2.7) | 9.0–30.0 | |
| Exam 5 | 285 | 27.5 (2.8) | 17.0–30.0 | |
| Exam 6 | 272 | 27.1 (3.3) | 5.0–30.0 |
Notes: TMT = Trail Making Test; HVLT = Hopkins Verbal Learning Test; MMSE = Mini-Mental State Examination.
The p value based on tests of trend in decline over time.
Assessing Order of Mean Declines Across Domains
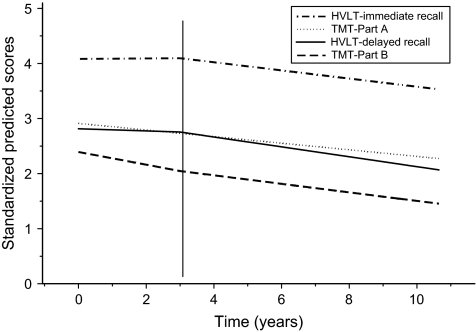
Table 3 presents annual mean rates of decline for each of the four domains, estimated from REGCM. For example, speed on the TMT, Part A, declined an average of 0.032 units per year, or a reduction of 0.8 (nearly one) circle connected per minute from a total of 25 (0.032 × 25). Because the three remaining outcomes showed significant shifts in rates of decline from Exams 3 onward (p = .03 for TMT, Part B, p < .01 for both HVLT measures), we presented rates before and after Exam 3. On the TMT, Part B, there was a modest attenuation in rate of decline after Year 3. Specifically, during the first 3 years, women showed a reduction of 0.73 (−0.029 × 25) circles connected per minute per year, and during the second 6 years, a reduction of 0.5 circles connected per minute per year. On the HVLT measures, there was no significant decline during the first 3 years, followed by an annual rate of decline of 0.4 words per year on immediate recall and nearly 0.3 words per year on delayed recall. When multiplying this rate for Years 3–9 (6 years), women will have declined by an average of nearly 2 of 12 possible words to be recalled or 18%. Relative, or joint, comparison of standardized estimates of decline across domains is presented in Table 3 and Figure 1 and shows that the TMT, Part B, declined fastest for the first 3 years (−0.119), with slight attenuation from Year 3 onward (−0.082). Rates of decline from Year 3 onward in the HVLT delayed and immediate recall were similar to that of TMT, Part B. Part A declined at half the initial rate of TMT, Part B, for the 9-year interval (−0.60).
Table 3.
Mean Annual Rates of Decline for Each of Four Cognitive Tests, From Random-Effects Growth Curve Models Adjusting For Age, Race, Education, and Comorbidity (N = 395)
| Annual Rate of Decline Over 9 Years |
||||||
| Slope (95% CI) | Standardized Slope (95% CI)* | p Value | Slope (95% CI) | Standardized Slope (95% CI) | p Value | |
| TMT, Part A (units per min) | −0.032 (−0.038, −0.026) | −0.060 (−0.072, −0.048) | <.001 | — | − | − |
| Annual Rate of Decline in Years 1–3 | Annual Rate of Decline in Years 3–9 | |||||
| TMT, Part B (units per min) | −0.029 (−0.035, −0.023) | −0.119 (−0.143, −0.095) | <.001 | −.020 (−0.028, −0.012) | −0.082 (−0.113, −0.051) | <.001 |
| HVLT immediate recall (no. of words correctly recalled) | 0.025 (−0.135, 0.186) | 0.005 (−0.026, 0.036) | .76 | −0.416 (−0.671, −0.161) | −0.083 (−0.134, −0.032) | .001 |
| HVLT delayed recall (no. of words correctly recalled) | −0.059 (−0.137, 0.019) | −0.025 (−0.054, 0.004) | .14 | −0.267 (−0.390, −0.144) | −0.101 (−0.148, −0.054) | <.001 |
Notes: CI = confidence interval; TMT = Trail Making Test; HVLT = Hopkins Verbal Learning Test.
Rates of decline for three of the four outcomes differed before and after Year 3.
Standardized slope estimates, that is, estimated regression coefficient divided by standard deviation of its corresponding outcome at baseline.
Figure 1.
Mean rates of decline across cognitive domains, including the Trail Making Test, Part A (dotted line); HVLT immediate recall (dashed and dotted line); HVLT delayed recall (solid line), and the Trail Making Test, Part B (long dashed line), from univariate random-effects models.
We observed variability in individuals’ speed at baseline and in rates of decline on TMT, Parts A and B, suggesting multiple sources of variation, beyond standard covariates included (p < .001; variance estimates for random intercept and random slope). Higher baseline speeds on the TMT were associated with less decline (significant inverse association between random intercept and random slope; p < .01). On HVLT measures, there was no heterogeneity in rates of decline.
Incident Impairment
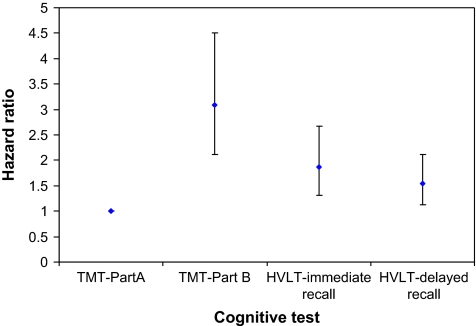
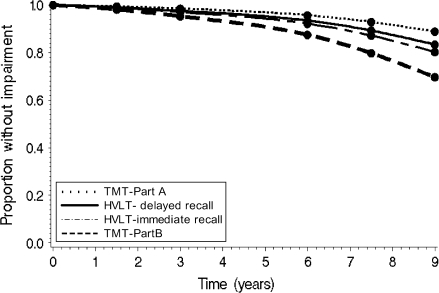
Analyses were then conducted to model the order of transitions to cognitive impairment across each domain among those without baseline impairments. Of 322 participants, 49% developed one or more impairments, as defined earlier, on a cognitive test during the 9-year interval. Specifically, 37%, 28%, 26%, and 21% of women developed incident impairment on TMT, Part B; HVLT immediate recall; HVLT delayed recall; and TMT, Part A, respectively. Figure 2 shows that the risk of impairment on the TMT, Part B, was 3 times higher than that for the TMT, Part A (95% confidence interval [CI]: 2.1–4.5, p < .01). Risks of impairment on HVLT delayed and immediate recalls were 1.9 (95% CI: 1.3–2.7, p < .01) and 1.6 (95% CI: 1.1–2.1, p < .01) times higher, respectively, after adjusting for covariates and participants’ baseline hazard on the TMT, Part A. The order of risks of incident impairment across cognitive tests was consistent with patterns observed using continuous data (Figure 1).
Figure 2.
Hazard ratios and corresponding 95% confidence intervals examining risk for incident impairment on each cognitive test, based on a multivariate discrete-time survival model, adjusting for age, education, race, and number of chronic diseases. The Trail Making Test, Part A, served as the reference test.
Figure 3.
Proportion of the sample remaining cognitively intact over time for each cognitive test from the multivariate discrete-time Cox proportional hazard models, based on a mean age of 73.8 years, mean education of 13 years, and average of 1.5 chronic diseases.
Clinical Transitions to Global Impairment
We compared whether clinical transition to global cognitive impairment on the MMSE was higher in groups with domain-specific impairments versus those who remained cognitively healthy. The largest, Healthy Group (N = 163) had minimal impairment on the MMSE (1.9%). The second largest, EF First Group (N = 75) had significantly higher MMSE impairment (25%) than the Healthy Group (p values <.01). MMSE impairment in the DI First Group (1 out of 11; 9%) was negligible. The DR First Group represented 12.5% (N = 40) of all incident impairments in delayed recall, indicating that most of the remaining 82.5% of incident DR impairments co-occurred with or after incident impairments in EF. Those developing incident impairments in EF and DR concurrently, and DR only, were most likely to experience global cognitive impairment (44.4% and 40%, respectively), which is consistent with Figure 1 in suggesting that these individuals had progressed.
DISCUSSION
This prospective study of initially cognitively intact older women observed that cognitive declines occurred in EF 3 years before mean declines in memory. When memory declines became evident, rates matched declines in EF. Transitions to clinical deficits in EF, or executive dysfunction, developed first in nearly 24% of all older women. Executive dysfunction developed more often than any other ability, accounting for 37% of the 49% developing any domain-specific cognitive impairment. Furthermore, progression to primary executive dysfunction, prior to the onset of memory or other impairments, was of sufficient magnitude to be linked to risk for global cognitive impairment. Furthermore, the aggregation of EF and memory impairments nearly doubled (44%) the risk for global MMSE impairment, signaling elevated risk for dementia. These findings suggest that many individuals presenting to clinicians with measurable memory declines may have already undergone unmeasured changes in EF. Such cognitive limitations place older adults at elevated risk for dementia, institutionalization, and mortality.
These findings highlight the potential importance of executive dysfunction early in some neurodegenerative processes, prior to the onset of disease-related memory changes. Although some memory and EF decline may likely have occurred prior to the period of observation here, we nevertheless observed significant EF declines that preceded memory declines by about 3 years, on average. EF is important to efficient storage and retrieval strategies (37), and its decline may promote or exacerbate neurodegenerative and age-related memory changes. There is substantial biologic evidence to support the causal role that changes in EF may play prior to significant declines in memory with age. From a neurobiological standpoint, frontal-subcortical pathways are particularly vulnerable to aging with disproportionately greater loss in prefrontal cortical circuits and structures than in other cortical areas (38,39). These reductions in both gray and white matter have been associated with cognition in older adults and with the test of EF used here (40–42). However, the degree to which these changes reflect the confluence of “normal” and neurodegenerative diseases remains to be determined. The substantial variability observed in individual basal levels and rates of EF decline suggests multiple contributors.
These results lend validity to the clinical import of EF, a skill involved in problem-solving; prioritizing goals; and updating, coordinating, and carrying out a sequence of actions. This skill is important to health maintenance, such as when revising one's daily medication regimen, prioritizing timely payment of utility bills, and avoiding impulse purchases. EF has been previously associated with instrumental activities of daily living (IADL) function and impairment (11,12).
These findings in a community-based sample of older women cannot speak directly to dementia risk but are informed by clinical studies demonstrating the import of EF during the prodromal and early stages of AD (17,37,43). Prospective research in those with preclinical AD showed that executive dysfunction followed or co-occurred with memory impairment (16,44). Here, in addition to observing that mean declines in memory followed those in EF, we found that clinical transitions to memory impairment occurred in 28% of the sample, with memory impairment being observed first in only 12.5% of these older women. Progression to impaired recall often co-occurred with executive dysfunction and was associated with increased risk for global cognitive impairment on the MMSE. These findings are consonant with recent studies showing that some individuals with MCI exhibit deficits in EF prior to the onset of memory impairments (20,45).
Psychomotor speed declined at modest linear rates for the 9-year interval, with substantial variability in individual basal levels and rates of decline. Mental speed has been variously considered a marker of biological aging (46,47). It may represent a basal neural function that reflects the integrity of multiple neural and physiologic systems.
Overall, this prospective study of cognitive declines may inform the challenges of identifying the boundaries between normal aging and the earliest stages of dementia and further speak to the broadening definition of MCI (45,48). Studies now recognize a form of MCI characterized by deficits in attention in the absence of objective memory deficits (20,45). Other larger studies have identified preclinical changes in cognition between 1.5 and 9 years prior to dementia onset (44,49,50), with delayed recall and naming being most predictive. In one study, a measure of EF similar to that used here declined most rapidly in individuals who developed AD 1.5 years later (44). Most recently, a 4-year, longitudinal study of individuals who were normal or had MCI at baseline showed the expected differences in rates of memory decline among those later converting to AD (43). These individuals also exhibited baseline differences in EF and general knowledge, suggesting that some decline in EF may have preceded MCI status.
Definitions in prospective cohort studies have increasingly acknowledged a nonamnestic category of cognitive declines (20). Amnestic and nonamnestic categories of MCI may represent different individual hierarchies in the clinical progression of AD pathology, or, different underlying age processes and pathologies (eg, vascular changes). The nonamnestic MCI category identifies attentional changes without changes in memory; those in this study who exhibited executive dysfunction prior to memory decline and impairment would fit this category. These trajectory analyses across domains suggest that increasing focus should be placed on characterizing EF decline in one's sixth decade, its sources of variability across individuals, and impairment in conjunction with memory in community-dwelling older adults.
Numerous limitations of this study warrant consideration. First among them is that this study was relatively small and involved only women, thus requiring generalization to men. Nevertheless, women are more long-lived than men (51) and, therefore, at heightened risk for AD (52) and live longer with AD (53). Second, selective loss to follow-up of the most impaired over time, as found in most population-based studies, risks underestimation of the true rates of decline. However, its impact here was minimized through the use of random-effects models and high levels of retention through the 9-year interval using in-home assessment, as needed. Nevertheless, we observed increasing drop out in later exams. Such drop out will lead to a conservative estimate of our findings. A review of those who dropped out revealed that such loss was surprisingly equivalent across levels (0,1,2 or more domains) and types of cognitive impairment.
Finally, perhaps the most challenging issue surrounding the study of EF is the broad range of tasks and definitions ascribed to it (54). At the core of EF is the ability to address novel situations and to prospectively plan, initiate, and carry out a course of goal-directed actions. This complex ability is fundamental to independence. Understanding which of those three key components of EF, set shifting, measured here, inhibiting, and working memory (54,55), are most vulnerable with age is important to future intervention efforts.
Strengths of this study include its population-based design, prospective examination of an initially cognitively intact cohort entering the target age range for cognitive and functional impairment; repeated cognitive assessments at intervals designed to minimize practice effects, analytic methods that accounted for heterogeneity in rates of decline and shared variance across measures, and 9-year longitudinal design of sufficient duration to observe declines.
In summary, this study provides evidence that the onset and rates of decline differ across cognitive domains and earliest declines appear in a component of EF. We offer initial evidence of hierarchical onsets of domain-specific cognitive impairments among older women who are at greater risk than men for the development of AD (52). Clinically significant impairments occurred most often in EF and then in EF and delayed recall combined. These deficits were associated with increased rates of global cognitive impairment. Similarly, when comparing rates of decline, EF declines here preceded memory declines. It is not yet clear if this is a causal relationship or a marker of underlying pathology causing both EF and memory impairments to emerge. If the former is true, EF decline may warrant intervention in order to halt progression of cognitive impairment broadly. These findings hold immediate implications for the import of developing interventions to prevent declines in EF and, with these interventions, screening and early detection. There are presently no known large-scale pharmacologic or behavioral interventions directed toward the prevention of executive dysfunctions.
FUNDING
This work was supported by grant 1 RO1 AG19825-02 and by R01 AG11703-10.
Acknowledgments
We would like to thank Dr. Marilyn Albert for providing important feedback on an earlier draft of this article.
References
- 1.Schaie KW, Willis SL, O’Hanlon AM. Perceived intellectual performance change over seven years. J Gerontol. 1994;49:P108–P118. doi: 10.1093/geronj/49.3.p108. [DOI] [PubMed] [Google Scholar]
- 2.Giambra LM, Arenberg D, Kawas C, Zonderman AB, Costa PT., Jr Adult life span changes in immediate visual memory and verbal intelligence. Psychol Aging. 1995;10:123–139. doi: 10.1037//0882-7974.10.1.123. [DOI] [PubMed] [Google Scholar]
- 3.Dixon RA, Wahlin A, Maitland SB, Hultsch DF, Hertzog C, Backman L. Episodic memory change in late adulthood: generalizability across samples and performance indices. Mem Cognit. 2004;32:768–778. doi: 10.3758/bf03195867. [DOI] [PubMed] [Google Scholar]
- 4.Schaie KW, Maitland SB, Willis SL, Intrieri RC. Longitudinal invariance of adult psychometric ability factor structures across 7 years. Psychol Aging. 1998;13:8–20. doi: 10.1037/0882-7974.13.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert M, Jones K, Savage C, et al. Predictors of cognitive change in older persons: MacArthur Studies of Successful Aging. Psychol Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 7.Ratcliff G, Dodge H, Birzescu M, Ganguli M. Tracking cognitive functioning over time: ten-year longitudinal data from a community-based study. Appl Neuropsychol. 2003;10:76–88. doi: 10.1207/S15324826AN1002_03. [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 9.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 10.Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: six-year longitudinal findings in the Berlin Aging Study (BASE) Psychol Aging. 2003;18:318–331. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- 11.Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. J Gerontol B Psychol Sci Soc Sci. 1999;54:S262–S270. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- 12.Grigsby J, Kaye K, Baxter J, Shetterly SM, Hamman RF. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. J Am Geriatr Soc. 1998;46:590–596. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 13.Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 14.Moss M, Albert M, Butters N, Payne M. Differential patterns of memory loss among patients with Alzheimer's disease, Huntington's disease, and alcoholic Korsakoff's syndrome. Arch Neurol. 1986;42:239–246. doi: 10.1001/archneur.1986.00520030031008. [DOI] [PubMed] [Google Scholar]
- 15.Small BJ, Fratiglioni L, Viitanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and 6-year follow-up of a population-based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- 16.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 17.Grady CL, Haxby JV, Horwitz B, et al. Longitudinal study of the early neuropsychological and cerebral metabolic changes in dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1988;10:576–596. doi: 10.1080/01688638808402796. [DOI] [PubMed] [Google Scholar]
- 18.Fabrigoule C, Rouch I, Taberly A, et al. Cognitive process in preclinical phase of dementia. Brain. 1998;121(Pt 1):135–141. doi: 10.1093/brain/121.1.135. [DOI] [PubMed] [Google Scholar]
- 19.Danigelis N, McIntosh B. Resources and the productive activity of elders: race and gender as contexts. J Gerontol. 1993;48 doi: 10.1093/geronj/48.4.s192. S203. [DOI] [PubMed] [Google Scholar]
- 20.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the Women's Health and Aging Study. J Clin Epidemiol. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Motor Skills. 1958;8:271–276. [Google Scholar]
- 25.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2nd ed. New York: Oxford University Press; 1991. [Google Scholar]
- 26.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test—Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 27.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5:125–142. [Google Scholar]
- 28.Rubin D. Estimating casual effects of treatments in randomized and non-randomized studies. J Ed Psychol. 1974;66:688–701. [Google Scholar]
- 29.Hebert LE, Wilson RS, Gilley DW, et al. Decline of language among women and men with Alzheimer's disease. J Gerontol B Psychol Sci Soc Sci. 2000;55:P354–P360. doi: 10.1093/geronb/55.6.p354. [DOI] [PubMed] [Google Scholar]
- 30.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. J Am Med Assoc. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 31.Smith PL. Splines as a useful and convenient statistical tool. Am Statistician. 1979;33:57–62. [Google Scholar]
- 32.Lesaffre E, Verbeke G. Local influence in linear mixed models. Biometrics. 1998;54:570–582. [PubMed] [Google Scholar]
- 33.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's Older Americans Normative Studies: updated AVLT norms for ages 56 to 97. J Clin Exp Neuropsychol. 1992;6(suppl):83–104. [Google Scholar]
- 34.Prentice RL, Gloeckler LA. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- 35.Hedeker D, Gibbons RD. A random-effects ordinal regression model for multilevel analysis. Biometrics. 1994;50:933–944. [PubMed] [Google Scholar]
- 36.Hedeker D, Gibbons RD. MIXOR: a computer program for mixed-effects ordinal regression analysis (MIXGSUR: a computer program for mixed-effects grouped-time survival analysis) Comput Methods Programs Biomed. 1996;49:157–176. doi: 10.1016/0169-2607(96)01720-8. [DOI] [PubMed] [Google Scholar]
- 37.Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer's disease. A longitudinal study. Brain. 1991;114(Pt 6):2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- 38.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Raz N, Gunning FM, Head D, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal JA, Emery CF, Madden DJ. Cardiovascualr and behavorial effects of aerobic exercise training in healthy older men and women. J Gerontol Med Sci. 1989;44:M147–M157. doi: 10.1093/geronj/44.5.m147. [DOI] [PubMed] [Google Scholar]
- 41.Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 42.O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- 43.Albert M, Blacker D, Moss MB, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21:158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- 44.Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58:853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- 45.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 46.Anstey KJ, Luszcz MA, Sanchez L. A reevaluation of the common factor theory of shared variance among age, sensory function, and cognitive function in older adults. J Gerontol B Psychol Sci Soc Sci. 2001;56:P3–P11. doi: 10.1093/geronb/56.1.p3. [DOI] [PubMed] [Google Scholar]
- 47.MacDonald SW, Hultsch DF, Dixon RA. Performance variability is related to change in cognition: evidence from the Victoria Longitudinal Study. Psychol Aging. 2003;18:510–523. doi: 10.1037/0882-7974.18.3.510. [DOI] [PubMed] [Google Scholar]
- 48.Morris JC. Mild cognitive impairment is early-stage Alzheimer disease: time to revise diagnostic criteria. Arch Neurol. 2006;63:15–16. doi: 10.1001/archneur.63.1.15. [DOI] [PubMed] [Google Scholar]
- 49.Amieva H, Jacqmin-Gadda H, Orgogozo JM, et al. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain. 2005;128(Pt 5):1093–1101. doi: 10.1093/brain/awh451. [DOI] [PubMed] [Google Scholar]
- 50.Saxton J, Lopez OL, Ratcliff G, et al. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- 51.Population Projections Program, Population Division. Washington, DC 20233: US Census Bureau; 2000. [Google Scholar]
- 52.Green R. Diagnosis and Management of Alzheimer's Disease and Other Dementias. Caddo, OK: Professional Communications; 2001. 2001. [Google Scholar]
- 53.Perls T. Centenarians who avoid dementia. Trends Neurosci. 2004;27:633–636. doi: 10.1016/j.tins.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Salthouse TA, Berish DE, Siedlecki KL. Construct validity and age sensitivity of prospective memory. Mem Cognit. 2004;32:1133–1148. doi: 10.3758/bf03196887. [DOI] [PubMed] [Google Scholar]
- 55.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]