Abstract
Background
Older persons often complain of fatigue, but the functional consequences of this symptom are unclear. The aim of the present study was to evaluate fatigue and its association with measures of physical function and disability in a representative sample of the older population.
Methods
Cross-sectional data from a population-based sample of 1,055 Italian men and women aged 65 and older were analyzed. Fatigue was defined according to two questions evaluating whether participants felt that “everything was an effort” and/or they “could not get going” on three or more days in the past week. Objective measures of physical function were handgrip strength, the Short Physical Performance Battery (SPPB), and 400-m walking speed. Disability was defined as the inability to complete the 400-m walk test and self-reported difficulty in activities of daily living (ADL) and instrumental activities of daily living (IADL).
Results
The prevalence of fatigue was higher in women (29%) than in men (15%). In age-adjusted analyses, fatigued men and women had weaker handgrip strength, lower SPPB score, slower walking speed, and higher mobility, ADL, and IADL disability than nonfatigued persons. Further adjustment for health behaviors, diseases, inflammatory markers, and thyroid function generally reduced the relationship between fatigue and functional outcomes, but fatigue remained significantly associated with SPPB score, walking speed, and mobility and IADL disability.
Conclusions
Older persons who report fatigue had significantly poorer functional status than those who did not report this symptom. The causal link between fatigue and these outcomes should be further investigated.
Keywords: Fatigue, Aging, Disability, Physical function
IN older adults, fatigue is a commonly reported symptom. Different definitions have sought to represent fatigue (1,2), and for use in the present study, it is conceptualized as “the awareness of a decreased capacity for physical and/or mental activity due to an imbalance in the availability, utilization, and/or restoration of resources needed to perform activity” (3). Typically, fatigue is studied in patient populations with an index condition such as cancer, HIV infection, or multiple sclerosis. Indeed, a review of symptoms associated with cancer, AIDS, heart disease, chronic obstructive pulmonary disease, and renal disease found that between 32% and 90% of patients report fatigue (4). However, although fatigue is often attributed to underlying disease, in a large study of the general population only about one third of all fatigue cases could be explained by a diagnosed medical condition (5).
The prevalence of fatigue in nondisease–specific community-dwelling older adults has not been well established. General population surveys indicate that fatigue increases with advancing age (6,7), although not consistently (8). In those studies, it was difficult to identify the prevalence of fatigue specifically for older individuals. However, in a sample of 199 older ambulatory residents of a long-term care facility, 47% reported moderate and severe fatigue (9). Furthermore, in a primary care sample of 124 older adults the prevalence of fatigue was 27% (10).
Although functional consequences of fatigue have not been well characterized, studies in older adults have shown that fatigue is associated with restricted activity and disability. In a study of 754 nondisabled community-dwelling older adults, Gill and colleagues (11) showed that fatigue was the leading reason for restricted activity. In fact, among 24 prespecified reasons, 65.5 episodes of fatigue per 100 person-months of restricted activity were reported, whereas “pain or stiffness in joints” was the second leading cause associated with about half the rate (35.7 episodes per 100 person-months of restricted activity) (11). In addition, Avlund and colleagues (12) found that “tiredness” predicted adverse events such as the onset of disability, future hospitalization, and use of home care help (13) in an older nondisabled population. However, tiredness in these studies was evaluated in relation to task-specific activities of daily living (ADL), and the functional consequences of nontask-specific tiredness or fatigue are unclear.
The present study used a measure of general fatigue to examine its distribution in a representative sample of older adults and to investigate the association of fatigue with measures of physical function and disability. Those classified as fatigued reported feeling that they could not get going and/or that everything was an effort on 3 or more days during a week (see the Methods section). Different measures of physical function were examined to understand whether fatigue was associated with each stage of the disablement process. In the framework of the disablement process (14–16), pathology is proposed as the first step in the model leading to impairments (e.g., low strength), functional limitation (e.g., slow walking speed), and ultimately, disability (e.g., inability to perform ADL). Typically, age-associated diseases are used to measure pathology, but markers of inflammation and thyroid function were examined as well in the current study to assess the subclinical disease that might be associated with fatigue and physical function (17–20).
METHODS
Study Design and Participants
This study used baseline data from the Invecchiare in Chianti study (InCHIANTI; Aging in the Chianti area), a prospective population-based study of a representative sample of people living in the Chianti geographic area (Tuscany, Italy). Baseline data were collected between September 1998 and March 2000. A detailed description of the population sample and data collection has been previously published (21). The study conformed to the ethical principles contained in the Declaration of Helsinki, and the Ethical Committee of the Italian National Institute of Research and Care of Aging approved the InCHIANTI study protocol.
The study population consisted of 1,155 participants aged 65–102 years randomly selected using a multistage stratified sampling method. The study population represents 91.7% of the initial target population of 1,260 eligible persons, with participation rates higher in women and with older persons (21). For the purpose of the analyses presented here, participants with a diagnosis of dementia (n = 82) were excluded. Of the remaining participants, 18 participants had missing responses to the questions on fatigue. Therefore, 1,055 participants were included in the analyses.
Fatigue
Fatigue was operationalized using two questions from the Center for Epidemiologic Studies-Depression scale (CES-D) (22). The participants were asked to consider their experience in the past week related to two statements: (a) “I feel that everything I did was an effort” and (b) “I could not get going.” Possible answers were (a) rarely or none of the time (less than 1 day), (b) some or a little of the time (1–2 days), (c) occasionally or a moderate amount of time (3–4 days), (d) all of the time (5–7 days). Those reporting three or more days to either question were classified as being fatigued.
Measure of Impairment
Maximum handgrip strength was measured with a handheld dynamometer (JAMAR, model #BK-7498; Fred Sammons, Inc., Burr Ridge, IL). Participants performed the task twice with each hand. The best result from either hand was used for this analysis.
Measures of Functional Limitations
Lower extremity function was assessed with the Short Physical Performance Battery (SPPB) (23,24), which evaluates balance, strength, and gait. In short, participants were first asked to stand with their feet in side-by-side, semitandem, and tandem balance positions for 10 seconds each. Participants were then asked to walk a distance of 4 m at their usual pace. Finally, participants were asked to rise from a chair and return to the seated position five times as quickly as possible while keeping their arms folded across their chest. Based on normative data (23), scores in each of these tasks were categorized along a range from 0 to 4. The sum of the three subscores yields the total SPPB score, ranging from 0 to 12 (best function).
Walking ability and endurance were measured with the 400-m walk test in which participants were asked to complete 20 laps of 20 m each as fast as possible and were allowed maximally two rest stops to complete the test. Exclusion criteria are described elsewhere (25). In those completing the 400-m walk, walking speed (m/s) was calculated. The time to walk 400 m is negatively correlated with maximum oxygen consumption (VO2max) in elderly people (26).
Measures of Disability
Mobility disability was defined as being unable to complete the 400-m walk test. Furthermore, those reporting any difficulty in one or more of six ADL items (washing face and arms, dressing and undressing, eating by yourself, using the toilet, getting in and out of bed, and controlling urination and bowel movements) were categorized as having ADL disability. Those expressing any difficulty in one or more of eight instrumented activities of daily living (IADL) items (daily shopping, cooking a simple meal, heavy housework, using the telephone, doing laundry, using public transportation, taking medication correctly, and managing house finances) were categorized as having IADL disability.
Covariates
Covariates included age, gender, and education (<6 years vs ≥ 6 years). Health behavior variables included smoking (never, former, and current) and being sedentary (those answering hardly any physical activity, mostly sitting/some walking, or light exercise 2–4 h/wk when asked to describe their level of physical activity during the last year) (27).
Height and weight were measured and body mass index (kg/m2) was calculated. A geriatrician used data from physical examination, laboratory, and self-report measures to assess the presence of hypertension, coronary heart disease (angina or acute myocardial infarction), congestive heart failure, stroke, peripheral artery disease, diabetes, chronic bronchitis/emphysema, hip fracture, cancer, and arthritis. The total number of these 10 comorbid diseases was also included as a covariate. Cognitive function was evaluated with the Mini-Mental State Examination ranging from 0 to 30 (best) (28). Symptoms of depression were evaluated with the CES-D total score (22), which was modified by excluding the two items that were used to categorize fatigue. As the original questionnaire has 20 questions, this was done by reducing the full range of the total score from 0 to 60 (worst) by 6 points. Correlation of the original and the rescaled total score was r = 0.99. Self-reported poor/very poor health was identified as well as self-reported poor/very poor sleep quality. The latter was derived from the Pittsburgh Sleep Quality Index questionnaire (29).
Biomarkers
Blood sampling was performed at the study clinic after a 15-minute rest period in the morning following a 12-hour fast. Aliquots of serum and plasma were stored at −80 C° until analysis. Hemoglobin level was analyzed using the autoanalyzer SYSMEX SE-9000 (Sysmex Corporation, Kobe, Japan). High-sensitivity C-reactive protein (CRP) was measured in ethylenediaminetetraacetic acid anticoagulated plasma using the BNII nephelometer (Dade Behring Inc., Deerfield, IL). Serum interleukin-6 (IL-6) was assessed with ultrasensitive enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). Serum tumor necrosis factor-α (TNF-α) was measured by an ultrasensitive solid-phase sandwich ELISA (R&D Systems).
Thyroid function was measured by thyroid-stimulating hormone (TSH), free thyroxine (fT4), and free triiodothyronine (fT3). Plasma levels of TSH, fT3, and fT4 were determined with commercial kits (Vitros TSH, FT3 and FT4 Reagent, respectively, Ortho-Clinical Diagnostics, Johnson & Johnson Medical S.p.A Section, Milan, Italy) by chemiluminescent assay.
Statistical Analyses
Gender-stratified analyses were performed. Log-transformed CRP, IL-6, TNF-α, TSH, fT3, and fT4 were used in the analyses because of their nonnormal distribution. Age-adjusted proportions and means were calculated using linear and logistic regression models, as appropriate, for all sociodemographic, clinical, biological, and functional characteristics by fatigue status (Table 1 and Figure 1). The associations of fatigue with functional impairment (handgrip), functional limitation (SPPB, 400-m walk speed), and disability (inability to walk 400 m, ADL, and IADL) were analyzed with linear and logistic regression analysis (Table 2). Covariates were included in these latter analyses if the p value observed in the bivariate analysis was lower than .1. Gender differences in the effect of fatigue on the various measures in the disability process were examined on the total sample using the same models as in Table 2, entering gender and the Gender × Fatigue interaction term. All statistical analyses were performed using Stata version 9.2 (StataCorp, College Station, TX), and a 5% level of significance was applied.
Table 1.
Age-Adjusted Distribution of Participant Characteristics Stratified by Fatigue Status and Gender
| Men |
Women |
|||||
| Nonfatigued (N = 393) | Fatigued (N = 71) | p value | Nonfatigued (N = 419) | Fatigued (N = 172) | p value | |
| Age (years) | 73.4 (0.3) | 76.4 (0.9) | <.01 | 74.9 (0.4) | 75.6 (0.5) | .24 |
| Education, 6 yr or more (%)* | 37.9 | 32.3 | .40 | 16.5 | 19.2 | .45 |
| Health behaviors | ||||||
| Smoking (%) | ||||||
| Never | 28.1 | 29.5 | .85 | 85.5 | 74.8 | <.01 |
| Former | 50.5 | 46.0 | 8.1 | 12.2 | ||
| Current | 20.9 | 24.0 | 6.2 | 12.3 | ||
| Sedentary (%) | 46.5 | 66.4 | <.01 | 74.5 | 81.5 | .07 |
| Clinical conditions | ||||||
| MMSE score | 26.0 (0.1) | 25.6 (0.3) | .27 | 24.9 (0.1) | 24.7 (0.2) | .46 |
| BMI | 27.0 (0.2) | 27.5 (0.4) | .24 | 27.6 (0.2) | 28.3(0.4) | .11 |
| Hypertension (%) | 57.1 | 53.1 | .54 | 65.1 | 57.9 | .10 |
| Coronary heart disease (%) | 11.0 | 22.4 | <.01 | 8.1 | 11.3 | .22 |
| Congestive heart failure (%) | 22.2 | 30.0 | .16 | 22.2 | 18.9 | .38 |
| Stroke (%) | 7.6 | 8.9 | .71 | 4.0 | 6.8 | .15 |
| Peripheral artery disease (%) | 15.9 | 22.4 | .18 | 10.4 | 11.3 | .74 |
| Diabetes (%) | 13.7 | 16.2 | .60 | 11.0 | 7.5 | .21 |
| Chronic bronchitis/emphysema (%) | 16.8 | 30.6 | <.01 | 4.2 | 4.1 | .94 |
| Hip fracture (%) | 1.8 | 3.0 | .47 | 2.5 | 4.8 | .14 |
| Cancer (%) | 4.3 | 4.4 | .98 | 6.6 | 8.2 | .50 |
| Arthritis (%) | 17.2 | 16.3 | .84 | 36.4 | 38.5 | .63 |
| Number of comorbid conditions (range: 0–10) | 1.7 (0.1) | 2.1 (0.2) | .02 | 1.7 (0.1) | 1.7 (0.1) | .92 |
| CES-D score, rescaled (without 2 fatigue items) | 8.0 (0.3) | 14.6 (0.7) | <.01 | 12.1 (0.4) | 19.3 (0.6) | <.01 |
| Sleep quality, poor or very poor (%) | 16.1 | 30.7 | <.01 | 28.2 | 49.4 | <.01 |
| Self-rated health, poor and very poor (%) | 3.3 | 16.2 | <.01 | 3.7 | 18.4 | <.01 |
| Biomarkers | ||||||
| Hemoglobin (g/dL) | 14.5 (0.1) | 14.4 (0.2) | .72 | 13.2 (0.1) | 13.2 (0.1) | .84 |
| CRP (mg/dL)† | 5.3 (0.6) | 11.4 (1.6) | .03 | 4.3 (0.3) | 5.7 (0.5) | .31 |
| IL-6 (pg/mL)† | 2.5 (0.3) | 4.4 (0.8) | .07 | 1.7 (0.1) | 2.2 (0.2) | .37 |
| TNF-α (pg/mL)† | 3.9 (0.3) | 4.0 (0.7) | .82 | 3.6 (0.3) | 3.8 (0.5) | .91 |
| TSH (mIU/L)† | 1.53 (0.1) | 1.56 (0.3) | .80 | 2.0 (0.3) | 2.6 (0.4) | .58 |
| fT4 (ng/dL)† | 1.42 (0.01) | 1.47 (0.4) | .22 | 1.48 (0.02) | 1.54 (0.03) | .43 |
| fT3 (pg/mL)† | 4.4 (0.04) | 4.3 (0.09) | .74 | 4.3 (0.03) | 4.2 (0.05) | .08 |
Notes: MMSE = Mini-Mental State Examination; BMI = body mass index; CES-D = Center for Epidemiologic Studies-Depression scale; CRP = C-reactive protein; IL = interleukin; TNF = tumor necrosis factor; TSH = thyroid-stimulating hormone; fT4 = free thyroxine; fT3 = free triiodothyronine.
Results below the age variable are age-adjusted means (standard errors) and proportions; p value denotes comparison of nonfatigued and fatigued.
Results presented are age-adjusted nontransformed values, but p values show result of analysis performed on log-transformed values.
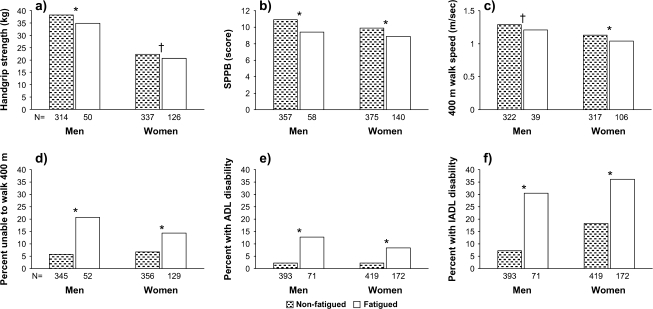
Figure 1.
Age-adjusted distribution of physical function and disability by fatigue status and gender. Results are presented as mean (a–c) or proportions (d–f). *p < .01 and †p = .02 for comparison of nonfatigued (filled bars) and fatigued (open bars).
Table 2.
Association (Regression Coefficients and Odds Ratios) of Fatigue With Functional Impairment, Limitations, and Disability
| Impairment | Functional Limitation |
Disability |
||||
| Handgrip (kg) |
SPPB (0–12) |
400-m Walk (m/s) |
Unable to Walk (0 = no, 1 = yes) |
ADL Disability (0 = no, 1 = yes) |
IADL Disability (0 = no, 1 = yes) |
|
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Men | ||||||
| Model 1 | −3.19 (−5.88, −0.50) | −1.29 (−1.89, −0.70) | −0.07 (−0.13, −0.01) | 4.01 (1.70, 9.47) | 5.71 (2.26, 14.43) | 5.15 (2.60, 10.20) |
| p = .020 | p < .001 | p = .027 | p = .002 | p < .001 | p < .001 | |
| Model 2 | −2.82 (−5.79, 0.15) | −1.19 (−1.81, −0.56) | −0.09 (−0.15, −0.02) | 3.67 (1.34, 10.10) | 5.07 (1.66, 15.50) | 4.08 (1.87, 8.89) |
| p = .063 | p < .001 | p = .007 | p = .012 | p = .004 | p < .001 | |
| Model 3 | −2.35 (−5.46, 0.76) | −1.05 (−1.70, −0.41) | −0.07 (−0.14, −0.00) | 3.30 (1.02, 10.70) | 2.38 (0.62, 9.12) | 2.79 (1.06, 7.34) |
| p = .138 | p = .001 | p = .042 | p = .047 | p = .207 | p = .038 | |
| Women | ||||||
| Model 1 | −1.54 (−2.94, −0.15) | −0.99 (−1.45, −0.53) | −0.08 (−0.12, −0.05) | 2.22 (1.14, 4.32) | 3.76 (1.76, 8.05) | 2.68 (1.66, 4.33) |
| p = .030 | p < .001 | p < .001 | p < .001 | p = .001 | p < .001 | |
| Model 2 | −0.45 (−2.02, 1.12) | −0.65 (−1.15, −0.16) | −0.05 (−0.10, −0.01) | 2.47 (1.11, 5.50) | 4.14 (1.78, 9.62) | 2.10 (1.24, 3.54) |
| p = .057 | p = .010 | p = .015 | p = .026 | p = .001 | p = .005 | |
| Model 3 | −0.27 (−1.93, 1.38) | −0.73 (−1.25, −0.21) | −0.05 (−0.10, −0.01) | 2.78 (1.19, 6.54) | 5.14 (1.86, 14.21) | 2.04 (1.11, 3.75) |
| p = .745 | p = .006 | p = .021 | p = .019 | p = .002 | p = .022 | |
Notes: SPPB = Short Physical Performance Battery; Unable to walk = unable to perform 400-m walk test; ADL = activities of daily living; IADL = instrumental activities of daily living; OR = odds ratio; CI = confidence interval.Model 1: adjusted for age, smoking, and sedentary; Model 2: Model 1 + number of comorbidities, chronic bronchitis/emphysema, coronary heart disease, rescaled Center for Epidemiologic Studies-Depression scale score, and sleep quality; Model 3: Model 2 + log-transformed C-reactive protein, interleukin-6, and free triiodothyronine.
RESULTS
Overall, participants had a mean age of 74.5 years (standard deviation = 7.0; range: 65–98). Fatigue was associated with older age in men, but not in women. The prevalence of fatigue was higher in women than in men (29.1% vs 15.3%). Adjusting for age, fatigued men and women had poorer self-rated health and quality of sleep and higher total CES-D score (Table 1) than nonfatigued men and women. In men, fatigue was associated with a greater likelihood of being sedentary (women: p = .07), having more comorbid conditions, higher prevalence of coronary heart disease and chronic bronchitis/emphysema, and higher CRP level. Fatigued women were more likely to be former or present smokers than nonfatigued women (Table 1).
Age-adjusted measures of physical function according to fatigue status are reported and compared in Figure 1. Fatigued men and women had significantly poorer performance on all measures of objective functional performance and had more disability compared with nonfatigued participants. In particular, the SPPB score was 1.5 and 1.0 points lower for fatigued men and women, respectively, compared with nonfatigued participants. Furthermore, fatigued men and women had 0.08 and 0.09 m/s lower walking speed, respectively, during the 400-m walk compared with nonfatigued participants. The prevalence of IADL disability in fatigued men and women was 23.3% and 17.9% higher, respectively, than in nonfatigued participants.
Self-reported fatigue remained significantly associated with poorer handgrip strength, lower SPPB score, slower walking speed, inability to walk 400 m, and ADL and IADL disability even after being sedentary status and smoking were included in the regression models as covariates (Table 2). Other covariates with an observed p value below .1 from the bivariate models (Table 1) were grouped into comorbid conditions (total number, chronic bronchitis/emphysema, coronary heart disease, total CES-D score, and sleep quality index) and biomarkers (CRP, IL-6, and fT3) in the Models 2 and 3, respectively. After considering these additional covariates, the strength of the associations between fatigue and functional outcomes was generally reduced. However, statistical significance remained except for handgrip strength (both genders) in Models 2 and 3 and for ADL disability (men) in Model 3 (Table 2). Although differences can be observed in the magnitude of the estimates for men and women in Table 2, further analyses that combined men and women revealed that the Gender × Fatigue interaction terms were not significant. Exploring further which of the markers of inflammation or thyroid function were more responsible for the largest reduction in the strength of the association in Model 3 showed that, in general, they work similarly but the tendency was that inflammatory factors have a greater impact (data not shown).
DISCUSSION
The present study evaluated fatigue and its association with physical function in a representative older population without a focus on any specific disease. In age-adjusted analyses, it was found that fatigued men and women could be distinguished from nonfatigued men and women on multiple variables that have been previously associated with fatigue. This is in line with the findings by Avlund and colleagues (30), which showed that tiredness in nondisabled older persons is a result of multiple potentially modifiable factors such as comorbidity, cognitive decline, and depressive mood. Furthermore, the large differences between fatigued and nonfatigued study participants in SPPB and 400-m walk is noteworthy, as a previous study identified a difference of 1 point in the SPPB and approximately 0.1 m/s in walking speed as substantial meaningful changes (31). Although fatigue is ultimately a perception, this study validates its importance by demonstrating a strong association between fatigue and handgrip strength, SPPB, 400-m walking speed, inability to walk 400 m, and ADL and IADL disability. These findings are consistent with previous research demonstrating that in nondisabled older persons, fatigue, expressed as tiredness when performing ADL, predicts functional limitation (32) and disability (12).
The association of fatigue with poor functional performance and disability remained significant even after controlling for comorbid conditions. This finding may suggest that fatigue is not entirely explained by disease status, but rather may have a specific and different pathophysiological pathway. Subclinical processes may be important as suggested by the reduction of the association of fatigue with poor physical function after adjusting for markers of inflammation and thyroid function in Model 3 (Table 2). It is conceivable that subclinical impairments increase the fatigability of various tissues that might contribute to poorer physical function (33,34).
Fatigue is often associated with anemia and cancer, but in the present study no difference was observed between fatigued and nonfatigued persons with regard to presence of lower hemoglobin levels or a cancer diagnosis. This may be explained by the fact the data are from a representative population rather than a specific disease population (35). However, both fatigued men and women had a much higher CES-D score, indicating more symptoms of depression in those who are fatigued. This is similar to findings from other studies showing an association of fatigue with depression (8). Lastly, it should be noted that a high percentage of fatigued participants were sedentary (men: 66%; women: 82%), suggesting the potential benefit of intervening with exercise, an intervention suggested by Evans and Lambert (2).
When interpreting the present results, some limitations of the study should be considered. First, due to the cross-sectional design, this study cannot address whether the observed relationships are causal. For example, ADL disability may cause fatigue but fatigue may also lead to ADL disability. Second, as described in the Methods section, fatigue was operationalized using two CES-D items that might not capture all elements of fatigue as previously defined (3). Measuring fatigue in this way may serve as a proxy for depression. However, the associations between fatigue and functional limitation or disability remained significant after adjusting for the total rescaled CES-D score. Furthermore, a question from the Pittsburgh Sleep Quality Index questionnaire (29) was evaluated as an alternative measure of fatigue. Similar results were obtained using this definition, but the context in which this questionnaire was introduced (directing the participant's attention to the domain of sleep problems) reduced face validity for using this as a fatigue measurement. Moreover, using only one of the CES-D items (“Everything I did was an effort”) produced similar results. Ultimately, to capture the inertial and endurance aspects of fatigue, both questions from the CES-D were used in this study. This choice is supported by literature applying these items to measure exhaustion in frailty and using the same cut points (36). Currently available fatigue instruments typically assess one or more dimensions of the fatigue experience (37). These unidimensional or multidimensional scales evaluate such characteristics as severity, impact, situation specificity, quality, duration, frequency, possible triggers, and/or distress. Each instrument is designed to evaluate one or more of these dimensions in a particular population according to an author's individual conceptualization of fatigue. As such, it is not surprising that so many scales exist, nor is this diversity necessarily unwarranted. Rather, what is most important is to choose the appropriate tool for the question under investigation. The current study aimed to evaluate the dimension of fatigue impact in a general population. Because impact is directly related to function, we believe our instrument was an appropriate choice, though certainly not the only possible one.
These findings suggest that, in a representative population of older adults, there is a high prevalence of fatigued persons who are generally in poorer health than those who are nonfatigued. Furthermore, the report of fatigue is significantly related to poorer functioning according to objective measures of physical function and disability even when adjusted for important covariates. This lends further support to the notion that objective measures may play a role in assessing fatigue while acknowledging that fatigue may largely be a subjective experience.
FUNDING
The InCHIANTI study was supported as a targeted project (ICS 110.1/RS97.71) by the Italian Ministry of Health, in part by the U.S. National Institute on Aging (263 MD 9164 13 and 263 MD 821336), and in part by the Intramural Research Program, National Institute on Aging, National Institutes of Health (NIH). Dr Cesari's work was supported by the University of Florida Institute on Aging and the Claude D. Pepper Older Americans Independence Center (NIH Grant 1 P30 AG028740).
Acknowledgments
Part of the paper was presented at the workshop “Unexplained Fatigue in the Elderly,” June 25–26, 2007, Bethesda, MD. Work performed at Laboratory of Epidemiology, Demography, and Biometry, National Institute on Aging, National Institutes of Health, Bethesda, MD.
References
- 1.Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology (Williston Park) 1998;12:369–377. [PubMed] [Google Scholar]
- 2.Evans WJ, Lambert CP. Physiological basis of fatigue. Am J Phys Med Rehabil. 2007;86:S29–S46. doi: 10.1097/phm.0b013e31802ba53c. [DOI] [PubMed] [Google Scholar]
- 3.Aaronson LS, Teel CS, Cassmeyer V, et al. Defining and measuring fatigue. Image J Nurs Sch. 1999;31:45–50. doi: 10.1111/j.1547-5069.1999.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 4.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Walker EA, Katon WJ, Jemelka RP. Psychiatric disorders and medical care utilization among people in the general population who report fatigue. J Gen Intern Med. 1993;8:436–440. doi: 10.1007/BF02599621. [DOI] [PubMed] [Google Scholar]
- 6.Lerdal A, Wahl A, Rustoen T, Hanestad BR, Moum T. Fatigue in the general population: a translation and test of the psychometric properties of the Norwegian version of the fatigue severity scale. Scand J Public Health. 2005;33:123–130. doi: 10.1080/14034940410028406. [DOI] [PubMed] [Google Scholar]
- 7.Loge JH, Ekeberg O, Kaasa S. Fatigue in the general Norwegian population: normative data and associations. J Psychosom Res. 1998;45:53–65. doi: 10.1016/s0022-3999(97)00291-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen MK. The epidemiology of self-perceived fatigue among adults. Prev Med. 1986;15:74–81. doi: 10.1016/0091-7435(86)90037-x. [DOI] [PubMed] [Google Scholar]
- 9.Liao S, Ferrell BA. Fatigue in an older population. J Am Geriatr Soc. 2000;48:426–430. doi: 10.1111/j.1532-5415.2000.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 10.Wijeratne C, Hickie I, Brodaty H. The characteristics of fatigue in an older primary care sample. J Psychosom Res. 2007;62:153–158. doi: 10.1016/j.jpsychores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 12.Avlund K, Rantanen T, Schroll M. Tiredness and subsequent disability in older adults: The role of walking limitations. J Gerontol A Biol Sci Med Sci. 2006;61:1201–1205. doi: 10.1093/gerona/61.11.1201. [DOI] [PubMed] [Google Scholar]
- 13.Avlund K, Damsgaard MT, Schroll M. Tiredness as determinant of subsequent use of health and social services among nondisabled elderly people. J Aging Health. 2001;13:267–286. doi: 10.1177/089826430101300206. [DOI] [PubMed] [Google Scholar]
- 14.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 15.Nagi S. Some conceptual issues in disability and rehabilitation. In: Sussman M, editor. Sociology & rehabilitation. Washington, DC: American Sociological Association; 1965. pp. 100–113. [Google Scholar]
- 16.Nagi S. Disability concepts revisited: implications for prevention. In: Pope A, Tarlov A, editors. Disability in America: toward a national agenda for prevention. Washington, DC: National Academy Press; 1991. pp. 304–327. [Google Scholar]
- 17.Tousoulis D, Antoniades C, Stefanadis C. Assessing inflammatory status in cardiovascular disease. Heart. 2007;93:1001–1007. doi: 10.1136/hrt.2006.088211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 19.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 20.Andersen-Ranberg K, Jeune B, Hoier-Madsen M, Hegedus L. Thyroid function, morphology and prevalence of thyroid disease in a population-based study of Danish centenarians. J Am Geriatr Soc. 1999;47:1238–1243. doi: 10.1111/j.1532-5415.1999.tb05205.x. [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 22.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 25.Shumway-Cook A, Guralnik JM, Phillips CL, et al. Age-associated declines in complex walking task performance: the Walking InCHIANTI toolkit. J Am Geriatr Soc. 2007;55:58–65. doi: 10.1111/j.1532-5415.2006.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54:127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 27.Patel KV, Coppin AK, Manini TM, et al. Midlife physical activity and mobility in older age: the InCHIANTI study. Am J Prev Med. 2006;31:217–224. doi: 10.1016/j.amepre.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Avlund K, Rantanen T, Schroll M. Factors underlying tiredness in older adults. Aging Clin Exp Res. 2007;19:16–25. doi: 10.1007/BF03325206. [DOI] [PubMed] [Google Scholar]
- 31.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 32.Avlund K, Sakari-Rantala R, Rantanen T, Pedersen AN, Frandin K, Schroll M. Tiredness and onset of walking limitations in older adults. J Am Geriatr Soc. 2004;52:1963–1965. doi: 10.1111/j.1532-5415.2004.52529_2.x. [DOI] [PubMed] [Google Scholar]
- 33.Helbostad JL, Leirfall S, Moe-Nilssen R, Sletvold O. Physical fatigue affects gait characteristics in older persons. J Gerontol A Biol Sci Med Sci. 2007;62:1010–1015. doi: 10.1093/gerona/62.9.1010. [DOI] [PubMed] [Google Scholar]
- 34.McNeil CJ, Rice CL. Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci. 2007;62:624–629. doi: 10.1093/gerona/62.6.624. [DOI] [PubMed] [Google Scholar]
- 35.Aapro MS, Cella D, Zagari M. Age, anemia, and fatigue. Semin Oncol. 2002;29:55–59. doi: 10.1053/sonc.2002.33534. [DOI] [PubMed] [Google Scholar]
- 36.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 37.Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res. 2004;56:157–170. doi: 10.1016/S0022-3999(03)00371-4. [DOI] [PubMed] [Google Scholar]