Abstract
Over the last two decades, aging research has expanded to include not only age-related disease models, and conversely, longevity and disease-free models, but also focuses on biological mechanisms related to the aging process. By viewing aging on multiple research frontiers, we are rapidly expanding knowledge as a whole and mapping connections between biological processes and particular age-related diseases that emerge. This is perhaps most true in the field of genetics, where variation across individuals has improved our understanding of aging mechanisms, etiology of age-related disease, and prediction of therapeutic responses. A close partnership between gerontologists, epidemiologists, and geneticists is needed to take full advantage of emerging genome information and technology and bring about a new age for biological aging research. Here we review current genetic findings for aging across both disease-specific and aging process domains. We then highlight the limitations of most work to date in terms of study design, genomic information, and trait modeling and focus on emerging technology and future directions that can partner genetic epidemiology and aging research fields to best take advantage of the rapid discoveries in each.
Keywords: Aging, Genetics, Epidemiology
THE field of aging research now spans multiple frontiers that have begun to map the connections between biological processes and particular age-related diseases. This is certainly true in the field of genetics, where variation across individuals has improved our understanding of aging mechanisms, etiology of age-related disease, and even prediction of therapeutic responses (1). Traditional genetic approaches have led to new models of aging mechanisms and disease pathogenesis through identification of mutations related to aging phentoypes. Studies of large families with multiple affected members identified mutations in lamin A (LMNA) and helicase (RECQL2) genes that cause premature aging in Werner's syndrome and Hutchinson-Gilford progeria (2–4). Missense mutations in the amyloid precursor protein and presenilin 1 and presenilin 2 genes cause early-onset familial Alzheimer's disease (AD) in a rare set of families (5–8). Other examples exist for Parkinson's disease and cancers (9–15). Most of these findings characterize rare early-onset manifestations of later onset diseases and have been extremely important in understanding the biological pathways relevant for these diseases and for the aging process more generally. Unfortunately, approaches that were successful for these rare familial forms have not been as successful for more common late-onset traits that are considered to be of more complex etiology. Yet, even these complex forms of age-related disease and aging per se have a significant genetic contribution including late-onset AD: 50%–80% (16), prostate cancer: 58% (17), heart rate: 13%–23%, (18) systolic blood pressure: 38%–46% (19), bone mineral density in premenopausal women: 46%–92% (20), and general life span: 20%–50% (21–24).
These heritability estimates argue for a greater effort to identify genetic contributions to disease and aging processes. So far, variants of the Apolipoprotein E (APOE) gene have shown the most consistent associations with age-related diseases and longevity (25–41), although several other genes have accumulating, yet currently inconsistent evidence (42). The promising future of genetic epidemiology in aging research relies on the potential for novel findings in genes such as these to direct areas of prediction, prevention, and therapy. For example, variants of methylenetetrahydrofolate reductase gene have been associated with cognitive decline, cardiovascular disease outcomes, osteoarthritis, and longevity, implicating this pathway, which includes folate and homocysteine metabolism, in aging processes (43–48). In particular, folate and vitamin B12 supplementation have been shown to significantly improve baseline serum homocysteine levels in individuals homozygous for the C677T polymorphism (49–52), implicating genetic associations in better targeting and preventive therapy. Candidate genes for age-related chronic conditions, including CVD, AD, Parkinson's disease, and cancers have been well studied, but few genes have been established as consistent genetic risk factors for these diseases and fewer have been related to aging more generally. This is likely due to the limitations in design and technology discussed in this article. As these methods improve, genetic studies of age-related diseases will yield consistent results and ultimately help target specific biological mechanisms and lead to better treatment and prevention. Although not a formal meta-analysis per gene, Table 1 provides a snapshot of the current literature for genetic associations with longevity and highlights age-related diseases or phenotypes that are known to be associated with these genes.
Table 1.
Summary of Chronic Disease-Related Genes With a Relationship to Aging and Longevity in Human Studies
| Gene | Cited Polymorphism | Association with Longevity |
Chronic Disease* | |
| Evidence For | Evidence Against | |||
| ACE | intron16ins/del | (28, 29, 53–55) | (27, 56–61) | CVD, AD |
| AGT | M235T | (25, 61) | CVD | |
| APOA1 | G-75A | (62) | CVD/lipid metabolism | |
| APOA4 | Gln360His | (63) | CVD/lipid metabolism | |
| Asp127Ser | (62) | |||
| APOB | 3′-APOB VNTR allele | (64–67) | (28, 30, 68) | CVD/lipid metabolism |
| Haplotype | (67) | |||
| APOC1 | HpaI site | (54) | CVD/lipid metabolism | |
| APOC3 | SstI cut site (G3238C) | (30) | (62) | CVD/lipid metabolism |
| T-455C | (69) | |||
| C-641A | (70) | (41) | ||
| APOE | E4/e2 isoforms | (25–41) | (53, 54, 58, 60, 71, 72) | CVD, AD, stroke |
| CETP | Ile405Val | (73, 74) | (41, 75–77) | CVD/lipid metabolism |
| FII | G20210A | (59) | CVD | |
| FGB | G-455A | (61, 78, 79) | CVD | |
| FVL | Arg506Gln | (61, 80–83) | CVD | |
| Factor VII | R353Q | (25, 84, 85) | (61, 78, 85) | CVD |
| GPIa | C807T | (59) | CVD | |
| GPIIIa | T1563C | (59) | CVD | |
| MTHFR | C677T (Ala222Val) | (25, 83, 86–90) | (54, 59, 61, 91, 92) | CVD, AD, osteoporosis |
| MTR | D919G | (93) | (61) | CVD |
| MTP | G-493T and Q95H | (94, 95) | (41, 96–98) | CVD/lipid metabolism |
| PAI-1 | 4G/5G insertion | (78, 79) | (61, 99, 100) | CVD |
| PON1 | Gln192Arg | (101-104) | (41, 105) | CVD/lipid metabolism |
| T-107C | (105) | (101) | ||
| Ht combinations, GWAS SNPs | (102, 106, 107) | |||
| REN | (108) | CVD | ||
| TAFI | G-438A | (79) | (61, 79) | CVD |
| TP53 | Arg72Pro | (25, 109–112) | Cancer | |
Notes: Genes were included based on a Pubmed search with the following terms: gene | genetic & longevity | aging | age-related. Hits from this search were further investigated and additional references cited when appropriate. Final inclusion depended on relevance to particular disease processes versus aging mechanisms (Table 2). The search was first performed in fall 2006 and updated in March 2008. SNP = single nucleotide polymorphism; GWAS = genomewide association study.
This column shows disease or biological processes previously related to the gene cited to provide biological context.
Biological markers of aging processes such as inflammation, oxidative stress, and DNA repair have been shown to be heritable, and these may therefore be good targets for genetic research as well (113,114). Several genes have already been implicated in these models of aging in either human or animal studies, with perhaps most consistency among the interleukin genes for inflammation and superoxide dismutase genes for oxidative stress. However, relatively little work compared with disease outcomes has been done, and no clear pattern has emerged given the limitations discussed below. Table 2 provides a summary of current findings for genetic associations with longevity and highlights biological mechanisms related to aging that are known to be associated with these genes.
Table 2.
Summary of Genes Involved in Specific Biologic Processes With a Relationship to Aging and Longevity in Human Studies
| Gene | Related Biologic Process | Polymorphism | Association With Aging and/or Longevity |
|
| Evidence For | Evidence Against | |||
| CAT | Oxidative stress | C-262T | (115) | |
| FOXO1A | Insulin resistance | T97347C | (116, 117) | |
| GWAS SNPs | (107) | |||
| GH1 | Insulin resistance | A1663T | (118) | |
| GHRHR | Insulin resistance | Intronic SNP | (118) | |
| GSTP1 | Oxidative stress | I105V | (119) | |
| GSTM1 | Oxidative stress | Deletion | (119, 120) | (121–123) |
| GSTT1 | Oxidative stress | Deletion | (119, 120, 122, 124) | (121) |
| IGF-1R | Insulin resistance | G1013A | (116) | |
| IL6 | Inflammation | C-174G | (125–129) | (79, 126, 130–132) |
| C-634G | (133) | |||
| VNTR | (134) | |||
| IL2 | Inflammation | T-330G | (135) | (128) |
| IL8 | Inflammation | A->T intron 1 | (128) | |
| IL12 | Inflammation | A->C exon 8 | (128) | |
| IL18 | Inflammation | A-607C | (133) | |
| IFNG | Inflammation | T874A | (135, 136) | (128, 132, 136) |
| IL1A | Inflammation | C’-889T | (131, 137) | |
| IL1B | Inflammation | C-511T | (129) | (131, 137) |
| +3953 | (131) | |||
| IL1RN | Inflammation | VNTR | (131, 137) | |
| IL10 | Inflammation | G-1082A | (129, 135, 136, 138) | (128, 131–133, 138) |
| T-819C | (133) | (136) | ||
| C-592A | (136) | |||
| INS-R | Insulin resistance | 2-SNP haplotype | (117) | |
| INS | Insulin resistance | VNTR | (118) | |
| IRS-1 | Insulin resistance | G972A | (118, 139) | (116, 117) |
| KL | Insulin resistance | KL-VS | (140, 141) | (41) |
| mtDNA | Energy metabolism | C5178A (D) | (142) | (142, 144) |
| G9055A (K) | (143, 145) | |||
| C150T (J) | (146, 147) | |||
| Haplogroup J | (148, 149, 150) | |||
| PARP | DNA metabolism/repair | Various mutations | (108, 151, 152) | |
| PI3KCB | Insulin resistance | T-359C | (116) | (117) |
| PI3KCG | Insulin resistance | (117) | ||
| PPARGC1A | Insulin resistance | (117) | ||
| SIRT1 | Caloric restriction | 5 SNPs | (153) | |
| SIRT3 | Caloric restriction | G477T | (154) | |
| VNTR- intron 5 | (155) | |||
| SOD1 | Oxidative stress | Animal models | (156, 157) | |
| SOD2 | Oxidative stress | Val16Ala | (25, 158, 159) | (108, 158) |
| TNFA | Inflammation | -308 G/A | (129) | (131, 138, 160, 161) |
| T-1031C | (133) | |||
| TNFB | Inflammation | C869T | (133) | |
| G252A | (160) | |||
| TLR4 | Inflammation | Asp299Gly | (162) | |
Note: Genes were included based on a Pubmed search with the following terms: gene | genetic & longevity | aging | age-related. Hits from this search were further investigated and additional references cited when appropriate. Final inclusion depended on relevance to aging mechanisms versus particular diseases (Table 1). The search was first performed in fall 2006 and updated in March 2008. SNP = single nucleotide polymorphism; GWAS = genomewide association study; mtDNA = mitochondrial DNA.
FUTURE DIRECTIONS
Although relationships between genes and aging are emerging, the field must still overcome critical limitations in sample sizes, genomic coverage, phenotype definitions, study design, and statistical approaches. As an example, the body of literature on the relationship between angiotensin converting enzyme (ACE) gene variation and stroke phenotypes has reported mixed results for many relatively small case-control studies for a variety of reasons (163–166). First, differences in study design may have been responsible for inconsistent results across studies, due to lack of precision (small sample sizes), underlying phenotypic heterogeneity (inappropriately mixing stroke subtypes), or varying ethnic compositions (166). Another major limitation may be that often only direct associations are considered, where well-studied polymorphisms within the ACE gene (eg, Alu insertion/deletion) are tested as potentially causal and no other variation in the gene is considered. Thus, unmeasured causal variants poorly correlated with these genotyped polymorphisms will be missed among detected associations. DNA variation catalogs such as the HapMap project (www.hapmap.org) now offer excellent single nucleotide polymorphism (SNP) correlation data that enable measurement of all common variation per gene or the selection of a particular variation that can serve as proxy information for all common variation within the gene. Finally, most published studies have focused on marginal associations, rather than modeling potential interactions. Some propose that the ACE effects may only be detectable through joint evaluation with important interacting factors. For example, Gao and colleagues reported no marginal associations between polymorphisms in ACE, APOE, and Fgβ and odds of ischemic stroke, yet found that individuals with “unfavorable” genotypes at all three loci had almost four times greater odds of stroke compared with those with other genotype combinations (163).
These limitations are indicative of the need for new paradigms as the fields of genetic epidemiology and aging merge. New strategies to exploit the available genomic technology and to use more efficient study designs and increase sample sizes are needed. Further, different models of how genes influence aging beyond sequence variation alone, such as epigenetic contributions, should be pursued. Phenotype characterization, whether more precise definitions or new concepts of what constitute a genetic “trait,” will be a key area for advancement. Yet, none of these can contribute without advanced statistical approaches to model the complexity that will result from expansion in each of these areas. We elaborate on each of these below.
Genetic Measurement
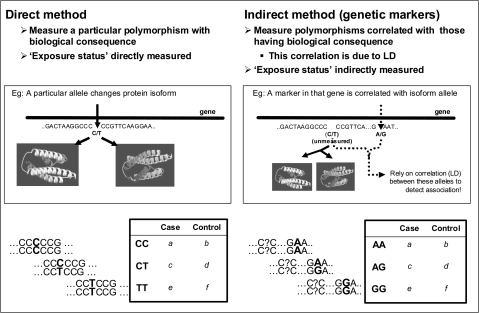
Genetic studies in aging are essentially based on general epidemiologic principles that relate an exposure to disease risk or quantitative values. The measurement of exposure can correspond directly to the factor of interest (eg, amount of alcohol consumed in a week) or can serve as an indirect proxy of that exposure (eg, reported drinks per week). Genetic exposures similarly can be thought of as direct, if the polymorphism genotyped in the study has a direct consequence on the biology and risk for disease. For example, the APOE ϵ4 variant codes for an isoform that is directly related to biological consequences that lead to vascular and cognitive diseases. However, most polymorphisms in the genome with direct consequences are not yet known, and studies typically rely on polymorphic “markers” in the genome that can serve as correlated proxies for causal variants. These markers often have no known biological consequences themselves but are highly correlated with the genomic sequence surrounding them and therefore serve as indirect measures of the rest of the nearby sequence that may contain a biologically relevant polymorphism (Figure 1). Continuing the APOE example, if other polymorphisms in the APOE gene were genotyped instead of the ϵ4 variant, those correlated with that variant would indirectly show association with diseases such as AD and CVD (167,168). A major limitation of previous genetic studies in aging has been the failure to measure enough polymorphisms per gene to achieve adequate coverage of all common variation within that gene.
Figure 1.
Direct versus indirect genetic association tests.
Genetic markers at known locations in the genome act as surrogates for surrounding sequence by exploiting a population genetic property called “linkage disequilibrium” (LD). Under this property, genetic variation in very small genomic distances remains together as one unit over several generations and appears correlated at the population level. Past studies that have only genotyped one, or very few, polymorphisms have not fully exploited the underlying LD structure of a particular gene; thus, only the sequence correlated with genotyped polymorphisms has been represented in studies, leaving the rest of the gene or genome unmeasured. Until recently, more complete gene coverage was difficult to achieve because this requires genotyping of all polymorphisms or prior knowledge of the correlation structure so that efficient surrogate markers can be chosen to represent the unmeasured variation. The most common type of marker is a SNP, where identical sequences in a population vary by only one base pair. There are approximately 10 million SNPs in the human genome, and the location, prevalence, and LD information between SNPs have been cataloged for more than 6 million of these in the International HapMap Project (169). Because these factors differ greatly by race, HapMap has examined these data separately in populations with European, African, and Asian ethnicity (170). Algorithms to identify the most efficient set of SNP markers (often called tagSNPs) are readily available (171–173). This has revolutionized the design opportunities for genetic studies, making it timely and cost effective to measure nearly all variation in a gene or even the whole genome.
The newly found ability to query the whole genome in this way has led many to question whether candidate gene-focused studies themselves are the main limitation of previous work. In these instances, not only may variation in chosen candidates be missed but also, in fact, entire genes with potential relevance are unmeasured. A growing trend is to query the entire genome using an efficient set of tagSNPs, in what is termed a genomewide association study (GWAS). A typical GWAS measures between 500,000 and 1 million SNPs, which act as correlated surrogates for 70%–92% of the remaining unmeasured common variation in European Caucasians depending on genotyping platform (169). These new advances allow us to identify genes implicating novel biological pathways to aging and age-related diseases that may not have been considered as a candidate gene. For example, six independent GWAS have recently reported an association between a 100-kb region on chromosome 9q21 and type 2 diabetes mellitus, coronary artery disease, myocardial infarction (MI), and coronary heart disease (174–179). This genomic region contains no annotated genes, yet lies adjacent to tumor suppressor genes CDKN2A and CDKN2B, which express proteins p16INK4a and p16INK4b, respectively. This is potentially exciting for aging research because animal and human studies have shown increased expression of these proteins in aging tissue (180–182).
The emergence of GWAS has inevitably been accompanied by new challenges, given the multiplicity of genotypes measured. Most importantly, for one to detect association while maintaining precision and controlling type I error, sample sizes on the order of thousands to tens of thousands are needed (174). For most U.S. research studies, these large sample sizes are unrealistic and only possible via collaborative efforts. However, careful consideration of sampling schemes, source populations, and phenotype characterization is needed before the pooling of studies can occur. Measurement of nearly a million SNPs remains an expensive endeavor, and many strategies are currently employed to most efficiently use genotyping funds. For example, staged approaches where the entire genome is queried on a subset of participants and top signals are genotyped in the remaining set are now common (183). However, choice of proportion to use in initial versus follow-up stages can be critical for the ultimate power of the study (184,185). Statistical methods and computational resources to handle this amount of data are currently limited and are an emerging field of methodological research.
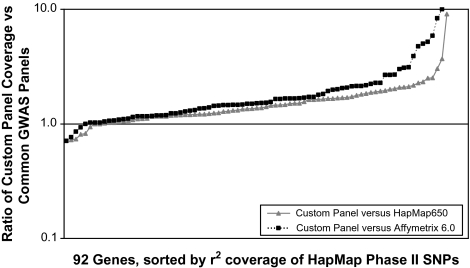
Although GWAS technology is an exciting new tool, even 1 million SNPs do not represent the entire genome because they rely on indirect association between markers and disease and therefore depend on the correlation between those markers and truly causal variants in the genome that are not directly measured. Current GWAS panels do not completely cover all variation within each gene and in fact miss some genes altogether. This is particularly true for populations or ethnicities not included in the original panel design because the markers are chosen based on genomic correlation structures specific to particular ethnicities. For example, Figure 2 shows gene coverage of common genetic variation, defined as any SNP with a minor allele frequency of at least 5%, for a study of muscle-related candidate genes among African Americans, using a tailored custom candidate gene SNP panel, versus the coverage of those same genes obtained via currently used GWAS panels. Several genes are completely unrepresented in the GWAS panel, and most are greatly underrepresented compared with a custom approach (often with several-fold lesser coverage). Therefore, focused candidate gene studies that choose optimal marker sets to represent strong biological candidate genes in particular populations remain the most powerful measurement approach for specific genes. Recent genotype imputation methods improve on the GWAS power by borrowing from known population-specific correlation structures to impute genotypes for SNPs that were not actually on the study panel, and thus increase the coverage of unmeasured SNPs that can be achieved (186–188). However, some truly associated genes may still be missed in GWAS, and among the discoveries that are made, careful replication and often additional genotyping is needed to capture and characterize the full genetic variation. The aging field will best benefit from a combination of GWAS and more finely tuned candidate gene studies with information flowing in both directions.
Figure 2.
Coverage comparison between a custom-made single nucleotide polymorphism (SNP) panel for 92 candidate genes and current genomewide association panels. The y axis shows the ratio of coverage for the custom panel versus either the Illumina 650 panel or the Affymetrix 6.0 panel. Coverage is defined as the percent of common (>5%) Yoruban HapMap Phase II SNPs that are in high linkage disequilibrium with genotyped SNPs on the panel (r2 ≥ .8) and thus are “covered” by the panel even if they are not genotyped. Points falling under 1 indicated that genomewide association studies have better coverage at that particular gene, whereas points falling above 1 indicate that the custom-made panel yields better coverage at that gene.
Phenotype Measurement
Measurement error and misclassification always plague epidemiologic research, as even nondifferential misclassification can introduce noise and reduce power. The potential for error in phenotype measurement in age-related traits is high, given the reliance on measurement tools that are too blunt, such as self-reports, or very sensitive, but too expensive to carry out in large numbers. Further, many age-related traits, for example, sarcopenia, are often dichotomized for research and treatment purposes, neglecting the true continuous nature of the underlying phenotype and decreasing power to detect genes that contribute to this distribution. The phenotype problem is further exacerbated by the complex nature of most aging phenotypes that often present as a result of many underlying causes.
Considering the large sample sizes needed to accommodate the number of genomic measurements in current studies, lack of precision and validity on phenotype measurement is now a major limitation in estimating genetic effects because sample size requirements are further inflated with increasing misclassification. Movement to more precise phenotype measurement tools is necessary to minimize the sacrifice in power due to misclassification, yet these are often prohibitively expensive for large-scale use. For example, lower extremity muscle strength in older adults is commonly measured with a handheld dynamometer (HHD) that is inexpensive and portable, allowing use in large samples. However, HHD lacks precision and validity compared with stationary Biodex dynamometers, which are expensive and require participants to come into a clinic (189). Epidemiologic techniques such as regression calibration or direct adjustment of measurement error can be quite useful in these situations (190,191), although they have not been applied widely in aging or genetic epidemiologic research. For example, the known correlation between HHD measures and Biodex measures from a small set of participants can be used to correct odds ratios (or other risk ratios) estimated using the HHD on a much larger set of participants.
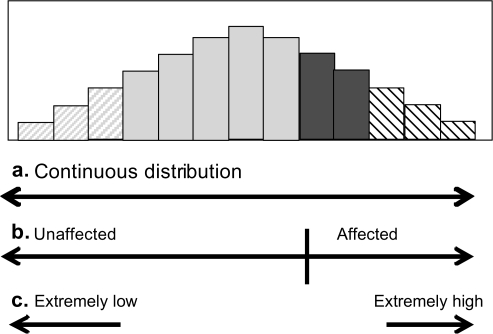
The practice of dichotomizing continuous phenotypes for treatment and research is another implicit source of misclassification. Continuing our muscle strength example, many classify individuals into “weak/not weak” categories, based on scores that populate a full distribution (Figure 3). This necessarily means that two individuals with very similar strength values may be classified differently, if they are just on either side of the threshold. Use of the full distribution, rather than this dichotomy, would remove this type of misclassification and take advantage of the actual measurements. One offshoot of this thinking has been to consider only those at the extremes of a continuous distribution (eg, top and bottom 10%) (192,193). Although this is appealing, finding these individuals, controlling for important confounders, and allowing for interactions in analysis become a daunting task. For this reason, extreme sampling or analysis techniques are often used only for screening, with the full distribution used to confirm and accommodate more sophisticated analyses (193).
Figure 3.
Possible phenotype definitions for an underlying continuous trait.
Beyond these measurement concerns, it is possible that the genetic “trait” of interest does not correspond to a disease classification, but rather to particular features related to disease. For example, genetic risk may be confined to subcategories of disease, such as frail individuals whose clinical syndrome is driven by sarcopenia, rather than anemia or reduced energy metabolism. Alternatively, genetic variation may contribute to the continuous distribution of a biologically relevant measure across the general population. Often, such features are considered “endophenotypes,” assumed to be more powerful given their continuous nature and proximity to the genetic cause (194). This has been the idea behind the study of “endophenotypes” in psychiatry and may help move the field of aging genetic epidemiology forward. For example, more effort is now focused on particular biological features or mechanisms related to frailty, such as serum levels of inflammatory markers.
Finally, the field may achieve the greatest advances by removing the disease paradigm all together and focusing on aging mechanisms, such as the efficiency of a biological system or the trajectory of an aging process that is quantitative in nature and may be more proximal to the genetic cause. Although practitioners may not immediately see the relevance of identifying genes with no specificity to disease, it is likely that such a paradigm is more true to the biology of aging and will more directly affect our broad understanding of aging. For example, identifying novel genes that have a role in free radical detoxification would inform mechanisms of oxidative stress and thus improve the understanding of a host of other disease-specific models of pathogenesis.
Other Genetic Frontiers
Genetic involvement in biological aging is not limited to inherited SNP variation of nuclear DNA. In fact, we all inherit multiple copies of mitochondrial DNA (mtDNA) from our mothers. Sequence variation of mtDNA, which codes for the critical energy production functions of the mitochondria, has been associated with longevity (148) (see Table 2). Methods to measure and analyze mtDNA in conjunction with nuclear DNA are needed at a much greater scale. Further, even nuclear DNA contains variation other than SNPs. The most common include repeat variants, where a particular segment of sequence is repeated multiple times in row and copy number variation (CNV) where more or less than the expected two parental copies exist for one person at a particular genomic region, due to nondysjunctions (>2 copies) or deletions (<2 copies), among other causes. CNVs are now easier to identify with whole-genome genotyping, panels and recent discoveries have shown large variability across individuals (195,196). This can have downstream implications in the timing or amount of expression of particular genes. Methods to measure both genotype and CNV are now in development and will add an important component to genetic studies of aging.
Somatic mutations that are not inherited, but rather accumulate over the life span, are likely a very important part of the aging process. Damage to DNA is inevitable during cellular replication and a clear relationship exists between aging and reduced DNA repair function (197,198). Thus, sequence variation in genes controlling DNA repair have been associated with aging, and genomic regions particularly susceptible to mutation may accumulate errors at a faster rate and therefore accelerate the aging process. For example, mtDNA has been shown to be more susceptible to oxidative damage leading to somatic mutations than nuclear DNA, leading to reduced energy production and accelerated aging (199–204). Another highly cited example of such susceptibility is the telomere region of each chromosome, which is maintained via telomerase enzyme activity. Studies have observed that increased oxidative stress may trigger loss of telomeres and telomerase function and can lead to genomic instability and single- and double-stranded DNA breaks and ultimately cause premature aging, whereas conservation of telomeres has been associated with longevity (205–207). Shortened telomere length has been associated with increased mortality and risk of cancer and CVD (208,209).
Finally, the amount of methylation, or other chemical modifications of the DNA, may be an important contributor to aging mechanisms. Such modifications, often termed epigenetic, do not involve the DNA sequence itself, but are rather chemical modifications on top of the sequence, such as methylation of cytosines and acetylation or phosphorylation of histones (210). These chemical signals are stable across DNA replication and control the timing and amount of gene expression in each tissue of the body by restricting or allowing access to promoter architecture. There is growing evidence that these modifications change with age, implying a role in aging biology (211–214). It is possible that accumulation of somatic DNA mutations does not occur at a high enough rate during the life span to induce common aging diseases, but epigenetic changes may occur at a frequency that could contribute to these effects. Very few studies have demonstrated epigenetic changes in humans with age, due to technical and biosample limitations, but as measurement tools improve, this will be yet another area of active research. A recent study has shown differences in local and global methylation by age by examining the similarity in methylation patterns between monozygotic (MZ) twins aged 3 years and MZ twins aged 50 years. Although these analyses were not in the same individuals (the same twins were not followed longitudinally), the similarity in methylation patterns between young twins compared with the dissimilar patterns among older twins argues strongly for age-related changes in the epigenome (215). Interestingly, this may be a way of connecting cumulative environmental damage with genetic mechanisms because it may be the epigenetic chemistry that is modified by environments, providing a biological connection between genetic and exposure-focused epidemiology. Efforts are now in place to improve measurement and to catalog variably methylated sites in the genome in several human tissues (216) to provide the foundations for monitoring age-related changes in the epigenome and connecting methylation to age-related phenotypes (210).
Analytic Complexity
Advances in genetic measurement and trait modeling bring new analytic challenges. Most analyses focus solely on the unadjusted marginal effects of one genotype at a time, using 1-degree of freedom score statistics such as the Cochran-Armitage trend test (217,218). This has several limitations including the potential misspecification of the genetic inheritance model, the lack of control of confounders, and the lack of modeling of important interactions. Because trend tests assume incremental effects of each allele on the trait (eg, additive or log-additive), they may not be appropriate for recessive, dominant, or other genetic inheritance models. Many have chosen to focus on 2-degree of freedom tests that estimate effects for each genotype separately; however, this may slightly reduce power, per test. More sophisticated modeling that is commonplace in other areas of aging epidemiology, such as controlling for confounders and assessing interactions, is often not pursued until after marginal effects in these simple unadjusted analyses are detected. It may be argued that confounding is not a grave concern for genetic risk factors because they are not time varying and were likely determined before any confounder. One important exception is genetic ancestry, which does determine marker genotype status as well as trait status and can therefore confound associations between markers and trait phenotypes. Thus, genetic ancestry is now commonly assessed using measured genotypes among study participants (219–222). Any genetic “outliers” can be removed from subsequent analyses, or, if subgroups of ancestry are observed, group membership can be adjusted for in the association analyses as a confounder or by adjusting the test statistic accordingly (220,223,224).
It is likely that many gene effects occur in complicated scenarios of gene-gene or gene-environment interactions. Estimation of effects without accommodating the underlying model is likely to attenuate estimates and contribute to false negatives. The field is now peppered with methods aimed at tackling this problem from the data mining, neural network, and classification perspectives, which all focus primarily on model building (225–229). The challenge becomes one of picking the best high-order interaction model from the almost-infinite number of possible combinations, realizing the large sample sizes needed in both discovery and validation phases. Many have resorted to only pursuing this kind of model building once a marginal genetic effect has been identified, which is likely to guarantee the importance of such models by limiting false positives but will miss many interactive relationships that do not yield a marginal signal. Some who pursue these methods circumvent the false-positive concern by seeding model selection according to known biological pathways or gene ontology (230). This may be quite fruitful, but is again likely to miss new discoveries and it puts much weight on prior knowledge.
A major analytic challenge has become the appropriate treatment of the multiple comparisons incurred in modern genetic epidemiology, which may include thousands to millions of genetic markers, modeled independently, interactively, and often for multiple phenotypes. Traditional focus on controlling type I errors for each statistical test has pursued control of the familywise error rate (FWER) across a family of tests, such that the probability of a single significant finding across M tests when all are truly null does not exceed a specified level (eg, 5%). This is the concept behind the Bonferroni and Sidak methods (231), and strong FWER control prevents unnecessary follow-up of false positives, making replication in subsequent studies more likely. However, this has been considered too conservative for most genetic applications, resulting in very low power and missed true-positive associations, for at least two reasons. First, genetic markers are correlated in the genome due to population genetic history and therefore are not independent. Many circumvent this limitation while controlling FWER through permutation procedures that estimate empirical p values that accommodate multiple tests while keeping the original correlation structure of the observed data intact (232). However, this is computationally intensive and often not feasible for large samples with large numbers of markers. As an alternative, methods exist to control FWER by estimating the effective number of independent tests as a single parameter based on the observed genotype data (233) or by accounting for the asymptotic distribution of the test statistics via simulation or numerical integration (234,235).
The second criticism of FWER control as an approach to the multiple comparison problem is the notion that one actually desires to control the probability of at least one significant result, assuming all tests are null. In fact, many would argue that we do not want to make such an assumption and instead have a prior belief that some of our genetic markers are indeed true positives. With this in mind, many support simply judging each association estimate separately and focusing on biological plausibility and replication. Others choose to control the false discovery rate rather than FWER (236,237). In this context, one protects the proportion of tests considered to be significant that are false positives, similar to controlling the positive predictive value (or its inverse) in a diagnostic testing framework(236,237). Finally, Bayesian methods have also been proposed that place uninformative (or informative) priors on a set of genetic markers and estimate a common effect across those markers, thus reducing the multiplicity problem (238,239).
Emerging genetic associations have shown that many effects are of modest size and that most data sets (even with thousands of individuals) are underpowered to detect these effects, especially if one requires very stringent significance criteria. Therefore, inappropriate focus on type I errors will almost surely result in increased type II errors (loss of power), and this must be carefully considered when applying any of these multiple comparison approaches.
The future is wide open regarding how to best incorporate such a wealth of genetic information into complex model building. The overall goal is to incorporate measurements from each of the emerging areas discussed into convergent models of aging, and this is an analytic challenge on the frontier of statistical aging and genetics research.
SUMMARY
This is an exciting time for aging research and for genetic epidemiology. Several advances have been made but were limited by the available human genome information, samples, and phenotype measures. Traditional genetic epidemiology designs focused on a single or very few genetic markers per gene. This approach relates to using a poor proxy for an unmeasured risk factor. It is likely that many true associations have been missed due to this incomplete marker information. Following the “indirect” association paradigm and using emerging human genome information, we are now able to adequately represent the common genetic variation in genes and across the whole genome using only a subset of well-chosen and easily genotyped SNPs. Many studies mentioned in earlier sections have been limited to relatively small samples, despite full-scale recruiting efforts, and have focused on disease outcomes with likely multiple causes, rather than on quantitative endophenotypes. Thus, detection of small marginal effects has been difficult, and replication across studies has been unlikely. We must focus on more precise measurements of continuous traits of interest and much larger and richer study samples that are adequately powered to detect small risk effects and interactions. The movement toward consortia focused on combined efforts, standardized measurements, and quantitative traits will provide a richer set of information as we move forward. Successful partnership of aging and genetic epidemiology can lead to discovery of new pathways for disease treatment and better identification of at-risk individuals, as well as new insights into the aging process that can guide behavior and treatment to maintain health with age.
FUNDING
We would like to acknowledge National Institutes of Health funding for contributions to this work including the Claude D. Pepper Older Americans Independence Center (P30-AG021334), T32-AG000247, T32-AG000120, R01-AG20688, and R01-ES015211.
Acknowledgments
We would also like to thank Brock Beamer for helpful comments.
References
- 1.Melzer D, Hurst AJ, Frayling T. Genetic variation and human aging: progress and prospects. J Gerontol A Biol Sci Med Sci. 2007;62:301–307. doi: 10.1093/gerona/62.3.301. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Lee L, Kudlow BA, et al. LMNA mutations in atypical Werner's syndrome. Lancet. 2003;362:440–445. doi: 10.1016/S0140-6736(03)14069-X. [DOI] [PubMed] [Google Scholar]
- 4.Yu CE, Oshima J, Fu YH, et al. Positional cloning of the Werner's syndrome gene. Science. 1996;272:258–262. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]
- 5.Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 6.Tanzi RE, Vaula G, Romano DM, et al. Assessment of amyloid beta-protein precursor gene mutations in a large set of familial and sporadic Alzheimer disease cases. Am J Hum Genet. 1992;51:273–282. [PMC free article] [PubMed] [Google Scholar]
- 7.Kamino K, Orr HT, Payami H, et al. Linkage and mutational analysis of familial Alzheimer disease kindreds for the APP gene region. Am J Hum Genet. 1992;51:998–1014. [PMC free article] [PubMed] [Google Scholar]
- 8.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 9.Leroy E, Boyer R, Polymeropoulos MH. Intron-exon structure of ubiquitin c-terminal hydrolase-L1. DNA Res. 1998;5:397–400. doi: 10.1093/dnares/5.6.397. [DOI] [PubMed] [Google Scholar]
- 10.Leroy E, Anastasopoulos D, Konitsiotis S, Lavedan C, Polymeropoulos MH. Deletions in the Parkin gene and genetic heterogeneity in a Greek family with early onset Parkinson's disease. Hum Genet. 1998;103:424–427. doi: 10.1007/s004390050845. [DOI] [PubMed] [Google Scholar]
- 11.Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 12.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 13.Tonin P, Weber B, Offit K, et al. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med. 1996;2:1179–1183. doi: 10.1038/nm1196-1179. [DOI] [PubMed] [Google Scholar]
- 14.Neuhausen SL, Mazoyer S, Friedman L, et al. Haplotype and phenotype analysis of six recurrent BRCA1 mutations in 61 families: results of an international study. Am J Hum Genet. 1996;58:271–280. [PMC free article] [PubMed] [Google Scholar]
- 15.Simard J, Tonin P, Durocher F, et al. Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat Genet. 1994;8:392–398. doi: 10.1038/ng1294-392. [DOI] [PubMed] [Google Scholar]
- 16.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 17.Page WF, Braun MM, Partin AW, Caporaso N, Walsh P. Heredity and prostate cancer: a study of World War II veteran twins. Prostate. 1997;33:240–245. doi: 10.1002/(sici)1097-0045(19971201)33:4<240::aid-pros3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Singh JP, Larson MG, O’Donnell CJ, et al. Heritability of heart rate variability: the Framingham Heart Study. Circulation. 1999;99:2251–2254. doi: 10.1161/01.cir.99.17.2251. [DOI] [PubMed] [Google Scholar]
- 19.Brown WM, Beck SR, Lange EM, et al. Age-stratified heritability estimation in the Framingham Heart Study families. BMC Genet. 2003;4(Suppl 1):S32. doi: 10.1186/1471-2156-4-S1-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pocock NA, Eisman JA, Hopper JL, et al. Genetic determinants of bone mass in adults. A twin study. J Clin Invest. 1987;80:706–710. doi: 10.1172/JCI113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JH, Flaquer A, Costa R, et al. Genetic influences on life span and survival among elderly African-Americans, Caribbean Hispanics, and Caucasians. Am J Med Genet A. 2004;128:159–164. doi: 10.1002/ajmg.a.30062. [DOI] [PubMed] [Google Scholar]
- 22.Yashin AI, Iachine IA, Harris JR. Half of the variation in susceptibility to mortality is genetic: findings from Swedish twin survival data. Behav Genet. 1999;29:11–19. doi: 10.1023/a:1021481620934. [DOI] [PubMed] [Google Scholar]
- 23.McGue M, Vaupel JW, Holm N, Harvald B. Longevity is moderately heritable in a sample of Danish twins born 1870-1880. J Gerontol. 1993;48:B237–B244. doi: 10.1093/geronj/48.6.b237. [DOI] [PubMed] [Google Scholar]
- 24.Herskind AM, McGue M, Holm NV, et al. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870-1900. Hum Genet. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- 25.Stessman J, Maaravi Y, Hammerman-Rozenberg R, et al. Candidate genes associated with ageing and life expectancy in the Jerusalem longitudinal study. Mech Ageing Dev. 2005;126:333–339. doi: 10.1016/j.mad.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Davignon J, Bouthillier D, Nestruck AC, Sing CF. Apolipoprotein E polymorphism and atherosclerosis: insight from a study in octogenarians. Trans Am Clin Climatol Assoc. 1987;99:100–110. [PMC free article] [PubMed] [Google Scholar]
- 27.Blanche H, Cabanne L, Sahbatou M, Thomas G. A study of French centenarians: are ACE and APOE associated with longevity? C R Acad Sci III. 2001;324:129–135. doi: 10.1016/s0764-4469(00)01274-9. [DOI] [PubMed] [Google Scholar]
- 28.Schachter F, Faure-Delanef L, Guenot F, et al. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- 29.Seripa D, Franceschi M, Matera MG, et al. Sex differences in the association of apolipoprotein E and angiotensin-converting enzyme gene polymorphisms with healthy aging and longevity: a population-based study from Southern Italy. J Gerontol A Biol Sci Med Sci. 2006;61:918–923. doi: 10.1093/gerona/61.9.918. [DOI] [PubMed] [Google Scholar]
- 30.Louhija J, Miettinen HE, Kontula K, et al. Aging and genetic variation of plasma apolipoproteins. Relative loss of the apolipoprotein E4 phenotype in centenarians. Arterioscler Thromb. 1994;14:1084–1089. doi: 10.1161/01.atv.14.7.1084. [DOI] [PubMed] [Google Scholar]
- 31.Stakias N, Liakos P, Tsiapali E, Goutou M, Koukoulis GN. Lower prevalence of epsilon 4 allele of apolipoprotein E gene in healthy, longer-lived individuals of Hellenic origin. J Gerontol A Biol Sci Med Sci. 2006;61:1228–1231. doi: 10.1093/gerona/61.12.1228. [DOI] [PubMed] [Google Scholar]
- 32.Zubenko GS, Stiffler JS, Hughes HB, 3rd, Fatigati MJ, Zubenko WN. Genome survey for loci that influence successful aging: sample characterization, method validation, and initial results for the Y chromosome. Am J Geriatr Psychiatry. 2002;10:619–630. [PubMed] [Google Scholar]
- 33.Rontu R, Ojala P, Hervonen A, et al. Apolipoprotein E genotype is related to plasma levels of C-reactive protein and lipids and to longevity in nonagenarians. Clin Endocrinol (Oxf) 2006;64:265–270. doi: 10.1111/j.1365-2265.2006.02455.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Wang G, Yang C, Li X. Apolipoprotein E gene polymorphism and its association with human longevity in the Uygur nationality in Xinjiang. Chin Med J (Engl) 2001;114:817–820. [PubMed] [Google Scholar]
- 35.Vogt MT, Cauley JA, Kuller LH. Apolipoprotein E phenotype, arterial disease, and mortality among older women: the study of osteoporotic fractures. Genet Epidemiol. 1997;14:147–156. doi: 10.1002/(SICI)1098-2272(1997)14:2<147::AID-GEPI4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Rea IM, Mc Dowell I, McMaster D, et al. Apolipoprotein E alleles in nonagenarian subjects in the Belfast Elderly Longitudinal Free-living Ageing Study (BELFAST) Mech Ageing Dev. 2001;122:1367–1372. doi: 10.1016/s0047-6374(01)00278-0. [DOI] [PubMed] [Google Scholar]
- 37.Panza F, Solfrizzi V, Torres F, et al. Decreased frequency of apolipoprotein E epsilon4 allele from Northern to Southern Europe in Alzheimer's disease patients and centenarians. Neurosci Lett. 1999;277:53–56. doi: 10.1016/s0304-3940(99)00860-5. [DOI] [PubMed] [Google Scholar]
- 38.Eggertsen G, Tegelman R, Ericsson S, Angelin B, Berglund L. Apolipoprotein E polymorphism in a healthy Swedish population: variation of allele frequency with age and relation to serum lipid concentrations. Clin Chem. 1993;39:2125–2129. [PubMed] [Google Scholar]
- 39.Frisoni GB, Louhija J, Geroldi C, Trabucchi M. Longevity and the epsilon2 allele of apolipoprotein E: the Finnish Centenarians Study. J Gerontol A Biol Sci Med Sci. 2001;56:M75–M78. doi: 10.1093/gerona/56.2.m75. [DOI] [PubMed] [Google Scholar]
- 40.Cauley JA, Eichner JE, Kamboh MI, Ferrell RE, Kuller LH. Apo E allele frequencies in younger (age 42-50) vs older (age 65-90) women. Genet Epidemiol. 1993;10:27–34. doi: 10.1002/gepi.1370100104. [DOI] [PubMed] [Google Scholar]
- 41.Novelli V, Viviani Anselmi C, Roncarati R, et al. Lack of replication of genetic associations with human longevity. Biogerontology. 2008;9:85–92. doi: 10.1007/s10522-007-9116-4. [DOI] [PubMed] [Google Scholar]
- 42.Christensen K, Johnson TE, Vaupel JW. The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet. 2006;7:436–448. doi: 10.1038/nrg1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strain JJ, Dowey L, Ward M, Pentieva K, McNulty H. B-vitamins, homocysteine metabolism and CVD. Proc Nutr Soc. 2004;63:597–603. doi: 10.1079/pns2004390. [DOI] [PubMed] [Google Scholar]
- 44.Cronin S, Furie KL, Kelly PJ. Dose-related association of MTHFR 677T allele with risk of ischemic stroke: evidence from a cumulative meta-analysis. Stroke. 2005;36:1581–1587. doi: 10.1161/01.STR.0000169946.31639.af. [DOI] [PubMed] [Google Scholar]
- 45.Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3:292–299. doi: 10.1111/j.1538-7836.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 46.Hong X, Hsu YH, Terwedow H, et al. Association of the methylenetetrahydrofolate reductase C677T polymorphism and fracture risk in Chinese postmenopausal women. Bone. 2007;40:737–742. doi: 10.1016/j.bone.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riancho JA, Valero C, Zarrabeitia MT. MTHFR polymorphism and bone mineral density: meta-analysis of published studies. Calcif Tissue Int. 2006;79:289–293. doi: 10.1007/s00223-006-0143-y. [DOI] [PubMed] [Google Scholar]
- 48.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 49.Coppola A, D’Angelo A, Fermo I, et al. Reduced in vivo oxidative stress following 5-methyltetrahydrofolate supplementation in patients with early-onset thrombosis and 677TT methylenetetrahydrofolate reductase genotype. Br J Haematol. 2005;131:100–108. doi: 10.1111/j.1365-2141.2005.05732.x. [DOI] [PubMed] [Google Scholar]
- 50.Pastore A, De Angelis S, Casciani S, et al. Effects of folic acid before and after vitamin B12 on plasma homocysteine concentrations in hemodialysis patients with known MTHFR genotypes. Clin Chem. 2006;52:145–148. doi: 10.1373/clinchem.2005.056119. [DOI] [PubMed] [Google Scholar]
- 51.Kapiszewska M, Kalemba M, Wojciech U, Milewicz T. Uracil misincorporation into DNA of leukocytes of young women with positive folate balance depends on plasma vitamin B12 concentrations and methylenetetrahydrofolate reductase polymorphisms. A pilot study. J Nutr Biochem. 2005;16:467–478. doi: 10.1016/j.jnutbio.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 52.McNulty H, Dowey le RC, Strain JJ, et al. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C->T polymorphism. Circulation. 2006;113:74–80. doi: 10.1161/CIRCULATIONAHA.105.580332. [DOI] [PubMed] [Google Scholar]
- 53.Forero DA, Pinzon J, Arboleda GH, et al. Analysis of common polymorphisms in angiotensin-converting enzyme and apolipoprotein e genes and human longevity in Colombia. Arch Med Res. 2006;37:890–894. doi: 10.1016/j.arcmed.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Galinsky D, Tysoe C, Brayne CE, et al. Analysis of the apo E/apo C-I, angiotensin converting enzyme and methylenetetrahydrofolate reductase genes as candidates affecting human longevity. Atherosclerosis. 1997;129:177–183. doi: 10.1016/s0021-9150(96)06027-3. [DOI] [PubMed] [Google Scholar]
- 55.Arias-Vasquez A, Sayed-Tabatabaei FA, Schut AF, et al. Angiotensin converting enzyme gene, smoking and mortality in a population-based study. Eur J Clin Invest. 2005;35:444–449. doi: 10.1111/j.1365-2362.2005.01515.x. [DOI] [PubMed] [Google Scholar]
- 56.Nacmias B, Bagnoli S, Tedde A, et al. Angiotensin converting enzyme insertion/deletion polymorphism in sporadic and familial Alzheimer's disease and longevity. Arch Gerontol Geriatr. 2007;45:201–206. doi: 10.1016/j.archger.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Panza F, Solfrizzi V, D’Introno A, et al. Angiotensin I converting enzyme (ACE) gene polymorphism in centenarians: different allele frequencies between the North and South of Europe. Exp Gerontol. 2003;38:1015–1020. doi: 10.1016/s0531-5565(03)00154-2. [DOI] [PubMed] [Google Scholar]
- 58.Zuliani G, Cherubini A, Volpato S, et al. Genetic factors associated with the absence of atherosclerosis in octogenarians. J Gerontol A Biol Sci Med Sci. 2002;57:M611–M615. doi: 10.1093/gerona/57.9.m611. [DOI] [PubMed] [Google Scholar]
- 59.Hessner MJ, Dinauer DM, Kwiatkowski R, Neri B, Raife TJ. Age-dependent prevalence of vascular disease-associated polymorphisms among 2689 volunteer blood donors. Clin Chem. 2001;47:1879–1884. [PubMed] [Google Scholar]
- 60.Choi YH, Kim JH, Kim DK, et al. Distributions of ACE and APOE polymorphisms and their relations with dementia status in Korean centenarians. J Gerontol A Biol Sci Med Sci. 2003;58:227–231. doi: 10.1093/gerona/58.3.m227. [DOI] [PubMed] [Google Scholar]
- 61.Bladbjerg EM, Andersen-Ranberg K, de Maat MP, et al. Longevity is independent of common variations in genes associated with cardiovascular risk. Thromb Haemost. 1999;82:1100–1105. [PubMed] [Google Scholar]
- 62.Garasto S, Rose G, Derango F, et al. The study of APOA1, APOC3 and APOA4 variability in healthy ageing people reveals another paradox in the oldest old subjects. Ann Hum Genet. 2003;67(Pt 1):54–62. doi: 10.1046/j.1469-1809.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 63.Merched A, Xia Y, Papadopoulou A, Siest G, Visvikis S. Apolipoprotein AIV codon 360 mutation increases with human aging and is not associated with Alzheimer's disease. Neurosci Lett. 1998;242:117–119. doi: 10.1016/s0304-3940(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 64.De Benedictis G, Falcone E, Rose G, et al. DNA multiallelic systems reveal gene/longevity associations not detected by diallelic systems. The APOB locus. Hum Genet. 1997;99:312–318. doi: 10.1007/s004390050364. [DOI] [PubMed] [Google Scholar]
- 65.De Benedictis G, Carotenuto L, Carrieri G, et al. Age-related changes of the 3′APOB-VNTR genotype pool in ageing cohorts. Ann Hum Genet. 1998;62(Pt 2):115–122. doi: 10.1046/j.1469-1809.1998.6220115.x. [DOI] [PubMed] [Google Scholar]
- 66.Jiang WX, Qiu CC, Cheng ZH, Niu WQ. [Comparative study of APOB gene 3′VNTR polymorphisms between natural longevity and controls in Uighur nationality] Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2006;23:523–527. [PubMed] [Google Scholar]
- 67.Jiang W, Qiu C, Cheng Z, et al. Correlation between haplotype of apolipoprotein B gene and natural longevity persons in Uygur nationality. Sci China C Life Sci. 2007;50:80–87. doi: 10.1007/s11427-007-0008-2. [DOI] [PubMed] [Google Scholar]
- 68.Varcasia O, Garasto S, Rizza T, et al. Replication studies in longevity: puzzling findings in Danish centenarians at the 3′APOB-VNTR locus. Ann Hum Genet. 2001;65(Pt 4):371–376. doi: 10.1017/S0003480001008715. [DOI] [PubMed] [Google Scholar]
- 69.Anisimov SV, Volkova MV, Lenskaya LV, et al. Age-associated accumulation of the apolipoprotein C-III gene T-455C polymorphism C allele in a Russian population. J Gerontol A Biol Sci Med Sci. 2001;56:B27–B32. doi: 10.1093/gerona/56.1.b27. [DOI] [PubMed] [Google Scholar]
- 70.Atzmon G, Rincon M, Schechter CB, et al. Lipoprotein genotype and conserved pathway for exceptional longevity in humans. PLoS Biol. 2006;4:e113. doi: 10.1371/journal.pbio.0040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asada T, Yamagata Z, Kinoshita T, et al. Prevalence of dementia and distribution of ApoE alleles in Japanese centenarians: an almost-complete survey in Yamanashi Prefecture, Japan. J Am Geriatr Soc. 1996;44:151–155. doi: 10.1111/j.1532-5415.1996.tb02431.x. [DOI] [PubMed] [Google Scholar]
- 72.Asada T, Kariya T, Yamagata Z, Kinoshita T, Asaka A. Apolipoprotein E allele in centenarians. Neurology. 1996;46:1484. doi: 10.1212/wnl.46.5.1484. [DOI] [PubMed] [Google Scholar]
- 73.Vergani C, Lucchi T, Caloni M, et al. I405V polymorphism of the cholesteryl ester transfer protein (CETP) gene in young and very old people. Arch Gerontol Geriatr. 2005;43:213–221. doi: 10.1016/j.archger.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 74.Barzilai N, Atzmon G, Schechter C, et al. Unique lipoprotein phenotype and genotype associated with exceptional longevity. Jama. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- 75.Arai Y, Hirose N, Yamamura K, et al. Deficiency of choresteryl ester transfer protein and gene polymorphisms of lipoprotein lipase and hepatic lipase are not associated with longevity. J Mol Med. 2003;81:102–109. doi: 10.1007/s00109-002-0407-6. [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez E, Mateo I, Infante J, et al. Cholesteryl ester transfer protein (CETP) polymorphism modifies the Alzheimer's disease risk associated with APOE epsilon4 allele. J Neurol. 2006;253:181–185. doi: 10.1007/s00415-005-0945-2. [DOI] [PubMed] [Google Scholar]
- 77.Cellini E, Nacmias B, Olivieri F, et al. Cholesteryl ester transfer protein (CETP) I405V polymorphism and longevity in Italian centenarians. Mech Ageing Dev. 2005;126:826–828. doi: 10.1016/j.mad.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Mannucci PM, Mari D, Merati G, et al. Gene Polymorphisms Predicting High Plasma Levels of Coagulation and Fibrinolysis Proteins: A Study in Centenarians. Arterioscler Thromb Vasc Biol. 1997;17:755–759. doi: 10.1161/01.atv.17.4.755. [DOI] [PubMed] [Google Scholar]
- 79.Reiner AP, Diehr P, Browner WS, et al. Common promoter polymorphisms of inflammation and thrombosis genes and longevity in older adults: the cardiovascular health study. Atherosclerosis. 2005;181:175–183. doi: 10.1016/j.atherosclerosis.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 80.Mari D, Mannucci PM, Duca F, Bertolini S, Franceschi C. Mutant factor V (Arg506Gln) in healthy centenarians. Lancet. 1996;347:1044. doi: 10.1016/s0140-6736(96)90181-6. [DOI] [PubMed] [Google Scholar]
- 81.Kristensen SR, Andersen-Ranberg K, Bathum L, Jeune B. Factor V Leiden and venous thrombosis in Danish centenarians. Thromb Haemost. 1998;80:860–861. [PubMed] [Google Scholar]
- 82.Heijmans BT, Westendorp RG, Knook DL, Kluft C, Slagboom PE. The risk of mortality and the factor V Leiden mutation in a population-based cohort. Thromb Haemost. 1998;80:607–609. [PubMed] [Google Scholar]
- 83.Faure-Delanef L, Quere I, Zouali H, Cohen D. Human longevity and R506Q factor V gene mutation. Thromb Haemost. 1997;78:1160. [PubMed] [Google Scholar]
- 84.Meiklejohn DJ, Riches Z, Youngson N, Vickers MA. The contribution of factor VII gene polymorphisms to longevity in Scottish nonagenarians. Thromb Haemost. 2000;83:519. [PubMed] [Google Scholar]
- 85.Tan Q, Yashin AI, Bladbjerg EM, et al. Variations of cardiovascular disease associated genes exhibit sex-dependent influence on human longevity. Exp Gerontol. 2001;36:1303–1315. doi: 10.1016/s0531-5565(01)00102-4. [DOI] [PubMed] [Google Scholar]
- 86.Kluijtmans LA, Whitehead AS. Reduced frequency of the thermolabile methylenetetrahydrofolate reductase genotype in the elderly. Atherosclerosis. 1999;146:395–397. doi: 10.1016/s0021-9150(99)00143-4. [DOI] [PubMed] [Google Scholar]
- 87.Heijmans BT, Gussekloo J, Kluft C, et al. Mortality risk in men is associated with a common mutation in the methylene-tetrahydrofolate reductase gene (MTHFR) Eur J Hum Genet. 1999;7:197–204. doi: 10.1038/sj.ejhg.5200283. [DOI] [PubMed] [Google Scholar]
- 88.Matsushita S, Muramatsu T, Arai H, Matsui T, Higuchi S. The frequency of the methylenetetrahydrofolate reductase-gene mutation varies with age in the normal population. Am J Hum Genet. 1997;61:1459–1460. doi: 10.1086/301640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Todesco L, Angst C, Litynski P, et al. Methylenetetrahydrofolate reductase polymorphism, plasma homocysteine and age. Eur J Clin Invest. 1999;29:1003–1009. doi: 10.1046/j.1365-2362.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- 90.Zuliani G, Volpato S, Mecocci P, Cherubini A. The A677V MTHFR allele is not associated with carotid atherosclerosis in octogenarians. Stroke. 2000;31:990–991. doi: 10.1161/01.str.31.4.983-f. [DOI] [PubMed] [Google Scholar]
- 91.Harmon DL, McMaster D, Shields DC, Whitehead AS, Rea IM. MTHFR thermolabile genotype frequencies and longevity in Northern Ireland. Atherosclerosis. 1997;131:137–138. doi: 10.1016/s0021-9150(97)06096-6. [DOI] [PubMed] [Google Scholar]
- 92.Brattstrom L, Zhang Y, Hurtig M, et al. A common methylenetetrahydrofolate reductase gene mutation and longevity. Atherosclerosis. 1998;141:315–319. doi: 10.1016/s0021-9150(98)00154-3. [DOI] [PubMed] [Google Scholar]
- 93.Linnebank M, Fliessbach K, Kolsch H, Rietschel M, Wullner U. The methionine synthase polymorphism c.2756Aright curved arrow G (D919G) is relevant for disease-free longevity. Int J Mol Med. 2005;16:759–761. [PubMed] [Google Scholar]
- 94.Puca AA, Daly MJ, Brewster SJ, et al. A genome-wide scan for linkage to human exceptional longevity identifies a locus on chromosome 4. Proc Natl Acad Sci U S A. 2001;98:10505–10508. doi: 10.1073/pnas.181337598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geesaman BJ, Benson E, Brewster SJ, et al. Haplotype-based identification of a microsomal transfer protein marker associated with the human lifespan. Proc Natl Acad Sci U S A. 2003;100:14115–14120. doi: 10.1073/pnas.1936249100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nebel A, Croucher PJ, Stiegeler R, et al. No association between microsomal triglyceride transfer protein (MTP) haplotype and longevity in humans. Proc Natl Acad Sci U S A. 2005;102:7906–7909. doi: 10.1073/pnas.0408670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bathum L, Christiansen L, Tan Q, et al. No evidence for an association between extreme longevity and microsomal transfer protein polymorphisms in a longitudinal study of 1651 nonagenarians. Eur J Hum Genet. 2005;13:1154–1158. doi: 10.1038/sj.ejhg.5201468. [DOI] [PubMed] [Google Scholar]
- 98.Beekman M, Blauw GJ, Houwing-Duistermaat JJ, et al. Chromosome 4q25, microsomal transfer protein gene, and human longevity: novel data and a meta-analysis of association studies. J Gerontol A Biol Sci Med Sci. 2006;61:355–362. doi: 10.1093/gerona/61.4.355. [DOI] [PubMed] [Google Scholar]
- 99.Benes P, Muzik J, Benedik J, et al. The C766T low-density lipoprotein receptor related protein polymorphism and coronary artery disease, plasma lipoproteins, and longevity in the Czech population. J Mol Med. 2001;79:116–120. doi: 10.1007/s001090100196. [DOI] [PubMed] [Google Scholar]
- 100.Lottermoser K, Dusing R, Ervens P, et al. The plasminogen activator inhibitor 1 4G/5G polymorphism is not associated with longevity: a study in octogenarians. J Mol Med. 2001;79:289–293. doi: 10.1007/s001090100192. [DOI] [PubMed] [Google Scholar]
- 101.Christiansen L, Bathum L, Frederiksen H, Christensen K. Paraoxonase 1 polymorphisms and survival. Eur J Hum Genet. 2004;12:843–847. doi: 10.1038/sj.ejhg.5201235. [DOI] [PubMed] [Google Scholar]
- 102.Bonafe M, Marchegiani F, Cardelli M, et al. Genetic analysis of Paraoxonase (PON1) locus reveals an increased frequency of Arg192 allele in centenarians. Eur J Hum Genet. 2002;10:292–296. doi: 10.1038/sj.ejhg.5200806. [DOI] [PubMed] [Google Scholar]
- 103.Rea IM, McKeown PP, McMaster D, et al. Paraoxonase polymorphisms PON1 192 and 55 and longevity in Italian centenarians and Irish nonagenarians. A pooled analysis. Exp Gerontol. 2004;39:629–635. doi: 10.1016/j.exger.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 104.Marchegiani F, Marra M, Spazzafumo L, et al. Paraoxonase activity and genotype predispose to successful aging. J Gerontol A Biol Sci Med Sci. 2006;61:541–546. doi: 10.1093/gerona/61.6.541. [DOI] [PubMed] [Google Scholar]
- 105.Campo S, Sardo MA, Trimarchi G, et al. Association between serum paraoxonase (PON1) gene promoter T(-107)C polymorphism, PON1 activity and HDL levels in healthy Sicilian octogenarians. Exp Gerontol. 2004;39:1089–1094. doi: 10.1016/j.exger.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 106.Tan Q, Christiansen L, Bathum L, et al. Genetic association analysis of human longevity in cohort studies of elderly subjects: an example of the PON1 gene in the Danish 1905 birth cohort. Genetics. 2006;172:1821–1828. doi: 10.1534/genetics.105.050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lunetta KL, D’Agostino RB, Sr., Karasik D, et al. Genetic correlates of longevity and selected age-related phenotypes: a genome-wide association study in the Framingham Study. BMC Med Genet. 2007;8(Suppl 1):S13. doi: 10.1186/1471-2350-8-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Benedictis G, Carotenuto L, Carrieri G, et al. Gene/longevity association studies at four autosomal loci (REN, THO, PARP, SOD2) Eur J Hum Genet. 1998;6:534–541. doi: 10.1038/sj.ejhg.5200222. [DOI] [PubMed] [Google Scholar]
- 109.Bonafe M, Olivieri F, Mari D, et al. P53 codon 72 polymorphism and longevity: additional data on centenarians from continental Italy and Sardinia. Am J Hum Genet. 1999;65:1782–1785. doi: 10.1086/302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Heemst D, Mooijaart SP, Beekman M, et al. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp Gerontol. 2005;40:11–15. doi: 10.1016/j.exger.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 111.Bojesen SE, Nordestgaard BG. The common germline Arg72Pro polymorphism of p53 and increased longevity in humans. Cell Cycle. 2008;7:158–163. doi: 10.4161/cc.7.2.5249. [DOI] [PubMed] [Google Scholar]
- 112.Orsted DD, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. Tumor suppressor p53 Arg72Pro polymorphism and longevity, cancer survival, and risk of cancer in the general population. J Exp Med. 2007;204:1295–1301. doi: 10.1084/jem.20062476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Maat MP, Bladbjerg EM, Hjelmborg JB, et al. Genetic influence on inflammation variables in the elderly. Arterioscler Thromb Vasc Biol. 2004;24:2168–2173. doi: 10.1161/01.ATV.0000143856.01669.e7. [DOI] [PubMed] [Google Scholar]
- 114.Wang XL, Rainwater DL, VandeBerg JF, Mitchell BD, Mahaney MC. Genetic contributions to plasma total antioxidant activity. Arterioscler Thromb Vasc Biol. 2001;21:1190–1195. doi: 10.1161/hq0701.092146. [DOI] [PubMed] [Google Scholar]
- 115.Christiansen L, Petersen HC, Bathum L, et al. The catalase -262C/T promoter polymorphism and aging phenotypes. J Gerontol A Biol Sci Med Sci. 2004;59:B886–B889. doi: 10.1093/gerona/59.9.b886. [DOI] [PubMed] [Google Scholar]
- 116.Bonafe M, Barbieri M, Marchegiani F, et al. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab. 2003;88:3299–3304. doi: 10.1210/jc.2002-021810. [DOI] [PubMed] [Google Scholar]
- 117.Kojima T, Kamei H, Aizu T, et al. Association analysis between longevity in the Japanese population and polymorphic variants of genes involved in insulin and insulin-like growth factor 1 signaling pathways. Exp Gerontol. 2004;39:1595–1598. doi: 10.1016/j.exger.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 118.van Heemst D, Beekman M, Mooijaart SP, et al. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell. 2005;4:79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 119.Pesch B, Dusing R, Rabstein S, et al. Polymorphic metabolic susceptibility genes and longevity: a study in octogonarians. Toxicol Lett. 2004;151:283–290. doi: 10.1016/j.toxlet.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 120.Christiansen L, Brasch-Andersen C, Bathum L, Kruse TA, Christensen K. A longitudinal study of the effect of GSTT1 and GSTM1 gene copy number on survival. Mech Ageing Dev. 2006;127:597–599. doi: 10.1016/j.mad.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 121.Yamamura K, Hirose N, Arai Y. Contribution of glutathione S-transferase M1 to longevity. J Am Geriatr Soc. 2001;49:338–339. doi: 10.1046/j.1532-5415.2001.49303346.x. [DOI] [PubMed] [Google Scholar]
- 122.Taioli E, Mari D, Franceschi C, et al. Polymorphisms of drug-metabolizing enzymes in healthy nonagenarians and centenarians: difference at GSTT1 locus. Biochem Biophys Res Commun. 2001;280:1389–1392. doi: 10.1006/bbrc.2001.4280. [DOI] [PubMed] [Google Scholar]
- 123.Muiras ML, Verasdonck P, Cottet F, Schachter F. Lack of association between human longevity and genetic polymorphisms in drug-metabolizing enzymes at the NAT2, GSTM1 and CYP2D6 loci. Hum Genet. 1998;102:526–532. doi: 10.1007/s004390050735. [DOI] [PubMed] [Google Scholar]
- 124.Gaspari L, Pedotti P, Bonafe M, et al. Metabolic gene polymorphisms and p53 mutations in healthy centenarians and younger controls. Biomarkers. 2003;8:522–528. doi: 10.1080/13547500310001627519. [DOI] [PubMed] [Google Scholar]
- 125.Christiansen L, Bathum L, Andersen-Ranberg K, Jeune B, Christensen K. Modest implication of interleukin-6 promoter polymorphisms in longevity. Mech Ageing Dev. 2004;125:391–395. doi: 10.1016/j.mad.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 126.Bonafe M, Olivieri F, Cavallone L, et al. A gender–dependent genetic predisposition to produce high levels of IL-6 is detrimental for longevity. Eur J Immunol. 2001;31:2357–2361. doi: 10.1002/1521-4141(200108)31:8<2357::aid-immu2357>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 127.Rea IM, Ross OA, Armstrong M, et al. Interleukin-6-gene C/G 174 polymorphism in nonagenarian and octogenarian subjects in the BELFAST study. Reciprocal effects on IL-6, soluble IL-6 receptor and for IL-10 in serum and monocyte supernatants. Mech Ageing Dev. 2003;124:555–561. doi: 10.1016/s0047-6374(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 128.Ross OA, Curran MD, Meenagh A, et al. Study of age-association with cytokine gene polymorphisms in an aged Irish population. Mech Ageing Dev. 2003;124:199–206. doi: 10.1016/s0047-6374(02)00132-x. [DOI] [PubMed] [Google Scholar]
- 129.Cederholm T, Persson M, Andersson P, et al. Polymorphisms in cytokine genes influence long-term survival differently in elderly male and female patients. J Intern Med. 2007;262:215–223. doi: 10.1111/j.1365-2796.2007.01803.x. [DOI] [PubMed] [Google Scholar]
- 130.Capurso C, Solfrizzi V, D’Introno A, et al. Interleukin 6-174 G/C promoter gene polymorphism in centenarians: no evidence of association with human longevity or interaction with apolipoprotein E alleles. Exp Gerontol. 2004;39:1109–1114. doi: 10.1016/j.exger.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 131.Wang XY, Hurme M, Jylha M, Hervonen A. Lack of association between human longevity and polymorphisms of IL-1 cluster, IL-6, IL-10 and TNF-alpha genes in Finnish nonagenarians. Mech Ageing Dev. 2001;123:29–38. doi: 10.1016/s0047-6374(01)00338-4. [DOI] [PubMed] [Google Scholar]
- 132.Pes GM, Lio D, Carru C, et al. Association between longevity and cytokine gene polymorphisms. A study in Sardinian centenarians. Aging Clin Exp Res. 2004;16:244–248. doi: 10.1007/BF03327391. [DOI] [PubMed] [Google Scholar]
- 133.Okayama N, Hamanaka Y, Suehiro Y, et al. Association of interleukin-10 promoter single nucleotide polymorphisms -819 T/C and -592 A/C with aging. J Gerontol A Biol Sci Med Sci. 2005;60:1525–1529. doi: 10.1093/gerona/60.12.1525. [DOI] [PubMed] [Google Scholar]
- 134.Panza F, Capurso C, D’Introno A, et al. Differences in allele frequencies of ACE I/D polymorphism between Northern and Southern Europe at different ages. Atherosclerosis. 2007;193:455–457. doi: 10.1016/j.atherosclerosis.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 135.Candore G. Polymorphisms of IL-2, IL-10 and IFN-, genes in Sicilian elderly: implication for longevity. Biogerontology. 2002;3(Suppl 1):36–37. [Google Scholar]
- 136.Lio D, Scola L, Crivello A, et al. Gender-specific association between -1082 IL-10 promoter polymorphism and longevity. Genes Immun. 2002;3:30–33. doi: 10.1038/sj.gene.6363827. [DOI] [PubMed] [Google Scholar]
- 137.Cavallone L, Bonafe M, Olivieri F, et al. The role of IL-1 gene cluster in longevity: a study in Italian population. Mech Ageing Dev. 2003;124:533–538. doi: 10.1016/s0047-6374(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 138.Lio D, Scola L, Crivello A, et al. Inflammation, genetics, and longevity: further studies on the protective effects in men of IL-10 -1082 promoter SNP and its interaction with TNF-alpha -308 promoter SNP. J Med Genet. 2003;40:296–299. doi: 10.1136/jmg.40.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Almind K, Inoue G, Pedersen O, Kahn CR. A common amino acid polymorphism in insulin receptor substrate-1 causes impaired insulin signaling. Evidence from transfection studies. J Clin Invest. 1996;97:2569–2575. doi: 10.1172/JCI118705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- 141.Rosenblatt KP, Kuro OM. Klotho, an aging-suppressor gene. Horm Res. 2007;67(Suppl 1):191–203. [Google Scholar]
- 142.Tanaka M, Gong J, Zhang J, et al. Mitochondrial genotype associated with longevity and its inhibitory effect on mutagenesis. Mech Ageing Dev. 2000;116:65–76. doi: 10.1016/s0047-6374(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 143.Ross OA, McCormack R, Curran MD, et al. Mitochondrial DNA polymorphism: its role in longevity of the Irish population. Exp Gerontol. 2001;36:1161–1178. doi: 10.1016/s0531-5565(01)00094-8. [DOI] [PubMed] [Google Scholar]
- 144.Yao YG, Kong QP, Zhang YP. Mitochondrial DNA 5178A polymorphism and longevity. Hum Genet. 2002;111:462–463. doi: 10.1007/s00439-002-0826-z. [DOI] [PubMed] [Google Scholar]
- 145.Ivanova R, Lepage V, Charron D, Schachter F. Mitochondrial genotype associated with French Caucasian centenarians. Gerontology. 1998;44:349. doi: 10.1159/000022041. [DOI] [PubMed] [Google Scholar]
- 146.Niemi AK, Moilanen JS, Tanaka M, et al. A combination of three common inherited mitochondrial DNA polymorphisms promotes longevity in Finnish and Japanese subjects. Eur J Hum Genet. 2005;13:166–170. doi: 10.1038/sj.ejhg.5201308. [DOI] [PubMed] [Google Scholar]
- 147.Zhang J, Asin-Cayuela J, Fish J, et al. Strikingly higher frequency in centenarians and twins of mtDNA mutation causing remodeling of replication origin in leukocytes. Proc Natl Acad Sci U S A. 2003;100:1116–1121. doi: 10.1073/pnas.242719399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.De Benedictis G, Rose G, Carrieri G, et al. Mitochondrial DNA inherited variants are associated with successful aging and longevity in humans. Faseb J. 1999;13:1532–1536. doi: 10.1096/fasebj.13.12.1532. [DOI] [PubMed] [Google Scholar]
- 149.Dato S, Passarino G, Rose G, et al. Association of the mitochondrial DNA haplogroup J with longevity is population specific. Eur J Hum Genet. 2004;12:1080–1082. doi: 10.1038/sj.ejhg.5201278. [DOI] [PubMed] [Google Scholar]
- 150.Niemi AK, Hervonen A, Hurme M, et al. Mitochondrial DNA polymorphisms associated with longevity in a Finnish population. Hum Genet. 2003;112:29–33. doi: 10.1007/s00439-002-0843-y. [DOI] [PubMed] [Google Scholar]
- 151.Cottet F, Blanche H, Verasdonck P, et al. New polymorphisms in the human poly(ADP-ribose) polymerase-1 coding sequence: lack of association with longevity or with increased cellular poly(ADP-ribosyl)ation capacity. J Mol Med. 2000;78:431–440. doi: 10.1007/s001090000132. [DOI] [PubMed] [Google Scholar]
- 152.Muiras ML, Muller M, Schachter F, Burkle A. Increased poly(ADP-ribose) polymerase activity in lymphoblastoid cell lines from centenarians. J Mol Med. 1998;76:346–354. doi: 10.1007/s001090050226. [DOI] [PubMed] [Google Scholar]
- 153.Flachsbart F, Croucher PJ, Nikolaus S, et al. Sirtuin 1 (SIRT1) sequence variation is not associated with exceptional human longevity. Exp Gerontol. 2006;41:98–102. doi: 10.1016/j.exger.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 154.Rose G, Dato S, Altomare K, et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38:1065–1070. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- 155.Bellizzi D, Rose G, Cavalcante P, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85:258–263. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 156.Parker JD, Parker KM, Sohal BH, Sohal RS, Keller L. Decreased expression of Cu-Zn superoxide dismutase 1 in ants with extreme lifespan. Proc Natl Acad Sci U S A. 2004;101:3486–3489. doi: 10.1073/pnas.0400222101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parkes TL, Elia AJ, Dickinson D, et al. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat Genet. 1998;19:171–174. doi: 10.1038/534. [DOI] [PubMed] [Google Scholar]
- 158.Taufer M, Peres A, de Andrade VM, et al. Is the Val16Ala manganese superoxide dismutase polymorphism associated with the aging process? J Gerontol A Biol Sci Med Sci. 2005;60:432–438. doi: 10.1093/gerona/60.4.432. [DOI] [PubMed] [Google Scholar]
- 159.Hiroi S, Harada H, Nishi H, et al. Polymorphisms in the SOD2 and HLA-DRB1 genes are associated with nonfamilial idiopathic dilated cardiomyopathy in Japanese. Biochem Biophys Res Commun. 1999;261:332–339. doi: 10.1006/bbrc.1999.1036. [DOI] [PubMed] [Google Scholar]
- 160.Ross OA, Curran MD, Rea IM, et al. HLA haplotypes and TNF polymorphism do not associate with longevity in the Irish. Mech Ageing Dev. 2003;124:563–567. doi: 10.1016/s0047-6374(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 161.Soto-Vega E, Richaud-Patin Y, Llorente L. Human leukocyte antigen class I, class II, and tumor necrosis factor-alpha polymorphisms in a healthy elder Mexican Mestizo population. Immun Ageing. 2005;2:13. doi: 10.1186/1742-4933-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Balistreri CR, Candore G, Colonna-Romano G, et al. Role of Toll-like receptor 4 in acute myocardial infarction and longevity. Jama. 2004;292:2339–2340. doi: 10.1001/jama.292.19.2339. [DOI] [PubMed] [Google Scholar]
- 163.Gao X, Yang H, ZhiPing T. Association studies of genetic polymorphism, environmental factors and their interaction in ischemic stroke. Neurosci Lett. 2006;398:172–177. doi: 10.1016/j.neulet.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 164.Gormley K, Bevan S, Markus HS. Polymorphisms in genes of the renin-angiotensin system and cerebral small vessel disease. Cerebrovasc Dis. 2007;23:148–155. doi: 10.1159/000097052. [DOI] [PubMed] [Google Scholar]
- 165.Banerjee I, Gupta V, Ganesh S. Association of gene polymorphism with genetic susceptibility to stroke in Asian populations: a meta-analysis. J Hum Genet. 2007;52:205–219. doi: 10.1007/s10038-006-0098-x. [DOI] [PubMed] [Google Scholar]
- 166.Ariyaratnam R, Casas JP, Whittaker J, et al. Genetics of ischaemic stroke among persons of non-European descent: a meta-analysis of eight genes involving approximately 32,500 individuals. PLoS Med. 2007;4:e131. doi: 10.1371/journal.pmed.0040131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fallin D, Cohen A, Essioux L, et al. Genetic analysis of case/control data using estimated haplotype frequencies: application to APOE locus variation and Alzheimer's disease. Genome Res. 2001;11:143–151. doi: 10.1101/gr.148401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Martin ER, Gilbert JR, Lai EH, et al. Analysis of association at single nucleotide polymorphisms in the APOE region. Genomics. 2000;63:7–12. doi: 10.1006/geno.1999.6057. [DOI] [PubMed] [Google Scholar]
- 169.Frazer KA, Ballinger DG, Cox DR, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.The International HapMap Consortium, Integrating ethics and science in the International HapMap Project. Nat Rev Genet. 2004;5:467–475. doi: 10.1038/nrg1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stram DO, Haiman CA, Hirschhorn JN, et al. Choosing haplotype-tagging SNPS based on unphased genotype data using a preliminary sample of unrelated subjects with an example from the Multiethnic Cohort Study. Hum Hered. 2003;55:27–36. doi: 10.1159/000071807. [DOI] [PubMed] [Google Scholar]
- 172.de Bakker PI, Yelensky R, Pe’er I. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 173.Carlson CS, Eberle MA, Rieder MJ, et al. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 176.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McPherson R, Pertsemlidis A, Kavaslar N, et al. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 180.Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 181.Krishnamurthy J, Ramsey MR, Ligon KL, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 182.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zondervan KT, Cardon LR. Designing candidate gene and genome-wide case-control association studies. Nat Protoc. 2007;2:2492–2501. doi: 10.1038/nprot.2007.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang H, Thomas DC, Pe’er I, Stram DO. Optimal two-stage genotyping designs for genome-wide association scans. Genet Epidemiol. 2006;30:356–368. doi: 10.1002/gepi.20150. [DOI] [PubMed] [Google Scholar]
- 185.Satagopan JM, Venkatraman ES, Begg CB. Two-stage designs for gene-disease association studies with sample size constraints. Biometrics. 2004;60:589–597. doi: 10.1111/j.0006-341X.2004.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39:906–913. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 187.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin–rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 188.Nicolae DL. Testing untyped alleles (TUNA)-applications to genome-wide association studies. Genet Epidemiol. 2006;30:718–727. doi: 10.1002/gepi.20182. [DOI] [PubMed] [Google Scholar]
- 189.Martin HJ, Yule V, Syddall HE, et al. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? A comparison with the gold standard Bodex dynamometry. Gerontology. 2006;52:154–159. doi: 10.1159/000091824. [DOI] [PubMed] [Google Scholar]
- 190.Greenland S. Basic methods for sensitivity analysis of biases. Int J Epidemiol. 1996;25:1107–1116. [PubMed] [Google Scholar]
- 191.Spiegelman D, McDermott A, Rosner B. Regression calibration method for correcting measurement-error bias in nutritional epidemiology. Am J Clin Nutr. 1997;65(Suppl 4):1179S–1186S. doi: 10.1093/ajcn/65.4.1179S. [DOI] [PubMed] [Google Scholar]
- 192.Schork NJ, Nath SK, Fallin D, Chakravarti A. Linkage disequilibrium analysis of biallelic DNA markers, human quantitative trait loci, and threshold-defined case and control subjects. Am J Hum Genet. 2000;67:1208–1218. doi: 10.1086/321201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arking DE, Pfeufer A, Post W, et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 194.Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 195.Estivill X, Armengol L. Copy number variants and common disorders: filling the gaps and exploring complexity in genome-wide association studies. PLoS Genet. 2007;3:1787–1799. doi: 10.1371/journal.pgen.0030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rodriguez-Revenga L, Mila M, Rosenberg C, Lamb A, Lee C. Structural variation in the human genome: the impact of copy number variants on clinical diagnosis. Genet Med. 2007;9:600–606. doi: 10.1097/gim.0b013e318149e1e3. [DOI] [PubMed] [Google Scholar]
- 197.King CM, Gillespie ES, McKenna PG, Barnett YA. An investigation of mutation as a function of age in humans. Mutat Res. 1994;316:79–90. doi: 10.1016/0921-8734(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 198.Weirich-Schwaiger H, Weirich HG, Gruber B, Schweiger M, Hirsch-Kauffmann M. Correlation between senescence and DNA repair in cells from young and old individuals and in premature aging syndromes. Mutat Res. 1994;316:37–48. doi: 10.1016/0921-8734(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 199.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Richter C. Oxidative damage to mitochondrial DNA and its relationship to ageing. Int J Biochem Cell Biol. 1995;27:647–653. doi: 10.1016/1357-2725(95)00025-k. [DOI] [PubMed] [Google Scholar]
- 201.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 202.Fukunaga M, Yielding KL. Deletion of mitochondrial genetic markers in yeast by ethidium and the photoaffinity probe, ethidium azide. Jpn J Med Sci Biol. 1979;32:219–223. doi: 10.7883/yoken1952.32.219. [DOI] [PubMed] [Google Scholar]
- 203.Ashok BT, Ali R. The aging paradox: free radical theory of aging. Exp Gerontol. 1999;34:293–303. doi: 10.1016/s0531-5565(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 204.Fleming JE, Miquel J, Cottrell SF, Yengoyan LS, Economos AC. Is cell aging caused by respiration-dependent injury to the mitochondrial genome? Gerontology. 1982;28:44–53. doi: 10.1159/000212510. [DOI] [PubMed] [Google Scholar]
- 205.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Joeng KS, Song EJ, Lee KJ, Lee J. Long lifespan in worms with long telomeric DNA. Nat Genet. 2004;36:607–611. doi: 10.1038/ng1356. [DOI] [PubMed] [Google Scholar]
- 207.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 208.Wright WE, Shay JW. Telomere-binding factors and general DNA repair. Nat Genet. 2005;37:116–118. doi: 10.1038/ng0205-116. [DOI] [PubMed] [Google Scholar]
- 209.Wright WE, Shay JW. Telomere biology in aging and cancer. J Am Geriatr Soc. 2005;37(Suppl 9):S292–S294. doi: 10.1111/j.1532-5415.2005.53492.x. [DOI] [PubMed] [Google Scholar]
- 210.Bjornsson HT, Fallin MD, Feinberg AP. An integrated epigenetic and genetic approach to common human disease. Trends Genet. 2004;20:350–358. doi: 10.1016/j.tig.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 211.Mays-Hoopes LL. Age-related changes in DNA methylation: do they represent continued developmental changes? Int Rev Cytol. 1989;114:181–220. doi: 10.1016/s0074-7696(08)60861-x. [DOI] [PubMed] [Google Scholar]
- 212.Issa JP, Ottaviano YL, Celano P, et al. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet. 1994;7:536–540. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- 213.Cooney CA. Are somatic cells inherently deficient in methylation metabolism? A proposed mechanism for DNA methylation loss, senescence and aging. Growth Dev Aging. 1993;57:261–273. [PubMed] [Google Scholar]
- 214.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 215.Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Eckhardt F, Beck S, Gut IG, Berlin K. Future potential of the Human Epigenome Project. Expert Rev Mol Diagn. 2004;4:609–618. doi: 10.1586/14737159.4.5.609. [DOI] [PubMed] [Google Scholar]
- 217.Armitage P. Tests for linear trends in proportions and frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- 218.Cochran W. Some methods for strengthening the common chi-square tests. Biometrics. 1954;10:417–451. [Google Scholar]
- 219.Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 220.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 221.Pritchard JK, Stephens M, Rosenberg NA, Donnelly P. Association mapping in structured populations. Am J Hum Genet. 2000;67:170–181. doi: 10.1086/302959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Devlin B, Roeder K, Wasserman L. Genomic control a new approach to genetic-based association studies. Theor Popul Biol. 2001;60:155–166. doi: 10.1006/tpbi.2001.1542. [DOI] [PubMed] [Google Scholar]
- 224.Devlin B, Roeder K, Wasserman L. Genomic control for association studies: a semiparametric test to detect excess-haplotype sharing. Biostatistics. 2000;1:369–387. doi: 10.1093/biostatistics/1.4.369. [DOI] [PubMed] [Google Scholar]
- 225.Unneberg P, Stromberg M, Sterky F. SNP discovery using advanced algorithms and neural networks. Bioinformatics. 2005;21:2528–2530. doi: 10.1093/bioinformatics/bti354. [DOI] [PubMed] [Google Scholar]
- 226.Breiman L, Friedman JH, Stone CJ, Olshen RA. Classification and Regression Trees. In: New ED, editor. United States of America: Chapman and Hall; 1984. 368. [Google Scholar]
- 227.Hastie T, Tibshrani R, Friedman JH. Springer Series in Statisitics. 1st ed. Vol. 1. New York, NY: Springer; 2003. The Elements of Statistical Learning. 552. [Google Scholar]
- 228.Kooperberg C, Ruczinski I. Identifying interacting SNPs using Monte Carlo logic regression. Genet Epidemiol. 2005;28:157–170. doi: 10.1002/gepi.20042. [DOI] [PubMed] [Google Scholar]
- 229.Curtis D, North BV, Sham PC. Use of an artificial neural network to detect association between a disease and multiple marker genotypes. Ann Hum Genet. 2001;65(Pt 1):95–107. doi: 10.1046/j.1469-1809.2001.6510095.x. [DOI] [PubMed] [Google Scholar]
- 230.Larsen P, Almasri E, Chen G, Dai Y. A statistical method to incorporate biological knowledge for generating testable novel gene regulatory interactions from microarray experiments. BMC Bioinformatics. 2007;8:317. doi: 10.1186/1471-2105-8-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sidak Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc. 1967;62:626–633. [Google Scholar]
- 232.Westfall PH, Zaykin DV, Young SS. Multiple tests for genetic effects in association studies. Methods Mol Biol. 2002;184:143–168. doi: 10.1385/1-59259-242-2:143. [DOI] [PubMed] [Google Scholar]
- 233.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Conneely KN, Boehnke M. So Many Correlated Tests, So Little Time! Rapid Adjustment of P Values for Multiple Correlated Tests. Am J Hum Genet. 2007;81:1158–1168. doi: 10.1086/522036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Seaman SR, Muller-Myhsok B. Rapid simulation of P values for product methods and multiple-testing adjustment in association studies. Am J Hum Genet. 2005;76:399–408. doi: 10.1086/428140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Storey JD. The positive false discovery rate: a Bayesian interpretation and the q-value. Annals of Statistics. 2003;31:2013–2035. [Google Scholar]
- 237.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc. B. 1995;57:289–300. [Google Scholar]
- 238.Thomas DC, Witte JS, Greenland S. Dissecting effects of complex mixtures: who's afraid of informative priors? Epidemiology. 2007;18:186–190. doi: 10.1097/01.ede.0000254682.47697.70. [DOI] [PubMed] [Google Scholar]
- 239.Greenland S. Methods for epidemiologic analyses of multiple exposures: a review and comparative study of maximum-likelihood, preliminary-testing, and empirical-Bayes regression. Stat Med. 1993;12:717–736. doi: 10.1002/sim.4780120802. [DOI] [PubMed] [Google Scholar]