Abstract
Background
Physical activity counseling increases physical activity among older people, but its effectiveness on mobility, that is, maintaining the ability to move independently, is unknown. We studied the effect of physical activity counseling on mobility among older people and evaluated whether counseling-induced benefits persist after cessation of the intervention.
Methods
In a 2-year, single-blinded, randomized controlled study, 632 sedentary participants aged 75–81 years were randomly assigned into the intervention (n = 318) or control (n = 314) group. The intervention group received a single individualized physical activity counseling session with a supportive telephone contact every 4 months for 2 years. The outcome measures—perceived difficulty in advanced (walking 2 km) and basic (walking 0.5 km) mobility—were gathered semiannually during the intervention and the 1.5-year postintervention follow-up.
Results
The proportion of participants with difficulties in advanced mobility at the beginning and end of the intervention was 34% and 38%, respectively, in the intervention group. In the control group, the corresponding proportions were 32% and 45%. The treatment effect was significant at the 2-year follow-up (odds ratio [OR] 0.84, 95% confidence interval [CI]: 0.70–0.99; p = .04) and remained significant 1.5 years postintervention (OR 0.82, 95% CI: 0.68–0.99; p = .04). The effect on basic mobility postintervention was parallel but nonsignificant (OR 0.87, CI: 0.69–1.09; p = .22).
Conclusions
Among older people, a single individualized physical activity counseling session with a supportive phone contact every 4 months for 2 years had a positive effect on mobility, an important factor for maintaining independence in the community in old age.
Keywords: Aging, Disability, Mobility limitation, Physical activity, Promotion
INDEPENDENT mobility is important for continuing to live in the community in old age. Among older people, difficulties in mobility, such as limitations in walking, increase the risk for further disability and the development of dependency (1–3). Observational longitudinal studies indicate that physical activity prevents the progression of mobility limitation and development of dependency in old age (4,5), and earlier randomized controlled trials (RCTs) have shown that physical activity counseling with continued phone support increases physical activity among older people in the short term (6–10). However, whether physical activity counseling, through increasing physical activity, prevents or slows down the age-related declines in mobility observed in many individuals is unknown.
We studied whether a session of individualized physical activity counseling coupled with telephone support for 2 years would help reduce the proportion of people with mobility limitation in old age. Our hypothesis was that by increasing physical activity, incident mobility limitation may be prevented and existing difficulties in mobility ameliorated. In addition, we evaluated whether counseling-induced benefits would persist 1.5 years after cessation of the intervention.
METHODS
Design
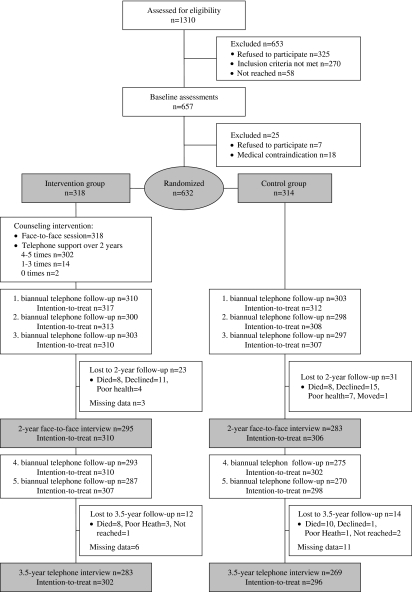
The design of the Screening and Counseling for Physical Activity and Mobility project has been reported in detail elsewhere (11) and is summarized briefly here. The present study was a 2-year, single-blinded, RCT with a 1.5-year follow-up after the intervention on the effects of customer-oriented physical activity counseling among older sedentary people. The flow of the study is presented in Figure 1. Interviews on mobility were carried out at baseline, and four times at 6-month intervals during the 2-year counseling intervention and three times during the 1.5-year postintervention follow-up. The Ethical Committee of the Central Finland Health Care District approved the study. All the participants gave their written informed consent prior to the study. This study is registered as ISRCTN07330512.
Figure 1.
Study flow according to mobility outcomes.
Participants
The target population consisted of all the 75- to 81-year-old residents of Jyväskylä, Central Finland, who were living in the city center area in March 2003 (N = 1,310). Contact information for the target population was gathered from the Finnish population register. To be eligible for the study, persons had to be able to walk 500 m without assistance, have a Mini-Mental State Examination (MMSE) (12) score greater than 21, be only moderately physically active or sedentary (at most 4 hours of walking or 2 hours of other exercise weekly), and have no severe medical contraindications for physical activity as assessed by the study nurse and, where necessary, further evaluated by a physician.
The final study group consisted of 632 persons (75% women) who were randomly assigned to the intervention group (n = 318) or control group (n = 314). Each week after completion of the baseline assessments, participants were allocated to groups, using a randomization ratio of 1:1, by drawing lots. The randomization was performed by a trial administrator. Allocation concealment was achieved by drawing names from opaque envelopes for 40–50 persons at the same time. The study nurses and interviewers who collected the data and the assistants who recorded the data were blinded to group allocation.
Measurements
The primary outcome of this study, “mobility limitation,” was studied using a structured interview on perceived difficulty in walking 2 km (advanced mobility) and 0.5 km (basic mobility). The questions were formulated as follows: “Do you have difficulty in walking 2 km/0.5 km? Five alternative response options were given: (a) able to manage without difficulty, (b) able to manage with some difficulty, (c) able to manage with great deal of difficulty, (c) able to manage only with help of another person, and (a) unable to manage even with help. Participants reporting some or great deal of difficulty and need for help of another person or inability were categorized as having difficulty, that is, mobility limitation. Self-reported difficulties have been found to be reliable and valid measures to capture mobility disability among older people (2,13,14).
Habitual physical activity was assessed using a previously validated 7-point scale (15): (a) mainly resting, (b) most activities performed sitting down, (c) light physical activity 1–2 h/wk, (d) moderate physical activity 3 h/wk, (e) moderate physical activity at least 4 h/wk, (f) strenuous physical exercise several times a week, and (g) competitive sports several times a week. Persons who belonged to the two highest categories were excluded from the study before randomization as they would not have benefited from our physical activity counseling intervention. To study the effects of the intervention on the changes in habitual physical activity from baseline to the 2-year follow-up, we categorized participants into (a) those whose activity level remained moderate or above, or who increased their activity level from sedentary (light physical activity 1–2 h/wk at the most) to at least moderate, and into (b) those who remained sedentary or who reduced their physical activity from at least moderate to sedentary.
Information on self-reported physician-diagnosed chronic conditions lasting for more than 3 months and prescription medication was collected during the face-to-face interviews conducted in the participants’ homes and then double checked during the study center visit by a nurse. Depression was measured with the Center for Epidemiological Studies Depression scale (CES-D) (16) and cognitive impairment was assessed with the MMSE (12). Body mass index (BMI, kg/m2) was calculated from body weight and height, measured at the laboratory, by dividing weight in kilograms by height squared in meters.
Intervention
Approximately 2 weeks after randomization, each participant in the intervention group received a single individualized motivational face-to-face physical activity counseling session by a physiotherapist who was specifically trained for the task and who did not take part in the data collection process (11). The counseling approach was based on the social cognitive theory of health behavior (17) and the motivational interviewing technique (18). The average duration of this session was 50 minutes. The topics covered during the counseling session included the person's present level of physical activity and interest in beginning or maintaining physical activity or exercise, that is, willingness to be active in performing everyday chores, to exercise on their own, or to participate in exercise classes. In addition, potential barriers to exercise and strategies to overcome these were considered. The physiotherapist and the participant together designed a personal physical activity plan that the person could carry out on her own after the counseling session, for example, in an exercise center. The counseling session was followed up with telephone contacts by the same physiotherapist to support compliance and behavior change. Telephone contact took place on average every 4 months during the 2-year intervention. In addition to personal counseling, the intervention group was invited to participate in two voluntary lectures on topics such as home calisthenics and disability prevention. The control group received no encouragement to increase their physical activity but had access to the same exercise facilities as the intervention group (11).
Statistical Analysis
Calculation of sample size was based on our pilot sample. We estimated that about 60% of the target population were experiencing, or were at increased risk for, mobility limitation. The significance level was set at 5% and power at 80%. A within-person correlation of .4 was assumed. To allow for 10% attrition, the total sample size needed was about 630. Comparisons of discrete baseline characteristics were performed using chi-square tests and comparisons of continuous variables were done using independent samples t test. All significances were two tailed and set at p less than .05 level. Logistic regression was used to analyze the changes in physical activity. These analyses were performed with SPSS, version 14.0.
To analyze the biannual 2-year follow-up and 1.5-year postintervention follow-up data on our primary outcomes, advanced and basic mobility, generalized estimating equation models were constructed (19) using SAS (version 9.1), GENMOD procedure. These models allow us to analyze whether the participants in the intervention group have a lower incidence or higher rate of recovery from task difficulty compared with the control group, that is, changes from no difficulty to difficulty, and from difficulty to no difficulty, are taken into account. Accordingly, the interaction term tested represents the difference in time-related change in the proportion of participants reporting difficulty in the intervention versus control group. Separate models were constructed for basic and advanced mobility. Results are expressed as odds ratios (OR) with 95% confidence intervals (CI). CIs not including 1 were considered as statistically significant. For cases with missing values at any points following the baseline measurements, data were imputed with the multiple imputation procedure implemented in SAS by using information on the other mobility tasks and baseline information on age, gender, long-term diseases, prescription medications, muscle power, walking speed, BMI, MMSE, and CES-D scores. We did not impute values for persons who died during the intervention or 1.5-year postintervention follow-up. The number of imputed observations at different measurement points during the 2-year intervention ranged from 17 to 41 (3%–7%) and during the 1.5-year follow-up after the intervention from 46 to 63 (7%–10%). Number needed to treat (NNT) was calculated for evaluating the efficacy of the trial at the 2-year follow-up point (20).
RESULTS
Program Feasibility
Ninety-one percent of the follow-up sample completed the mobility interview at every semiannual follow-up point during the counseling intervention. During the intervention, 16 participants died and 38 withdrew from the study (Figure 1). The baseline characteristics of the intervention and control groups were comparable (Table 1). At baseline, about 30% of the intervention group and 28% of the control group reported having sustained some form of injury during the previous year. After 2 years, the numbers were similar, with no statistical differences between the groups. This indicates that the intervention did not cause excessive adverse events.
Table 1.
Baseline Characteristics by Randomization Group
| Characteristics | Interventiona (N = 318) | Controla (N = 314) |
| Mean ± SD | Mean ± SD | |
| Age (y) | 77.6 ± 1.9 | 77.6 ± 1.9 |
| Number of chronic diseases | 3.0 ± 2.0 | 3.0 ± 2.0 |
| Number of prescription medications | 4.0 ± 2.7 | 4.1 ± 2.8 |
| MMSE | 27.1 ± 2.0 | 27.0 ± 2.2 |
| BMI | 28.3 ± 4.5 | 28.4 ±4.5 |
| Years of education | 9.1 ± 4.0 | 9.3 ± 4.4 |
| % | % | |
| Women | 74.5 | 75.2 |
| Ability to walk 2 km without difficulties | 66.2 | 68.1 |
| Ability to walk 0.5 km without difficulties | 86.5 | 84.7 |
| CES-D score ≥16 | 19.4 | 20.0 |
| Physical activityb | ||
| Mainly resting | 0 | 0 |
| Most activities performed sitting down | 0.6 | 1.6 |
| Light physical activity 1–2 h/wk | 23.6 | 23.6 |
| Moderate physical activity 3 h/wk | 51.6 | 48.7 |
| Moderate physical activity ≥4 h/wk | 24.2 | 26.2 |
Notes: MMSE = Mini-Mental State Examination; BMI = body mass index (kg/m2); CES-D = Center for Epidemiological Studies Depression scale; SD = standard deviation.
No statistically significant differences between the groups.
Persons who belonged to the two highest categories (6–7) were excluded from the study before randomization.
Physical activity counseling increased physical activity and reduced the decline in physical activity significantly in the intervention group compared with the control group (21). The proportion of participants who increased their activity level from sedentary to at least moderate or remained at least moderately active during the 2-year intervention was significantly higher in the intervention compared with the control group (83% vs 72%, OR 2.0, 95% CI: 1.3–3.0). Similarly, the proportion of those who reduced their physical activity level from at least moderate to sedentary or who remained sedentary was lower in the intervention than in the control group (17% vs 28%, OR 0.51, 95% CI: 0.3–0.8).
Mobility
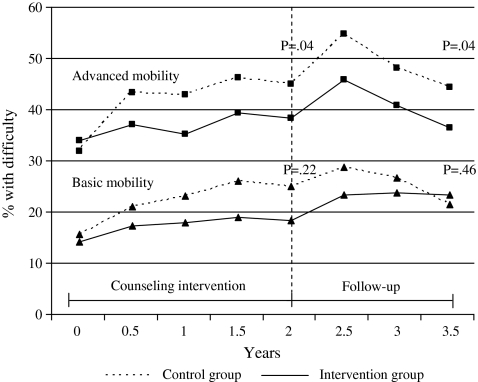
The proportion of participants reporting difficulties in advanced and basic mobility increased in both groups during the intervention (Figure 2). In advanced mobility, the proportions of participants with difficulty in the intervention group were 34% in the beginning and 38% at the end of the intervention, whereas in the control group, the corresponding proportions were 32% and 45%. The treatment effect was significant (OR 0.84, 95% CI: 0.70–0.99) (Table 2). The positive effect of the intervention at the 2-year follow-up was mainly due to prevention of difficulty among those without difficulty at baseline and to a lesser extent due to recovery from baseline difficulty. About 52% of those without difficulty at baseline remained so in the intervention group versus 47% of those in the control group, whereas 9% of those with difficulty at baseline recovered in the intervention group versus 8% in the control group. The effect on basic mobility was parallel but nonsignificant at the end of the 2-year intervention (OR 0.87, 95% CI: 0.69–1.09). In advanced mobility, the treatment effect remained significant (OR 0.82, 95% CI: 0.68–0.99) after the postintervention 1.5-year follow-up, whereas in basic mobility, the effect gradually disappeared (OR 1.09, 95% CI: 0.87–1.37). (Table 2; Figure 2) The sensitivity analyses performed suggested no substantial differences in effects due to imputation.
Figure 2.
Proportion of Participants With Difficulty in Advanced and Basic Mobility According to Randomized Groups at Semiannual Follow-Up Points During the Counseling Intervention and 1.5-Year Postintervention Follow-Up.
Note. p value indicates the statistical significance of the treatment effects (Group × Time interaction) observed in generalized estimating equation models.
Table 2.
Treatment Effects of Physical Activity Counseling Intervention on Advanced and Basic Mobility Among 75- to 81-Year-Old People After the 2-Year Intervention (2-Year Follow-Up) and 1.5-Year Postintervention Follow-Up (3.5-Year Follow-Up)
| 2-year Follow-Up | 3.5-year Follow-Up | |||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Advanced mobilitya | 0.84 | 0.70–0.99 | .04 | 0.82 | 0.68–0.99 | .04 |
| Basic mobilityb | 0.87 | 0.69–1.09 | .22 | 1.09 | 0.87–1.37 | .46 |
Notes: CI = confidence interval; OR = odds ratio, represents the extent of the treatment effects (Group × Time interaction).
Difficulties in walking 2 km.
Difficulties in walking 0.5 km.
At the 2-year follow-up point, the NNT for advanced mobility was 15. This indicates that to prevent one person from developing difficulty or to recover from baseline difficulty, 15 persons had to receive counseling.
DISCUSSION
This study showed that a single physical activity counseling session with supportive phone contacts for 2 years had a positive effect on advanced mobility among older sedentary community-dwelling people. The effect on basic mobility was not statistically significant, although a parallel positive trend was observed.
To our knowledge, this is the first RCT to investigate the long-term effects of individualized physical activity counseling on mobility outcomes among older people. In the earlier RCTs on individualized physical activity counseling among older people aged 60 and older (6–9), the follow-ups have ranged from 6 to 12 months and mobility limitations have not been studied as outcomes. In our study, we followed up mobility limitations biannually for a total of 3.5 years.
Our hypothesis was that physical activity counseling would increase physical activity that in turn would be reflected as perceived improvements in mobility. We found that our intervention increased physical activity and slowed down the decrease in physical activity of the intervention group. Earlier studies have shown that specific exercise interventions improve muscle function, postural control, and walking speed (22–25), all of which are on the causal pathway to disability (26,27). Mood benefits and increased social activation may also partly explain the positive effects on self-reported mobility, especially as the counseling was motivational with the emphasis on self-efficacy for more active behavior (11,21). These mediating factors merit further investigation.
Majority of the beneficial effect of the intervention on mobility took place via the prevention of new difficulty among those without difficulty at baseline, whereas the ameliorating effect on difficulty was smaller. This suggests that people with mobility difficulties probably need a different kind of intervention than that studied here to improve their mobility. Further studies are clearly needed.
Mobility is a critical component for the maintenance of independence in old age and difficulties in mobility often precede more severe disability (3). Promoting mobility by physical activity may be an effective means of supporting independent living among older people. After our low-cost 2-year counseling program, we found a significant 19% reduction in the risk of difficulty in advanced mobility with the NNT of 15. By comparison, it was estimated recently that 254 women aged 70–74 need to be screened with bone densitometry and treated, 51 of them by bisphosphonates for 5 years to avert one hip fracture (28). As our primary outcome was perceived difficulty, a subjective evaluation of one's mobility in one's everyday environment, and the study was population based, our results can be considered clinically relevant and significant, and can be generalized to older community-dwelling people. It has been argued recently (29) that conducting a clinical trial in older individuals may lead to disease-specific improvements that have only little impact on the day-to-day function of the individual. Particularly among older people, it is crucial to include an assessment of functional outcomes, reflecting the overall health status of the individual, in RCTs (29).
Our study has some limitations that must be considered when interpreting the results. Activation of the participants in our control group, due to the physical activity–related interviews or to potential personal interaction between intervention and control group members, may cause underestimation of the results. In addition, reporting bias due to social desirability may affect the results, although the physiotherapist who carried out the counseling did not participate in the data collection process. Furthermore, we are unable to draw conclusions about the superiority of different exercise promotion strategies. A recent study among 30- to 50-year-old people found that leaflets were equally efficacious in increasing physical activity as personalized counseling (30). However, older people have more health-related and other perceived barriers to physical activity than younger people and may therefore require more personalized approaches.
The strength of our study is the randomized controlled design with multiple observations on mobility over a total follow-up period of 3.5 years. In addition, our intervention was inexpensive, acceptable, and efficacious and could easily be adapted to various settings in other countries. However, local circumstances, such as community resources and differences in the attitudes toward physical activity, should be taken into account.
CONCLUSION
Among older people, a single individual physical activity counseling session with supportive contact every 4 months for 2 years had a positive effect on mobility and may thus help to maintain functional independence in this age group.
CONFLICT OF INTEREST
None disclosed.
Acknowledgments
This study was funded by the Ministry of Education, Finland; Ministry of Social Affairs and Health, Finland; Juho Vainio Foundation, Finland; Finnish Cultural Foundation, Finland; City of Jyväskylä, Finland; and University of Jyväskylä, Finland.
References
- 1.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 4.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48:493–498. doi: 10.1111/j.1532-5415.2000.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 5.Boyle PA, Buchman AS, Wilson RS, Bienias JL, Bennett DA. Physical activity is associated with incident disability in community-based older persons. J Am Geriatr Soc. 2007;55:195–201. doi: 10.1111/j.1532-5415.2007.01038.x. [DOI] [PubMed] [Google Scholar]
- 6.Halbert JA, Silagy CA, Finucane PM, Withers RT, Hamdorf PA. Physical activity and cardiovascular risk factors: effect of advice from an exercise specialist in Australian general practice. Med J Aust. 2000;173:84–87. doi: 10.5694/j.1326-5377.2000.tb139250.x. [DOI] [PubMed] [Google Scholar]
- 7.Kerse N, Elley CR, Robinson E, Arroll B. Is physical activity counseling effective for older people? A cluster randomized, controlled trial in primary care. J Am Geriatr Soc. 2005;53:1951–1956. doi: 10.1111/j.1532-5415.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 8.Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M465–M470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto BM, Goldstein MG, Ashba J, Sciamanna CN, Jette A. Randomized controlled trial of physical activity counseling for older primary care patients. Am J Prev Med. 2005;29:247–255. doi: 10.1016/j.amepre.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Hillsdon M, Foster C, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD003180.pub2. (1):CD003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leinonen R, Heikkinen E, Hirvensalo M, et al. Customer-oriented counseling for physical activity in older people: study protocol and selected baseline results of a randomized-controlled trial (ISRCTN 07330512) Scand J Med Sci Sports. 2007;17:156–164. doi: 10.1111/j.1600-0838.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Mänty M, Heinonen A, Leinonen R, et al. Construct and predictive validity of a self-reported measure of preclinical mobility limitation. Arch Phys Med Rehabil. 2007;88:1108–1113. doi: 10.1016/j.apmr.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Fried LP, Salive ME. Disability as a public health outcome in the aging population. Annu Rev Public Health. 1996;17:25–46. doi: 10.1146/annurev.pu.17.050196.000325. [DOI] [PubMed] [Google Scholar]
- 15.Grimby G. Physical activity and muscle training in the elderly. Acta Med Scand Suppl. 1986:233–237. doi: 10.1111/j.0954-6820.1986.tb08956.x. 711. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 17.Bandura A. The Exercise of Control. New York: WH Freeman;; 1997. [Google Scholar]
- 18.Rollnick S, Mason P, Butler C. Health Behavior Change. A Guide for Practitioners. London, UK: Churchill Livingstone;; 1999. [Google Scholar]
- 19.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;42:13–22. [Google Scholar]
- 20.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pakkala I, Read S, Leinonen R, Hirvensalo M, Lintunen T, Rantanen T. The effects of physical activity counseling on mood among 75- to 81-year-old people: a randomized controlled trial. Prev Med. 2008;46:412–418. doi: 10.1016/j.ypmed.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 23.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59:48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- 24.Howe TE, Rochester L, Jackson A, Banks PM, Blair VA. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004963.pub2. (4):D004963. [DOI] [PubMed] [Google Scholar]
- 25.Karinkanta S, Heinonen A, Sievanen H, et al. A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int. 2007;18:453–462. doi: 10.1007/s00198-006-0256-1. [DOI] [PubMed] [Google Scholar]
- 26.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54:439–467. [PubMed] [Google Scholar]
- 27.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 28.Jarvinen TL, Sievanen H, Khan KM, Heinonen A, Kannus P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ. 2008;336:124–126. doi: 10.1136/bmj.39428.470752.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhasin S, Cress E, Espeland MA Working Group on Functional Outcome Measures for Clinical Trials, Functional outcomes for clinical trials in frail older persons: time to be moving. J Gerontol A Biol Sci Med Sci. 2008;63:160–164. doi: 10.1093/gerona/63.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinmonth AL, Wareham NJ, Hardeman W, et al. Efficacy of a theory-based behavioural intervention to increase physical activity in an at-risk group in primary care (ProActive UK): a randomised trial. Lancet. 2008;371:41–48. doi: 10.1016/S0140-6736(08)60070-7. [DOI] [PubMed] [Google Scholar]