Abstract
To determine if B6D2F1 mice represent a suitable model of oxidative stress–mediated impaired endothelium-dependent dilation (EDD) with aging, mice were studied at 6.9 ± 0.3 and 31.9 ± 0.6 months. EDD to acetylcholine (ACh) was 26% (p < .001) and 12% (p < .001) lower, respectively, in isolated carotid (n = 10–11) and femoral (n = 10) arteries from older mice, and reductions in arterial pressure to systemic ACh infusion were smaller in older mice (n = 6–10; p < .01). Nitrotyrosine was marked in aorta of older mice (p < .05, n = 4). Superoxide production in carotid arteries was greater (p < .05), and TEMPOL restored dilation in carotid arteries and systemically in older mice. NG-nitro-l-arginine methyl ester (l-NAME) reduced carotid artery dilation in young more than older mice, whereas TEMPOL restored the effects of l-NAME in older mice. Carotid artery stiffness was increased in older compared with young mice (p = .04). Our results provide the first comprehensive evidence that B6D2F1 mice are a useful model for investigating mechanisms of reduced nitric oxide–dependent, oxidative stress–associated EDD and increased arterial stiffness with aging.
Keywords: Superoxide, Arterial stiffness, Carotid artery, Femoral artery, Nitrotyrosine
IMPAIRED endothelium-dependent dilation (EDD) is a central feature of vascular endothelial dysfunction and is associated with increased risk of future cardiovascular events (1–4). EDD is reduced in older adults in the absence of clinical cardiovascular disease or major risk factors (1–4), suggesting an independent adverse effect of aging on endothelial function. The age-associated reduction in EDD is observed in both peripheral conduit arteries (5) as well as in central (6) and peripheral (7–10) resistance vessels. It is not known if this reflects systemic arterial endothelial dysfunction because of the difficulty in studying the latter in humans.
The mechanisms underlying impaired EDD with aging in humans are incompletely understood, but several lines of evidence support a prominent role for oxidative stress. These include increased vascular endothelial abundance of nitrotyrosine, a cellular marker of oxidative stress, in healthy older adults (11), and the ability of acute infusions of antioxidants to restore EDD to normal (i.e, young adult) or near-normal levels in older adults (5,8). Oxidative stress impairs EDD in older adults, at least in part, by reducing the bioavailability of nitric oxide (NO), a potent endothelium-derived signaling molecule (8). The decrease in NO-mediated dilation with aging is not caused by reduced vascular smooth muscle sensitivity to NO because dilation in response to “NO donors” such as sodium nitroprusside (SNP, i.e, “endothelium-independent dilation”) is preserved in healthy older adults (5,7,8,12).
The clinical importance of age-associated reductions in EDD combined with the inherent limitations of studying mechanistic processes in humans, particularly in healthy older adults, have lead to the use of animal models to address issues on this topic. Much of the work to date has been performed on the Fischer 344 rat model of aging (13–16). However, increasing interest in mouse models has resulted in recent investigations using C57BL/6 mice (17–20), the most commonly used mouse model of aging (21).
Despite its utility, there are some potential limitations associated with the use of the C57BL/6 mouse to study changes in EDD with aging. First, the results of the initial studies using this model have been inconsistent, with either reduced (19,20) or unchanged (17,18) EDD observed in arteries of older mice. Second, in 2006 the National Institute on Aging announced that C57BL/6 mice of various ages would be available for purchase from their subsidized colonies only by investigators with active grants in which use of these mice is approved (NOT-AG-06-012 (22)), thus limiting their availability to a small group of specifically funded investigators. There also may be legitimate concerns about basing observations solely on this single inbred strain of mice (23). Collectively, these points suggest that it may be useful to establish other mouse models of impaired EDD with aging.
The long-lived B6D2F1 hybrid mouse may represent a suitable model of vascular endothelial dysfunction with aging. Older B6D2F1 mice demonstrate increased arterial stiffness (24), another clinically important expression of vascular aging that frequently covaries with impaired EDD in humans (25). Concerns with overuse of inbred strains like the C57BL/6 mouse also would be lessened with the use of this model. However, it is unknown if senescent B6D2F1 mice demonstrate oxidative stress–dependent reductions in EDD as observed with human aging.
Accordingly, the overall goal of the present study was to test this hypothesis. Because the B6D2F1 hybrid has a longer lifespan than the C57BL/6 mouse (10% survival at 39 vs 32 months, respectively), we chose to study the older mice at approximately 30 months, an age that corresponds to a 50%–75% survival rate. This is a survival rate similar to that observed in the C57BL/6 mouse at 24–26 months of age (26). Our first aim was to determine if older B6D2F1 mice exhibit reduced EDD in central (carotid) and peripheral (femoral) arteries studied ex vivo compared with young adult animals. If so, our second aim was to determine if older B6D2F1 mice also demonstrate reduced systemic arterial EDD in the conscious state in vivo. The third aim was to determine if these reductions in EDD in older B6D2F1 mice are associated with evidence of oxidative stress and reduced NO bioavailability. A fourth aim was to determine if endothelium-independent dilation (EID) is preserved in older B6D2F1 mice in the face of reductions in EDD, as is the case with human aging. Finally, given the aforementioned evidence of arterial stiffness in older B6D2F1 mice (24), we took the opportunity to determine if the hypothesized age-associated reductions in carotid and femoral artery EDD in our B6D2F1 mice would be associated with evidence of increased stiffness in one or both of these arteries.
METHODS
Animals
Young (6–9 months) and older (29–34 months) male B6D2F1 mice were obtained from the National Institute on Aging rodent colony. All mice were housed in an animal care facility at the University of Colorado at Boulder (UCB) on a 12:12 light:dark cycle and fed standard rodent chow ad libitum. All animal procedures conformed to the Guide to the Care and Use of Laboratory Animals (National Institutes of Health publication no. 85-23, revised 1996) and were approved by the UCB Animal Care and Use Committee.
EDD and EID in Isolated Carotid and Femoral Arteries Studied Ex Vivo: Effects of Oxidative Stress and NO Bioavailability
Measurements of EDD and EID in isolated vessels studied ex vivo were performed using a modification of the methods described previously by d'Uscio and colleagues as well as Muller-Delp and colleagues (14,27). Mice were euthanized by exsanguination via cardiac puncture while under isoflurane anesthesia. Both the right and left carotid arteries (n = 7–11 per group) were excised and placed in myograph chambers (DMT Inc., Atlanda, GA) containing EDTA-buffered physiological saline solution (PSS), cannulated onto glass micropipettes and secured with nylon (11-0) suture. Both right and left femoral arteries (n = 5–11 per group) were excised from the mouse and placed in cold (4°C) PSS until carotid artery studies were completed. Once cannulated, both carotid and femoral arteries were warmed to 37°C and pressurized to 50 mm Hg intraluminal pressure and allowed to equilibrate for 1 hour. All arteries then were submaximally preconstricted with phenylepherine (2 μM) because the arteries do not exhibit spontaneous tone. Increases in luminal diameter in response to increasing concentrations of the endothelium-dependent dilator acetylcholine (ACh: 1 × 10−9 to 1 × 10−4 M) and endothelium-independent dilator SNP (1 × 10−10 to 1 × 10−4 M) were determined. We chose to conduct all follow-up responses solely in the carotid artery because it displayed a greater degree of dysfunction in the older mice. Responses to ACh were repeated in the presence of the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 0.1 mM, 30-minute incubation) to determine the contribution of NO. To determine the role of oxidative stress in age-related declines in vascular function, these same responses were repeated in the contralateral vessel following a 60-minute incubation in the presence of the superoxide dismutase (SOD) mimetic TEMPOL (1 mM) (17,28,29).
Data presentation.—
All functional data are presented on a percent basis. Preconstriction was calculated as percentage of maximal diameter according to the following formula:
Vasodilator responses were recorded as actual diameters and expressed as a percentage of maximal response according to the following formula:
where Dm is maximal inner diameter at 50 mm Hg, Ds is the steady-state inner diameter recorded after the addition of drug, and Db is the steady-state inner diameter following preconstriction before the first addition of drug (14).
Systemic EDD and EID in the Conscious In Vivo State: Effects of Oxidative Stress
Measurements of systemic EDD and EID in the conscious in vivo state were performed using the model established previously by Cernadas and colleagues (30). Mice (n = 6–10 per group) were anesthetized (3 mg/kg, intraperitoneally) with xylazine (1.5 mg/mL), ketamine (7.5 mg/mL), and acepromazine (0.005 mg/mL), and right carotid artery and dual left jugular venous catheters were surgically implanted (microrenthane tubing ID 0.025, Braintree Scientific, Braintree, MA). Heparinized saline-filled catheters (31) were tunneled to the dorsal surface of the neck and secured under the skin. The following day, catheters were externalized and mice were placed in a restrainer (Braintree Scientific). Carotid artery blood pressure was measured using an external pressure transducer (Becton Dickinson, Franklin Lakes, NJ) coupled to a Gould amplifier and Windaq data acquisition system with mice in the conscious state. Mean arterial pressure was measured preinfusion and in response to intravenous infusion of ACh (0.05, 0.1, 0.15, 0.25, 0.5, and 0.75 mg/kg) and SNP (0.05, 0.1, 0.2, 0.4, 0.6, and 0.8 mg/kg). Doses were administered via jugular catheters using a Harvard pump (Kd Scientific, Holliston, MA) (15 μL/min). The magnitude of the reductions in mean arterial pressure from baseline in response to ACh was taken as a measure of systemic EDD, whereas the reductions in arterial pressure in response to SNP were used as a measure of systemic EID (30). Following ACh, TEMPOL was administered to experimentally reduce superoxide concentrations as described above. TEMPOL (100 mg/kg, bolus) was infused 15 minutes prior to ACh dose responses (32). All dose responses were repeated three times, and the mean data for each mouse were used in the analysis. The effects of NO bioavailability on age-related differences in EDD could not be determined in this model because doses of l-NAME sufficient to block NO systemically caused increases in mean arterial pressure detrimental to the health of the animal.
Nitrotyrosine
Thoracic aortas were used to determine nitrotyrosine abundance as a marker of vascular oxidative stress because the carotid arteries of the mice do not yield sufficient protein for Western blot analysis (30). The vessels were excised, cleared of surrounding tissues while maintained in 4°C physiological saline, quickly frozen in liquid nitrogen, and stored at −80°C. The frozen aortas were pulverized over liquid nitrogen and homogenized in ice-cold RIPA lysis buffer (0.5 M Tris–HCl, pH 7.4, 1.5 M NaCl, 2.5% deoxycholic acid, 10% NP-40, 10 mM EDTA, 1 mM NaF, 1 mM sodium ortovanadate, 1 mM phenylmethylsulfonylfluoride) containing protease (Protease Inhibitor Cocktail Tablet, Roche, Indianapolis, IN) and phosphatase (0.01% phosphatase inhibitor cocktail, Sigma, St. Louis, MO) inhibitors (17,33,34). Protein concentrations of the lysates were determined by BCA colorimetric protein assay (Pierce, Rockford, IL). Fifteen micrograms of protein containing 1 M DTT was loaded into polyacrylamide gels, separated by electrophoresis, and transferred onto a nitrocellulose membrane. The membrane was blocked in 5% nonfat dry milk in Tris-buffered saline (TBS) with 0.05% Tween (TBST) for 1 hour. Following blocking, the membrane was washed with TBST and nitrotyrosine expression was measured by standard western blotting techniques using a monoclonal primary antibody (Abcam, Cambridge, MA), HRP-conjugated secondary antibody (Jackson Immunological, West Grove, PA) and Supersignal ECL (Pierce). Bands were visualized using a digital acquisition system (ChemiDoc-It, UVP Inc, Upland, CA), and quantification was performed using VisionWorks software (UVP Inc). Data are presented normalized to GAPDH protein expression to account for differences in protein loading (n = 4 aortas per age group). Nitrotyrosine can have bands at 180, 55, and 25 kDa, and although we did not see the 180-kDa band in our mouse aortic lysates, the 55- and 25-kDa bands displayed a similar pattern. For analysis, the 55- and 25-kDa bands were quantified and normalized to GAPDH protein expression, and the mean normalized value for each sample was used in the statistical analysis. Blots were replicated in triplicate.
Dihydroethidium Staining for Superoxide
Dihydroethidium (DHE) staining was performed on frozen unfixed vessel sections to evaluate in situ superoxide in carotid arteries (35,36). Carotid arteries from young and older mice (n = 5 per group) were excised and frozen in optimal cutting temperature media (Fisher Scientific, Pittsburgh, PA) in liquid nitrogen–cooled isopentane. Serial sections (7 μm) were rehydrated with PBS or PBS containing 1 mM TEMPOL for 10 minutes. A 2 μM DHE solution, with and without TEMPOL, was then applied to the sections with unaltered PBS applied to one section as a control. The sections were then incubated at 37°C in a dark humid chamber for 30 minutes. The slides were rinsed and allowed to air-dry in the dark before 4',6-diamidino-2-phenylindole (DAPI) mounting medium and a cover slip were applied. Images were obtained under light of 550 nm using a Nikon Eclipse E600W microscope and analyzed using MetaMorph 7.1 imaging software. Because the background autofluorescence of carotid artery sections was higher in young compared with older mice (911 ± 49 vs 390 ± 41 AU), the data are presented normalized to the fluorescence of the DHE sample according to the following formula: [DHEi − (DHE + TEMPOL)i]/DHEi × 100, where DHE is the fluorescent intensity of the section incubated in DHE alone and (DHE + TEMPOL)i is the fluorescent intensity of the section incubated in DHE following a 10-minute pretreatment with TEMPOL.
Stiffness Measurements in Isolated Carotid and Femoral Arteries
Carotid (n = 9–11 per group) and femoral (n = 5–10 per group) arteries were incubated in Ca2+-free PSS for 1 hour. Lumen diameter and medial wall thickness were measured in response to increases in intraluminal pressure (5 and 20–200 mm Hg, in 20 mm Hg increments). Passive pressure–diameter relations were expressed relative to lumen diameter measured at 100 mm Hg to account for age-associated differences in baseline diameter. Circumferential stress, stretch, and incremental stiffness were calculated as previously described (14). Wall thickness was measured at each pressure increment and used in the calculation of circumferential stress.
Statistics
For animal and vessel characteristics and aortic nitrotyrosine abundance, group differences were determined by one-way analysis of variance (ANOVA). For all dose responses, group differences were determined by repeated measures ANOVA. For variables in which a significant interaction or main effect for group were found, one-way ANOVA was used to determine group differences at individual doses or pressures. Dunnett's T3 post hoc test for unequal variances was used where appropriate. Potential bivariate relations of interest between variables were assessed using Pearson product–moment correlation analyses. Data are presented as mean ± standard error of the mean. Significance was set at p < .05.
RESULTS
Age, Body Mass, and Resting Arterial Blood Pressure
The mean ages at which mice were studied were 6.9 ± 0.3 months (young) and 31.9 ± 0.6 months (older). Body mass was not different between age groups (young: 34.6 ± 0.6 vs older: 33.7 ± 0.7 g, p = .34). Conscious mean arterial pressure was not different in young and older mice (young: 130 ± 1 vs older: 136 ± 3 mm Hg, p = .26).
Lumen Diameter and Medial Wall Thickness in Isolated Carotid and Femoral Arteries
Lumen diameter was greater in the carotid arteries of the older animals (young: 403 ± 4 vs older: 431 ± 8 μm, p = .004), whereas medial wall thickness (young: 39 ± 7 vs older: 41 ± 6 μm, p = .35) and the medial wall to lumen ratio (wall thickness/lumen diameter × 100) (young: 9.4 ± 2.1 vs older: 9.5 ± 1.6, p = .35) did not differ between groups. Lumen diameter was not different in the femoral arteries of the young and older mice (young: 411 ± 5 vs older: 417 ± 11, p = .60), whereas medial wall thickness (young: 31 ± 5 vs older: 40 ± 8, p = .03) and the medial wall to lumen ratio (young: 7.7 ± 1.3 vs older: 10.0 ± 1.8, p = .03) were greater in the older mice.
Isolated Carotid and Femoral Arteries of Older B6D2F1 Mice Demonstrate Impaired EDD With Preserved EID
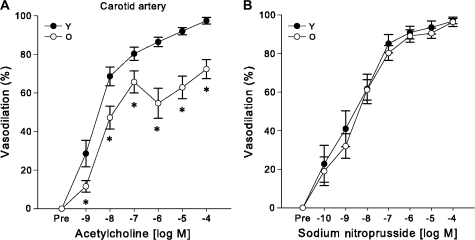
Preconstriction, expressed as a percent of maximal diameter, to phenylephrine was not different in the carotid arteries from young and older mice (p = .33). The subsequent maximal carotid artery dilation in response to ACh was 26% lower in the older mice (p < .001) (Figure 1A), whereas there was no significant difference in sensitivity (The half maximal inhibitory concentration (IC50); young: 6 × 10−9 ± 3 × 10−9 vs older: 1.8 × 10−8 ± 1.2 × 10−8 M, p = .34). There were no differences in dilation to SNP in the carotid arteries of young and older mice (p = .51, Figure 1B).
Figure 1.
Reduced endothelium-dependent dilation, with preserved endothelium-independent dilation in the carotid arteries of older (O, 29–34 months) compared with young (Y, 6–9 months) B6D2F1 mice. (A) Dose–response dilations to the endothelium-dependent vasodilator, acetylcholine, in isolated carotid arteries from young (n = 10) and older (n = 11) mice. (B) Dose–response dilations to the endothelium-independent vasodilator sodium nitroprusside (Y: n = 7, O: n = 10). For dose–response relations with significant (p < .05) main effects for group or interaction, asterisk denotes significant (p < .05) differences between age groups at particular doses.
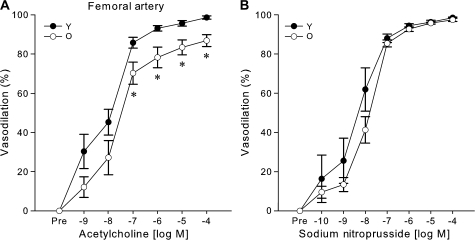
Preconstriction to phenylephrine was not different in the femoral arteries from young and older mice (p = .83). The subsequent maximal femoral artery dilation in response to ACh (1 × 10−4 M) was 12% lower in the older mice (p < .001) (Figure 2A), whereas there was no significant difference in sensitivity to ACh (IC50: young: 1 × 10−8 ± 0.4 × 10−8 vs older: 4 × 10−8 ± 1 × 10−8 M, p = .10). There were no differences in dilation to SNP in the femoral arteries of young and older mice (p = .18, Figure 2B).
Figure 2.
Reduced endothelium-dependent dilation, with preserved endothelium-independent dilation in the femoral arteries of older (O, 29–34 months) compared with young (Y, 6–9 months) B6D2F1 mice. (A) Dose–response dilations to the endothelium-dependent vasodilator, acetylcholine, in isolated femoral arteries from young (n = 10) and older (n = 10) mice. (B) Dose–response dilations to the endothelium-independent vasodilator sodium nitroprusside (Y: n = 7, O: n = 8). For dose–response relations with significant (p < .05) main effects for group or interaction, asterisk denotes significant (p < .05) differences between age groups at particular doses.
Collectively, these results demonstrate that EDD is reduced in carotid and femoral arteries of older B6D2F1 mice, whereas EID is preserved.
Older B6D2F1 Mice Demonstrate Impaired Systemic EDD With Preserved Systemic EID in the Conscious In Vivo State
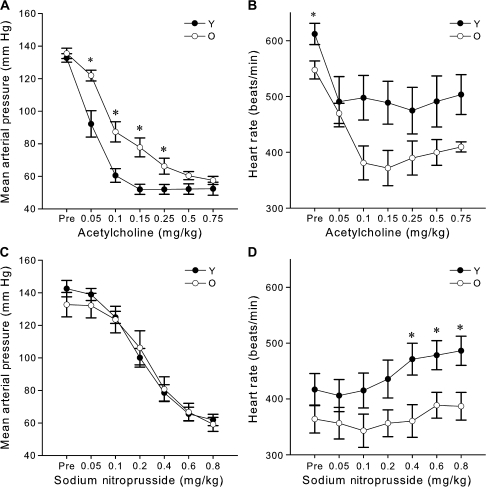
There were no differences in mean arterial pressure between the young and older mice before infusion (p = .26, Figure 3A). Incremental intravenous infusion of ACh reduced mean arterial pressure in a curvilinear, dose-dependent manner in both groups with saturation of the response occurring at the higher doses (p = .001, Figure 3A). The reductions in mean arterial pressure from baseline during the steep portion of the dose–response curve (0.05, 0.10, 0.15, 0.25 mg/kg ACh) were smaller in the older mice (p < .05, Figure 3A). Older mice were less sensitive to systemic infusion of ACh (IC50—young: 0.04 ± 0.01, older: 0.11 ± 0.02, p = .03). Pre-ACh infusion, heart rate was lower in the older compared with young mice (Figure 3B, p = .02). Heart rate also tended to be lower in older mice during the infusion of ACh (p = .06, Figure 3B). In contrast, the reductions in mean arterial pressure in response to incremental infusion of SNP were similar in the young and older mice (p = .72, Figure 3C). Pre-SNP infusion, heart rate did not differ between young and older mice (p = .2, Figure 3D), whereas during the infusion heart rate was lower in the older mice at the highest doses of SNP (p < .03, Figure 3D). Prior infusion of ACh reduced heart rate approximately 33% in both young and older mice, likely due to a prolonged direct effect of ACh on heart rate (37). Nevertheless, similar differences (50–60 beats per minute) in preinfusion heart rate between young and older mice were found for both ACh and SNP responses. These observations demonstrate that systemic EDD is reduced in older B6D2F1 mice in vivo in the conscious state, whereas systemic EID is preserved.
Figure 3.
Reduced systemic endothelium-dependent dilation, with preserved systemic endothelium-independent dilation of older (O, 29–34 months) compared with young (Y, 6–9 months) B6D2F1 mice. (A) Dose–response reductions in mean arterial pressure to intravenous administration of the endothelium-dependent vasodilator, acetylcholine (ACh), in young (n = 6) and older (n = 6) mice. (B) Heart rate responses to the intravenous infusion of ACh in young (n = 5) and older (n = 5) mice. (C) Dose–response reductions in mean arterial pressure to intravenous administration of the endothelium-independent vasodilator sodium nitroprusside (SNP; Y: n = 8, O: n = 6). (D) Heart rate responses to the intravenous infusion of SNP in young (n = 12) and older (n = 10) mice. For dose–response relations with significant (p < .05) main effects for group or interaction, asterisk denotes significant (p < .05) differences between age groups at particular doses.
Vascular Oxidative Stress Develops With Increased Superoxide Production in Older B6D2F1 Mice and Mediates Impaired EDD via Reduced NO Bioavailability
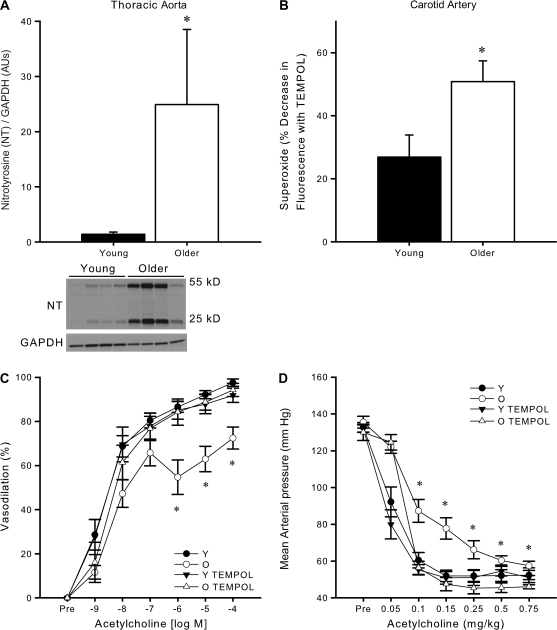
Abundance of nitrotyrosine in the thoracic aorta was marked in the older mice, but low in the young mice (p = .04, Figure 4A). Consistent with this, carotid arteries from older mice demonstrated a greater relative reduction in the fluorescent signal elicited by DHE when pretreated with TEMPOL (p = .02, Figure 4B), indicative of increased superoxide production in older compared with young mice. These data provide molecular evidence for the development of vascular oxidative stress linked to increased superoxide production with aging in B6D2F1 mice.
Figure 4.
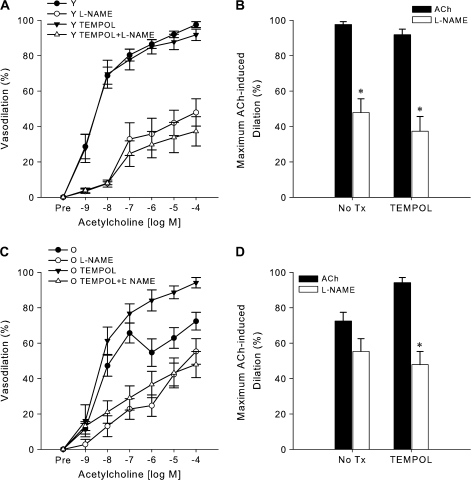
Vascular oxidative stress develops in older B6D2F1 mice and mediates impaired endothelium-dependent dilation. (A) Increased nitrotyrosine abundance in the thoracic aorta of older mice measured by western blot analysis. Data are normalized to GAPDH protein expression to account for differences in protein loading. The summary graph represents the mean quantification of the 55- and 25-kDa bands (n = 4 per group). The 55- and 25-kDa bands of a representative blot are shown below the summary graph. Asterisk denotes a significant group difference (p < .05). (B) Increased superoxide in carotid arteries of older mice indicated by a larger reduction in dihydroethidium staining in older compared with young mice following inhibition with the superoxide dismutase mimetic, TEMPOL (n = 5 per group). Asterisk denotes a significant group difference (p < .05). (C) Dose–response dilations to acetylcholine (ACh) in the absence (n = 10 per group) or presence (n = 9 per group) of TEMPOL in isolated carotid arteries of young (Y) and older (O) mice. Asterisk denotes significant (p < .05) differences between TEMPOL-treated and untreated responses in older mice. (D) Systemic endothelium-dependent dilation to intravenous ACh infusion in the absence (n = 6 per group) or presence (n = 6 per group) of TEMPOL. Asterisk denotes significant (P < .05) differences between TEMPOL-treated and untreated responses in O mice.
To examine the role of vascular oxidative stress in the reduced EDD of older B6D2F1 mice, we measured dilation in response to ACh in the absence versus presence of the SOD mimetic TEMPOL. TEMPOL did not affect the baseline diameter of the isolated arteries (young: p = 1.0, older: p = 1.0) or blood pressure (young: p = .5, older: p = .3) in young or older mice. TEMPOL restored EDD in the isolated carotid (young vs older: p = .51, Figure 4C) and femoral (young vs older: p = .78, data not shown) arteries of older mice to levels observed in young animals, as well as in the intact conscious animals in vivo (young vs older with TEMPOL: p = 1.0, Figure 4D). These findings indicate that the reductions in EDD in both isolated large conduit arteries and systemically in the older animals were mediated by oxidative stress.
In young mice, carotid artery dilation in response to ACh was reduced by 51% after pretreatment with the NO synthase inhibitor l-NAME compared with ACh alone (p < .001, Figure 5A). In contrast, the reduction in carotid artery dilation to ACh after pretreatment with l-NAME was much less in the older mice and was observed only at selective doses (10−8 to 10−6 M ACh, p < .05, Figure 5C). Following pretreatment with TEMPOL, the l-NAME–associated reduction of the carotid artery dilatory response to ACh was only slightly augmented in the young mice (from 51% to 59%, p = .78 with vs without TEMPOL, Figure 5B), whereas in the older mice TEMPOL administration evoked a much larger l-NAME–associated reduction in the dilatory response to ACh (from 22% to 53%, p = .03 with vs without TEMPOL, Figure 5D).
Figure 5.
Reducing superoxide (oxidative stress) restores endothelium-dependent dilation in carotid arteries of older B6D2F1 mice by improving nitric oxide (NO) bioavailability. Dose–response dilations of carotid arteries to acetylcholine (ACh) in the absence (n = 10 per group) or presence (n = 7 per group) of the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME) and/or the superoxide dismutase mimetic TEMPOL in (A) young (Y) and (C) older (O) mice. Maximal dilation to ACh in the carotid arteries of (B) young and (D) older mice following either no treatment (No Tx: n = 10 per group) or pretreatment (n = 7 per group) with TEMPOL in the absence or presence of l-NAME. Asterisk denotes significant (p < .05) differences in maximal dilation following l-NAME inhibition (p < .05).
Collectively, these observations demonstrate that the impaired EDD in isolated carotid arteries from older B6D2F1 mice is mediated in part by oxidative stress–associated reductions in NO bioavailability.
Isolated Carotid, but not Femoral, Arteries of Older B6D2F1 Mice Demonstrate Increased Stiffness
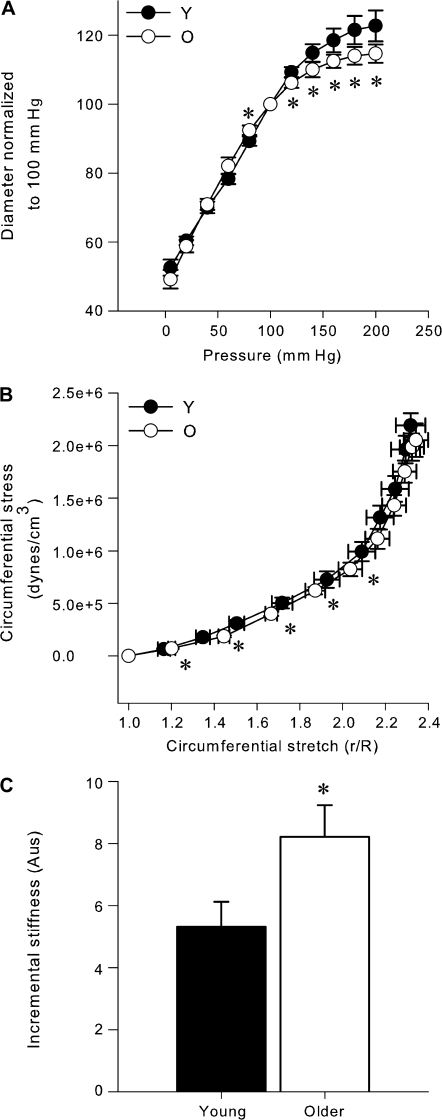
The passive distention of isolated carotid arteries in response to the higher intraluminal pressures was less in carotid arteries of the older mice compared with the young controls (p < .05, Figure 6A). Circumferential stretch was greater in the carotid arteries of the older mice at 20–, 40–, 60–, 80–, and 100–mm Hg intraluminal pressures (p < .05, Figure 6B), whereas there were no differences in circumferential stress (p = .47, Figure 6B). As a result, incremental stiffness was 35% greater in the carotid arteries of the older mice (p = .04, Figure 6C). In contrast to the carotid arteries, there was no increase in the incremental stiffness in the femoral arteries of older mice (young: 15.1 ± 3.2 vs older: 14.3 ± 3.7 AUs, p = .84).
Figure 6.
Increased stiffness in isolated carotid arteries of older B6D2F1 mice. (A) Passive pressure–diameter relations in isolated carotid arteries from young (Y: n = 10) and older (O: n = 10) mice. There was a significant (p < .05) interaction between group and pressure. Asterisk denotes significant (p < .05) differences between age groups at given pressures. (B) The circumferential stress and stretch calculated from the pressure–diameter relations (n = 10 per group). There was a significant (p < .05) interaction between group and pressure for circumferential stretch. Asterisk denotes significant (p < .05) differences in stretch between age groups at particular pressures. No significant differences were found for circumferential stress. (C) Incremental stiffness is greater in older B6D2F1 mice compared with young mice (n = 10 per group). Asterisk denotes significant differences (p < .05).
Correlations
In the pooled group of young and older animals, isolated carotid and femoral artery EDD were not significantly related to lumen diameter, wall thickness, or stiffness (p > .05). There also were no significant relations within the groups of young and older animals.
DISCUSSION
In the present study we used a combination of ex vivo and in vivo studies to demonstrate reductions in EDD in older B6D2F1 mice. Specifically, we showed that EDD, as determined by responses to the endothelium-dependent dilator ACh, was reduced in a large elastic artery (carotid), in a large muscular artery (femoral), and in the systemic arterial circulation of older animals. We also demonstrated that the decreased EDD in older B6D2F1 mice is associated with vascular oxidative stress and increased superoxide production and that oxidative stress reduces EDD in these animals by decreasing NO bioavailability. Finally, we showed that, as is the case with human aging, EID is preserved in older B6D2F1 mice in the face of reductions in EDD. Overall, our results establish the first experimental support for the idea that B6D2F1 mice, a long-lived mouse model of aging, represent a useful model for investigating the mechanisms of age-associated oxidative stress–mediated impaired EDD.
Aging and EDD in Isolated Arteries
Previous studies of the effects of aging on large central artery EDD using rodent models, particularly mouse models, have reported inconsistent results. In the C57BL/6 mice, impaired EDD has been observed in older animals in some cases (19,20), but not others (18). In one study in which impaired EDD was observed, an age-associated decrease in EID (i.e, vascular smooth muscle sensitivity to NO) also was found in the older animals (19). Age-related reductions in large elastic artery EDD also have been reported in 129/SvJ wild-type mice (38) and F344 rats (16). Based on this previous work, we believe that the dilatory response of the carotid arteries of older B6D2F1 mice to ACh represents an effective model of reduced large elastic artery EDD with aging.
In the present study, we found a decrease in femoral artery EDD in older B6D2F1 mice. To our knowledge, this is the first report of reduced EDD in a large muscular conduit artery with aging in rodents. An earlier study in female Ro-Ro Wistar rats found that aortic, but not femoral, rings of older animals demonstrated reduced EDD compared with young animals (39). This is of interest because in the present study we found a greater % reduction in EDD with aging in the carotid, compared with the femoral artery. Together, the observations from these two studies suggest the possibility of greater age-related declines in EDD in large elastic arteries than in large muscular arteries, at least in rodents. The previous study on Wistar rats (39) postulated that such differences may provide a basis for the differential anatomical development of atherosclerosis with aging.
Aging and EDD In Vivo
In the present investigation, we used the reduction in mean arterial pressure to intravenous administration of ACh to demonstrate reductions in systemic arterial EDD in older B6D2F1 mice, an experimental approach used previously in young and older F344 rats (30). In the latter study, the hypotensive responses to bradykinin, another endothelium-dependent dilator, also were smaller in the older animals. Reduced dilatory responses in resistance vessels may be responsible for our observations of impaired systemic arterial EDD with aging, although this cannot be directly shown using the present data. Consistent was this idea, decreased dilation in response to ACh has been observed in skeletal muscle and mesenteric arterioles of F344 rats with aging (14,15,40), as well as in forearm resistance vessels of older humans (7–10,41).
It is possible that these in vivo responses to ACh infusion were influenced by arterial baroreflexes, which act to maintain mean arterial pressure within proper limits. However, the reductions in heart rate to ACh and SNP were greater in older mice, whereas the reductions in blood pressure were smaller or similar in older and young mice in response to ACh and SNP, respectively. This dissociation of events suggests that a baroreflex-related heart rate response cannot explain differences in the blood pressure responses to ACh and SNP in the young and older animals. Importantly, the smaller reduction in blood pressure in response to ACh in the older animals in vivo is consistent with the smaller vasodilatory response in the isolated carotid and femoral arteries of the older animals, whereas both the in vivo blood pressure and isolated artery vasodilatory responses to SNP were similar in the young and older animals. Indeed, the observation that the hypotensive response to intravenous SNP was similar in young and older B6D2F1 mice in the present investigation, as observed previously in F344 rats (30), indicates that differences in arterial baroreflexes do not explain the impaired dilatory response to ACh in the older animals.
Thus, although we recognize that the experiments are not definitive, the results of the in vivo studies are consistent with the idea of age-associated reductions in systemic EDD. This is important because it would be difficult to study these responses in humans given the inherent risks associated with systemic infusions of vasodilators, particularly for older adults.
It should be noted that the arterial pressures we recorded in our conscious animals were dampened to a large degree by the length of tubing that extended from the mouse and the relatively large volume of fluid in the external pressure transducer. Although every effort was made to minimize the length of this fluid column, the dampening of the signal resulted in an attenuation of the pulse pressure to ∼2 to 10 mm Hg compared with pulse pressures of 30–45 mm Hg recorded in anesthetized B6D2F1 mice using an indwelling pressure transducer (24). As a result, the systolic and diastolic pressures recorded likely were not accurate and we, therefore, reported only the arithmetic mean pressures recorded at baseline and during the dose responses. Importantly, we repeated all dose responses three times and found the measured pressures to be highly reproducible between trials. As such, we used the mean values from the three trials.
Aging and EDD: Oxidative Stress and NO Bioavailability
Multiple lines of evidence from ex vivo and in vivo studies in both humans and experimental animals indicate that vascular oxidative stress develops with aging and is responsible for the associated reductions in EDD (5,8,16,34,42,43). We recently demonstrated increased nitrotyrosine, a cellular marker of oxidative stress, in endothelial cells obtained from the brachial artery of older compared with young healthy men (11). The present finding of increased nitrotyrosine in the aortas of older B6D2F1 mice is consistent with these observations in humans. Moreover, administration of antioxidants improves EDD in both conduit arteries and resistance vessels of older humans (5,8). The results of the present study that TEMPOL, a SOD mimetic, restored EDD in isolated conduit arteries and in the systemic arterial circulation of our older animals demonstrate that oxidative stress plays a similar role with aging in B6D2F1 mice. Our results with TEMPOL administration also are consistent with observations from the present study (Figure 4B) and previous investigations (16) that superoxide bioavailability is increased in arteries of older animals, as well as findings from studies using pharmacological inhibition of superoxide and transgenic models (16,44). Taken together, the results of the present study indicate that the B6D2F1 mouse is an appropriate model of oxidative stress–mediated impaired EDD with aging.
Investigations in humans have demonstrated that oxidative stress impairs the dilatory responses to ACh in part via a reduction in NO bioavailability (8). Results of some (43,45), although not all (46), previous investigations in rodents are consistent with these observations in humans. In the present study, our experiments using l-NAME to inhibit NO production indicate that reduced NO bioavailability is a key mechanism by which oxidative stress reduces carotid artery dilation in response to ACh. As such, our findings provide support for the idea that the B6D2F1 mouse is a useful model of oxidative stress–associated reductions in NO bioavailability and EDD with aging.
Aging and EID
In our experiments on isolated carotid and femoral arteries and the systemic arterial circulation, we found that the responses to SNP were similar in young and older B6D2F1 mice. These findings suggest that the impaired dilatory responses to ACh in the older animals were not caused by differences in the sensitivity of vascular smooth muscle cells to NO, but rather to a reduced capacity for endothelium-dependent production or bioavailability of NO. Our results are consistent with findings of preserved dilatory responses to SNP in humans despite age-associated impairments in ACh-mediated EDD (5,9–12,42). Maintained dilatory responses to SNP with aging also have been observed in some (14,16,30), but not all (19), previous studies in rodents. The fact that EID was consistently preserved in our older animals in both our isolated vessel preparations as well as our in vivo model despite reductions in EDD in each of these conditions may represent an advantage of the B6D2F1 mouse.
Aging and Arterial Stiffness
The stiffness of the large elastic arteries in the cardiothoracic circulation, including the carotid artery, increases with aging even in healthy adult humans (47–49). In contrast, peripheral conduit artery stiffness does not increase with aging in humans (47,50). Increased stiffness of large elastic arteries also has been documented ex vivo and in vivo in older mice (51), including older B6D2F1 mice (24). In the present study, we found increased stiffness in carotid, but not femoral, arteries from older B6D2F1 mice. The isolated arteries were studied under conditions of no vascular smooth muscle tone, suggesting that although the medial wall thickness and wall to lumen ratio was unaffected by advancing age, the mechanical properties of the carotid arteries were altered in our older animals. Interestingly, the opposite effects were found in the femoral artery, such that medial wall thickness and the wall to lumen ratio both were increased in older mice, but incremental stiffness remained similar to the young mice. Considered together, previous findings (24) and the present results indicate that the B6D2F1 mouse is an appropriate model of age-associated increases in large elastic artery stiffness in the absence of peripheral artery stiffening.
Physiological and Clinical Significance
We believe that the present findings may have both physiological and clinical implications for arterial aging. Cardiovascular diseases remain a leading cause of death in modern societies, and vascular disorders have a key role in clinical cardiovascular diseases (25). Vascular aging represents an important risk factor for cardiovascular diseases (4,25). Impaired EDD is, in turn, a central feature of vascular aging (25). Recently, there has been widespread use of both wild-type and transgenic mouse models for the study of the mechanisms of cardiovascular diseases. The emerging understanding of the important role of vascular aging in these processes (25,52,53) together with restrictions related to the current use of C57BL/6 mice establish the importance of developing additional mouse models of vascular aging, including impaired EDD. Our findings provide the first systematic support for the B6D2F1 mouse in this context.
CONCLUSIONS
In conclusion, the results of the present study indicate that the B6D2F1 mouse may represent a new and useful model of impaired EDD with aging. Our results show that EDD is reduced in isolated carotid and femoral arteries, as well as in the systemic arterial circulation, of older compared with young adult B6D2F1 mice. Moreover, our findings suggest that older B6D2F1 mice demonstrate vascular oxidative stress associated with increased superoxide production, that this state of oxidative stress causes reductions in EDD, and that reduced NO bioavailability is an important mechanism involved. Finally, our data show that large elastic artery stiffness is increased in older B6D2F1 mice in the absence of increases in peripheral artery stiffness. Overall, our results suggest that the B6D2F1 mouse can be used effectively to study key properties of vascular aging observed in humans.
FUNDING
This work was supported by National Institutes of Health awards AG006537, AG013038, AG022241, AG000279, AG029337, and AG015897.
References
- 1.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 2.Vita JA, Keaney JF., Jr Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 3.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 4.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 5.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egashira K, Inou T, Hirooka Y, et al. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88:77–81. doi: 10.1161/01.cir.88.1.77. [DOI] [PubMed] [Google Scholar]
- 7.Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 8.Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 9.DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 10.DeSouza CA, Clevenger CM, Greiner JJ, et al. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–262. doi: 10.1113/jphysiol.2002.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 12.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 13.Weindruch R, Masoro EJ. Concerns about rodent models for aging research. J Gerontol. 1991;46:B87–B88. doi: 10.1093/geronj/46.3.b87. [DOI] [PubMed] [Google Scholar]
- 14.d'Uscio LV, Smith LA, Katusic ZS. Hypercholesterolemia impairs endothelium-dependent relaxations in common carotid arteries of apolipoprotein e-deficient mice. Stroke. 2001;32:2658–2664. doi: 10.1161/hs1101.097393. [DOI] [PubMed] [Google Scholar]
- 15.Sun D, Huang A, Yan EH, et al. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H2249–H2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown KA, Chu Y, Lund DD, Heistad DD, Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2006;290:H2600–H2605. doi: 10.1152/ajpheart.00676.2005. [DOI] [PubMed] [Google Scholar]
- 17.Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension. 2006;48:1072–1079. doi: 10.1161/01.HYP.0000247302.20559.3a. [DOI] [PubMed] [Google Scholar]
- 18.Wang YX, Halks-Miller M, Vergona R, et al. Increased aortic stiffness assessed by pulse wave velocity in apolipoprotein E-deficient mice. Am J Physiol Heart Circ Physiol. 2000;278:H428–H434. doi: 10.1152/ajpheart.2000.278.2.H428. [DOI] [PubMed] [Google Scholar]
- 19.Blackwell KA, Sorenson JP, Richardson DM, et al. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol. 2004;287:H2448–H2453. doi: 10.1152/ajpheart.00248.2004. [DOI] [PubMed] [Google Scholar]
- 20.Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol. 2007;27:1941–1946. doi: 10.1161/ATVBAHA.107.146852. [DOI] [PubMed] [Google Scholar]
- 21.Sprott RL, Ramirez I. Current inbred and hybrid rat and mouse models for gereontological research. ILAR J. 1997;38:104–109. doi: 10.1093/ilar.38.3.104. [DOI] [PubMed] [Google Scholar]
- 22.Announcing Changes in Availability of the NIA Aged Rodent Colony. Office of Biological Resources and Resource Development; National Institute on Aging; 2006. NOT-AG-06–012. [Google Scholar]
- 23.Miller RA, Austad S, Burke D, et al. Exotic mice as models for aging research: polemic and prospectus. Neurobiol Aging. 1999;20:217–231. doi: 10.1016/s0197-4580(99)00038-x. [DOI] [PubMed] [Google Scholar]
- 24.Reddy AK, Li YH, Pham TT, et al. Measurement of aortic input impedance in mice: effects of age on aortic stiffness. Am J Physiol Heart Circ Physiol. 2003;285:H1464–H1470. doi: 10.1152/ajpheart.00004.2003. [DOI] [PubMed] [Google Scholar]
- 25.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 26.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 27.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Hein TW, Wang W, Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res. 2003;92:322–329. doi: 10.1161/01.res.0000056759.53828.2c. [DOI] [PubMed] [Google Scholar]
- 29.Qamirani E, Ren Y, Kuo L, Hein TW. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005;25:995–1001. doi: 10.1161/01.ATV.0000159890.10526.1e. [DOI] [PubMed] [Google Scholar]
- 30.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, et al. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in humans and experimental animals: part 2: blood pressure measurement in experimental animals: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Arterioscler Thromb Vasc Biol. 2005;25:e22–e33. doi: 10.1161/01.ATV.0000158419.98675.d7. [DOI] [PubMed] [Google Scholar]
- 32.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- 33.Suda O, Smith LA, d'Uscio LV, Peterson TE, Katusic ZS. In vivo expression of recombinant vascular endothelial growth factor in rabbit carotid artery increases production of superoxide anion. Arterioscler Thromb Vasc Biol. 2005;25:506–511. doi: 10.1161/01.ATV.0000153284.81572.f0. [DOI] [PubMed] [Google Scholar]
- 34.Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 35.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 36.Somers MJ, Mavromatis K, Galis ZS, Harrison DG. Vascular superoxide production and vasomotor function in hypertension induced by deoxycorticosterone acetate-salt. Circulation. 2000;101:1722–1728. doi: 10.1161/01.cir.101.14.1722. [DOI] [PubMed] [Google Scholar]
- 37.Sears CE, Choate JK, Paterson DJ. Inhibition of nitric oxide synthase slows heart rate recovery from cholinergic activation. J Appl Physiol. 1998;84:1596–1603. doi: 10.1152/jappl.1998.84.5.1596. [DOI] [PubMed] [Google Scholar]
- 38.Francia P, delli Gatti C, Bachschmid M, et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 39.Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Luscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- 40.Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- 41.Taddei S, Virdis A, Mattei P, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 42.Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Physiol Heart Circ Physiol. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- 44.Tatchum-Talom R, Martin DS. Tempol improves vascular function in the mesenteric vascular bed of senescent rats. Can J Physiol Pharmacol. 2004;82:200–207. doi: 10.1139/y04-010. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan JC, Loomis ED, Collins M, Imig JD, Inscho EW, Pollock JS. Age-related alterations in NOS and oxidative stress in mesentericarteries from male and female rats. J Appl Physiol. 2004;97:1268–1274. doi: 10.1152/japplphysiol.00242.2004. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka H, DeSouza CA, Seals DR. Arterial stiffness and hormone replacement use in healthy postmenopausal women. J Gerontol A Biol Sci Med Sci. 1998;53:M344–M346. doi: 10.1093/gerona/53a.5.m344. [DOI] [PubMed] [Google Scholar]
- 48.Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003;57:861–868. doi: 10.1016/s0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- 49.Moreau KL, Gavin KM, Plum AE, Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension. 2005;45:1107–1112. doi: 10.1161/01.HYP.0000165678.63373.8c. [DOI] [PubMed] [Google Scholar]
- 50.Seals DR, Tanaka H, Clevenger CM, et al. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol. 2001;38:506–513. doi: 10.1016/s0735-1097(01)01348-1. [DOI] [PubMed] [Google Scholar]
- 51.Hartley CJ, Reddy AK, Madala S, Entman ML, Michael LH, Taffet GE. Noninvasive ultrasonic measurement of arterial wall motion in mice. Am J Physiol Heart Circ Physiol. 2004;287:H1426–H1432. doi: 10.1152/ajpheart.01185.2003. [DOI] [PubMed] [Google Scholar]
- 52.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 53.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]