Abstract
Functionally significant stretch-activated ion channels have been clearly identified in excitable cells. Although single-channel studies suggest their expression in other cell types, their activity in the whole-cell configuration has not been shown. This discrepancy makes their physiological significance doubtful and suggests that their mechanical activation is artifactual. Possible roles for these molecules in nonexcitable cells are acute cell-volume regulation and, in epithelial cells, the complex adjustment of ion fluxes across individual cell membranes when the rate of transepithelial transport changes. We report the results of experiments on isolated epithelial cells expressing in the basolateral membrane stretch-activated K+ channels demonstrable by the cell-attached patch-clamp technique. In these cells, reversible whole-cell currents were elicited by both isosmotic and hyposmotic cell swelling. Cation selectivity and block by inorganic agents were the same for single-channel and whole-cell currents, indicating that the same entity underlies single-channel and whole-cell currents and that the single-channel events are not artifactual. In these cells, when the rate of apical-membrane NaCl entry increases, the cell Na+ content and volume also increase, stimulating the Na+,K+-ATPase at the basolateral membrane, i.e., both Na+ extrusion and K+ uptake increase. We speculate that, under these conditions, the parallel activation of basolateral K+ channels (by the swelling) elevates conductive K+ loss, tending to maintain the cell K+ content constant (“pump-leak parallelism”). This study describes a physiologically relevant stretch-activated channel, at both the single-channel and whole-cell levels, in a nonneural cell type.
Acute cell-volume decrease after swelling has been shown to involve the activation of K+ and/or Cl− channels in the plasma membrane of many cell types (including epithelial cells). Activation of one of these channels suffices if there is a sufficiently high native conductance for the other one, allowing the efflux of K+ and Cl− ions, with obligatory osmotic loss of water, thus leading to cell-volume decrease. The mechanisms by which the increase in cell volume is sensed and transduced are unknown. Possibilities include dilution of an intracellular messenger, followed by signal amplification by a signaling cascade, dilution of intracellular macromolecules, thus changing the chemical activity of regulatory protein(s) (“macromolecular crowding”) or direct or indirect mechanical activation by changes in tension of the plasma membrane and perhaps the cytoskeleton. These and other possibilities have been the subjects of insightful and comprehensive recent reviews (1, 2).
Ion channels activated by plasma-membrane stretch have been shown with the on-cell patch-clamp technique in numerous cell types and experimental conditions (for reviews, see refs. 3–6; for examples in epithelial cells, see refs. 7–10). However, the physiological significance of these observations has been questioned because of difficulties in reproducing the current activation in the whole-cell configuration (11). It is possible that in on-cell patches there is decoupling of the cell membrane from the cytoskeleton that causes an artifactual response of the channel proteins in the patch to membrane stretch. In this context, mechanical overstimulation of patches has been shown to either increase or decrease channel mechanosensitivity (12). It has been shown that osmoreceptive hypothalamic neurons express a stretch-inactivated K+ channel (13, 14), and some experimental results support the notion that cell swelling in the whole-cell configuration does indeed result in activation of putative mechanosensitive channels (15). However, there has been no study showing that similar experimental perturbations activate both single-channel and whole-cell currents.
In this study, we compared the effects of membrane stretch in the same cells with both on-cell patches and the whole-cell configuration, by using isolated polarized Necturus gall bladder (NGB) epithelial cells (16, 17), a preparation that allows for easy access to the basolateral membrane. These cells express a basolateral-membrane K+ conductance (GKb) activated by cell swelling and inhibited by cell shrinkage, in parallel with a Cl− conductance inhibited by cell swelling (18). Our aims were to identify biophysically the channel(s) responsible for the swelling-activated GKb and to test whether K+ currents with consistent properties could be elicited in the on-cell and in the whole-cell configurations of the patch-clamp technique by both hyposmotic and isosmotic swelling. Such an elicitation would constitute a demonstration, by both single-channel and whole-cell current measurements, of a stretch-activated channel with physiological significance in a nonneural cell.
MATERIALS AND METHODS
Cell Isolation.
Isolated, polarized NGB epithelial cells were obtained as described (16). Briefly, excised gall bladders were opened longitudinally and pinned serosal side up on Sylgard–coated Petri dishes (Dow-Corning). The cells were dissociated by a 1.5-h incubation at room temperature in physiologic salt solution (PSS; in mM, 90 NaCl/10 Hepes⋅NaOH/2.5 KCl/1.8 CaCl2/1.0 MgCl2/0.5 sodium phosphate/5 glucose, pH 7.60; 195 milliosmol/kg) containing collagenase type IV and protease type XIV (1 mg/ml each; Sigma). After the incubation, the tissue was washed with ice-cold nominally Ca2+- and Mg2+–free PSS containing 0.5 mM EDTA. Then, the cells were resuspended in PSS and exposed to hyaluronidase (1 mg/ml; Sigma) for 5 min. This last step was necessary to attain gigaohm seals. After the exposure to hyaluronidase, the cells were washed twice with PSS, resuspended, and maintained at 4°C until used (within 6 h).
Electrophysiology.
Single, polarized NGB cells were patched on the basolateral membrane. Whole-cell and single-channel currents were recorded with an Axopatch 200A (Axon Instruments, Foster City, CA). Data acquisition and analysis were performed with pclamp 6.0 (Axon Instruments). Single-channel currents were filtered at 1 kHz and acquired at 5 kHz, whereas most whole-cell current traces were filtered at 2 kHz and acquired at 5 kHz. For whole-cell experiments, the pipette solution contained (in mM) 90 KCl, 1.0 EGTA, 1.0 MgCl2, 10 Hepes⋅KOH, 2.0 Na2ATP (pH 7.40; osmolality = ≈190 milliosmol/kg). The bath solution was PSS without phosphate. Increases in cell volume were elicited by a 28% reduction in bath osmolality by NaCl reduction (HYPO medium) relative to isosmotic medium (ISO) or by applying positive pressure to the pipette. For single-channel experiments, the pipette solution contained (in mM) 92.5 KCl, 1.8 CaCl2, 1.0 MgCl2, 10 Hepes, and 7.8 glucose (pH 7.40; osmolality ≈200 milliosmol/kg). The reference electrode was an Ag–AgCl wire in a bath solution/3% agar bridge. The pipettes (borosilicate glass; World Precision Instruments, Sarasota, FL) were pulled with a multistage P-87 Flaming-Brown micropipette puller (Sutter Instruments, San Rafael, CA) and fire polished. All experiments were carried out at room temperature.
Statistics.
Data shown are means ± SEM. Statistical comparisons were done with Student’s t test. Differences were considered significant at P < 0.05.
RESULTS
Stretch-Activated and Swelling-Activated Channels Are Present in the Basolateral Membrane of NGB Cells.
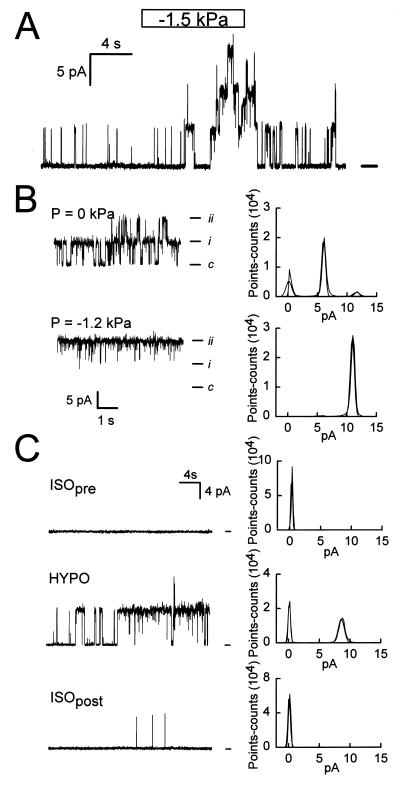
Cell-attached patches of the basolateral-membrane domain of single polarized epithelial cells from NGB were obtained by gentle pipette suction (the suction pressure, applied via a syringe connected to a water manometer, was up to 6 cm H20 = 0.6 kPa). Of 33 seals in which the resistance was at least 5 GΩ, K+ channels were evident in 18. The open probability (Po) of these channels was low under basal conditions (<0.1). Occasionally, Cl− channels were observed as well (data not shown). Records from cell-attached patches are shown in Fig. 1. With symmetric high K+ solutions, the channel is linear and has a conductance of ≈140 pS. In the cell-attached mode, applying suction to the pipette interior caused a rapid increase in current, which reversed when the pressure was returned to 0 (Fig. 1A). Both the increase and the decrease in current levels at the beginning and the end of the pressure pulse, respectively, indicated single-channel events of the same amplitude as those observed in the basal condition. The response depicted could be reproduced several times in the same patch, with pipette pressures as high as 3 kPa (not shown). We conclude that the increase in current elicited by membrane stretch is caused by an increase in Po of channels already active, albeit with a low Po, in the basal state (Fig. 1B). As shown in Fig. 1C, an increase in channel activity also could be obtained in cell-attached patches when cells were exposed to HYPO solution. In contrast with the apical-membrane maxi-K+ channels (19), the basolateral-membrane K+ channels are insensitive to membrane voltage and [Ca2+]i (data not shown).
Figure 1.
Single-channel activity in the basolateral membrane domain of polarized epithelial cells isolated from NGB. (A) Cell-attached patch, Vp (pipette voltage) = 0. There is low-level K+ channel activity under control conditions, with a large increase after rapid application of −1.5 kPa to the pipette interior (syringe connected to water manometer). Note the rapid reversibility of the effect. (B) Changes in steady-state channel activity by −1.2 kPa. (B, Right) Current histograms are depicted; bin = 0.24 pA. (C) Effect of hyposmotic cell swelling (HYPO Δ osmolality = −28%) on K+ channel activity. (C, Right) Current histograms are depicted; bin = 0.20 pA. The bath solution was changed slowly; HYPO data shown were obtained near maximum swelling.
Similar Whole-Cell Currents Are Observed with Hyposmotic and Isosmotic Swelling in Isolated Polarized NGB Cells.
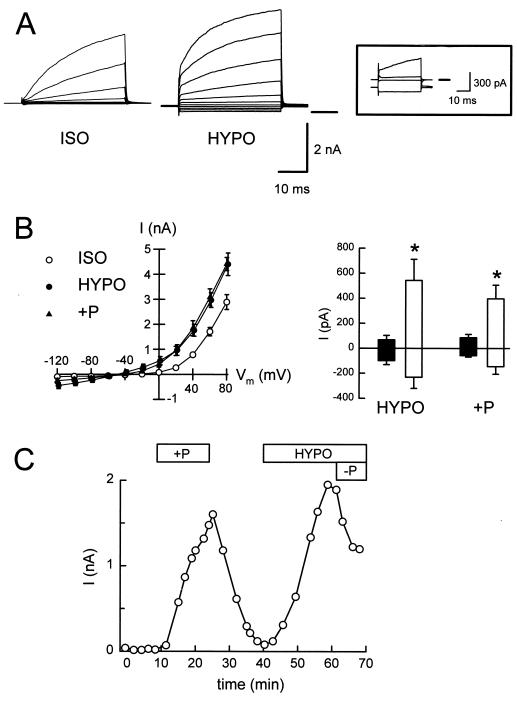
Next, we examined whether the activation by stretch and swelling of single-channels in the basolateral membrane of NGB cells was a real cell response or an artifact caused by patch formation (i.e., membrane decoupling from the cytoskeleton). To test this possibility, we measured whole-cell currents after breaking the cell-attached patches. The relevant currents are IK, measured at ECl [−3 mV in ISO and 4 mV in HYPO solution] and ICl, measured at EK (−88 mV). The electrodiffusive Na+ permeability of both apical and basolateral membranes is negligible (20, 21). From intracellular-microelectrode studies in the assembled epithelium, the K+ current is likely to correspond to both apical-membrane maxi-K+ channels (19) and to basolateral-membrane channels with different properties (17, 22), whereas the increase in K+ conductance by cell swelling is entirely basolateral (18). In the absence of protein kinase A or protein kinase C activation, the Cl− current is exclusively basolateral (23, 24). With a KCl-based pipette solution and a NaCl-based bath solution, whole-cell currents were activated by transient exposure to 28% HYPO solution (hyposmotic swelling) or to pipette pressure increases ranging from 0.5 to 3 kPa (isosmotic swelling). Either maneuver resulted in moderate cell swelling, without membrane detachment or appearance of blebs (assessed by modulation-contrast microscopy at ×400). Typical current records are shown in Fig. 2A. The summaries in Fig. 2B show that the currents have reversal potentials (Er) consistent with K+ selectivity (permeability ratios PNa/PK = 0.18 for hyposmotic and PNa/PK = 0.13 for isosmotic swelling) and that IK increased significantly in both hyposmotic and isosmotic swelling. In contrast, ICl did not change significantly in either condition. The currents depicted in Fig. 2B (Right) correspond to data obtained as those shown in Fig. 2A (Insert). At more positive voltages, the relative increase in IK is smaller because of the supralinear contribution of apical-membrane maxi-K+ channels activated by membrane depolarization (19). Fig. 2C shows that the effect of positive pipette pressure is reversible and that the current activated by hyposmotic swelling is turned off by decreasing the pipette pressure. These results are consistent with the single-channel studies and suggest that the K+ channel is directly or indirectly activated by membrane stretch. The lack of morphological changes denoting membrane disruption and the reversibility of the current activation in the whole-cell configuration rule out the possibility that the channel activation observed in on-cell patches results from membrane decoupling from the cytoskeleton (11, 12).
Figure 2.
Whole-cell currents in isolated polarized NGB epithelial cells. Control solutions were KCl-based (pipette), and NaCl-based (bath), respectively. Increases in cell volume were elicited by a 28% reduction in bath osmolality (NaCl reduction; HYPO) or by applying positive pressure to the pipette (0.5–3 kPa; +P). (A) Whole-cell currents, control, and HYPO. Pulse protocol: 20-mV steps from −120 mV to +80 mV for 40 ms, from a holding potential of −60 mV. Insert depicts control and ISO currents near ECl (0 mV, upward deflections) and near EK (−80 mV, downward traces). In this example, both currents were larger in HYPO. (B, Left) I–V relationships from six experiments. (B, Right) IK (upward bars; measured at ECl) increases significantly (asterisks) in HYPO or +P (open bars), relative to control (filled bars). ICl (downward bars; measured at EK) did not change significantly. (C) Time course of whole-cell currents at Vm = 0 mV (near ECl). Reversible effect of raising the pipette hydrostatic pressure (+P; 1 kPa). The current elicited by HYPO was reversed by decreasing the pipette pressure (−P; −1 kPa).
Swelling-Activated Whole-Cell Currents and Stretch-Activated Single-Channel Currents Have Similar Cationic Selectivity.
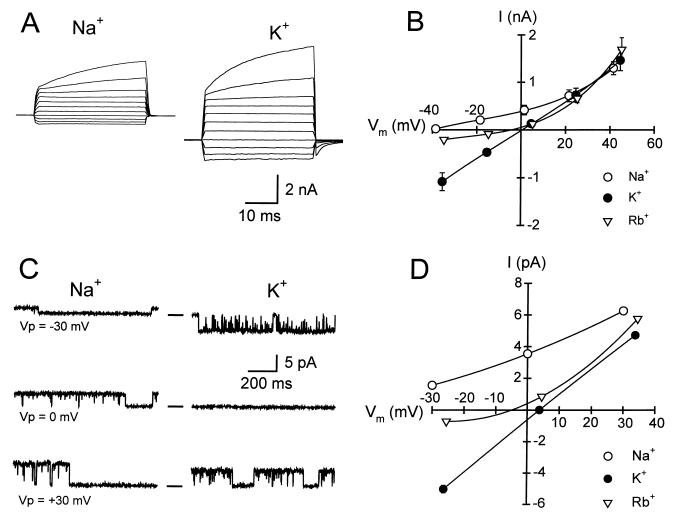
To determine the cationic selectivity of the whole-cell and single-channel currents, external Na+ was replaced mole by mole with K+ or Rb+. Fig. 3A shows typical whole-cell currents activated by hyposmotic swelling with Na+ or K+ in the bath. Fig. 3B shows the I–V plots for the whole-cell current in the presence of the different cations tested. The changes in Er, determined by interpolation, were −23.2 ± 6.1 mV in Rb+ and −41.7 ± 5.2 mV in Na+, compared with K+ bath solution. These results indicate that the channel responsible for the whole-cell swelling-activated current is highly permeable to K+ and has lower permeabilities to Rb+ and Na+: PNa/PK = 0.17 and PRb/PK = 0.39. Similar experiments were performed on patches excised after activating the channels in situ by applying suction to the pipette. Fig. 3C shows single-channel currents with Na+ or K+ in the bath, and Fig. 3D shows the I–V plots for single-channel currents with the same cations as those tested for the whole-cell currents. The changes in Er, determined by interpolation or extrapolation, were −14.7 ± 2.6 mV in Rb+ and −63.9 ± 4.8 mV in Na+, compared with K+ bath solution. These values yield the permeability ratios PNa/PK = 0.05 and PRb/PK = 0.55. Because of the expression of Cl− channels, one would expect a higher degree of ionic selectivity in the single-channel data. Thus, the whole-cell and single-channel reversal potential data are in good agreement.
Figure 3.
Cation selectivity of whole-cell (hyposmotic swelling) and single-channel (stretch) currents in isolated polarized NGB epithelial cells. The pipette solution contained KCl as main salt; the bath’s main salt (NaCl) was substituted with either KCl or RbCl mole-per-mole. (A) Whole-cell current records with NaCl bath and KCl pipette solutions (Na+/K+). Protocol as in Fig. 2. (B) I–V relationships from 13 (Na+/K+), 13 (K+/K+), and 3 (Rb+/K+) experiments. Er, determined by interpolation, changed by −23.2 ± 6.1 mV in Rb+ and −41.7 ± 5.2 mV in Na+, compared with K+ bath solution. (C) Single-channel currents in patches excised after activation of single-channel currents by pipette suction during exposure to NaCl- and KCl-based bath solutions. (D) I–V relationships. Er, determined by interpolation or extrapolation, changed by −14.7 ± 2.6 mV in Rb+ (n = 3) and −63.9 ± 4.8 mV in Na+ (n = 4), compared with K+ bath solution.
Swelling-Activated Whole-Cell Currents and Stretch-Activated Single-Channel Currents Have Similar Blocker Sensitivity.
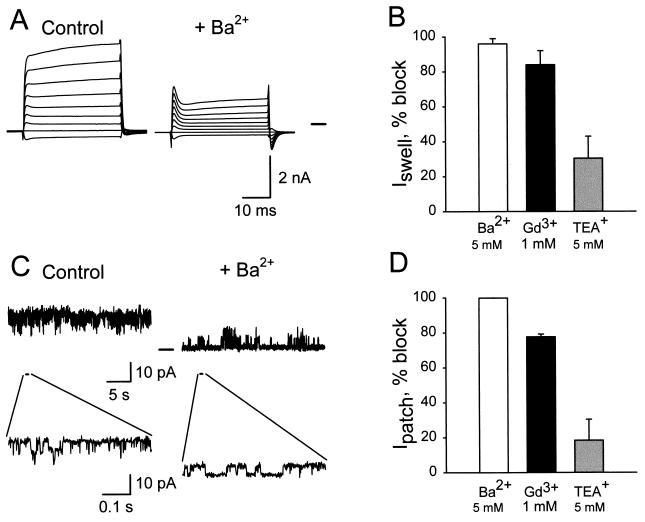
The effects of inorganic K+ channel blockers on whole-cell (Fig. 4 A and B) and single-channel currents (Fig. 4 C and D) also were examined. Exposure to 5 mM Ba2+ or 1 mM Gd3+ inhibited both whole-cell and single-channel currents, whereas there were no significant effect of 5 mM TEA+ or 10 μM Gd3+ (the latter not shown). Again, there was excellent agreement between the whole-cell and single-channel data.
Figure 4.
Effects of K+ channel blockers on whole-cell and single-channel currents. (A) Whole-cell currents after cell swelling by 28% HYPO solution, with NaCl bath and KCl pipette solution (control) and in the presence of 5 mM Ba2+ in the bath (+Ba2+). (B) Percentage of blocking of K+ currents by 5 mM Ba2+ (n = 5), 1 mM Gd3+ (n = 4), or 5 mM TEA+ (n = 4). The control current at ECl was 700 ± 120 pA. The effects of these concentrations of Ba2+ and Gd3+ were statistically significant, whereas there was no effect of TEA+. (C) Single-channel records on outside-out patches at 20 mV, control, and 5 mM Ba2+. Single-channel openings are clearly observed in the expanded traces. (D) Percentage of blocking by 5 mM Ba2+, 1 mM Gd3+, or 5 mM TEA+ (n = 3 experiments). The lowest current level in 5 mM Ba2+ was assumed to be 0. Blocks by 5 mM Ba2+ and 1 mM Gd3+ were statistically significant.
DISCUSSION
Although ion channels activated by plasma-membrane stretch have been demonstrated with the on-cell patch-clamp technique in numerous cell types and experimental conditions, their physiological relevance has been questioned because of difficulties in reproducing the current activation in the whole-cell configuration. We report here a K+ channel that is activated by both stretch and cell swelling on cell-attached patches of isolated, polarized NGB epithelial cells. We conclude that this channel is also responsible for the whole-cell currents elicited by isosmotic or hyposmotic cell swelling, based on the fact that both the ionic selectivity and blocker sensitivity of the stretch-activated channel match those of the whole-cell current elicited by hyposmotic swelling. Moreover, the whole-cell currents activated by either isosmotic or hyposmotic swelling are cation-selective and have similar PNa/PK values. The stretch-activated channels are located on the basolateral membrane. The apical membrane expresses maxi-K+ channels that are gated by membrane depolarization and increases in intracellular [Ca2+] (20), but they are insensitive to cell swelling, as shown by membrane-conductance measurements (18).
Channel activation seems to be independent of Ca2+ entry, because it is observed in cell-attached patches with a nominally Ca2+-free, EGTA-containing pipette solution (not shown). Also, in the epithelium in situ, intracellular [Ca2+] rises transiently after hyposmotic cell swelling, but chelation of intracellular Ca2+ does not inhibit the increase in GKb (18).
The mechanosensitive channel of NGB epithelial cells does not adapt, in contrast with the fast adaptation shown in Xenopus oocytes (25). This difference cannot be attributed to decoupling of the patch from the cytoskeleton in our experiments (see Results). Because the activation of GKb in the epithelium in situ is also monophasic and persistent for the entire duration of the stimulus (18), the lack of adaptation is functionally appropriate, i.e., the channels that remain open contribute to the maintenance of cell homeostasis during changes in solute entry, which can be long-lasting.
There are several mechanisms by which the mechanical force exerted on the plasma membrane could activate mechanosensitive channels. Sachs and Morris (6) empirically define a stretch-activated channel as one whose Po increases when the membrane is stretched. The mechanism of activation could be direct, if tension is conveyed to the channel by stretch of the membrane and/or cytoskeleton, or indirect, mediated by a stretch-sensitive chemical transducer, e.g., intracellular arachidonic acid (26) or released ATP (27). Membrane deformation has been shown to cause ATP release (28, 29), and hence it is conceivable that ATP could activate the K+ channel via purinergic receptors. This mechanism does not operate in our system, because ATP in the pipette did not activate the channels of on-cell patches, although in the same studies suction did (data not shown). However, we cannot rule out the involvement of another chemical transducer such as arachidonic acid.
The detection of cell volume could be related to changes in intracellular macromolecular crowding (30) or in cytosol ionic strength (31). Hyposmotic swelling, produced by reducing the extracellular osmolality, is the result of rapid osmotic water in-flow because of the high osmotic water permeability of both apical and basolateral membranes (32, 33). Concomitant with cell swelling, there are reductions in cell osmolality, ionic strength, and cell solute concentrations. Isosmotic swelling, produced by increasing the pressure of the pipette in the whole-cell configuration, is not accompanied by major changes in internal osmolality or ionic strength, or ion concentrations, but the concentrations of nondiffusible macromolecules decrease. Inasmuch as both isosmotic and hyposmotic swelling had the same effects, channel activation is unlikely to result from changes in intracellular osmolality, ionic strength, or concentrations of the main diffusible ions, but we cannot rule out channel activation by dilution of one or more macromolecules. However, in cell-attached patches, channels could be activated by pipette suction, i.e., without cytosol dilution. Taken together, these results suggest, but do not prove, a mechanical activation process.
We did not attempt to compare the mechanical force exerted on cell-attached patches and on the whole-cell membrane. Quantitative estimations of the changes in membrane tension from mechanical alterations of cell-attached patches and entire cells are, at best, difficult. This difficulty emanates from problems in assessing the exact geometry of the patch (and sometimes the cell) and the mechanical properties of components in series and in parallel with the channels themselves. Currently, there is no appropriate quantitative approach to estimate the change in tension on a channel by deformation of the cell or a membrane patch (for a detailed discussion, see ref. 6). Hence, it would be futile to attempt to apply Laplace’s law to idealized patches or cells. The presence of basolateral-membrane indentations and apical-membrane microvilli (18) prevents even the assessment of the threshold pressure at which “true” membrane stretch starts. Therefore, comparisons of either estimated tensions or current kinetics in on-cell and whole-cell configurations seem to us inappropriate at the present time.
In isolated NGB epithelial cells, activation of stretch-activated K+ channels in cell-attached patches is consistent with whole-cell current activation as well as with stimulation of the GKb in the epithelium in situ. Hence, stretch-activated K+ channels underlie the GKb that is activated by cell swelling in this tissue and perform a specific cell function. We speculate that increased NaCl entry across the apical membrane of NGB epithelial cells has two effects, both mediated by cell swelling. First, stimulation of the basolateral-membrane Na+,K+-ATPase results in increases of both Na+ efflux and K+ influx across the basolateral membrane. Second, activation of the K+ channel in the same membrane raises the electrodiffusive K+ efflux, helping maintain the cell K+ content. Hence, this channel contributes to adjust the rates of basolateral-membrane K+ transport so that uptake (via the Na+,K+-ATPase) and efflux (via the mechanosensitive channels) match each other when the rate of salt entry increases (34). These results show, by measurements of both single-channel and whole-cell currents, a functionally significant stretch-activated channel in a nonneural cell.
Acknowledgments
We thank G. A. Altenberg, O. P. Hamill, and S. A. Weinman for comments on preliminary versions of the manuscript, Kelli Spilker for technical support, and Deborah Robb for secretarial help. This work was supported by National Institutes of Health Grant DK-38734 and a minority supplement awarded to C.G.V.
ABBREVIATIONS
- NGB
Necturus gall bladder
- PSS
physiologic salt solution
- HYPO
hyposmotic medium
- ISO
isosmotic medium
References
- 1.Hallows K R, Knauf P A. In: Principles of Cell Volume Regulation. Strange K, editor. Boca Raton, FL: CRC; 1994. pp. 3–29. [Google Scholar]
- 2.Lang F, Busch G L, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 3.Morris C E. J Membr Biol. 1990;113:93–107. doi: 10.1007/BF01872883. [DOI] [PubMed] [Google Scholar]
- 4.Sackin H. Annu Rev Physiol. 1995;57:333–353. doi: 10.1146/annurev.ph.57.030195.002001. [DOI] [PubMed] [Google Scholar]
- 5.Hamill O P, McBride D W., Jr Pharmacol Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- 6.Sachs F, Morris C E. Rev Physiol Biochem Pharmacol. 1998;132:1–77. doi: 10.1007/BFb0004985. [DOI] [PubMed] [Google Scholar]
- 7.Sackin H. Am J Physiol. 1987;253:F1253–F1262. doi: 10.1152/ajprenal.1987.253.6.F1253. [DOI] [PubMed] [Google Scholar]
- 8.Ubl J, Murer H, Kolb H A. J Membr Biol. 1988;104:223–232. doi: 10.1007/BF01872324. [DOI] [PubMed] [Google Scholar]
- 9.Sackin H. Proc Natl Acad Sci USA. 1989;86:1731–1735. doi: 10.1073/pnas.86.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipovic D, Sackin H. Am J Physiol. 1992;262:F857–F870. doi: 10.1152/ajprenal.1992.262.5.F857. [DOI] [PubMed] [Google Scholar]
- 11.Morris C E, Horn R. Science. 1991;251:1246–1249. doi: 10.1126/science.1706535. [DOI] [PubMed] [Google Scholar]
- 12.Hamill O P, McBride D W. Annu Rev Physiol. 1997;59:621–633. doi: 10.1146/annurev.physiol.59.1.621. [DOI] [PubMed] [Google Scholar]
- 13.Oliet S H R, Bourque C W. Nature (London) 1993;364:341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- 14.Oliet S H R, Bourque C W. Neuron. 1996;16:175–181. doi: 10.1016/s0896-6273(00)80034-3. [DOI] [PubMed] [Google Scholar]
- 15.Cemerikic D, Sackin H. Am J Physiol. 1993;264:F697–F714. doi: 10.1152/ajprenal.1993.264.4.F697. [DOI] [PubMed] [Google Scholar]
- 16.Segal A S, Boulpaep E L, Maunsbach A B. Am J Physiol. 1996;270:C1843–C1863. doi: 10.1152/ajpcell.1996.270.6.C1843. [DOI] [PubMed] [Google Scholar]
- 17.Torres R J, Subramanyam M, Altenberg G A, Reuss L. J Gen Physiol. 1997;109:61–72. doi: 10.1085/jgp.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres R J, Altenberg G A, Copello J A, Zampighi G, Reuss L. Am J Physiol. 1996;270:C1864–C1874. doi: 10.1152/ajpcell.1996.270.6.C1864. [DOI] [PubMed] [Google Scholar]
- 19.Segal Y, Reuss L. J Gen Physiol. 1990;95:791–818. doi: 10.1085/jgp.95.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Os C H, Slegers J G. J Membr Biol. 1975;24:341–363. doi: 10.1007/BF01868631. [DOI] [PubMed] [Google Scholar]
- 21.Reuss L, Finn A L. J Membr Biol. 1975;25:141–161. doi: 10.1007/BF01868572. [DOI] [PubMed] [Google Scholar]
- 22.Copello J, Wehner F, Reuss L. Am J Physiol. 1993;264:C1128–C1136. doi: 10.1152/ajpcell.1993.264.5.C1128. [DOI] [PubMed] [Google Scholar]
- 23.Reuss L. Physiol Rev. 1989;69:503–545. doi: 10.1152/physrev.1989.69.2.503. [DOI] [PubMed] [Google Scholar]
- 24.Heming T A, Copello J, Reuss L. J Gen Physiol. 1994;103:1–18. doi: 10.1085/jgp.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamill O P, McBride D W., Jr Proc Natl Acad Sci USA. 1992;89:7462–7466. doi: 10.1073/pnas.89.16.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert I H. J Membr Biol. 1989;108:165–176. doi: 10.1007/BF01871027. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Roman R, Lidofsky S D, Fitz J G. Proc Natl Acad Sci USA. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grygorczyk R, Hanrahan J W. Am J Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson D R, Kennedy I, Burton T J. J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minton A P, Colclasure G C, Parker J C. Proc Natl Acad Sci USA. 1992;89:10504–10506. doi: 10.1073/pnas.89.21.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon C L, Basavappa S, Strange K. Am J Physiol. 1998;275:C416–C422. doi: 10.1152/ajpcell.1998.275.2.C416. [DOI] [PubMed] [Google Scholar]
- 32.Persson B E, Spring K R. J Gen Physiol. 1982;79:481–505. doi: 10.1085/jgp.79.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotton C U, Weinstein A M, Reuss L. J Gen Physiol. 1989;93:649–679. doi: 10.1085/jgp.93.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz S G. Renal Physiol Biochem. 1994;17:134–137. [PubMed] [Google Scholar]