Abstract
SecA is a multifunctional protein involved in protein translocation in bacteria. The structure of SecA on membrane is dramatically altered compared with that in solution, accompanying with functional changes. We previously reported the formation of a novel ring-like structure of SecA on lipid layers, which may constitute part of the preprotein translocation channel. In the present work, two dimensional crystallization of Escherichia coli SecA on lipid monolayers was performed to reveal the structural details of SecA on lipid layers and to investigate its function. The 2D crystals composed of ring-like structures were obtained by specific interaction between SecA and negatively charged lipid. The 2D projection map and 3D reconstruction from negative stained 2D crystals exhibited a distinct open channel-like structure of SecA, with an outer diameter of 7 nm and an inner diameter of 2 nm, providing the structural evidence for SecA importance in forming the part of the translocation channel. This pore structure is altered after transferring crystals to the SecB solution, indicating that the lipid-specific SecA structure has the SecB binding activity. The strategy developed here provides a promising technique for studying structure of SecA complex with its ligand on membrane.
Keywords: SecA, two-dimensional crystal, electron microscopy, preprotein translocation
The Escherichia coli translocase mediates the translocation of a large number of precursor proteins across the bacteria inner membranes (Wickner and Leonard, 1996). SecA, along with SecYEG and other Sec proteins, are intrinsic components of Escherichia coli translocase (Manting and Driessen, 2000; Wickner and Leonard, 1996). SecA is an obligatory component with multiple functions (de Keyzer et al., 2003). It binds precursor proteins, hydrolyzes ATP, and uses the energy of hydrolysis to translocate proteins across the membrane. To reveal the exact mechanism of SecA functions, structural information is necessary. Several structures of SecA from different species were obtained by X-ray structural analysis. Three forms of SecA from Bacillus subtilis have been reported, and showed different oligomeric packings and structure features(Hunt et al., 2002; Osborne et al., 2004; Zimmer et al., 2006). The SecA structures from Mycobacterium tuberculosis (Sharma et al., 2003) and Thermus thermophilus (Vassylyev et al., 2006) also showed different dimeric packing modes compared with antiparallel dimer of Bacillus subtilis SecA (Hunt et al., 2002). The diversity of SecA structures indicates the complexity of SecA structures, which may be related to the multiple functions of SecA. However, these crystals structures in soluble forms cannot explain the structural changes that take place when SecA interacts with membrane or other Sec proteins in the translocation process, which is more important for understanding SecA function mechanisms.
Although SecA is soluble and lacks any predicted classical transmembrane domains (Schmidt et al., 1988), it plays its important role mainly on or in membrane (Ulbrandt et al., 1992; Economou and Wickner, 1994). SecA interacts with SecB (Crane et al. 2005; Patel et al 2005; Randall et al. 2006) and binds to a membrane through its interaction with SecYEG and acidic phospholipids (Hendrick and Wickner, 1991). It may integrate into the membrane either by itself or together with other Sec proteins (Chen et al., 1996). Up to date, the nature of SecA interaction with membrane, particularly the structural aspects, remains unclear. Recent studies have indicated that SecA exhibits a significant conformational change upon interaction with lipids. Or et al. (2002) and Benach et al. (2003) have reported that SecA dimers dissociated into monomers upon interaction with negatively charged lipids by biochemical assays. Bu et al. (2003) showed that SecA adopted different monomer conformations on liposomes upon interaction with ATP or ADP, as measured by small-angle neutron-scattering study. However, neither method can give the direct structure information of SecA on membrane. The high resolution structure of SecA on membrane is crucial for elucidation of SecA functions in translocation process.
In our previous work (Wang et al., 2003), we found that SecA on negatively charged lipid layers assumed two characteristic forms by electron microscopy and by atomic force microscopic examination: a ring-like structure and a dumbbell-like structure. The dumbbell-like structure resembles the SecA X-ray structure in solution, while the novel ring-like pore structure is totally different. The ring-like structure implied that SecA may play a more important structural role in forming a translocation channel than was previously proposed (Chen et al., 1996). However, the physiological significance of SecA ring-like structure is still in debate (de Keyzer et al., 2003). In the present work, we found SecA ring-like structure can form highly-ordered packings on lipid layers, which provides an ideal system to get the medium or high resolution model for this novel structure form. More importantly, SecA projecting map was altered after SecB binding, which indicates the SecA ring-like structure is an active structure in binding with SecB.
SecA was purified from lysates of Escherichia coli BL21(λDE3)/pT7-SecA as described (Chen et al., 1996). SecB was purified as previously described (Weiss et al., 1988). The same lipid monolayer technique in our previous work (Wang et al., 2003) was used in the current work to obtain the two-dimensional crystal of SecA. Briefly, 16 μl of protein solution with concentration from 20 μg/ml to 200 μg/ml in 10 mM Tris-HCl, pH 7.4 buffer was placed in a small Teflon well (4 mm in diameter and 0.5 mm in depth) till the liquid surface bulged out, and the surface was coated by 0.5-1.0 μl lipid solution (∼1 mg/ml) in chloroform:methanol (3:1, v/v) with syringe. The whole system was then incubated in a sealed humid atmosphere at desired temperature for from 5 minutes up to 48 hours. After incubation, the lipid monolayer was picked up with hydrophobic carbon-coated copper grid, and was then negatively stained with several drops of uranyl acetate solution (1%, w/v). To investigate the SecB effect on SecA structures, after 2D crystals formation, the lipid monolayer with SecA crystals was picked up using a carbon-coated nickel grid, which was transferred to a drop of incubation buffer (10mM Tris-HCl, pH 7.4) with 70 μg/ml SecB. As controls, the crystals were also transfer to buffer without SecB protein. After 0.5 to 2 hours incubation at 4°C, the nickel grid was picked up and negatively stained. The specimens were examined in a Phillips CM120 microscope operated at 100 kV. The low-dose mode was used to take images on Kodak SO-163 films at 49,600 × magnification calibrated by catalase crystals. Images were digitized on Nikon Coolscan 9000ED scanner at a step size of 12.7 μm/pixel. Those images with sharp reflections judged by optical diffraction spectra were analyzed using MRC image processing software (Crowther et al., 1996), including Fourier transformation, indexing, lattice parameters refinement, lattice unbending, amplitude and phase extraction, origin refinement and combination, and lattice line fit. The 3D map was calculated using the CCP4 crystallographic programs, and the surface was displayed by DINO program (http://www.dino3d.org).
SecA ring-like molecules tended to pack together to form 2D crystals on phospholipid monolayers containing negatively charged lipids. Crystals were usually observed on E. coli lipid extract, DOPE/DMPG, DOPC/DMPG, egg PC/DMPS or pure DOPG monolayer, but not on neutral lipid (DOPC) or positively charged lipid (SA/DOPC) monolayer. These results suggest that 2D crystallization of SecA is induced by the specific interaction between protein and negatively charged lipids. Crystal formation was sensitive to the ionic strength. When the crystallization buffer contained 100 mM NaCl or more, the crystals were rarely observed. The crystals could form at a wide range of temperatures but with different stability. At low temperature (lower than 16°C), the crystals were stable and the best crystals were obtained after incubation for 24 hours (Figure 1A). In contrast, at 37°C, the ring-like structures emerged shortly after interaction with lipid (less than 5 min.) and formed low-ordered packings. After 1 hour incubation at 37°C, the ring-like structure disappeared and no crystal could be observed. That implies that at a physiological temperature (37 °C), the ring-like structure alone is not stable, and may need the interaction with other proteins, such as SecYEG, to stabilize it.
Figure 1. 2D crystals of SecA ring-like structures.
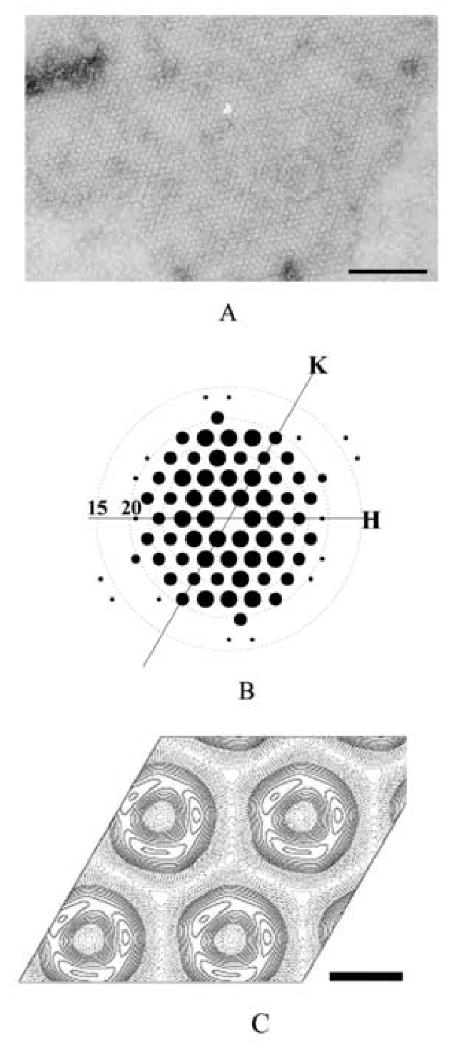
A. The negative stained 2D crystals of SecA formed on E. coli lipid monolayers. The crystallization condition was 10 mM Tris-Ac, pH 7.4, 200 μg/ml SecA, incubating with lipid monolayer composed of E. coli lipid extract for 24 hour at 4°C. The scale bar is 100 nm.
B. Computed Fourier transform of a SecA 2D crystal after two-round unbending. The resolution circles representing 20, 15 Å were indicated. Only spots with IQ less than 4 are displayed, and the size of the spot is inverse proportional to IQ: IQ 1 is largest and IQ 4 is smallest.
C. The projection map of SecA 2D crystals averaged from 10 untilted images without any symmetry imposed. The scale bar is 5 nm.
Electron micrographs of the best negatively stained crystals were diffracted to 2.5 nm. After unbending, the diffraction spots with intensity quality (IQ) less than 4 covered 95% of all possible reflections up to a resolution of 2.0 nm (Figure 1B). The diffraction pattern suggested a hexagonal arrangement. The space group of the crystal was determined to be p3 two-side layer group by the ALLSPACE program (Valpuesta et al., 1994) (Table 1). The lattice parameters were determined to be a =b=9.1 nm, γ=120°. Ten untilted images of negatively stained 2D crystals were analyzed and combined together to generate a data set with averaged reflections up to 2nm resolution. The averaged projection maps without any symmetry imposed were shown in Figure 1C. The basic unit of 2D crystal is a ring-like structure with an outer diameter of 7 nm and an inner diameter of 2 nm. One ring-like structure has three high-density domains in the projection map. One high density domain has the oval shape with the dimension 3 nm by 5 nm, which is much smaller than SecA monomer, so the p3 symmetry cannot come from SecA trimeric packing. The “apparent symmetry” in the crystal could derive from the three similar sized intrinsic domains in SecA molecules due to low resolution, which is usually observed in low resolution analysis on negative stained 2D crystal and will disappear after increasing the resolution (Wang et al. 2002).
Table1.
The symmetry analysis of SecA 2D crystal and SecA 2D crystal with SecB
| Original crystal | Crystal with SecB | |||
|---|---|---|---|---|
| Layer group | Phase resid.(No) v. other spots (90° random) | Phase resid.(No) v. other spots (90° random) | ||
| p1 | 20.1 | 68 | 22.7 | 58 |
| p2 | 49.1 | 34 | 42.5′ | 29 |
| p12_b | 59.4 | 20 | 54.4 | 16 |
| p12_a | 59.1 | 19 | 64.7 | 17 |
| p121_b | 60.3 | 20 | 55.3 | 16 |
| p121_a | 41.7 | 19 | 47.7 | 17 |
| c12_b | 59.4 | 20 | 54.4 | 16 |
| c12_a | 59.1 | 19 | 64.7 | 17 |
| p222 | 66.3 | 73 | 72.9 | 62 |
| p2221b | 52.6 | 73 | 50.3 | 62 |
| p2221a | 57 | 73 | 52 | 62 |
| p22121 | 66.2 | 73 | 57.3 | 62 |
| c222 | 66.3 | 73 | 72.9 | 62 |
| p4 | 63.1 | 78 | 62.9 | 65 |
| p422 | 62.3 | 171 | 66.6 | 140 |
| p4212 | 63.3 | 171 | 59.4 | 140 |
| p3 | 10.3* | 56 | 36 | 46 |
| p312 | 19.1* | 130 | 45.2 | 110 |
| p321 | 20.8* | 135 | 40.2 | 113 |
| p6 | 29.9′ | 146 | 42.4 | 121 |
| p622 | 28.6′ | 299 | 45.1 | 252 |
Probable
Acceptable
In order to better understand the overall architecture of SecA ring-like structure, 3D reconstruction was carried out from 49 images of crystals tilted -61 to 61°. The dataset contained three serials with a total of 26 images and 23 individual high tilt angle images (> 50°). Only reflections showing an intensity quality of 4 or better were used in the analysis. The extracted amplitude and phase data were then combined cumulatively from low tilt angle to high tilt angle without any symmetry imposed. The average phase error, based on the refinement of each new measurement along with all previously accumulated phases was 25°. Total 501 Fourier terms from 51 independent lattice lines were collected and used for 3D reconstruction. The surface representation shows a channel-like cylinder structure with the height of 5.5 nm (Figure 2). The characteristic feature is a 2 nm open cavity in the center. The enclosed volume contains molecular mass of about 200 kDa (assuming the protein density of 1.33g/cm3), confirming that the ring-like structure is a dimer of SecA. The low-resolution 3D model of SecA on membrane provides direct evidence that SecA alone may form preprotein translocation channel on membrane. The X-ray structure of an archaeal SecYEβ revealed a funnel-like cavity with a diameter of 20-25 Å at the cytoplasmic side (van den Berg, 2004), which is indicated to be the translocation channel. The pore size of SecYEβ is highly consistent with the SecA pore size reported here. Different from the closed channel structure of SecYEβ, the SecA channel is an open structure. We have found two forms of SecA on membrane: dumbbell-like structure and ring-like structure (Wang et al., 2003). The dumbbell-like structure is similar as the X-ray structure (Hunt et al., 2002) while the ring-like structure is totally different from the solution structure. Dramatic conformational change and subunit interaction change must happen to form the compact the channel-like structure. However, the current resolution is too low to reveal more information on monomer organization and structural changes compared with solution structure. The negative stained crystals can reach to 2.0 nm resolution, indicating the crystals are highly ordered. It is suitable for future cryo-EM study to get medium resolution structure, which is in progress.
Figure 2. 3D map of SecA ring-like structure.
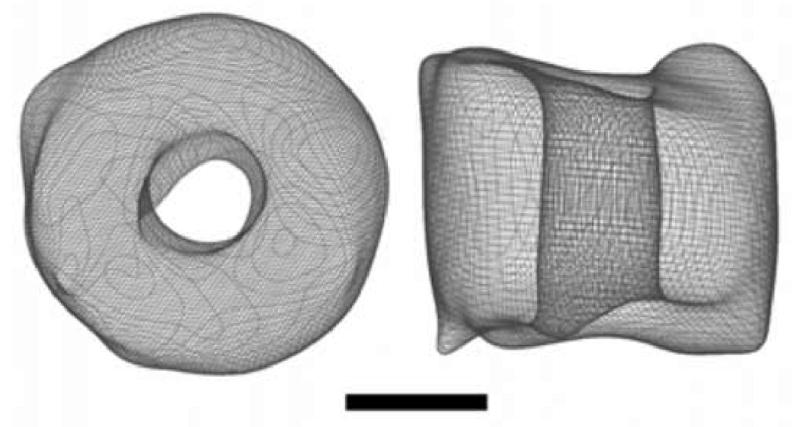
The left one is top view perpendicular to the membrane. The right one is side view. The 3D reconstruction resolution is 2 nm and from totally 59 images. The bar is 2.5 nm.
To determine whether the SecA ring-like structure is an active structure or not, we tested its ability to bind SecB protein as the test substrate, and examined the effect of SecB on SecA crystal structure. We transferred the pre-formed SecA 2D crystals to buffer with or without SecB protein, and examine the SecB effect on the crystals. Although during transfer process, some crystals showed some degrees of defects and distortions, the 2D crystals were readily observed and remained same with respect to the unit cell parameters. The resolution is slightly decreased compared with original crystals, and the best crystal can reach to 2.3nm after unbending. SecA crystals after incubation with buffer didn't show the symmetry change. It still showed strong p3 symmetry. However, after incubation with SecB, the crystals exhibited a considerable change in the crystal symmetry. Table 1 showed that the original p3 symmetry of the 2D crystals is lost after incubating with SecB. We calculated the averaged projection map from three images of SecA/SecB crystals without any symmetry imposed (Fig.3A). There were four high-density domains in the projection map. Compared with Figure 1C, the major difference between the projection maps with or without SecB was that one domain was split into two sub-domains and became slightly larger (as indicated by an arrow in Figure 3B). Based on the difference, we proposed that the SecA ring-like structure had one SecB binding site available towards solution (Figure 3C), and was active in binding with SecB. It is not yet clear whether the interaction site observed here corresponds to any of the three SecA-SecB interaction sites (Crane et al., 2005; Patel et al. 2006; Randall et al. 2005).
Figure 3. Effect of SecB on the projection map of SecA 2D crystals.
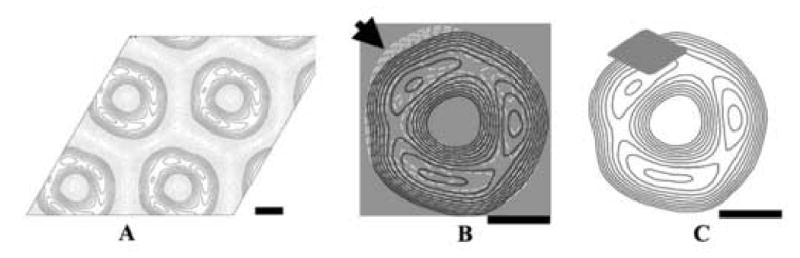
A. The projection map of SecA 2D crystals with SecB, averaged from 3 images without symmetry imposed. The scale bar is 2.5 nm.
B. Merging of the original SecA crystal structure with SecA/SecB crystal structure. The white dashed contour is the projection map of SecA/SecB. The dark solid contour is the projection map of SecA alone. The projection maps were rotated and translated according to the cross-correlation calculation, and then merged together. The main different domain is indicated by dark arrow. The scale bar is 2.5 nm.
C. The putative diagram of SecB binding to SecA ring-like structure. SecB is indicated as a grey rhombus. The scale bar is 2.5 nm.
In our previous work, we used electron microscopy and AFM to investigate the SecA structure on membrane, and showed the novel ring-like structure formation. In the present work, we obtained the 3D model for the SecA ring-like structures on membrane by electron crystallography. The SecA is an open channel with the center pore of 2 nm. This structure feature indicates that SecA can form the preprotein translocation channel or part of the translocation channel. Moreover, the pore structure is active in binding with SecB, indicating that it could also be involved in the recognition of SecB-preprotein complex in the targeting step. The possible multifunction of the SecA structure emphasizes the importance of SecA pore structure in protein translocation process in bacteria. The high resolution structure elucidation will help to better understand the nature and functions of SecA pore structures. The present 2D crystals may provide an ideal system for future structural analysis of SecA and SecA/SecB complex on the membrane.
Acknowledgments
We thank D. Oliver for providing the SecA-overexpressing strain; A. J. Drissen for providing SecB-overexpressing strain;. Jiang for maintenance of the EM and B. R. Baumstark for editing the manuscript. This work was supported by the research grants from the National Nature Science Foundation of China and the National Basic Research Program of China (2004 CB720005) (S. F. S. and Y. C.) and an NIH grant, GM 34766 (P.C. T).
Abbreviations
- EM
electron microscope
- 2D crystal
two-dimensional crystal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benach J, Chou YT, Fak JJ, Itkin A, Nicolae DD, Smith PC, Wittrock G, Floyd DL, Golsaz CM, Gierasch LM, Hunt JF. Phospholipid-induced monomerization and signal-peptide-induced oligomerization of SecA. J Biol Chem. 2003;278:3628–3638. doi: 10.1074/jbc.M205992200. [DOI] [PubMed] [Google Scholar]
- 2.Bu Z, Wang L, Kendall DA. Nucleotide binding induces changes in the oligomeric state and conformation of Sec A in a lipid environment: a small-angle neutron-scattering study. J Mol Biol. 2003;332:23–30. doi: 10.1016/s0022-2836(03)00840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X, Xu H, Tai PC. A significant fraction of functional SecA is permanently embedded in the membrane. SecA cycling on and off the membrane is not essential during protein translocation. J Biol Chem. 1996;271:29698–29706. doi: 10.1074/jbc.271.47.29698. [DOI] [PubMed] [Google Scholar]
- 4.Crane JM, Mao C, Lilly AA, Smith VF, Suo Y, Hubbel WL, Randall LL. Mapping of the docking of SecA onto the chaperone SecB by site-directed spin labeling: insight into the mechanism of ligand transfer during protein export. J Mol Bio. 2005;353:295–307. doi: 10.1016/j.jmb.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 5.Crowther RA, Henderson R, Smith JM. MRC image processing programs. J Struct Biol. 1996;116:9–16. doi: 10.1006/jsbi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 6.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 7.Hanein D, Matlack KE, Jungnickel B, Plath K, Kalies KU, Miller KR, Rapoport TA, Akey CW. Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell. 1996;87:721–732. doi: 10.1016/s0092-8674(00)81391-4. [DOI] [PubMed] [Google Scholar]
- 8.Hendrick JP, Wickner W. SecA protein needs both acidic phospholipids and SecY/E protein for functional high-affinity binding to the Escherichia coli plasma membrane. J Biol Chem. 1991;266:24596–24600. [PubMed] [Google Scholar]
- 9.Hunt JF, Weinkauf S, Henry L, Fak JJ, McNicholas P, Oliver DB, Deisenhofer J. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science. 2002;297:2018–2026. doi: 10.1126/science.1074424. [DOI] [PubMed] [Google Scholar]
- 10.de Keyzer J, van der Does C, Driessen AJ. The bacterial translocase: a dynamic protein channel complex. Cell Mol Life Sci. 2003;60:2034–2052. doi: 10.1007/s00018-003-3006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manting EH, Driessen AJ. Escherichia coli translocase: the unravelling of a molecular machine. Mol Microbiol. 2000;37:226–238. doi: 10.1046/j.1365-2958.2000.01980.x. [DOI] [PubMed] [Google Scholar]
- 12.Meyer TH, Menetret JF, Breitling R, Miller KR, Akey CW, Rapoport TA. The bacterial SecY/E translocation complex forms channel-like structures similar to those of the eukaryotic Sec61p complex. J Mol Biol. 1999;285:1789–1800. doi: 10.1006/jmbi.1998.2413. [DOI] [PubMed] [Google Scholar]
- 13.Or E, Navon A, Rapoport T. Dissociation of the dimeric SecA ATPase during protein translocation across the bacterial membrane. EMBO J. 2002;21:4470–4479. doi: 10.1093/emboj/cdf471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborne AR, Clemons WM, Jr, Rapoport TA. A large conformational change of the translocation ATPase SecA. Proc Natl Acad Sci U S A. 2004;101:10937–10942. doi: 10.1073/pnas.0401742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel CN, Smiht VF, Randall LL. Characterization of three areas of interactions stabilizing compleses between SecA and SecB, two proteins involved in protein export. Protein Sci. 2006;15:1379–1386. doi: 10.1110/ps.062141006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randall LL, Crane JM, Lilly AA, Liu G, Mao C, Patel CN, Hardy SJS. Asymmetric binding between SecA and SecB two symmetric proteins: Implication for function in export. J Mol Bio. 2005;348:479–489. doi: 10.1016/j.jmb.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt MG, Rollo EE, Grodberg J, Oliver DB. Nucleotide sequence of the secA gene and secA(Ts) mutations preventing protein export in Escherichia coli. J Bacteriol. 1988;170:3404–3414. doi: 10.1128/jb.170.8.3404-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma V, Arockiasamy A, Ronning DR, Savva CG, Holzenburg A, Braunstein M, Jacobs WR, Jr, Sacchettini JC. Crystal structure of Mycobacterium tuberculosis SecA, a preprotein translocating ATPase. Proc Natl Acad Sci U S A. 2003;100:2243–2248. doi: 10.1073/pnas.0538077100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulbrandt ND, London E, Oliver DB. Deep penetration of a portion of Escherichia coli SecA protein into model membranes is promoted by anionic phospholipids and by partial unfolding. J Biol Chem. 1992;267:15184–92. [PubMed] [Google Scholar]
- 20.Valpuesta JM, Carrascosa JL, Henderson R. Analysis of electron microscope images and electron diffraction patterns of thin crystals of phi 29 connectors in ice. J Mol Biol. 1994;240:281–287. doi: 10.1006/jmbi.1994.1445. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg B, Clemons WM, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 22.Vassylyev DG, Mori H, Vassylyeva MN, Tsukazaki T, Kimura Y, Tahirov TH, Ito K. Crystal Structure of the Translocation ATPase SecA from Thermus thermophilus Reveals a Parallel, Head-to-Head Dimer. J Mol Biol. 2006 doi: 10.1016/j.jmb.2006.09.061. doi:10.1016/j.jmb.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 23.Wang HW, Chen Y, Yang H, Chen X, Duan MX, Tai PC, Sui SF. Ring-like pore structures of SecA: implication for bacterial protein-conducting channels. Proc Natl Acad Sci U S A. 2003;100:4221–4226. doi: 10.1073/pnas.0737415100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HW, Sui SF. Two-Dimensional Assembly of Pentameric Rabbit C-Reactive Proteins on Lipid Monolayers. J Struct Biol. 2002;134:46–55. doi: 10.1006/jsbi.2001.4364. [DOI] [PubMed] [Google Scholar]
- 25.Weiss JB, Ray PH, Bassford PJ., Jr Purified secB protein of Escherichia coli retards folding and promotes membrane translocation of the maltose-binding protein in vitro. Proc Natl Acad Sci U S A. 1988;85:8978–8982. doi: 10.1073/pnas.85.23.8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickner W, Leonard MR. Escherichia coli preprotein translocase. J Biol Chem. 1996;271:29514–29516. doi: 10.1074/jbc.271.47.29514. [DOI] [PubMed] [Google Scholar]
- 27.Zimmer J, Li W, Rapoport TA. A Novel Dimer Interface and Conformational Changes Revealed by an X-ray Structure of B. subtilis SecA. J Mol Biol. 2006 doi: 10.1016/j.jmb.2006.08.044. doi:10.1016/j.jmb.2006.08.044. [DOI] [PubMed] [Google Scholar]


