Summary
The mechanism by which sphingosine-1-phosphate receptor-1 (S1P1) acts to promote lymphocyte egress from lymphoid organs is not defined. Here we showed that CCR7-deficient T cells left lymph nodes more rapidly than wild-type cells whereas CCR7 overexpressing cells were retained for longer. After treatment with FTY720, an agonist that causes down-modulation of lymphocyte S1P1, CCR7-deficient T cells were less effectively retained than wild-type T cells. Moreover, treatment with pertussis toxin to inactivate signaling via Gαi-protein coupled receptors restored egress competence to S1P1 deficient lymphocytes. We also found that T cell accumulation in lymph node cortical sinusoids required intrinsic S1P1 expression and was antagonized by CCR7. These findings suggest a model where S1P1 acts in the lymphocyte to promote lymph node egress by overcoming retention signals mediated by CCR7 and additional Gαi-coupled receptors. Furthermore, by simultaneously upregulating S1P1 and downregulating CCR7, T cells that have divided multiple times switch to a state favoring egress over retention.
Introduction
Lymphocyte egress from lymphoid organs is essential for immunsurveillance and for lymphocyte effector function. After entry into lymphoid tissues, lymphocytes spend several hours to a day migrating extensively within the tissue prior to exiting and returning to circulation (Cyster, 2005). Lymphocyte entry into lymph nodes occurs via high endothelial venules (HEVs) and requires the concerted action of selectins, chemokines and integrins (von Andrian and Mempel, 2003). The principal chemokine receptor involved in entry, CCR7, is highly expressed on T cells and the ligand, CCL21, is abundant on HEV and is present on stromal cells throughout the T zone. The second CCR7 ligand, CCL19, is also expressed by T zone stromal cells (Cyster, 2005). Recent work has shown that CCR7 and CCR7 ligands are not only involved in cell entry to lymphoid tissues but they also promote T cell motility within the T zone (Huang et al., 2007; Okada and Cyster, 2007; Worbs et al., 2007).
In contrast to the established cascade of molecular interactions required for cell entry from blood into tissues, the multistep requirements for egress have not been well defined. Egress occurs from lymph nodes and Peyer's patches into efferent lymphatics and from spleen into blood (Cyster, 2005). The exact site of egress in each of these tissues is not firmly established although egress from lymph nodes is typically thought to occur at the medullary sinuses that connect to the subcapsular space and the efferent lymphatic (Sanna et al., 2006; Wei et al., 2005). An additional sinus structure that begins in the T zone and joins with the medullary sinuses, termed the cortical sinusoid, was also suggested based on ultrastructural studies to be involved in egress (Kelly, 1975; Soderstrom and Stenstrom, 1969). However, the role of cortical sinusoids in egress has not been further explored.
Important insight into molecular requirements for lymphocyte egress came from the finding that the immunosuppressant molecule, FTY720, inhibited egress (reviewed in (Cyster, 2005; Rosen et al., 2007)). After in vivo treatment, FTY720 becomes phosphorylated and the phospho-form is an agonist on four of the five sphingosine-1-phosphate (S1P) receptors including S1P receptor-1 (S1P1), a receptor that signals via Gαi-containing heterotrimeric G-proteins (Rosen et al., 2007). Genetic studies have established that S1P1 is needed in the lymphocyte for normal egress to occur from thymus and peripheral lymphoid tissues (Allende et al., 2004; Matloubian et al., 2004). Recent work has shown that S1P is required for induction of egress (Pappu et al., 2007) and treatments that cause elevations in lymphoid tissue S1P abundance disrupt egress (Schwab et al., 2005). Despite these advances, the mechanism by which S1P1 acts in the lymphocyte to promote egress has been unclear.
In earlier work, we suggested that FTY720 acts to inhibit egress by downregulating and functionally antagonizing lymphocyte S1P1 (Matloubian et al., 2004). However, engagement of endothelial S1P1 can increase tight junction formation and it has been proposed that FTY720 may instead inhibit egress by closing egress portals (Sanna et al., 2006; Wei et al., 2005). Thus, the mechanism by which FTY720 and other S1P1 agonists inhibit lymphocyte egress is still debated (Rosen et al., 2007).
Two non-mutually exclusive models can be considered for how lymphocyte S1P1 promotes egress: (1) it functions to overcome signals that retain cells in the lymphoid tissue; (2) it transmits unique signals that promote migration or transmigration into exit structures. Here we have performed experiments that test the first of these models. We demonstrate that CCR7 and additional Gαi-coupled systems promoted T cell retention within lymphoid organs and that lymphocyte S1P1 functioned to overcome these retention signals. Inhibition of lymphocyte Gαi overcame FTY720 mediated retention, providing further evidence that this drug inhibits egress by acting on the lymphocyte. Lymph node cortical sinusoids were identified as a site where S1P1 acted during lymphocyte egress from lymph nodes. Together these studies provide evidence for a model where S1P1 functions to promote egress at least in part by overcoming signals that retain cells in the lymph node.
Results
CCR7 promotes T cell retention in lymph nodes
To test whether CCR7 can mediate T cell retention in lymph nodes, CCR7 deficient and wild-type splenocytes were co-transferred into wild-type recipient mice and equilibrated for one day. Circulating lymphocytes were then inhibited from further entry into lymph nodes by treating the mice with integrin-neutralizing antibodies (‘entry blockade’), and co-transferred cells were enumerated either at the treatment time (t = 0 h) or eight hours later (t = 8 h). Previous studies have shown that αL- and α4-containing integrins are not required for lymphocyte egress (Arnold et al., 2004; Lo et al., 2005) and treatment with these antibodies achieves a more complete blockade of entry into mucosal lymph nodes than is achieved by loss of CD62L function (Arbones et al., 1994; Berlin-Rufenach et al., 1999). CCR7 deficiency resulted in more rapid exit of T cells from mesenteric and peripheral lymph nodes relative to the co-transferred control T cells (Fig. 1A). B cell exit efficiencies were not affected by CCR7 deficiency (Suppl. Fig. 1). As another approach to examine the exit efficiency of CCR7-deficient cells, we generated mixed bone marrow chimeras and measured the frequency of wild-type and CCR7-deficient cells in lymph versus lymph nodes. In mice reconstituted with a mixture of wild-type CD45.1 and wild-type CD45.2 cells, the frequency of the two types of cells in lymph nodes and in lymph were similar (Fig. 1B). In contrast, in mice reconstituted with a mixture of wild-type CD45.1 cells and Ccr7-/- CD45.2 cells, there was an enrichment of Ccr7-/- cells in lymph compared to lymph nodes (Fig. 1B). The frequency of CD45.1 and CD45.2 B cells in lymph and lymph nodes was similar for both groups (Suppl. Fig. 1). These observations suggest that CCR7-deficient T cells exit into lymph more rapidly than wild-type T cells.
Figure 1. CCR7 affects T cell egress rates from lymph nodes.

(A) Wild-type and Ccr7-/- spleen cells were co-transferred to wild-type recipients and after 24 h, the mice were treated with integrin-blocking antibodies for 8 h. The data shown indicate the fraction of T cells remaining in recipient mesenteric (m) and peripheral (p) lymph nodes at 8 h compared to 0 hr. (B) Irradiated mice were reconstituted using mixtures of CD45.2+ Ccr7+/+ or Ccr7-/- bone marrow and CD45.1+ WT bone marrow and the representation of CD45.2+ cells in peripheral and mesenteric lymph nodes (LN) and in lymph (LYM) was determined. (C) Flow cytometric analysis of S1P1 on Ccr7-/- and Ccr7+/+ CD4 T cells from lymph nodes of mixed-bone marrow chimeras (left panel) and Ccr7+/- (middle panel) or Ccr7-Tg (right panel) CD4+ T cells together with co-transferred control cells. Shaded histograms show staining with control antibody. (D) Flow cytometric data showing CCR7 expression using anti-CCR7 mAb on Ccr7+/- and littermate control (upper panel) or Ccr7-Tg and littermate control (lower panel) lymph node CD4+ T cells. Shaded histograms show staining of Ccr7-/- cells. (E and F) Transfers and integrin blockade were performed as in A. In E the fraction of cells remaining after 14 h in the indicated lymph nodes was determined. F shows the ratio of transferred Ccr7+/- and Ccr7+/+ T cells in peripheral and mesenteric lymph nodes (LN) and in lymph (LYM) at 0 h. (G) Wild-type control and Ccr7-Tg cells were transferred to wild-type recipients and the mice were treated as in A. The data shown indicate the fraction of T cells remaining in the indicated lymph nodes at 12 h compared to 0 h of entry blockade. In A-B and E-G, bars indicate mean and points indicate data from individual mice. (H) Distribution of WT, Ccr7-/-, Ccr7+/- and Ccr7-Tg cells within peripheral lymph nodes. CFSE labeled T cells were transferred into wild-type recipients and one day later lymph node sections were stained to detect transferred cells (blue) and LYVE-1+ sinusoids (brown). Objective magnification, 5×.
Analysis of Ccr7-/- T cell distribution in lymph node sections revealed that the cells were mostly located near HEV and areas positive for the lymphatic marker, LYVE-1, and they were not distributed in the deeper T zone (Fig. 1H) as expected (Förster et al., 1999). Interestingly, the mean S1P1 surface expression was lower on CCR7-deficient T cells than on control T cells present in the same lymph nodes, although some of the CCR7-deficient cells did express normal amounts of the receptor (Fig. 1C). It seemed likely that the lower expression reflected increased exposure of the mispositioned cells to S1P because lymphocyte S1P1 is highly sensitive to down-modulation by S1P (Lo et al., 2005; Schwab et al., 2005).
In an attempt to distinguish whether CCR7-deficiency led to more rapid egress because the T cells were more poised to respond to exit promoting signals or because of their inability to distribute through the T zone and away from possible exit sites, we performed two further types of experiments. First, we asked whether Ccr7+/- T cells showed evidence of more rapid egress. Flow cytometric analysis confirmed that Ccr7+/- T cells have about half the normal amount of CCR7 (Fig. 1D). Using the adoptive transfer followed by entry blockade procedure, Ccr7+/- cells showed a trend toward more rapid egress compared to co-transferred wild-type control cells at 8 hours (not shown) and a significant difference after 14 hours (Fig. 1E). Flow cytometric analysis of lymph and lymph node cells from recipient mice that had not been antibody treated showed a greater frequency of Ccr7+/- cells in lymph compared to lymph nodes (Fig. 1F). However, in contrast to the Ccr7-/- cells, Ccr7+/- cells were distributed throughout the lymph node T zone (Fig. 1H) and they had normal amounts of surface S1P1 (Fig. 1C).
In the second type of experiment, we asked whether over-expression of CCR7 could lead to increased retention of cells in lymph nodes. Circulating peripheral T cells from transgenic mice over-expressing CCR7 under the control of a CD4 minigene (Kwan and Killeen, 2004) have ∼2 fold more CCR7 on their surface (Fig. 1D). Transgenic T cells were co-transferred with control T cells into wild-type mice, equilibrated for one day and further cell entry into lymph nodes was inhibited using integrin neutralizing antibodies. Co-transferred cells were enumerated at the time of entry blockade (t = 0 h) and twelve hours later (t = 12 h). A greater fraction of the CCR7 transgenic T cells were retained in mesenteric and peripheral lymph nodes compared to co-transferred control cells over the 12 hours (Fig. 1G). In contrast to CCR7-deficient T cells, the transgenic T cells were distributed throughout the T zone (Fig. 1H) and they exhibited normal surface S1P1 levels (Fig. 1C). Taken together these results indicate that CCR7 promotes T cell retention in lymph nodes and they suggest the involvement of mechanisms other than regulation of gross distribution within the T zone.
CCR7 deficiency promotes T cell egress in FTY720 treated hosts
To examine the possibility that S1P1 acts to promote lymphocyte egress by overcoming lymphoid tissue retention signals, we set out to test whether CCR7 deficiency reduced the dependence of T cells on S1P1 for undergoing egress. As a component of these studies, we further examined the relationship between FTY720 treatment and modulation of lymphocyte S1P1 function. We treated mice for the same short (4-5 hr) time period and with doses corresponding to the range previously used to define the IC50 for egress inhibition by the active FTY720 enantiomer (Rosen et al., 2003). Comparison of the effect of FTY720 on T cell S1P1 surface expression and on cell numbers in lymph and blood showed that there was a strong positive correlation (Fig. 2A, B). Notably, FTY720 at 0.05mg/kg caused approximately 50% S1P1 down-modulation, achieving amounts similar to cells with heterozygous deficiency in Edg1, the gene encoding S1P1 (Fig. 2C), and the treatment led to a similar decrease in T cell numbers in lymph and blood to the reductions observed in Edg1+/- mice (Fig. 2B and D). These findings provide support for the conclusion that FTY720-induced down-modulation of lymphocyte S1P1 contributes to the mechanism by which this drug reduces lymphocyte egress.
Figure 2. Dose sensitivity of FTY720-mediated S1P1 modulation and induction of lymphopenia.
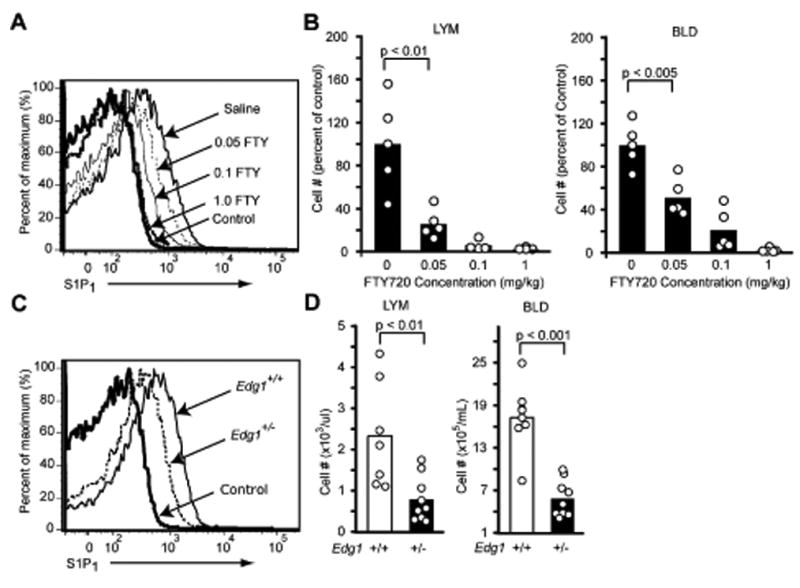
(A) FACS analysis of S1P1 on lymph node CD4+ T cells 4.5 h after in vivo treatment with the indicated amounts of FTY720. (B) Fraction of CD4+ T cells remaining in blood (BLD) and lymph (LYM) 4.5 h after treatment with the indicated amounts of FTY720 compared to saline treated controls. (C) FACS analysis of S1P1 on wild-type littermate control and Edg1+/- lymph node CD4+ T cells. (D) Number of CD4+ T cells in blood and lymph of wild-type and Edg1+/- mice. Data are representative of at least 3 mice for each condition. Bars represent means and dots individual mice.
To test whether S1P1 functioned in part to overcome CCR7 mediated retention, wild-type mice were reconstituted with a mixture of wild-type and CCR7-deficient bone marrow cells and they were then treated with FTY720 to modulate S1P1 function. The mice were also treated with integrin-blocking antibodies to ensure that any differences observed were not due to differences in lymph node entry. As expected, FTY720 strongly inhibited lymph node egress and reduced numbers of cells were present in lymph after one day of combined FTY720 treatment and entry blockade (Fig. 3A). However, amongst the T cells continuing to appear in lymph at this time there was a strong increase in the representation of CCR7-deficient cells (Fig. 3A, B). In contrast, there was no change in the proportion of CCR7-deficient B cells appearing in the lymph (Fig. 3A). To correct for differences in the extent of reconstitution by the CD45.1 and CD45.2 bone marrow cells in individual animals, the frequency of each T cell type in the lymph was normalized by that in the lymph nodes of the same animals and this confirmed that there was a significant overrepresentation of CCR7-deficient cells in lymph of FTY720 treated animals (Fig. 3C). Thus, although total numbers of T cells were reduced in the lymph of all the animals after FTY720 treatment, the CCR7-deficient cells were reduced by only 60-70% versus more than 90% for wild-type cells. The treatments did not cause detectable changes in lymph node T cell numbers over the period of these experiments. Taken together, these findings support a model where S1P1 functions within T cells in part to overcome CCR7-mediated retention.
Figure 3. FTY720-mediated inhibition of T cell egress is partially CCR7 dependent.
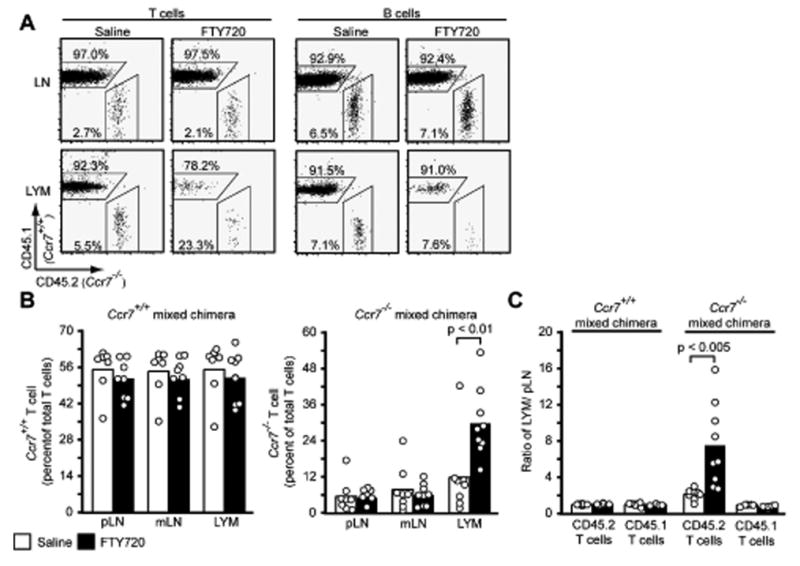
(A) Flow cytometric analysis of CD45.2+ Ccr7-/- and CD45.1+ WT T and B cells in mixed bone marrow chimeras treated for 24 h with saline or FTY720 and with integrin blocking antibodies for 20 h. Numbers indicate percent cells in the indicated gates out of total T or B cells. The means (±SD) of lymphocyte numbers in the samples were as follows: saline lymph=6.3×103/ul (± 4.5×103), n=8; FTY720 lymph=7.5×102/ul (± 9.7×102), n=9; saline LN=1.3×107 (± 4.0×106), n=8; FTY720 LN=1.6×107 (± 8.1×106), n=9. (B) Frequency of CD45.2+ T cells in the peripheral LN, mesenteric LN and lymph of mice that had been reconstituted as in A (Ccr7-/- mixed chimera) or with a mixture of CD45.2+ Ccr7+/+ and CD45.1+ WT bone marrow as a further control (Ccr7+/+ mixed chimera). (C) Same data as in B plotted as the ratio of each type of T cell in lymph versus peripheral lymph nodes for each animal. Bars in B and C represent mean and points individual animals and the data are pooled from three experiments.
Pertussis Toxin treatment promotes egress of S1P1-deficient cells
Though reproducible, CCR7-deficiency led to only a partial recovery in T cell egress into lymph of FTY720 treated mice. We next asked whether additional Gαi-coupled receptors were involved in retention by using a pertussis toxin (PTX) pulse-loading procedure that allows cell entry into lymph nodes during the first 2-3 hours after transfer, prior to Gαi inactivation by the PTX enzyme (Lo et al., 2005; Okada and Cyster, 2007) (see Methods). Cells pulse-loaded with PTX or the non-enzymatic (Oligomer B) subunit as control were co-transferred into saline or FTY720 treated recipients and after 3 hours further entry of cells into lymph nodes was blocked by treatment with integrin neutralizing antibodies. Cell numbers were measured at 0 and 21 h after integrin blockade. Using this approach we found that Gαi inactivation facilitated release of T cells (Fig. 4A) and B cells (Suppl. Fig. 1) from lymph nodes of FTY720 treated mice. When compared to saline treated hosts, a similar fraction of cells was released suggesting that the main effect of FTY720 had been overcome by lymphocyte PTX-treatment (Fig. 4A). The low numbers of cells that seed recipient lymphoid tissues in this adoptive transfer approach made lymph measurements difficult but amongst the small number of cells in lymph of FTY720 treated animals there was a strong bias in favor of PTX treated cells (Suppl. Fig. 2). Analysis of blood samples showed that greater numbers of transferred PTX-treated than control-treated cells were present at 0 h, perhaps due to inefficient entry into tissues (Fig. 4A). At 21 h the number PTX-treated cells in the blood of saline- and FTY720-treated recipients were comparable, consistent with similar extents of egress (Fig. 4A).
Figure 4. PTX treatment facilitates egress of FTY720 exposed and S1P1 deficient cells.
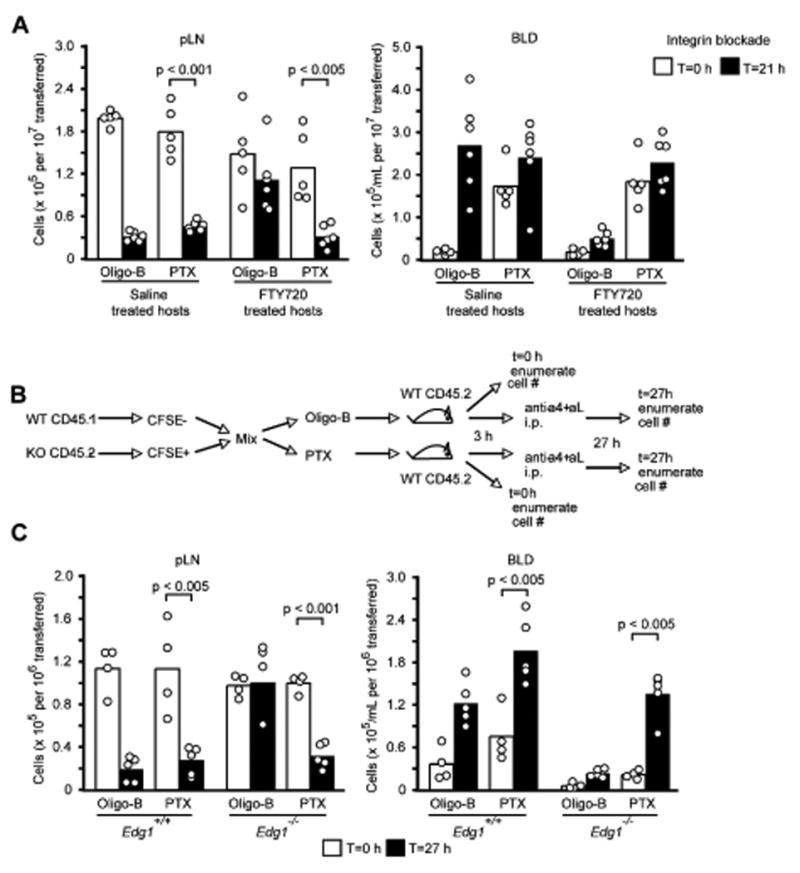
(A) Egress of PTX or oligomer B treated T cells from lymph nodes of FTY720 treated mice. Splenocytes were treated with PTX or with Oligomer B (Oligo-B) as a control and co-transferred into hosts pre-treated with saline or FTY720 4 h earlier. Three hours after transfer, the mice were treated with integrin-blocking antibodies. The number of remaining cells in lymph nodes 21 h after “entry blockade” was determined. Numbers shown are normalized for the number of input splenocytes. (B and C) PTX restores egress of S1P1-deficient lymphocytes. (B) Diagram of transfer experiment. (C) Number of transferred Oligomer B or PTX treated Edg1-/- or Edg1+/+ T cells in peripheral lymph nodes and blood at 0 h and 27 h of integrin blockade. Numbers shown are normalized for the number of CD62L-high, single-positive input thymocytes. Bars represent mean and points individual animals.
We then asked whether Gαi inhibition would restore egress competence to T cells that lacked S1P1 expression. T cells from the thymus of S1P1-deficient fetal liver chimeras were pulsed with PTX or oligomer B and then transferred to wild-type mice (Fig. 4B). After allowing 3 hours for cell entry into lymph nodes, further entry was blocked by integrin neutralization. One day later we found that a significant fraction of the PTX-treated but not of the oligomer B-treated T cells had been released from the lymph nodes and increased numbers of cells were detected in blood (Fig. 4C). These observations suggest that when Gαi signaling is inhibited S1P1 in T cells is not essential for egress from lymph nodes, and they support the idea that S1P1 promotes egress at least in part by counteracting retention signals mediated by Gαi-coupled receptors including CCR7.
LYVE-1+ cortical sinusoids and lymphocyte exit
For a direct interplay to occur between S1P1 and CCR7 during egress we speculated that both signals needed to be encountered in a common microenvironment. Although T cell egress from lymph nodes is usually considered to occur at the medullary sinuses, areas where CCR7 ligand abundance is low, ultrastructural studies have suggested that ‘upstream’ cortical sinusoids may also be involved (Kelly, 1975; Soderstrom and Stenstrom, 1969). Staining of lymph node sections for the lymphatic marker LYVE-1 (Jackson et al., 2001) revealed numerous positive cells in the macrophage-rich medullary region, as expected, and also identified LYVE-1+ sinusoids in the T zone or paracortex, often in areas adjacent to HEV and proximal to B cell follicles (Fig. 5A and B). LYVE-1 staining of sinusoids in the paracortical region has recently been reported (Hirakawa et al., 2005; Prevo et al., 2004). In some lymph node cross-sections, LYVE-1+ sinusoids could be observed extending from the T cell rich paracortical region to medullary areas (Fig. 5A). These paracortical structures typically contained numerous T cells and B cells (Fig. 5C). These combined observations lead us to categorize the LYVE-1+ structures in the T zone as being identical to the cortical sinusoids described in ultrastructural studies (Belisle and Sainte-Marie, 1981; Compton and Raviola, 1985; Kelly, 1975; Kowala and Schoefl, 1986; Soderstrom and Stenstrom, 1969). In contrast to their rich lymphocyte content, the cortical sinusoids contained few dendritic cells (Fig. 5C). Because dendritic cells undergo only very limited egress from lymphoid organs, their deficiency in the cortical sinusoids is consistent with these structures playing a role in lymphocyte egress.
Figure 5. S1P1 deficient cells are found less frequently within cortical sinusoids.
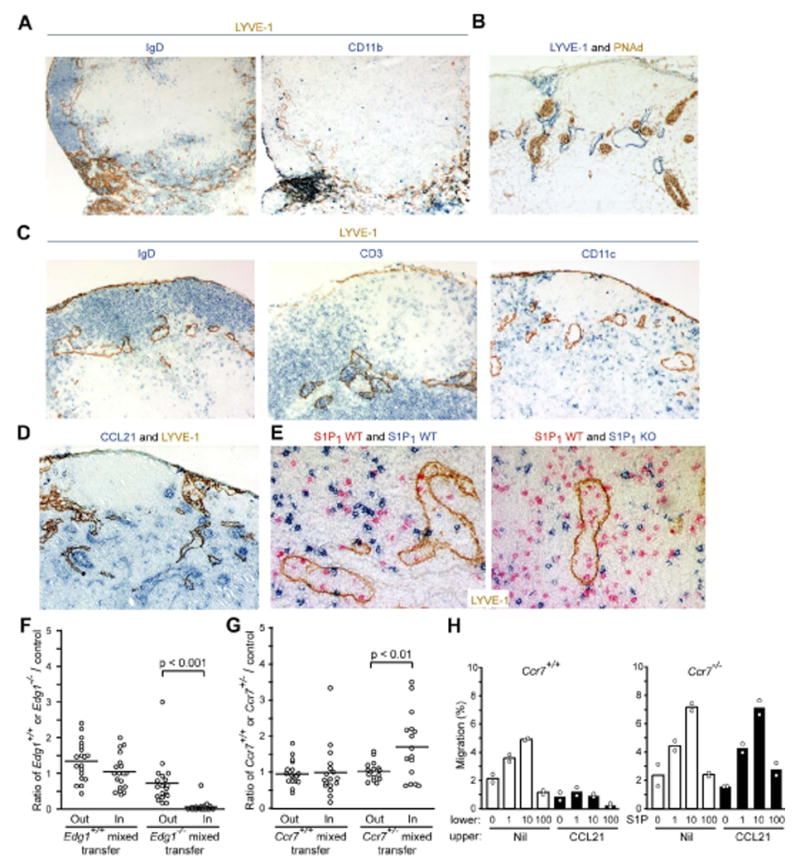
(A) Serial sections of lymph node stained for LYVE-1 (brown) and IgD (blue) or CD11b (blue) showing LYVE-1 expressing structures extending from cortical areas into the macrophage-rich medullary region. (B) Proximity of cortical LYVE-1+ structures (blue) to PNAd expressing HEV (brown). (C) LYVE-1+ cortical structures (brown) contain B cells (IgD, blue) and T cells (CD3, blue) but are relatively devoid of dendritic cells (CD11c, blue). (D) CCL21 expression (blue) in wild-type lymph nodes with respect to LYVE-1 (brown). (E) Effect of S1P1-deficiency on T cell appearance in cortical sinusoids. CD45.2+ thymocytes from wild-type or S1P1 deficient fetal liver chimeras were labeled with CFSE and each population was co-transferred with wild-type (CD45.1+) thymocytes into B6 (CD45.2+) mice. Thirty-six hours after transfer, recipient lymph nodes were sectioned and stained to detect the fetal liver chimera-derived (CSFE+) T cells (blue) and the co-transferred wild-type cells (CD45.1, red) and for LYVE-1 (E). Objective magnifications: (A) 5×; (B-D) 10×; (E) 20×. (F and G) Enumeration of (F) Edg1+/+ and Edg1-/- cells or (G) Ccr7+/+ and Ccr7+/- cells and co-transferred wild-type control T cells inside LYVE-1+ sinusoid structures (In) and in a 30 μm thick area surrounding each sinusoid (Out). Data are plotted as ratio of wild-type, Edg1-/- or Ccr7+/- cells to co-transferred control cells in each region. Each dot represents data from a single sinusoid cross-section and bars represent the means. (H) Transwell migration of Ccr7+/+ or Ccr7-/- CD4+ T cells in response to the indicated concentrations of S1P. Cells were added to the upper wells in the absence or presence of 1 μg/ml CCL21 as indicated. Data are representative of three experiments.
Cortical sinusoids were located in areas rich in the CCR7 ligand, CCL21 (Fig. 5D). It is currently not possible to stain for the S1P1 ligand, S1P. We therefore sought an alternative approach to determine whether S1P1 function was required at the level of the cortical sinusoid and tested the impact of S1P1 deficiency on the appearance of transferred T cells in cortical sinusoids. Thymocytes from Edg1-/- or Edg1+/+ fetal liver chimeras were transferred, together with allelically marked control cells, into wild-type recipients. One day later, few Edg1-/- cells could be detected within LYVE-1+ cortical sinusoids whereas similar numbers of Edg1+/+ cells were observed in cortical sinusoids compared to T cells co-transferred as internal controls (Fig. 5E and Suppl. Fig. 3A and B). Enumeration of wild-type and S1P1-deficient T cells within sinusoids and in a 30 μm thick region surrounding each structure confirmed that S1P1-deficient cell accumulation inside sinusoids was strongly inhibited (Fig. 5F). The ratio of S1P1-deficient to wild-type cells in the immediate vicinity of the sinusoid (0.73±0.63, Fig. 5F) was little different than the ratio in the central T zone (0.65±0.16, n=13). A similar frozen tissue analysis of T cell distribution within the medullary region could not be performed because the high density of LYVE-1 staining did not allow sinus and non-sinus areas to be distinguished. These findings indicate T cells require S1P1 for entry into or accumulation within LYVE-1+ cortical sinusoids but that the receptor is not critical for T cells to approach these structures.
We next asked whether CCR7 antagonized appearance of cells within sinusoids using Ccr7+/- cells because they enter lymph nodes with similar efficiency to wild-type cells and distribute through the T zone (Fig. 1H). Ccr7+/- T cells were found at a higher frequency inside LYVE-1+ sinusoids than in the immediately surrounding regions (Fig. 5G and Suppl. Fig. 3). In control experiments allelically distinguishable wild-type populations were equally represented in sinusoids and in the surrounding regions (Fig. 5G). These observations indicate that CCR7 antagonizes T cell entry into or accumulation inside LYVE-1+ sinusoids and they suggest that this effect cannot be explained solely by influences of CCR7 on cell proximity to these structures.
To further test for a direct interplay between CCR7 and S1P1, we tested the ability of CCL21 to retain cells against an S1P gradient in transwell migration assays. In the absence of CCL21, naïve T cells migrated to the lower chamber in response to S1P (Fig. 5H). However, when CCL21 was included with the cells in the upper chamber at 1 μg/ml, a concentration within the range estimated to exist in lymph node T zones (Luther et al., 2002), wild-type T cells could no longer migrate to S1P in the lower chamber (Fig. 5H). This retention-effect was fully dependent on CCR7 expression by the T cells (Fig. 5H). These results are consistent with the possibility that there is a direct interplay between lymphocyte CCR7 and S1P1 at the level of entry into or retention within LYVE-1+ cortical sinusoids.
T cells that have divided multiple times become S1P1hi and have reduced CCR7 function
Our findings identified an antagonistic relationship between CCR7 and S1P1 in determining the egress of naïve T cells. Activated T cells initially downregulate S1P1 transcription and are retained in the responding lymph node and by day three the daughter cells begin recovering S1P1 transcripts and S1P responsiveness (Matloubian et al., 2004). Activated T cells are also known to downregulate CCR7 function (Ansel et al., 1999; Hardtke et al., 2005). Here we examined the relationship between the extent of cell division and the reciprocal changes in S1P1 and CCR7 function following in vivo activation. We found that at day 3 of the OTII T cell response to ovalbumin (OVA) in adjuvant, S1P1 was re-expressed on a large fraction of the T cells that had undergone four or more divisions (Fig. 6A). Chemotaxis assays showed that recovery of S1P1 function was most prominent in cells that had divided four or more times (Fig. 6B). Reciprocally, CCR7 was downregulated in the activated cells as assessed by surface staining (Fig. 6C) and by transcript abundance (Fig. 6D), with the down-modulation being most substantial in the cells that had divided multiple times. CCL21 chemotactic responses were most strongly reduced in cells that had divided more than four times (Fig. 6E). These observations suggest that once antigen-engaged T cells have undergone four or more divisions, many of the cells acquire an S1P1hi phenotype and are functionally CCR7 low, a combination of changes that most likely reduces the propensity of the cells to migrate further within the T zone and instead favors prompt egress. Consistent with this proposal, there was an enrichment for T cells that had undergone more than 4 divisions amongst the divided T cells in blood and lymph compared to draining lymph node (Fig. 6F).
Figure 6. T cells that have divided multiple times upregulate S1P1 and downregulate CCR7.

Mice receiving CFSE-labeled OTII T cells were immunized s.c. with OVA in CFA or left unimmunized and draining lymph node cells were analyzed at day 3. (A) Flow cytometric analysis of S1P1 versus CFSE on OTII T cells. The line indicates the baseline determined by staining cells with a control antibody. (B) Transwell migration assay showing relationship between cell division (Div) number and recovery of S1P responsiveness. OTII T cells that had divided 0-3 times are shown by dashed lines, 4-8 times by thin solid lines and endogenous CD4+ T cells by a thick solid line. (C) Flow cytometric analysis of CCR7 versus CFSE on OTII T cells. (D) RT-QPCR analysis of CCR7 on sorted OTII T cells that had divided the indicated number of times based on CFSE dilution. (E) Transwell migration assay showing relationship between cell division number and reduced CCL21 responsiveness. Bars show mean±sd (n=4). (F) Overlay of CFSE profile in lymph node versus blood and lymph, showing enrichment for highly divided cells in circulation. Data are representative of at least 3 experiments.
Discussion
The above findings extend the functions of CCR7 beyond promoting cell entry, compartmentalization and motility within lymphoid organs to demonstrate that it also has a role in favoring lymphocyte retention within these tissues. The requirement for S1P1 in lymphocyte egress is partially relieved by removing CCR7 and more fully relieved by globally inhibiting lymphocyte Gαi. These findings support a model, elaborated upon below, where S1P1 promotes lymph node egress at least in part by overcoming Gi-mediated retention signals. LYVE-1+ cortical sinusoids are shown to be a site of interplay between S1P1 and CCR7 responses, with S1P1 promoting T cell accumulation inside sinusoids and CCR7 countering this activity. We also find that inhibition of lymphocyte Gαi largely overcomes the egress inhibitory effects of systemic FTY720 treatment, supporting the conclusion that FTY720 inhibits egress via effects on the lymphocyte. Coincident S1P1 upregulation and CCR7 downregulation occurs in activated T cells that have undergone several cell divisions and might be a mechanism to favor rapid egress of newly generated effector cells.
This study provides new insight regarding both anatomical and signaling aspects of T cell egress from lymph nodes. To integrate these findings and provide a framework for further discussion it is helpful to consider a provisional model for the early events leading to egress. During ‘random migration’ over stromal cells in the T zone (Bajenoff et al., 2006), T cells are likely to encounter LYVE-1+ egress structures. In this location they are suggested to be exposed to overlapping distributions of S1P and CCR7 ligand and possibly other retention factors. Each cell then makes a choice to respond to one or the other cue based on the dominance of the signal. Such decision making has been observed for neutrophils responding to overlapping cues in vitro (Foxman et al., 1999; Heit et al., 2002). The molecular basis for this type of interplay remains to be fully defined but seems likely to depend on the ability of a dominantly signaling receptor to compete successfully for cytoskeletal elements that are needed for achieving cell polarity and movement. Factors that influence which signal is dominant may include: the spatial orientation in which the cell encounters the two cues; the lag in recovery of full S1P1 surface expression following lymph node entry (Lo et al., 2005); possible partial desensitization of CCR7 by ligand (Kohout et al., 2004); changes in receptor abundance or function due to T cell activation status (Ansel et al., 1999; Hardtke et al., 2005; Matloubian et al., 2004); and changes in ligand abundance due to immune response status of the lymph node (Mueller et al., 2007). Partial reductions in receptor (or ligand) expression would be expected to have strong effects on the outcome of this cellular decision-making and the finding that S1P1 and CCR7 hemizygosity strongly influences egress efficiency is consistent with the model. The ability of small (2-3 fold) changes in receptor abundance to cause repositioning of cells in competing chemoattractant gradients has been observed in other systems (Reif et al., 2002). In cases where the CCR7 signal dominates, the cell is suggested to continue random migration in the T zone. In cases where the S1P1 signal dominates, the cell is suggested to localize within the LYVE-1+ sinusoid. Removal of both S1P and retention receptor responsiveness, for example by PTX treatment, would eliminate the competitive signaling and allow cell localization inside sinusoids by the remaining ‘random’ motility. The behavior of cells within cortical sinusoids has not yet been characterized but – as suggested long ago (Soderstrom and Stenstrom, 1969; Kelly, 1975) – there may be a general tendency for sinusoidal cells to move toward medullary sinuses and the efferent lymph. It is recognized that some alternatives to this model are not excluded by current findings and it will be important in future studies to further characterize the dynamics of S1P1-dependent events during T cell egress using real-time imaging procedures.
Cortical sinusoids were possibly first identified by Soderstrom and Stenston in their description of ‘mud streams’ of lymphocytes in the paracortical (T cell) zone that had a higher density of cells than the average parenchyma and that sometimes appeared as sinus-like structures continuous with the medullary sinus system (Soderstrom and Stenstrom, 1969). Further studies described paracortical sinuses near HEV and connecting with medullary sinuses that were suggested to allow cells to move from the T zone to the medulla (Belisle and Sainte-Marie, 1981; Kelly, 1975). Using LYVE-1 to identify these structures, we found that accumulation of lymphocytes within them was S1P1 dependent and that they contained few dendritic cells, consistent with their playing a role in lymphocyte egress. These experiments also provided functional evidence that S1P is available within or nearby cortical sinusoids. The cell types responsible for generating lymph S1P have not yet been determined though they were shown to be radiation resistant (Pappu et al., 2007) and may correspond to LYVE-1+ cells.
Our studies do not address to what extent egress occurs via cortical sinusoids versus other sites. In ultrastructural studies, cells can be observed traversing the wall of cortical sinusoids and medullary sinuses (Compton and Raviola, 1985; Heath and Spalding, 1987; Nicander et al., 1991). In addition, treatment with pharmacological S1P1 agonists has been suggested to reduce the entry of lymphocytes from the medulla into medullary sinuses (Mandala et al., 2002; Sanna et al., 2006; Wei et al., 2005). Moreover, our finding that transferred CCR7-deficient cells are enriched near LYVE-1+ medullary regions and have down-modulated S1P1 is consistent with cells encountering S1P in this region even before exiting the tissue. Future studies will need to address the extent to which the S1P1-dependent egress step occurs via cortical sinusoids, medullary sinuses and subcapsular sinuses. In addition to locally counteracting S1P1 responses, CCR7 may favor retention over egress by promoting T cell migration and dispersal within the T zone and away from egress sites. In this regard it should be noted that the nature and distribution of the additional Gαi-coupled receptor ligands that are implicated by our studies as promoting T cell retention in lymph nodes is not known. It seems possible that competition between S1P1 and these additional retention systems occurs in other regions of the lymph node such as the medulla.
The ability of T cells to exit lymph nodes even when their Gαi signaling is inhibited suggests that the lymph node egress structures may be unusually permissive for cell entry and consistent with this notion, imaging of lymph node medullary sinuses has suggested the presence of portals that may function as egress hotspots (Wei et al., 2005). Ultrastructural studies have suggested that cortical sinusoids may also have openings (Belisle and Sainte-Marie, 1981; He, 1985; Soderstrom and Stenstrom, 1969) though this has been debated (Heath and Spalding, 1987; Nicander et al., 1991). PTX strongly inhibits egress from the thymus indicating that there are unique requirements for egress from this organ, perhaps reflecting a less penetrable endothelial barrier (reviewed in Cyster, 2005). Although PTX-treatment substantially rescued the egress capability of S1P1-deficient cells, treated cells did not egress from lymph nodes with wild-type efficiency, as noted in a previous study (Lo et al., 2005). Further work will be needed to determine whether the reduction in egress of PTX-treated cells is due to their reduced motility (Huang et al., 2007; Okada and Cyster, 2006) or whether it indicates an additional contribution of S1P1 beyond overcoming Gi-retention signals, such as helping guide cells into regions rich in egress structures. Although we did not observe an effect of S1P1-deficiency on T cell localization near cortical sinusoids, it is possible that T cell migration toward these structures can be promoted by many signals, including S1P1. Moreover, our studies have not examined S1P1-deficient T cell migration into or within the medulla. Consistent with a possible role of this type, treatment with S1P1 agonists altered T cell motility in the medulla but not the T zone (Wei et al., 2005).
Based on the ability of FTY720 to cause marked down-modulation and functional inhibition of S1P1 on lymphocytes, FTY720 was suggested to inhibit lymphocyte egress by causing functional antagonism of S1P1 (Cyster, 2005; Matloubian et al., 2004). An alternative mechanism that has been proposed is agonist action on stromal cells at medullary egress sites to cause closing of egress portals (Sanna et al., 2006; Wei et al., 2005). In our current experiments we observe a tight relationship between the extent of S1P1 surface down-modulation induced by FTY720 on lymph node T cells and the extent of egress inhibition as measured by loss of cells from lymph and blood. A similar potency of FTY720 in causing downregulation of S1P1 on human lymphocytes was recently reported in an in vitro study (Maeda et al., 2007). Importantly, we find close similarity in the reduction in egress caused by S1P1 heterozygosity as caused by the dose of FTY720 that induces a ∼50% decrease in lymphocyte S1P1 abundance. These findings favor the conclusion that FTY720 inhibits egress by functional antagonism of lymphocyte S1P1. In further agreement with this mode of action is our finding that PTX treatment of the lymphocyte largely overcomes the egress-inhibitory effect of FTY720 treatment.
The identification of a role for CCR7 in mediating T cell retention within lymph nodes indicates that reduced CCR7 function can lead to decreased cell numbers in lymphoid organs through accelerated egress as well as reduced entry. For example, the poor ability of CCR7-deficient regulatory T cells to suppress T cell proliferation in lymph nodes may partly reflect inefficient retention of these cells (Schneider et al., 2007). Similarly, the reduced CCL21 expression in lymphoid tissues following certain viral infections (Mueller et al., 2007) might be a mechanism to promote release of T cells into circulation. Finally, our experiments suggest that the regulated egress of newly developing effector cells is controlled not just by down-modulation and re-expression of S1P1 (Lo et al., 2005; Matloubian et al., 2004) but also by the parallel changes in CCR7 function. Early after activation S1P1 transcripts and protein are markedly down-regulated, consistent with the initial retention of activated cells in the responding lymphoid tissue. However, after approximately four divisions, many of the cells not only upregulate S1P1 but they also lose much of their CCR7 function, a combination of changes that may help ensure these newly generated effector cells do not further scan the lymph node T zone for antigen but exit rapidly into circulation to travel to the site of infection.
Experimental Procedures
Mice and Adoptive Cell Transfer
CD45.2 C57BL/6 (B6) and CD45.1 B6 mice were from the National Cancer Institutes or a colony maintained at the University of California, San Francisco. Ccr7-/- mice (Forster et al., 1999) and CCR7 Tg mice (Kwan and Killeen, 2004) were 10 generations backcrossed to B6. OTII B6 mice were as described (Barnden et al., 1998). S1P1 deficient thymocytes were generated by reconstitution of irradiated B6-CD45.1 mice with Edg1-/- E12.5 fetal liver cells as described (Matloubian et al., 2004). Ccr7 wild-type and Ccr7-/- mixed bone marrow chimeras were made as described (Reif et al., 2002) using 50:50 mixtures of wild-type CD45.1 and littermate control or Ccr7-/- CD45.2 bone marrow. In some experiments, ∼2×107 cells per mL were labeled with 3.3 μM of carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen/Molecular Probes) or 5-(and-6)-(((4-chloromethyl)benzoyl)amino) tetramethylrhodamine (CMTMR, Invitrogen/Molecular Probes; (10 μM) in RPMI1640 containing 2% FCS for 20 minutes at 37°C then washed by spinning through a layer of fetal calf serum. For OTII transfers, mice that received 2×107 CFSE labeled spleen and lymph node cells 1 day before were immunized s.c. with 200 μg OVA (Sigma) in CFA (Sigma) as previously described (Matloubian et al., 2004). Lymph collection was performed as described (Matloubian et al., 2004). Briefly, under a stereomicroscope, lymph was drawn from the cysterna chyli using a fine borosilicate glass microcapillary pipette (Sutter Instrument). Cell numbers determined by flow cytometry were divided by the volume of collected lymph to determine the concentration. Protocols were approved by the Institutional Animal Care and Use Committee of the University of California San Francisco.
Antibodies and Treatments
The anti-αL (clone M17/4, rat IgG2a) hybridoma was from American Type Culture Collection and the anti-α4 (clone PS/2, rat IgG2b) hybridoma was kindly provided by David Erle (University of California, San Francisco). Antibodies were administered intraperitoneally at 100 μg per mouse in PBS. Cells were treated with 10 ng/mL of Oligomer B or PTX at 37°C for 10 min, washed twice in warm RPMI, 2% FCS, and 10 mM Hepes, and then transferred to recipient mice. For generation of a LYVE-1 specific mAb, DT569 cells (Ba/F3 cells stably transfected with mouse IL-2 plasmid) were transfected with mouse LYVE-1 inserted into the pEF vector with sequence encoding the preprolactin leader sequence and Flag epitope inserted in place of the codons for the first 24 amino acids of LYVE-1. Rats were immunized with transfected cells at RnD Systems and the resultant hybridoma supernatants were screened for reactivity to LYVE-1 transfected cells by flow cytometry. Mab 22 stained LYVE-1 transfected cells but not control DT569 cells transfected with Flag-tagged CCR7. This antibody is now available from RnD systems as clone 223322. Anti-CCR7 monoclonal antibody was obtained from BioLegend.
Chemotaxis Assays
Cells were washed in RPMI1640 with 0.5% fatty acid free BSA several times, resensitized for 30 minutes at 37°C then tested for transmigration across uncoated 5 μm transwell filters (Corning Costar Corp.) for 3 h to sphingosine-1-phosphate (S1P) (Sigma-Aldrich) or CCL21 (R&D Systems) in the bottom chamber as described (Reif et al., 2002). FTY720 was from a custom synthesis performed by SRI International (Menlo Park, CA) and was administered i.p. in saline.
Immunohistochemical and Flow Cytometric Analysis
7 μm cryostat sections were fixed and stained as described (Reif et al., 2002). CFSE labeled cells were visualized in sections with alkaline-phosphatase conjugated anti-fluorescein antibodies (Roche). Congenic transferred lymphocytes were visualized by staining with biotinylated antibodies to CD45.1 (clone A20) or CD45.2 (Clone 104). For visualization of LYVE-1+ structures in sections, either unconjugated or biotinylated Mab22 was used. Lymphocyte preparations were stained with various fluorochrome conjugated antibodies purchased from BD Pharmingen or anti-S1P1 as described (Lo et al., 2005) and data were acquired on an FACS LSRII (Beckton Dickinson) and analyzed with FlowJo software (Treestar).
Supplementary Material
Supplemental Figure 1. Effect of CCR7 deficiency and PTX treatment on B cell egress from lymph nodes. (A and B) Wild-type and CCR7-deficient B cells have similar egress efficiency. (A) Transfers and integrin blockade were performed as in Figure 1A, and the fraction of cells remaining after 8 h in the indicated lymph nodes was determined. (B) Irradiated mice were reconstituted using mixtures of CD45.2+ Ccr7+/+ or Ccr7-/- bone marrow and CD45.1+ WT bone marrow as described in Figure 1B and the representation of CD45.2+ cells in peripheral and mesenteric lymph nodes (LN) and in lymph (LYM) was determined. (C) Egress of PTX or oligomer B treated B cells from lymph nodes of FTY720 treated mice. Data are from the same animals shown in Figure 3B. The number of remaining cells in lymph nodes 21 h after “entry blockade” was determined. Numbers showed are normalized for the number of input splenocytes.
Supplemental Figure 2. PTX treatment facilitates egress of T lymphocytes into lymph of FTY720 treated mice. (A) Splenocytes were labeled with CFSE or CMTMR and treated with PTX or Oligo-B, respectively, and transferred into hosts pre-treated with FTY720 4 h earlier. Profiles show flow cytometric analysis of transferred cells in the lymph and lymph nodes of hosts 22 h after transfer. Numbers represent the percentages of transferred T cells in the indicated gates out of total T cells. Samples from three individual mice are shown. (B) Splenocytes were treated with PTX or Oligo-B, transferred into hosts pre-treated with saline or FTY720 and treated 3 h later with integrin-blocking antibodies as in figure 4. The frequency of transferred cells in the lymph 21 h after “entry blockade” was determined. Numbers shown are percent transferred T cells out of total lymph samples. Typical lymph collections were 1-3 μl. Bars represent means and dots individual mice. The mean (±SD) number of transferred T cells per μl of lymph were: 9.3±5.2 Oligo-B treated cells and 3.0±2.0 PTX treated cells in control (saline) treated hosts; 1.3±1.2 Oligo-B treated cells and 6.4±3.1 PTX treated cells in FTY720 treated hosts.
Supplemental Figure 3. Effect of S1P1-deficiency or reduced CCR7 signaling on T cell appearance in cortical sinusoids. (A and B) Further examples of the type of analysis shown in Fig. 5E. CD45.2+ thymocytes from S1P1-deficient (A) or wild-type (B) fetal liver chimeras were labeled with CFSE and each population was co-transferred with wild-type (CD45.1+) thymocytes into B6 (CD45.2+) mice. Thirty-six hours after transfer, recipient lymph nodes were sectioned and stained to detect the fetal liver chimera-derived (CSFE+) T cells (blue) and the co-transferred wild-type cells (CD45.1, red) and for LYVE-1. Data are representative of peripheral lymph nodes from at least three recipient mice of each type. (C and D) Examples of the histological analysis used to generate the data shown in Fig. 5G. CFSE labeled Ccr7+/+ (C) or Ccr7+/- (D) cells were co-transferred with CD45.1+ control cells into B6 mice. After 36 h immunohistochemistry was performed as described in A and B. White dashed line defines a 30um thick area surrounding the LYVE-1+ structure. Scale bar, 30 μm. Objective magnification: 20×.
Acknowledgments
We are grateful to Reinhold Forster, Martin Lipp, Richard Proia and Nigel Killeen for mice, Olivia Lam for assistance with the mouse colony, Joao Pereira and Ying Xu for technical assistance and Steve Rosen and members of the Cyster lab for comments on the manuscript. T.H.M.P. is supported by the Boyer Program in the Biochemical Sciences and the UCSF Medical Scientist Training Program. J.G.C. is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by grants from the NIH. The authors declare they have no financial conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine-1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Arnold CN, Butcher EC, Campbell DJ. Antigen-Specific Lymphocyte Sequestration in Lymphoid Organs: Lack of Essential Roles for alpha(L) and alpha(4) Integrin-Dependent Adhesion or Galpha(i) Protein-Coupled Receptor Signaling. J Immunol. 2004;173:866–873. doi: 10.4049/jimmunol.173.2.866. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration and territoriality in lymph nodes. Immunity. 2006 doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Belisle C, Sainte-Marie G. Tridimensional study of the deep cortex of the rat lymph node. III. Morphology of the deep cortex units. Anat Rec. 1981;199:213–226. doi: 10.1002/ar.1091990206. [DOI] [PubMed] [Google Scholar]
- Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1- deficient mice. J Exp Med. 1999;189:1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton CC, Raviola E. Structure of the sinus-lining cells in the popliteal lymph node of the rabbit. Anat Rec. 1985;212:408–423. doi: 10.1002/ar.1092120412. [DOI] [PubMed] [Google Scholar]
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Foxman EF, Kunkel EJ, Butcher EC. Integrating conflicting chemotactic signals. The role of memory in leukocyte navigation. J Cell Biol. 1999;147:577–588. doi: 10.1083/jcb.147.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood. 2005;106:1924–1931. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- He Y. Scanning electron microscope studies of the rat mesenteric lymph node with special reference to high-endothelial venules and hitherto unknown lymphatic labyrinth. Arch Histol Jpn. 1985;48:1–15. doi: 10.1679/aohc.48.1. [DOI] [PubMed] [Google Scholar]
- Heath TJ, Spalding HJ. Pathways of lymph flow to and from the medulla of lymph nodes in sheep. J Anat. 1987;155:177–188. [PMC free article] [PubMed] [Google Scholar]
- Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JH, Cardenas-Navia LI, Caldwell CC, Plumb TJ, Radu CG, Rocha PN, Wilder T, Bromberg JS, Cronstein BN, Sitkovsky M, et al. Requirements for T lymphocyte migration in explanted lymph nodes. J Immunol. 2007;178:7747–7755. doi: 10.4049/jimmunol.178.12.7747. [DOI] [PubMed] [Google Scholar]
- Jackson DG, Prevo R, Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–321. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- Kelly RH. Functional anatomy of lymph nodes. I. The paracortical cords. Int Arch Allergy Appl Immunol. 1975;48:836–849. doi: 10.1159/000231371. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS. Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem. 2004;279:23214–23222. doi: 10.1074/jbc.M402125200. [DOI] [PubMed] [Google Scholar]
- Kowala MC, Schoefl GI. The popliteal lymph node of the mouse: internal architecture, vascular distribution and lymphatic supply. J Anat. 1986;148:25–46. [PMC free article] [PubMed] [Google Scholar]
- Kwan J, Killeen N. CCR7 directs the migration of thymocytes into the thymic medulla. J Immunol. 2004;172:3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- Lo CG, Xu Y, Proia RL, Cyster JG. Cyclical modulation of sphingosine-1-phosphate receptor 1 surface expression during lymphocyte recirculation and relationship to lymphoid organ transit. J Exp Med. 2005;201:291–301. doi: 10.1084/jem.20041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing Activities of Homeostatic Chemokines CCL19, CCL21, and CXCL12 in Lymphocyte and Dendritic Cell Recruitment and Lymphoid Neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Matsuyuki H, Shimano K, Kataoka H, Sugahara K, Chiba K. Migration of CD4 T Cells and Dendritic Cells toward Sphingosine 1-Phosphate (S1P) Is Mediated by Different Receptor Subtypes: S1P Regulates the Functions of Murine Mature Dendritic Cells via S1P Receptor Type 3. J Immunol. 2007;178:3437–3446. doi: 10.4049/jimmunol.178.6.3437. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Hosiawa-Meagher KA, Konieczny BT, Sullivan BM, Bachmann MF, Locksley RM, Ahmed R, Matloubian M. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317:670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- Nicander L, Nafstad P, Landsverk T, Engebretsen RH. A study of modified lymphatics in the deep cortex of ruminant lymph nodes. J Anat. 1991;178:203–212. [PMC free article] [PubMed] [Google Scholar]
- Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18:278–285. doi: 10.1016/j.coi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Okada T, Cyster JG. CC Chemokine Receptor 7 Contributes to Gi-Dependent T Cell Motility in the Lymph Node. J Immunol. 2007;178:2973–2978. doi: 10.4049/jimmunol.178.5.2973. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of Lymphocyte Egress into Blood and Lymph by Distinct Sources of Sphingosine-1-Phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Prevo R, Banerji S, Ni J, Jackson DG. Rapid plasma membrane-endosomal trafficking of the lymph node sinus and high endothelial venule scavenger receptor/homing receptor stabilin-1 (FEEL-1/CLEVER-1) J Biol Chem. 2004;279:52580–52592. doi: 10.1074/jbc.M406897200. [DOI] [PubMed] [Google Scholar]
- Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, Cyster JG. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- Rosen H, Alfonso C, Surh CD, McHeyzer-Williams MG. Rapid induction of medullary thymocyte phenotypic maturation and egress inhibition by nanomolar sphingosine 1-phosphate receptor agonist. Proc Natl Acad Sci U S A. 2003;100:10907–10912. doi: 10.1073/pnas.1832725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–107. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P(1) antagonist in vivo. Nat Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204:735–745. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Soderstrom N, Stenstrom A. Outflow paths of cells from the lymph node parenchyma to the efferent lymphatics--observations in thin section histology. Scand J Haematol. 1969;6:186–196. doi: 10.1111/j.1600-0609.1969.tb01825.x. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med. 2007;204:489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Effect of CCR7 deficiency and PTX treatment on B cell egress from lymph nodes. (A and B) Wild-type and CCR7-deficient B cells have similar egress efficiency. (A) Transfers and integrin blockade were performed as in Figure 1A, and the fraction of cells remaining after 8 h in the indicated lymph nodes was determined. (B) Irradiated mice were reconstituted using mixtures of CD45.2+ Ccr7+/+ or Ccr7-/- bone marrow and CD45.1+ WT bone marrow as described in Figure 1B and the representation of CD45.2+ cells in peripheral and mesenteric lymph nodes (LN) and in lymph (LYM) was determined. (C) Egress of PTX or oligomer B treated B cells from lymph nodes of FTY720 treated mice. Data are from the same animals shown in Figure 3B. The number of remaining cells in lymph nodes 21 h after “entry blockade” was determined. Numbers showed are normalized for the number of input splenocytes.
Supplemental Figure 2. PTX treatment facilitates egress of T lymphocytes into lymph of FTY720 treated mice. (A) Splenocytes were labeled with CFSE or CMTMR and treated with PTX or Oligo-B, respectively, and transferred into hosts pre-treated with FTY720 4 h earlier. Profiles show flow cytometric analysis of transferred cells in the lymph and lymph nodes of hosts 22 h after transfer. Numbers represent the percentages of transferred T cells in the indicated gates out of total T cells. Samples from three individual mice are shown. (B) Splenocytes were treated with PTX or Oligo-B, transferred into hosts pre-treated with saline or FTY720 and treated 3 h later with integrin-blocking antibodies as in figure 4. The frequency of transferred cells in the lymph 21 h after “entry blockade” was determined. Numbers shown are percent transferred T cells out of total lymph samples. Typical lymph collections were 1-3 μl. Bars represent means and dots individual mice. The mean (±SD) number of transferred T cells per μl of lymph were: 9.3±5.2 Oligo-B treated cells and 3.0±2.0 PTX treated cells in control (saline) treated hosts; 1.3±1.2 Oligo-B treated cells and 6.4±3.1 PTX treated cells in FTY720 treated hosts.
Supplemental Figure 3. Effect of S1P1-deficiency or reduced CCR7 signaling on T cell appearance in cortical sinusoids. (A and B) Further examples of the type of analysis shown in Fig. 5E. CD45.2+ thymocytes from S1P1-deficient (A) or wild-type (B) fetal liver chimeras were labeled with CFSE and each population was co-transferred with wild-type (CD45.1+) thymocytes into B6 (CD45.2+) mice. Thirty-six hours after transfer, recipient lymph nodes were sectioned and stained to detect the fetal liver chimera-derived (CSFE+) T cells (blue) and the co-transferred wild-type cells (CD45.1, red) and for LYVE-1. Data are representative of peripheral lymph nodes from at least three recipient mice of each type. (C and D) Examples of the histological analysis used to generate the data shown in Fig. 5G. CFSE labeled Ccr7+/+ (C) or Ccr7+/- (D) cells were co-transferred with CD45.1+ control cells into B6 mice. After 36 h immunohistochemistry was performed as described in A and B. White dashed line defines a 30um thick area surrounding the LYVE-1+ structure. Scale bar, 30 μm. Objective magnification: 20×.


