Abstract
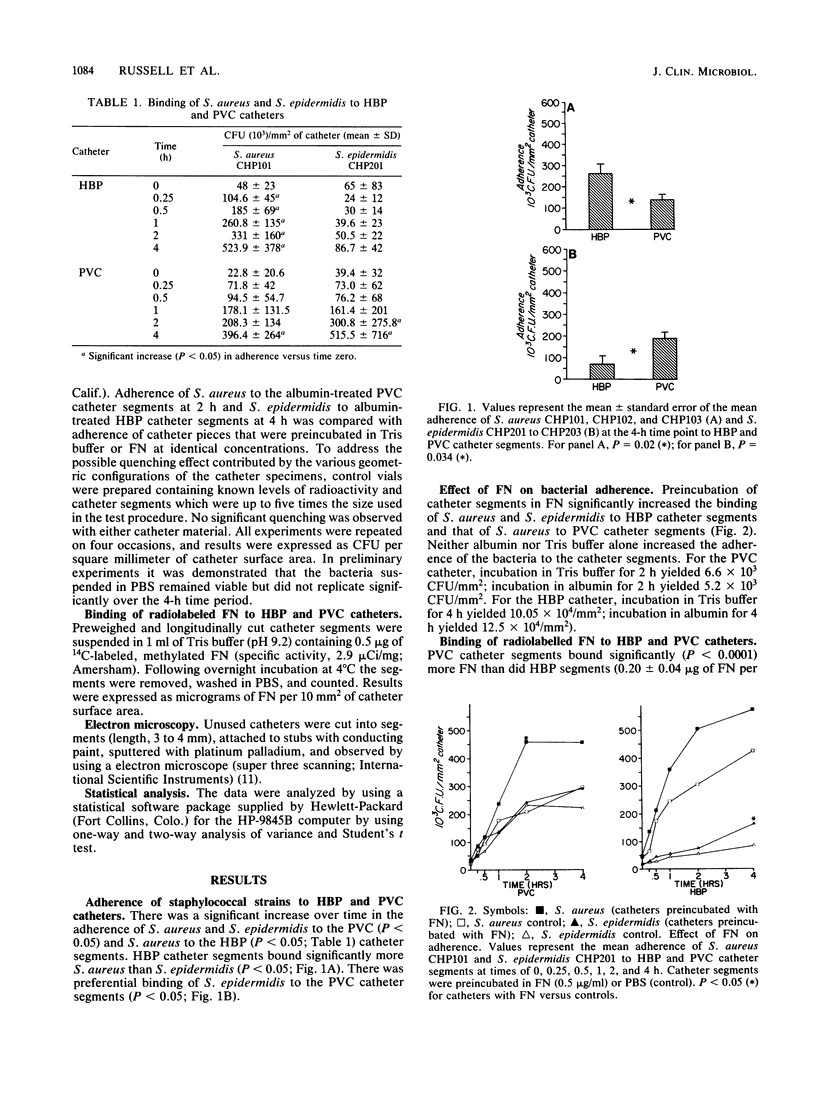
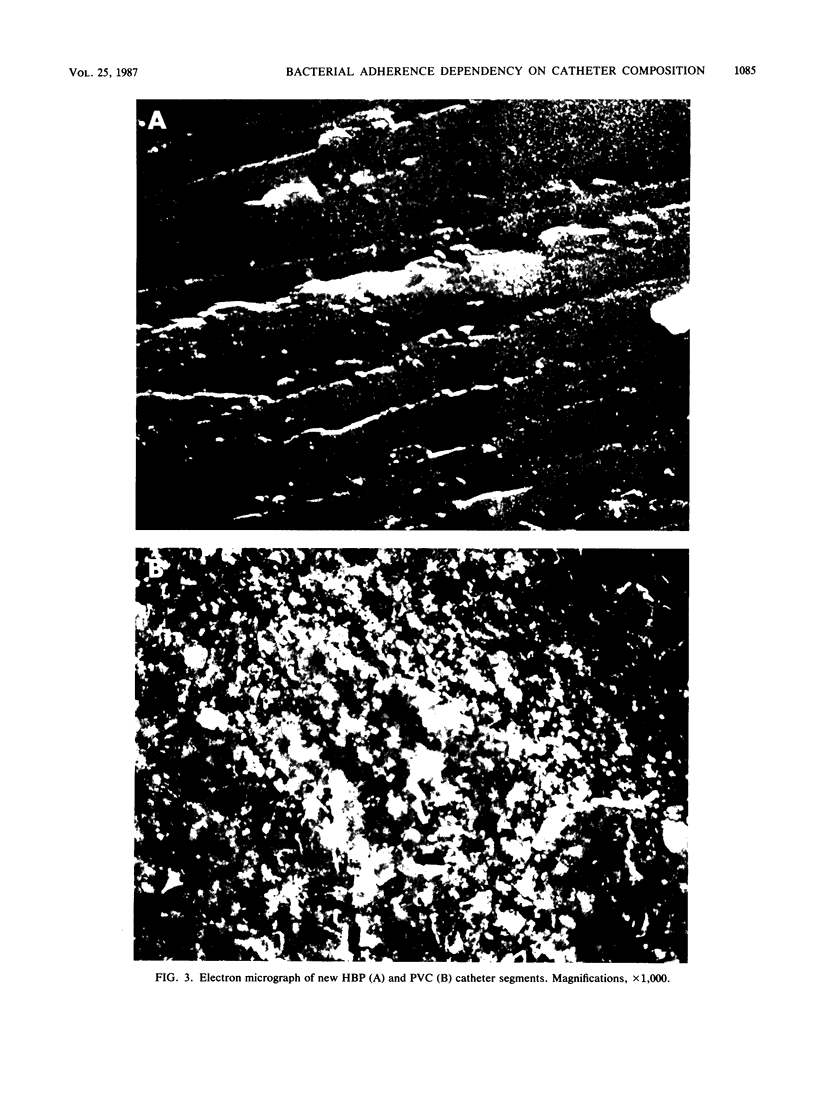
Intravenous hyperalimentation has improved the survival of premature infants. However, long-term placement of intravenous catheters may result in the development of catheter-related sepsis. Fibronectin in plasma contains binding sites for staphylococcal species as well as marked affinity for inert plastics and therefore may provide a substrate for bacterial adherence to indwelling catheters. We determined the adherence of labeled [( 3H]leucine) coagulase-positive (CPS) and coagulase-negative (CNS) staphylococci to untreated and fibronectin-coated polyvinyl chloride (PVC) and heparin-bonded polyurethane (HBP) catheter segments and quantitated the binding of 14C-labeled, purified fibronectin to these catheters. PVC catheter segments bound significantly more CNS than CPS (P less than 0.05), while HBP catheters bound more CPS than CNS (P less than 0.05). Fibronectin significantly increased the adherence of CPS to PVC catheters (P less than 0.05) and CNS to HBP catheters (P less than 0.05). PVC catheters bound more fibronectin (P less than 0.0001) than did HBP catheters. Catheter composition may influence the spectrum of nosocomial pathogens to which infants are susceptible through different bacterial adherences and interactions with adhesive proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Speer M. E., Rudolph A. J. Bacterial colonization of radial artery catheters. Pediatrics. 1980 Jan;65(1):94–97. [PubMed] [Google Scholar]
- Band J. D., Maki D. G. Infections caused by aterial catheters used for hemodynamic monitoring. Am J Med. 1979 Nov;67(5):735–741. doi: 10.1016/0002-9343(79)90727-7. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Bisno A. L., Parisi J. T., McLaughlin B., Hester M. G., Luther R. W. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann Intern Med. 1982 Jan;96(1):1–10. doi: 10.7326/0003-4819-96-1-1. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983 Apr;40(1):407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleri D. J., Corrado M. L., Seligman S. J. Quantitative culture of intravenous catheters and other intravascular inserts. J Infect Dis. 1980 Jun;141(6):781–786. doi: 10.1093/infdis/141.6.781. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Doran J. E., Raynor R. H. Fibronectin binding to protein A-containing staphylococci. Infect Immun. 1981 Sep;33(3):683–689. doi: 10.1128/iai.33.3.683-689.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espersen F., Clemmensen I. Isolation of a fibronectin-binding protein from Staphylococcus aureus. Infect Immun. 1982 Aug;37(2):526–531. doi: 10.1128/iai.37.2.526-531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey H., Burkhard G. Measurement of staphylococcal protein A and detection of protein A-carrying staphylococcus strains by a competitive ELISA method. J Immunol Methods. 1981;47(1):99–107. doi: 10.1016/0022-1759(81)90260-x. [DOI] [PubMed] [Google Scholar]
- Kido D. K., Paulin S., Alenghat J. A., Waternaux C., Riley W. D. Thrombogenicity of heparin- and non-heparin-coated catheters: clinical trail. AJR Am J Roentgenol. 1982 Nov;139(5):957–961. doi: 10.2214/ajr.139.5.957. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Bentley K. L., Schoen R. C. Adhesive substrates for fibronectin. J Cell Physiol. 1981 Dec;109(3):481–488. doi: 10.1002/jcp.1041090314. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Binding sites for streptococci and staphylococci in fibronectin. Infect Immun. 1984 Aug;45(2):433–436. doi: 10.1128/iai.45.2.433-436.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J Clin Microbiol. 1984 May;19(5):687–693. doi: 10.1128/jcm.19.5.687-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Proctor R. A., Prendergast E., Mosher D. F. Fibronectin mediates attachment of Staphylococcus aureus to human neutrophils. Blood. 1982 Apr;59(4):681–687. [PubMed] [Google Scholar]
- Scherer L. R., West K. W., Weber T. R., Kleiman M., Grosfeld J. L. Staphylococcus epidermidis sepsis in pediatric patients: clinical and therapeutic considerations. J Pediatr Surg. 1984 Aug;19(4):358–361. doi: 10.1016/s0022-3468(84)80252-3. [DOI] [PubMed] [Google Scholar]
- Sheth N. K., Franson T. R., Rose H. D., Buckmire F. L., Cooper J. A., Sohnle P. G. Colonization of bacteria on polyvinyl chloride and Teflon intravascular catheters in hospitalized patients. J Clin Microbiol. 1983 Nov;18(5):1061–1063. doi: 10.1128/jcm.18.5.1061-1063.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth N. K., Rose H. D., Franson T. R., Buckmire F. L., Sohnle P. G. In vitro quantitative adherence of bacteria to intravascular catheters. J Surg Res. 1983 Mar;34(3):213–218. doi: 10.1016/0022-4804(83)90062-8. [DOI] [PubMed] [Google Scholar]
- Sitges-Serra A., Puig P., Jaurrieta E., Garau J., Alastrue A., Sitges-Creus A. Catheter sepsis due to Staphylococcus epidermidis during parenteral nutrition. Surg Gynecol Obstet. 1980 Oct;151(4):481–483. [PubMed] [Google Scholar]
- Switalski L. M., Rydén C., Rubin K., Ljungh A., Hök M., Wadström T. Binding of fibronectin to Staphylococcus strains. Infect Immun. 1983 Nov;42(2):628–633. doi: 10.1128/iai.42.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy P. T., Lai L. W., Drake T. A., Sande M. A. Effect of fibronectin on adherence of Staphylococcus aureus to fibrin thrombi in vitro. Infect Immun. 1985 Apr;48(1):83–86. doi: 10.1128/iai.48.1.83-86.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P. E., Waldvogel F. A., Morgenthaler J. J., Nydegger U. E. Adsorption of fibronectin onto polymethylmethacrylate and promotion of Staphylococcus aureus adherence. Infect Immun. 1984 Sep;45(3):768–774. doi: 10.1128/iai.45.3.768-774.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P., Suzuki R., Waldvogel F. A., Morgenthaler J. J., Nydegger U. E. Foreign body infection: role of fibronectin as a ligand for the adherence of Staphylococcus aureus. J Infect Dis. 1984 Oct;150(4):546–553. doi: 10.1093/infdis/150.4.546. [DOI] [PubMed] [Google Scholar]
- Verbrugh H. A., Peterson P. K., Smith D. E., Nguyen B. Y., Hoidal J. R., Wilkinson B. J., Verhoef J., Furcht L. T. Human fibronectin binding to staphylococcal surface protein and its relative inefficiency in promoting phagocytosis by human polymorphonuclear leukocytes, monocytes, and alveolar macrophages. Infect Immun. 1981 Sep;33(3):811–819. doi: 10.1128/iai.33.3.811-819.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J. G., Weesner K. M. Staphylococcus aureus endocarditis and pericarditis in an infant with a central venous catheter. Clin Pediatr (Phila) 1984 Jan;23(1):46–47. doi: 10.1177/000992288402300109. [DOI] [PubMed] [Google Scholar]