How did the study come about?
High fertility and infant mortality rates have been features of India for several decades. A systematic study of the incidence of pregnancy and longitudinal observation of the babies was required to assess the scale of these problems, evaluate the causal factors and identify babies at high risk of mortality. Such longitudinal studies were lacking in India. Thus, in 1969-1973, Professor Rao and Inbaraj from the Christian Medical College, Vellore established a longitudinal study of maternal health and pregnancy outcomes. 1-5 It included mothers living within specified urban and rural areas of the North Arcot district. Besides measurements at birth, the babies were followed up during infancy for a variety of outcomes. Subsequently, the children were followed up between the ages of 6 to 8 years, and 10 to 15 years to study the effects of prenatal factors on physical growth, development and health during childhood and adolescence.
Following publications from a number of studies linking pre-natal factors and childhood growth to adult chronic diseases such as diabetes and coronary heart disease6-11, it was decided to re-trace the cohort in 1998 to 2002, then aged 26-32 years to study the relationship of maternal size, newborn size and trajectories of childhood growth to cardiovascular disease risk factors in these young south Indian adults.
What did the study cover?
The original study (1969-73) had five main objectives: (i) to study the relationship of birth weight and gestational age to infant mortality and the incidence of congenital defects; (ii) to study maternal blood pressure before and during pregnancy and the incidence of toxaemia; (iii) to assess the effects of parental consanguinity on reproductive outcomes; (iv) to examine the impact of family planning programmes on fertility; and (v) to estimate rates of fetal loss, and neonatal, infant and early childhood mortality1. The subsequent follow-up studies focused on the effects of prenatal factors, birthweight and gestational age on physical growth and development and mortality during childhood and adolescence.
For the follow-up in young adulthood (1998 -2002), the main objective was to study glucose tolerance, insulin resistance and insulin secretion, and a range of cardiovascular risk factors (body composition, blood pressure and plasma lipid concentrations) in relation to parental size, neonatal size and childhood growth.
Who is in the sample?
The original study sample was based on a population census carried out in defined areas of Vellore town and adjoining rural villages (KV Kuppam rural development block, one among 20 blocks) in the North Arcot (now Vellore) district in Tamilnadu state, situated at a distance of 160 km towards the west of Chennai(old name:Madras). These rural and urban areas consisted of 26 of 42 contiguous villages, and a third of Vellore town, representing different socio-economic strata, respectively 1-4 (Figure 1). All married nonpregnant women of childbearing potential in the study areas were identified and 20,626 women (rural: 11,628; urban: 8,998) were recruited. Women who were already pregnant were not recruited into the study. These women were visited once in 5 weeks to assess the menstrual status. During a visit, if a woman was not pregnant, a non-pregnancy follow-up was undertaken. If she was pregnant, she was included under a pregnancy follow-up. Upon delivery for such women, birth measurements were recorded and an infancy follow-up was started. (Refer table 1)
Figure 1.
Maps showing (a) Location of Vellore in India (b) Vellore district in Tamilnadu state showing KV Kuppam development block (rural area – dark grey shade) and Vellore town (urban area – light grey shade)
Table 1.
Maternal, birth and infant follow-up characteristics during the early phase of the study (1969-73)
| Category | Cohort age | Variables |
|---|---|---|
| Non-pregnancy follow-up | ||
| Socio-demographic characteristics |
Married women (15 – 45 years) |
Mother’s and father’s education mother’s and father’s occupation type of consanguinity religion housing conditions (roof, flooring, wall ownership) sanitation and water household size educational status of the highest educated member in the household |
| Parental stature | Height and weight of the father Non-pregnancy height and weight of the mother |
|
| Menstruation | Once in 5 weeks | Starting and ending date |
| Pregnancy follow-up | ||
| Prenatal factors | Once in 5 weeks | Antenatal care Illness during pregnancy |
| Obstetric characteristics |
mother’s age at delivery gravida and parity Sex of the newborn place of delivery attendant at delivery number of earlier fetal deaths birth internal gestational age number of hours since the termination, when the measurement was taken |
|
| Birth measurements | birth weight, crown–heel length, head Circumference, congenital malformation |
|
| Infant follow-up | 1, 2, 3, 6, 9 and 12 months |
breast feeding Weight, crown–heel length, head circumference morbidity reason for withdrawal from follow up Mortality (neonatal and post-neonatal) |
| Childhood | 6 – 8 years | Height, weight, IQ, morbidity, mortality |
| Adolescence | 10 – 15 years | Height, weight, blood pressures |
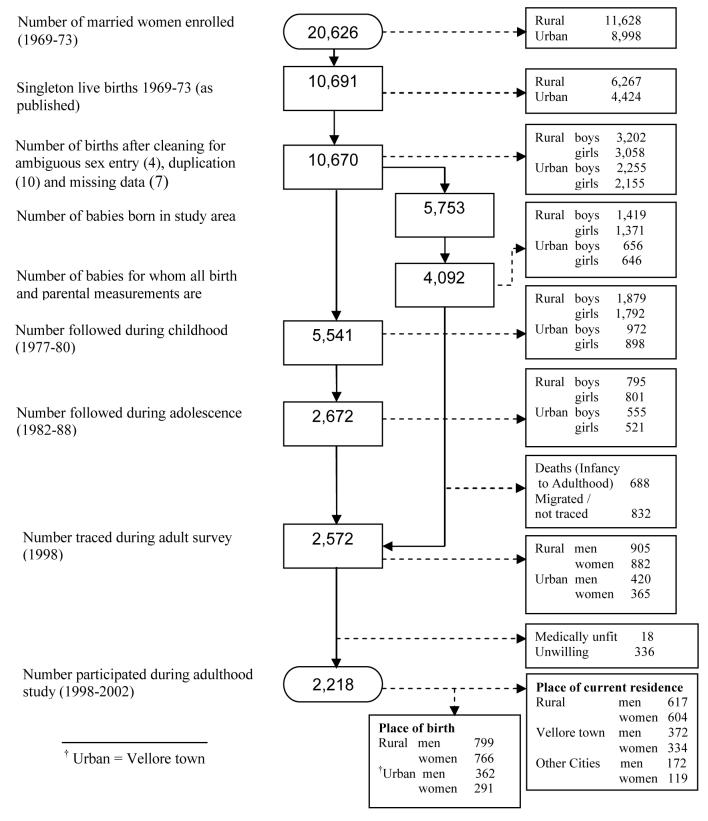
Thus, this birth cohort is defined as all children born to these women during the study period (1969-73), and comprised 10,670 single live born babies (rural: 6,260; urban: 4,410). Of these, 4,092 had complete birth and parental measurements (Figure 2). During 1998-2002, 2,572 of these 4,092 individuals were re-traced for the young adult study, and 2,218 (86.2%) participated (rural: 1,221; Vellore town: 706; major cities: 291 as per current place of residence). This sample of 2,218 subjects belong to 1939 mothers, i.e., about 20% of the subjects had at least one sibling.
Figure 2.
Flow chart showing different phases of Vellore birth cohort study
How often have they been followed up?
Pregnancy, Birth and Infancy (1969-74)
The eligible women who were included in the study were followed once in every five weeks before pregnancy, and those who conceived during the study period were also followed similarly until delivery.1-4 Live born babies were examined and measured at birth and followed at the end of each month up to the first three months and thereafter every three monthsz up to one year.
Childhood and Adolescence (1977-80 and 1982-88)
Follow-up studies at two time periods in childhood (6 to 8 years of age) and adolescence (10 to 15 years of age) focusing on physical and mental growth were carried out. During the childhood study period, 5541 children were observed with a maximum of two follow up measurements per child. About 2672 children from the original cohort were followed up on a yearly basis during the adolescent study period with a minimum of single observation per child to a maximum of six observations per child.
Young Adulthood (1998-2002)
The study team examined 2,218 subjects belonging to the original cohort during their early adulthood (26 to 32 years of age) and information on socio-economic, anthropometric and clinical aspects was collected 5.
What was the attrition like?
Of the 10,691 live births recorded, birth and parental measurements were available for 4,092. The primary reason for incomplete birth measurements was that many mothers, especially primiparas left the study area to return to their maternal residence for delivery as per traditional practice. As a consequence, firstborns are underrepresented. Infant height and weight measurements were made only up to 3 months instead of the planned 1, 2, 3, 6, 9 and 12 months. However, the other information such as head and chest circumferences, breast feeding pattern, morbidity and mortality was recorded as per the infancy follow-up plan. When the re-tracing survey was carried out prior to the young adult follow-up, it was found that out of the 4,092 babies, 688 had died during infancy, childhood and adolescence, and 832 had migrated out of the study area or could not be traced by the study team. Selected parental characteristics of the individuals studied (n=2,218) were compared with the remainder (8,452) from the original cohort.5 Characteristics such as parity (primiparas: studied 12% vs not studied 20%; p < 0.001) and education of the head of the household (illiterate: 9 and 9%; attended school ≤ 5th standard: 30 and 24%; 6–11th standard: 23 and 27%; college graduate: 3 and 4%; p < 0.001) were significantly different between the studied and not studied groups. However, there were no significant differences in parental weight and height between the respective groups.
Selected early variables of the individuals between the studied (n=2,218) and the remainder (n=1,874) for whom the birth and parental measurements were available (n=4,092) were also compared. Differences in birthweight (2813 g versus 2762 g, p = 0.002) and infant weight at 3 months (4859 g versus 4704 g, p < 0.001) were small but attained statistical significance because of large sample size. The body size during childhood and adolescent was similar between the studied and not studied groups.
Of the 2,218 subjects studied in the final time point, the height and weight measurements were available for 1638 (74%) subjects during infancy, 1532 (69%) subjects during childhood and 1743 (78%) subjects during adolescence. Complete measurements for all time points were available for 1093 (50%) subjects.
What has been measured?
Maternal nutrition, birth measurements and follow-up during infancy
Weight and height were measured for all women recruited into the study before pregnancy and their husbands. During pregnancy, morbidity status was recorded every month and (for a sub-sample) blood pressure in each trimester. Gestational age was estimated in completed weeks from the first day of the last menstrual period (LMP) to the date of delivery. Obstetric and socio-demographic characteristics were recorded in detail. Weight, crown-heel length, crown rump length, head circumference and chest circumference were measured in all live born babies within 48 hours of birth. These measurements were also obtained every month for the first three months, and selectively at 6, 9 and 12 months. Besides these, breast feeding practices, morbidity and mortality status and reason for withdrawal of infants during follow up were recorded. Table 1 provides the summary of the information collected during pre-pregnant, pregnant and infant period.
Physical growth during childhood and adolescence
During childhood, mortality and morbidity information for the first 6-8 years was collected retrospectively. Indices of physical growth (height and weight) and mental development (mental age, intelligence quotient) were obtained. During adolescence, physical growth (height and weight) and blood pressure measurements were obtained.
Cardiovascular risk factors in young adulthood
Between the ages of 26 to 32 years, information on socio-economic status, education, family history of disease, tobacco and alcohol consumption, diet and physical activity was obtained by questionnaire. Height, weight, waist and hip circumferences, and skinfold thicknesses were measured. Blood pressure was recorded and blood samples were taken for assay of fasting plasma glucose, insulin, fibrinogen and lipid concentrations and oral glucose tolerance test was carried out5 (Table 2). Subjects were classified as having impaired glucose tolerance (IGT), impaired fasting glucose (IFG) or diabetes (DM) according to WHO criteria.12 Besides, DNA samples were collected from these subjects for further genetic inquiry with reference to cardiovascular risk factors.
Table 2.
Socio-demographic, anthropometric and biochemical characteristics collected at young adulthood during 1999-2002 (Cohort age 26 – 32 years)
| Category | Variables |
|---|---|
| Socio-economic status | education occupation type of house number of rooms in the house number of people living in the house water and sanitation material possessions number of houses / dwelling owned farm land ownership (rural only) farm animals ownership (rural only) farm equipment ownership (rural only) |
| Diet | food frequency questionnaire (over 3 months) (animal proteins, Beverages, fruits and vegetables, high caloric foods) |
| Life style | physical activity intake of tobacco / narcotic in any form alcohol consumption |
| Medical history | blood pressure diabetes angina or heart pain heart attack or myocardial infarction stroke family history of illness consanguinity |
| Anthropometry | height, weight, waist, head circumference, hip |
| Skin folds | mid upper arm circumference, triceps, biceps, subscapular, supra–iliac, mid-thigh circumference, thigh skin fold |
| Blood pressure | systolic, diastolic, pulse |
| Blood Samples | plasma glucose (fasting, 30, 60, 120 minute) plasma insulin (fasting, 30, 60, 120 minute) plasma fibrinogen plasma lipids (cholesterol, Trig, HDL, LDL) |
What has the Vellore birth cohort study found?
The initial publications from the birth cohort highlighted the magnitude of the problems of maternal undernutrition, blood pressure, fertility and reproductive loss13-15, infant mortality, congenital malformations and low birthweight and preterm births in this south Indian population. It was found that the average body mass index (BMI) of pre-pregnant mothers was very low at 18.6 and 19.4 kg/m2 in rural and urban areas respectively.16 About 32% of singleton births weighed less than 2500 gram at birth and 18.4% were born before 37 weeks of gestation (for whom birthweight and gestational age was available).17 The general fertility rate was 172 per 1000 women in the childbearing age (rural 185.5; urban 154.4).14 A little over one-fifth of the pregnancies resulted in foetal loss (overall 23.0%; rural 23.1%; urban 22.9%).1 The incidence of congenital malformation was 9.5 per 1000 live births.18 The overall infant mortality rate was 85.9 per 1000 live births for whom the birthweight and gestational age was available. Highest mortality rates were seen in gestational ages under 28 weeks (369.2 per 1000 live births) or birthweight less than 1500g (568.2 per 1000 live births).18
Consanguineous marriages were extremely common. 47% and 29% of all marriages were consanguineous in the rural and urban areas respectively.4 When compared to non-consanguineous, consanguineous marriages showed slightly higher rates of foetal loss (rural 21.1% vs 22.9%; urban 20.8% vs 22.1%), congenital malformations (rural 7.7 vs 5.9; urban 9.7 vs 12.2) and infant mortality (rural 93.6 vs 106.7; urban 93.3 vs 106.9). Though the differences were statistically significant due to large sample size, the effect of consanguinity on reproductive outcomes was small.19
The relationship of birthweight (BW) and gestational age (GA) with respect to physical growth and development in childhood (6 to 8 years) was studied. Babies with slow intrauterine growth rate (BW ≤ 2250g and GA ≥ 37 weeks) had the lowest mean values for both body weight and stature in childhood; those with normal (BW > 2250g and GA ≥ 37 weeks) the highest, while babies with fast intrauterine growth rate (BW > 2250g and GA < 37 weeks) had intermediate mean values.20 The mean systolic and diastolic blood pressure levels in rural adolescents were stable over the age range 10-15 years in both sexes, while their urban counterparts showed a consistent increase with age.21 The temporal changes in the distribution of birthweights was studied comparing the original birth cohort (1969-73) with births that occurred during 1989-93 in the same study area. The mean birthweight showed a marginal increase of 70g from 2274.5g ± 500.2 to 2845.4g ± 451 and the proportion of low birthweight (< 2500g) reduced significantly from 27.2% to 15.9% in rural and 19.1% to 10.8% in urban. 22 The positive changes which occurred in some of the health indicators over 20 years i.e., between 1969-73 and 1989-93 are recorded as follows. The crude birth rate decreased from 35.9 to 24.0 and 37.8 to 21.1 per 1000 in rural and urban respectively. The infant mortality rate decreased from 102 to 58.9 and 106.6 to 62.8 per 1000 live births in rural and urban respectively. The average number of children in a family decreased from 3.5 to 2.2 and 3.6 to 2.3 in rural and urban respectively.23
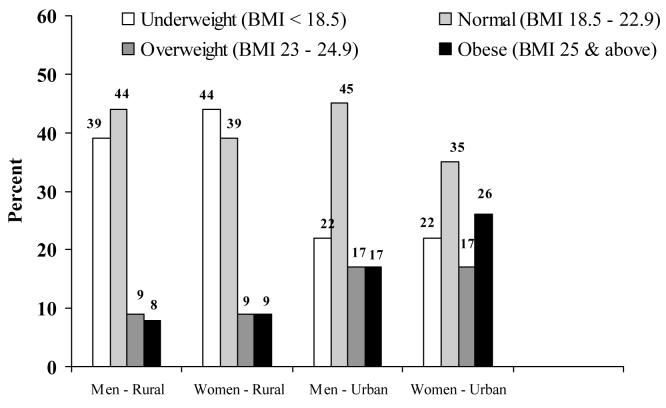
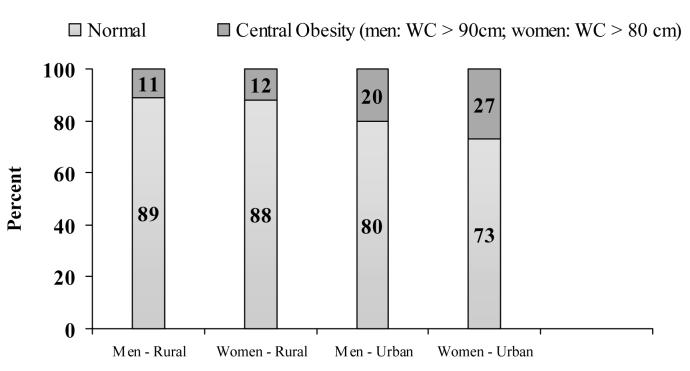
Recent work had focused on the prevalence of IGT and diabetes, and other cardiovascular risk factors and their associations with adult life style factors of this cohort. The prevalence of Type 2 DM and impaired glucose tolerance (IGT) was higher in urban areas than in rural (3.7% versus 2.1%; 18.9% versus 14.3%, respectively), while the prevalence of impaired fasting glycaemia (IFG) was similar in urban and rural populations (3.8% versus 3.4%)5. The prevalence of underweight, normal weight, overweight and obesity are displayed in figure 3 corresponding to BMI cut-offs at 18.5, 23.0, 25.0 kg/m2 for Asian adults as defined by IOTF.24 Figure 4 displays the prevalence of central obesity using waist circumference (90cm for men and 80cm for women) 24 in these subjects.
Figure 3.
Prevalence of under weight, normal weight, overweight and obesity in young adults
Figure 4.
Prevalence of central obesity in young adults
The relationships of IGT, diabetes, insulin resistance and insulin increment to parental size, size at birth and growth in infancy, childhood and adolescence were examined in detail. Shorter maternal height was associated with IGT in young adults. The highest risk of IGT or DM (OR 5.9, 95% CI 2.9-11.8) was in subjects in the lowest third of childhood BMI and the highest third of adult BMI. IGT/diabetes and insulin resistance was associated with rapid BMI gain between childhood/adolescence and adult life. Intergenerational effects on birthweight were also studied in the 1969-73 cohort and their offspring. A low birthweight (LBW mother had 2.8 times risk (95% CI 1.2 - 6.4) of delivering a LBW baby and a LBW father was twice as likely to produce a LBW baby (OR 2.2; 95% CI 1.0 - 4.8). Every 100g increase in maternal BW was associated with an increase in offspring BW of 14g; the equivalent figure for paternal BW was 18.1g (p<0.001 for both).25 Further analyses on the pattern of metabolic syndrome in the young adults and its relationship to birthweight and childhood BMI are forthcoming.
What are the main strengths and weaknesses?
Strengths
Birth cohort studies are rare in India, and the detailed birth and longitudinal childhood growth data in the Vellore cohort are unique in south India. Several of the nutritional features (low maternal BMI, low birthweight, child malnutrition, infant feeding methods), marriage practices (high rate of consanguineous marriages) and adult lifestyle characteristics (high rates of smoking and alcohol consumption and low physical activity levels in the urban cohort) in the Vellore cohort are distinct from developed countries.
The Vellore birth cohort is population-based and represents both rural and urban areas. Birth measurements other than birthweight were recorded, measurements were carried out prospectively by trained personnel with uniform criteria, and accurate gestational ages were available. Very few birth cohort studies have studied the effect of parental weight, height and BMI on long-term health outcomes in the offspring.
DNA samples stored from the young adult cohort subjects is an important resource for studies of the effects of genes and gene-environment interactions on the emerging epidemic of obesity, metabolic and cardiovascular diseases.
Weaknesses
Major weaknesses are that about half of the original births were delivered outside the study area due to customary practice. Although the study planned to collect measurements throughout infancy, in practice these measurements were made only up to three months in most cases. Like many other birth cohort studies, there was considerable attrition due to mortality and migration by the time of the adult follow-up – 21% of the original cohort took part in this study.
Can I get hold of the data? Where can I find more?
The study’s data is not freely available. Specific proposals on future collaboration would be welcomed. For further information contact investigators based in Vellore or Southampton.
Acknowledgements
The initial cohort study on pregnancy outcomes was financed by the US National Center for Health Statistics, Public Health Service, Department of Health, Education, and Welfare, Washington DC, USA. (Agreement No. 01-657-2 NCHS-IND- 7). We would like to record our gratitude to the late Dr. Jacob Yerushalmy, formerly Professor and Head of the Biostatistics Division, School of Public Health, University of California, Berkeley for his guidance as a Project Officer for this study. Subsequent follow up during childhood and adolescence was supported by the Indian Council of Medical Research. The adult follow-up study was funded by the British Heart Foundation (Grant No. RG\98001). We thank the men and women who took part in the study and their families as well as the field and laboratory staff for their contribution.
References
- 1.National Center for Health Statistics, Health Resources Administration, Public Health Service, US Department of Health, Education and Welfare Longitudinal Studies in Human Reproduction. 1976. (No. 01-657-2 NCHS-IND- 7)–Final Report. [Google Scholar]
- 2.Rao PSS, Inbaraj SG. Inbreeding in Tamil Nadu, south India. Soc Biol. 1977;24:281–288. doi: 10.1080/19485565.1977.9988298. [DOI] [PubMed] [Google Scholar]
- 3.Rao PSS, Inbaraj SG. Inbreeding effects on human reproduction in Tamil Nadu of south India. Ann Hum Genet. 1977;41:87–98. doi: 10.1111/j.1469-1809.1977.tb01964.x. [DOI] [PubMed] [Google Scholar]
- 4.Rao PSS, Inbaraj SG. Inbreeding effects on fetal growth and development. J Med Genet. 1980;17:27–33. doi: 10.1136/jmg.17.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghupathy P, Antonisamy B, Fall CHD, Geethanjali FS, Leary SD, Saperia J, Priya G, Rajarathinam A, Richard J. High prevalence of glucose intolerance even among young adults in south India. Diabetes Res Clin Pract. 2007;77:269–279. doi: 10.1016/j.diabres.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJP, Osmond C, Winter PD, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 7.Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith G Davey. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478–80. doi: 10.1016/S0140-6736(96)03482-4. [DOI] [PubMed] [Google Scholar]
- 8.Rich-Edwards JW, Stampfer MJ, Manson JE, et al. Birthweight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ. 1997;315:396–400. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leon DA, Lithell HO, Vagero D, et al. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915-29. BMJ. 1998;317:241–45. doi: 10.1136/bmj.317.7153.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50-60 years. BMJ. 1996;312:406–10. doi: 10.1136/bmj.312.7028.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hales CN, Barker DJP, Clark PMS, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–22. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Part I; Diagnosis and Classification of Diabetes Mellitus. World Health Organisation; Geneva: 1999. (WHO/NCD/NCS99.2) [Google Scholar]
- 13.Rao PSS, Inbaraj SG, Subramaniam VR. Blood pressure measures among women in south India. J of Epidemiol and Comm Health. 1994;38:49–53. doi: 10.1136/jech.38.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao PSS, Inbaraj SG. Some aspects of pregnancy terminations in a south Indian population. Indian J Med Res. 1973;61:1247–1255. [PubMed] [Google Scholar]
- 15.Rao PSS, Inbaraj SG. Extent of perinatal loss in south Indian urban and rural populations. Indian Pediatrics. 1975;12:221–227. [PubMed] [Google Scholar]
- 16.Samuel LK, Rao PSS. Socio-economic differentials in mothers at risk based on pre-pregnancy weights & heights. Indian J Med Res. 1992;96:159–167. [PubMed] [Google Scholar]
- 17.Rao PSS, Inbaraj SG. Birth measurements of south Indian infants. Indian J Med Res. 1982;76:214–223. [PubMed] [Google Scholar]
- 18.Rao PSS, Inbaraj SG. A prospective study of infant mortality and congenital malformation in relation to intra-uterine growth rates in south India. Indian J Med Res. 1978;67:245–254. [PubMed] [Google Scholar]
- 19.Rao PSS. Inbreeding in Tamilnadu and its effects on human reproduction. Medical Genetics in India. 1978;2:121–128. [Google Scholar]
- 20.Lakshmanudu M, Venkatalakshmi V, Rao PSS. Growth of south Indian children during 6-12 years of age in relation to birthweight and gestational age. Indian Pediatrics. 1988;25:237–243. [PubMed] [Google Scholar]
- 21.Lakshmanudu M, Mani K, Rao PSS. Blood pressure levels in south Indian adolescents. Indian Pediatrics. 1992;29:715–722. [PubMed] [Google Scholar]
- 22.Antonisamy B, Rao PSS, Sivaram M. Changing scenario of birthweight in south India. Indian Pediatrics. 1994;31:931–937. [PubMed] [Google Scholar]
- 23.Antonisamy B, Sivaram M, Richard J, Rao PSS. Trends in Intra-uterine growth of single livebirths in Southern India. Journal of Tropical Pediatrics. 1996;42:339–341. doi: 10.1093/tropej/42.6.339. [DOI] [PubMed] [Google Scholar]
- 24.WHO/IASO/IOTF The Asia Pacific Perspective: Redefining Obesity and Its Treatment. Health Communications Australia Pty Ltd; 2000. p. 18. [Google Scholar]
- 25.Agnihotri B, Antonisamy B, Priya G, Fall CHD, Raghupathy P. Intergenerational Study of Trends in Human Birth Weight across Two Successive Generations. Indian J Pediatr. 2008;75:1–7. doi: 10.1007/s12098-008-0066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]