Abstract
The balance of redox is pivotal for normal function and integrity of tissues. Ischemic insults occur as results of a variety of conditions, leading to an accumulation of reactive oxygen species (ROS) and an imbalanced redox status in the tissues. The oxidant stress may activate signaling mechanisms provoking more toxic events, and eventually cause tissue damage. Therefore, treatments with antioxidants, free radical scavengers and their mimetics, as well as gene transfer approaches to overexpress antioxidant genes represent potential therapeutic options to correct the redox imbalance. Among them, antioxidant gene transfer may enhance the production of antioxidant scavengers, and has been employed to experimentally prevent or treat ischemic injury in cardiovascular, pulmonary, hepatic, intestinal, central nervous or other systems in animal models. With improvements in vector systems and delivery approaches, innovative antioxidant gene therapy has conferred better outcomes for myocardial infarction, reduced restenosis after coronary angioplasty, improved the quality and function of liver grafts, as well as outcome of intestinal and cerebral ischemic attacks. However, it is crucial to be mindful that like other therapeutic armentarium, the efficacy of antioxidant gene transfer requires extensive preclinical investigation before it can be used in patients, and that it may have unanticipated short- or long-term adverse effects. Thus, it is critical to balance between the therapeutic benefits and potential risks, to develop disease-specific antioxidant gene transfer strategies, to deliver the therapy with an optimal time window and in a safe manner. This review attempts to provide the rationale, the most effective approaches and the potential hurdles of available antioxidant gene transfer approaches for ischemic injury in various organs, as well as the possible directions of future preclinical and clinical investigations of this highly promising therapeutic modality.
Keywords: ischemia/reperfusion, ischemic injury, oxidant stress, reactive oxygen species, antioxidant, gene transfer, gene therapy, liver, heart, lung, central nervous system, intestine, superoxide dismutase, catalase, glutathione peroxidase, heme oxygenase-1, xanthine oxidase, NADPH oxidase
1.0 Introduction
1.1 Enhanced oxidant stress during ischemic conditions
Reactive oxygen species (ROS) are largely generated from mitochondrial energy metabolism via oxidative phosphorylation in the respiratory chain of eukaryotes. Because of the existence of antioxidant enzymes, such as superoxide dismutase (SOD) and catalase, and antioxidants, such as the reduced form of glutathione (GSH), as well as vitamin C and E, the redox balance is well maintained. Upon injurious insults including ischemia, inflammation, drugs, alcohol intake, or environmental pollutants, there is increased production of superoxide anion (O2−.) or other ROS from various sources resulting in the disturbance of this delicate balance. The increase in ROS consumes endogenous antioxidant compounds, such as GSH, and induces expression of antioxidant enzymes in order to maintain the redox balance. When the injury is pronounced or persistent, compensatory responses become inadequate to correct the imbalanced redox state, giving rise to oxidant stress, with activation of subsequent signaling events leading to inflammatory responses and tissue damage. Cardiac, cerebral, pulmonary or intestinal ischemic attacks often take place secondary to arterial thrombosis or emboli from other sites. In these cases, enhanced oxidant stress exists along with chronic pathologic changes within the involved vascular wall and surrounding tissue. In the event of ischemia/reperfusion (I/R)-induced donor organ damage, oxidant stress depends on the donor conditions (living donor or cadaveric), preservation method and duration, the match of tissue typing, as well as the complexity of surgical procedure of implantation. More profound oxidant stress usually occurs when the blood supply is re-established for either ischemic tissue or implanted grafts. Thus, oxidant stress represents one of the major causes of ischemic injury, and antioxidant therapy may ameliorate the injury when it is properly delivered during an optimal time window and at right doses. A variety of antioxidants, scavengers, or scavenger mimetics have been evaluated in various ischemic conditions. This review aims to provide an update of preclinical anti-oxidative interventions in various organ systems in which ischemia is a common cause of tissue damage, such as brain, heart, lung, and intestine. For I/R-associated donor organ injury, the liver is used as an example for a better understanding of the complexity of gene transfer as a therapeutic paradigm.
1.2 Antioxidant enzymes
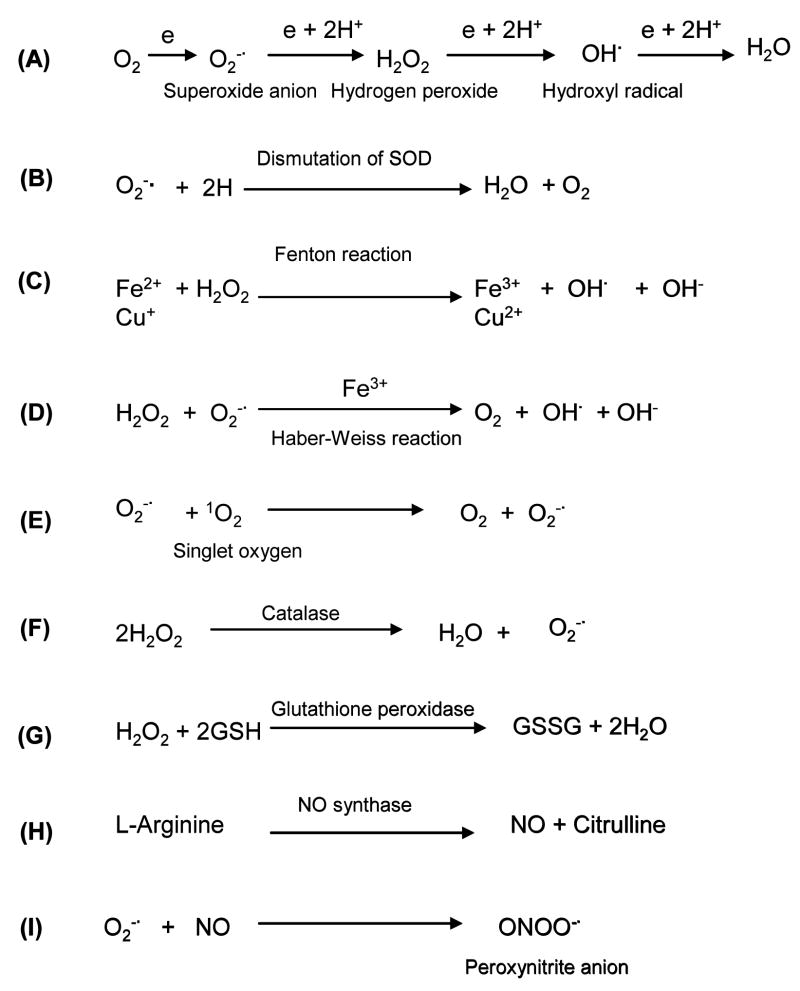
Antioxidant enzymes play a fundamental role in maintaining the delicate redox balance in the body and are essential in keeping the physiological function and in coping with oxidant stress from endogenous or exogenous sources. The gene expression of most antioxidant enzymes, such as SOD, glutathione peroxidase (Gpx), catalase or heme oxygenase-1 (HO-1), is inducible under inflammation, trauma or other stressful conditions, and this induction represents the key mechanism for the body in response to a variety of stressors. The following are common antioxidant enzymes chosen for gene delivery in preventing or treating ischemic conditions. The chemistry of their catalyzing reactions is shown in Fig. 1.
Fig. 1.
Chemical reactions involved in formation of reactive oxygen species (ROS) and actions of ROS scavengers. Common ROS include O2−., hydrogen peroxide (H2O2), hydroxyl radical (OH.) singlet molecular oxygen (1O2), nitric oxide (NO) and peroxynitrite anion (ONOO−.). SOD = superoxide dismutase (modified from Wu et al. 1999 [7]).
Superoxide dismutase (SOD), which catalyzes the dismutation of O2−. to hydrogen peroxide (H2O2), is a major ROS scavenger (Fig. 1). There are three isozymes of SOD, and each displays unique subcellular locations, and plays anti-oxidative roles in various compartments [1]. Cu/Zn-SOD is localized in the cytosol and nucleus of all cell types, and functions as the intracellular anti-oxidative system [2]. Manganese SOD (Mn-SOD) is exclusively localized in the mitochondria [3]. The unique extracellular distribution and secretory nature of extracellular SOD (EC-SOD) offer anti-oxidative protection against ROS not only in the cytosol but also in the extracellular space. Elevated SOD activity can be found in the cell lysates and culture medium after EC-SOD gene delivery [1,4,5]. This is physiologically important because O2−. existing in the extracellular space cannot cross the cell membrane to be removed by intracellular SOD [6].
Catalase, which is a potent scavenger of H2O2, provides another means of inhibiting oxidant stress [7]. It prevents the formation of more toxic HO−. when SOD is insufficient to remove O2−. overload. Insufficient catalase activity may lead to an accumulation of H2O2, which may in turn be converted into more toxic HO−. in the presence of Cu2+ and Fe2+ (Fenton reaction) or O2−. (Haber-Wiess reaction) [7,8] (Fig. 1). Thus, the addition or production of catalase will result in additional anti-oxidative activity against oxidative stress that is present under ischemic conditions. The combination of SOD and catalase gene delivery may achieve additive effects in reducing oxidant stress [9].
Glutathione peroxidase (Gpx) is a ubiquitous antioxidant enzyme that is located in both the mitochondria and the cytosol. Similar to catalase, Gpx facilitates the breakdown of H2O2 into water and oxygen. The enzyme consumes GSH as a substrate for H2O2 breakdown [7]. In in vitro studies, Gpx was shown to confer greater protection against oxidative stress than SOD, catalase, or the combination of SOD and catalase [10]. The primary defense against peroxide in the heart is via Gpx, which is depleted during ischemia [11]. In one study, herpes simplex virus (HSV) mediated Gpx overexpression in the striatum of rat brain was able to prevent subsequent ischemic damage caused by middle cerebral artery occlusion [10].
Heme oxygenase-1 (HO-1) uses NADPH as a cofactor to catalyze the oxidative degradation of heme to biliverdin, carbon monoxide and iron; and is a critical enzyme in heme metabolism [12]. Indeed, HO-1 is conserved across species. ROS are scavenged during the subsequent quick conversion of biliverdin into bilirubin catalyzed by biliverdin reductase [12]. Besides its action in heme metabolism, antioxidant role and cytoprotection may account for its potential antioxidant effect on ischemic damage of neural tissue [13], heart [14,15], kidneys [16], muscle [17] and liver [15,18–20]. Because of its function in vascular beds, a great amount of attention has been focused on inducing HO-1 expression or overexpression in the cardiovascular system to prevent ischemic myocardial injury [21].
2.0 Antioxidant Gene Transfer Vectors
The purpose of gene transfer is to introduce antioxidant enzyme genes into target organs in order to prevent or treat ischemic attack or to prevent I/R-associated donor organ damage in transplantation. To deliver functional genes to cells or tissues, viral vectors, such as adenoviral, adeno-associated (AAV), retroviral or lentiviral vectors, and non-viral vectors, for instance, liposomes, polymers, or other physical approaches or electroporation, can be used. Selecting a proper vector with a feasible delivery approach is the key to achieve sufficient and persistent transgene expression in target organs, because various viral or non-viral vectors exhibit different gene transfer efficiency, immunogenicity, toxicity and oncogenicity. For viral vectors, viral genetic background, integration site in the host genome, immunogenicity, foreign gene carrying capacity, organ tropism, infectivity, and readiness for genetic modification and for vector manipulation are the crucial factors to consider. For nonviral vectors, chemical structures of synthetic lipids or polymers, transfection efficiency, cytotoxicity, in vivo stability, tissue distribution, and feasible administration routes are the main issues to consider. The choice of the most effective vector should be tailored for a particular need. General features of common gene delivery systems are listed in Table 1 [22].
Table 1.
Characteristics of commonly used vectors in gene transfer
| Vector | Genome | Foreign gene- carrying capacity | Transfection or transduction | Genomic integration | Tropism (Examples) |
|---|---|---|---|---|---|
| Retrovirus | ssRNA | ~8.0 kb | Stable | Yes | Wide range of cells with species specificity |
| Adenovirus | dsDNA | ~7.5 kb | Transient | No | Liver, epithelial cells |
| Adeno- associated virus (AAV) | ssDNA | ~4.7 kb | Stable | No | Liver, brain, heart, muscle, bone marrow |
| SV40 | dsDNA | ~5.0kb | Stable | No | Liver, lung, spleen, lymphocytes |
| Lentivirus | ssRNA | ~5.0kb | Stable | Yes | Proliferating and non- proliferating cells |
| Herpes simplex virus (HSV) [160] | dsDNA | ~15.0kb | Stable | No | Neural tropism and spread in nerve system |
| Plasmid DNA | dsDNA | Depending on plasmid constructs | Transient | No | Most cell types |
| Liposome or polymer- mediated gene delivery | dsDNA | Depending on plasmid constructs | Transient | No | Formulation-dependent, and in most cell types |
Modified from Yen RD, et al. [22]. dsDNA = double stranded DNA; ssDNA = single stranded DNA.
One must be cognizant of the advantages and disadvantages of the various vector systems when preclinical trails are planned, because an ideal or universal gene delivery system has yet to emerge. For high gene transfer efficiency, viral vectors, such as adenoviral vector or AAV are generally used, especially for liver gene transfer because of their liver tropism [23]. However, the immune response against viral components is a major concern, and it may lead to the loss of infected recombinant viral vector and shut-down of the transgene expression [24]. AAV appears to be more promising for clinical uses than adenoviral vector because of less toxicity to the infected organ [25]. For long-term gene transfer, both retroviral vectors and lentiviral vectors can be considered because of their ability to integrate into the genome. However, low transduction efficiency in non-proliferative cells and the possibility of insertion-induced oncogenesis limit the usefulness of retroviral vectors [26]. On the other hand, lentiviral vectors have been used more frequently in recent years, due to their ability to transduce both proliferative and non-proliferative cells, lower frequency in causing insertion-elicited mutation, and improved vector safety profile [27]. For example, lentiviral vector encoding the human HO-1 gene under the control of the antioxidant response element has a special application in the development of oxidative stress-inducible gene therapy [28]. Moreover, HSV is used in the nervous system because of its natural neurotropism [29]. For non-viral vectors, hydrodynamic plasmid administration is widely used for high levels of liver transgene expression in rodents; however, it is not directly applicable for large animals or humans [30,31]. With significant modifications, minimally invasive and selective hydrodynamic gene transfer methods have been applied in pigs [32,33], non-human primates [34], and humans [33], however, the gene transfer efficiency needs to be improved. Nanoparticles made of lipids or polymers are commonly used non-viral vectors for in vivo gene transfer via topical or systemic administration [35]. They are less immunogenic, and may be more amendable for cell-specific or tissue-specific delivery than viral vectors [36]. Transient gene expression and relatively lower gene transfer efficiency are the common disadvantages [37]. Taken together, balancing the risks and benefits of gene therapy for a specific organ or disease process requires a thorough understanding of the vector systems as well as the pathophysiology of the condition.
3.0 Antioxidant Gene Therapy for Cardiovascular Disorders
3.1 Cellular mechanisms of oxidative stress injury in cardiovascular diseases
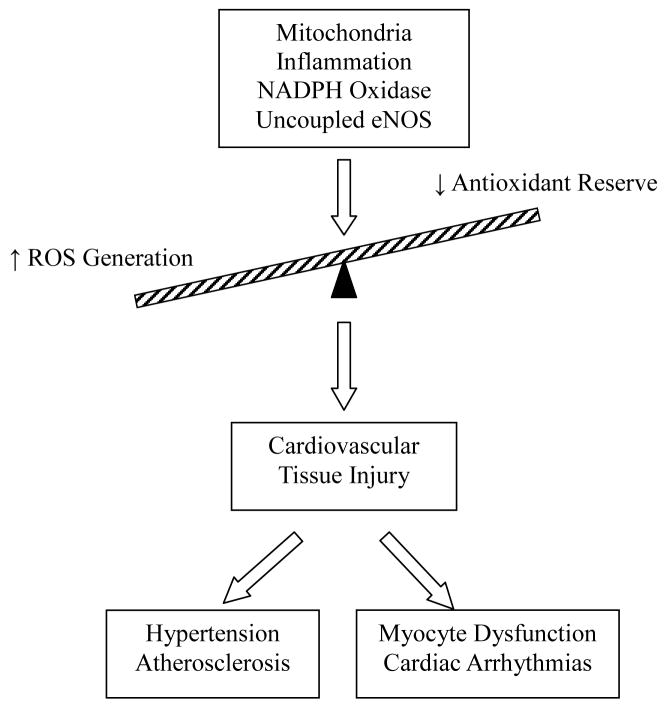
Cardiovascular diseases represent one of the most common disorders affecting Western societies. There is accumulating evidence to support the notion that oxidative injury plays a critical role in several cardiovascular diseases including myocardial infarction, myocardial I/R, atherosclerosis, endothelial dysfunction, restenosis, hypertension as well as cardiomyopathies and heart failure [38–42]. The oxidative stress-associated injury is a direct result of an imbalance between an increase in ROS production and a decrease in antioxidant reserve under various pathological processes (Fig. 2). To fully understand the potentials and challenges of antioxidant therapy, it is essential to appreciate the cellular mechanisms contributing to the imbalance which occurs during these processes [40,42].
Fig. 2.
Diagram depicting an imbalance in cellular redox state from an increase in the production of ROS and a decrease in antioxidant reserve under various pathological processes leading to tissue injury.
ROS generated in vascular endothelial cells were through NADPH oxidase (Nox), uncoupled endothelial NO synthase (NOS) and byproduct of mitochondrial respiration (Fig. 2). The overall effects of the oxidative stress result in an increase in Ca2+ entry into cardiac myocytes, alteration in gene expression and activation of distinct signal transduction pathways. The resultant Ca2+ overload thus triggers wide spread injury on subcellular organelles in cardiac myocytes, including sarcolemma, sarcoplasmic reticulum (SR), mitochondria, myofibrils and nucleus. Specifically, ROS lead to lipid peroxidation and protein oxidation. Lipid oxidation leads to disruption of the membrane lipid bilayer as well as the production of cytotoxic metabolites. On the other hand, protein oxidation causes structural oxidation of sulfhydryl groups or methionine residues leading to conformational changes, fragmentation and polymerization.
Redox-sensitive proteins contain highly conserved cysteine residues, and their oxidation, nitrosylation or the formation of disulfide bonds are crucial events in the redox signaling. Changes in the redox state of proteins play an important role in many cellular functions including gene transcription, cellular metabolism, ionic homeostasis and signal transduction. Previous studies have documented that activity of various ion regulatory proteins can be modulated by redox-dependent mechanisms. Redox-mediated alteration in the activity of ion channels and pumps results in a significant increase in intracellular Ca2+ during I/R injury. Specifically, ROS generation during I/R injury directly affects Ca2+-handling proteins including ryanodine receptors (RyR), SR Ca2+-ATPase (SERCA) as well as inositol 1,4,5-trisphosphate (IP3)-induced Ca2+ release channel [43]. Previous studies have shown that reagents that oxidize thiols activate cardiac RyR channels, but inhibit the SERCA pump. Moreover, the redox modulation of RyR activity is mediated by the redox modification of sulfhydryl groups of cysteine residues. Other key components of cardiac excitation–contraction coupling such as the SERCA and L-type Ca2+ channel are also subject to redox modulation. We and others have previously shown that the pore-forming subunit of the L-type Ca2+ channel contains functionally important free sulfhydryl groups that modulate gating of the channel. Exposure of the channels to thiol oxidizing agents results in a significant reduction in L-type Ca2+ current [44,45]. Furthermore, ROS, presumably acting as oxidants of the thiol groups, also decreased Ca2+ currents [46]. A significant increase in ROS generation during reperfusion of the ischemic heart resulting in changes in the activity of the major Ca2+-handling proteins in cardiac myocytes is likely to play an important role in I/R-related intracellular Ca2+ overload [43].
Exposure to ROS also triggers the activation of a distinct signaling pathway as well as alteration of gene expression. For example, H2O2 has been shown to upregulate the gene expression of growth factor in vascular endothelial cells and c-fos and c-jun in neonatal cardiomyocytes. Activation of these genes was shown to be associated with the activation of transcription factors including NF-κB and activator protein-1 (AP-1). Indeed, both NF-κB and AP-1 represent one of the redox-sensitive molecular targets, and the activation of these transcription factors is a prerequisite for induction of pro-inflammatory gene expression.
3.2 Antioxidant gene therapy for cardiovascular diseases
Despite the strong evidence supporting the link between oxidative stress and cardiac injury in various types of cardiovascular diseases, the efficacy of oral antioxidant therapy has largely been disappointing. It has been suggested that a more targeted delivery of antioxidant therapy via gene transfer may provide a means to accomplish this goal. Indeed, a large number of studies to date have provided exciting proof-of-concept that this targeted approach may be beneficial in different pathological conditions. These studies have utilized a wide array of well described animal models for cardiovascular diseases. Adenoviral vectors were used in most studies with a few exceptions where AAV or retroviral vectors were used.
HO-1 is one of the best characterized protective genes in cardiovascular diseases and represents one of the stress-inducible enzymes. The beneficial effects of HO-1 gene transfer have been documented in several animal models including hypertension in spontaneously hypertensive rats (SHR) [47], cardiac I/R injury [14,48,49] and carotid or femoral artery balloon angioplasty [50,51]. Specifically, one study using a rat model of cardiac I/R showed that HO-1 gene transfer resulted in the reduction in infarct size. This was accompanied by decreases in myocardial lipid peroxidation, proapoptotic protein Bax and proinflammatory cytokine interleukin-1β. In addition, there was a concomitant increase in antiapoptotic Bcl-2 protein level suggesting that HO-1 may exert its cardioprotective effects in part by reducing oxidative stress, associated inflammation and apoptotic cell death [48]. Treatment with HO-1 gene transfer was also associated with a reduction in fibrosis and ventricular remodeling as well as the restoration of left ventricular function and chamber dimensions after myocardial infarction.
Vascular injury is characterized by inflammation with a local reparative process, supporting a potential protective role for HO-1 in arterial repair. Previous studies have demonstrated that induction of HO-1 attenuated neointima formation after experimental vascular injury [50]. Induction of the HO-1 pathway reduced the severity of vascular injury by at least two adaptive mechanisms independent of nitric oxide [51]. HO-1 directly reduced vasoconstriction and inhibited cell proliferation during vascular injury. Expression of HO-1 in arteries stimulated vascular relaxation, mediated by guanylate cyclase and cGMP, independent of nitric oxide. In addition, HO-1 directly inhibited vascular smooth muscle cell growth, and the inhibition was mediated by cell-cycle arrest. In addition, HO-1 adenoviral gene transfer has been shown to decrease atherosclerotic lesions in an apoE mouse model of atherosclerosis [52]. These studies suggest that HO-1 may represent an important in vivo vasoprotective mediator that is capable of attenuating the pathophysiological remodeling response to endovascular injury.
Gene therapy with SOD including EC-SOD, Mn-SOD, Cu/Zn-SOD has been tested in a variety of animal models. EC-SOD has been used successfully to decrease blood pressure in SHR model [53], to reduce restenosis in balloon angioplasty models [54,55] as well as to reduce infarct size in a cardiac I/R model [56,57]. EC-SOD gene therapy markedly reduced infarct size in a rabbit model of myocardial infarction with 30 minutes of ischemia followed by reperfusion compared to control group. A more recent study further supported the proof-of-concept from earlier studies demonstrating that pre-emptive gene therapy using recombinant AAV delivery of EC-SOD protects heart against I/R injury, improves ventricular function, and prolongs survival in a rat model [57]. The EC-SOD gene was delivered 6 weeks prior to myocardial injury. Significant myocardial protection was documented by the decrease in infarct size at 24 hour post I/R, associated with an improved left ventricular function at 7 weeks post injury, as well as enhanced long-term survival in the EC-SOD-treated group. The study provides evidence for beneficial and cardioprotective effects of ‘pre-emptive’ gene therapy via expression of a secreted protein that affords a paracrine cardioprotective action against future injury [57].
Similarly, Mn-SOD and Cu/Zn-SOD have also been shown to result in similar beneficial effects in cardiac I/R injury [58,59]. The possible mechanisms underlying the cardioprotective effects of Mn-SOD and Cu/Zn-SOD have been investigated in a rat model of cardiac I/R injury using recombinant adenoviruses expressing Mn-SOD or Cu/Zn-SOD to modulate superoxide levels in the mitochondrial or cytoplasmic compartments, respectively. Ectopic expression of both Mn-SOD and Cu/Zn-SOD provided cardioprotection from I/R injury, as evidenced by a significant reduction in infarct size as well as apoptotic cell death compared to controls. Moreover, Mn-SOD and Cu/Zn-SOD expression significantly delayed the activation of NF-κB and AP-1 transcription factors following I/R injury. Further analysis of pro- and anti-apoptotic genes and protein expression revealed that ectopic SOD expression resulted in changes in gene expression consistent with protection against apoptosis. The study supports the notion that both mitochondrial and cytoplasmic-derived SOD isozymes provide cardioprotection against I/R injury, and that their beneficial actions are derived, at least in part, from the alteration in AP-1 and NF-κB activation resulting in an overall antiapoptotic effect [58].
One of the new exciting areas of intense investigation that merit closer examination is the use of antioxidant therapy in balloon injury. Percutaneous coronary intervention (PCI), commonly known as coronary angioplasty, is a widely used technique for the treatment of acute coronary syndrome as well as chronic ischemic heart diseases. The procedure is used to treat the stenotic coronary arteries due to atherosclerosis secondary to the build up of cholesterol-laden plaques. Previous studies have documented that balloon angioplasty induces an acute increase in systemic oxidative stress, local O2−. generation and decreased vascular SOD activity [60–62]. The procedure is associated with a significant incidence of restenosis. Therefore, drug-eluting coronary stents (or scaffolds) are widely used that are placed into narrowed, diseased coronary arteries allowing a slow release of drugs to block cell proliferation while the stents further prevent the recoil and restenosis of the vessels. Drug-eluting stents in current clinical use were approved by the FDA after clinical trials, showing that they were statistically superior to bare-metal stents for the treatment of native coronary artery narrowing, having lower rates of major adverse cardiac events. First-generation drug-eluting stents were coated with either rapamycin or paclitaxel to prevent restenosis. However, some studies have found an increased risk for late stent thrombosis [63,64]. Several novel stent systems are being investigated in order to address these issues. A recent study has demonstrated beneficial effects of EC-SOD gene therapy in the prevention of restenosis using balloon angioplasty in animal models [55]. Hence, local therapy using viral vectors directed toward oxidative stress may thus represent an attractive strategy against stent-induced vascular injury in atherosclerotic disease.
4.0 Antioxidant Gene Therapy for Pulmonary Diseases
4.1 Oxidant stress and the lungs
In the context of oxidative stress and injury, the lung represents a unique organ in the body since it is directly exposed to higher oxygen tensions as well as environmental oxidants. ROS can be generated either endogenously by metabolic reactions and lipid peroxidation products during activation of circulating inflammatory cells as well as exogenously from air pollutants or cigarette smoke. Most living cells, including lung cells, generate free radicals under normal conditions. Similar to the cardiovascular system, an imbalance of the oxidative stress and antioxidant mechanisms occurs during these pathologic states. Indeed, there is increasing evidence that ROS generation plays a major role in a wide range of pulmonary diseases including asthma, chronic obstructive pulmonary disease (COPD), I/R injury post transplantation, parenchymal lung diseases, for example, idiopathic pulmonary fibrosis and lung granulomatous diseases as well as malignancies. Asthma and COPD are inflammatory lung diseases that are characterized by systemic and chronic inflammation and oxidative stress. Moreover, it is now recognized that oxidative stress represents one of the main pathologic factors that perpetuate the disease progression in COPD.
4.2 Antioxidant gene therapy for pulmonary diseases
Recent studies have suggested that antioxidant enzymes delivered to the lung can improve alveolar epithelial and/or endothelial cell function. In many instances, gene transfer was the avenue leading to localized expression of these antioxidant enzymes [65]. In addition, nonviral vectors using immunotargeting liposomes or transposon have also been used successfully in different species. These studies have documented the beneficial effects of antioxidant enzymes in a wide range of pulmonary diseases including I/R injury post transplantation, pulmonary toxicity post irradiation and viral infection.
Lung transplantation has become an accepted therapy for end-stage pulmonary diseases by offering the possibility of improved quality of life as well as life expectancy. However, transplanted lungs are especially prone to I/R injury as compared to other transplant organs, since the lungs received the entire cardiac output and are exposed to higher oxygen tension and ROS. Despite advances in tissue preservation and improvement in surgical techniques, I/R injury remains a common complication post lung transplantation. I/R injury has been suggested to be one of the major risk factors for the development of primary acute graft failure post transplantation and is characterized by endothelial dysfunction, capillary leak, and an intense neutrophilic inflammatory reaction in the lung parenchyma. Moreover, the development of I/R injury has been correlated with the later progression of obliterative bronchiolitis. Clinically, I/R injury is seen as a progressive deterioration in gas exchange, opacification of the lung on chest X-ray, and increased pulmonary vascular resistance within 24 hours post transplantation.
Even though the precise cellular mechanisms of I/R injury post transplantation have not been fully elucidated, it has been recognized that inflammation and ROS-induced endothelial dysfunction represent a critical component in the pathophysiology of lung I/R injury [66,67]. Several other mechanisms have also been identified including adhesion and sequestration of activated leukocytes through disrupted endothelium followed by cytotoxic disruption of lung parenchyma, complement activation, platelet activation, increased endothelin-1 expression, and release of inflammatory cytokines [68].
Several therapeutic strategies aimed at disrupting a variety of these pathways have been shown to attenuate I/R injury in animal models of lung transplantation. Enhanced pulmonary endothelial oxidative stress is associated with profound vascular leak and extravasation of inflammatory cells, leading to necrosis and programmed cell death in lung grafts. Accordingly, recent studies have shown that immunotargeting of catalase, conjugated with an antibody to platelet-endothelial cell adhesion molecule-1, to donor rats augmented the antioxidant capacity of the pulmonary endothelium, reduced oxidative stress and I/R injury, and improved the function of transplanted lung grafts [67]. One recent study has taken the advantage of the preferential expression of angiotensin-converting enzyme (ACE) in pulmonary capillaries [69]. Conjugates of ACE monoclonal antibody (MAb) 9B9 with catalase (9B9-CAT) were evaluated in vivo using lung I/R injury in rats. The treated animals exhibited a significantly lower degree of lung injury compared with control rats including decreased serum levels of endothelin-1 and decreased expression of inducible NOS. Taken together, these studies support the potential protective effects of antioxidative enzymes of the pulmonary endothelium [67,69].
Additional studies have tested the roles of indoleamine-2,3-dioxygenase (IDO), a unique cytosolic enzyme possessing both T cell-suppressive and antioxidant properties, in I/R injury in a rat model of lung transplantation [70,71]. Nonviral gene transfer approaches were used including sleeping beauty transposon that can mediate long-term transgene expression [70] as well as targeted delivery with cationic polymer [71]. It was shown that enhanced IDO activity prevented endothelial cell apoptosis, reduced vascular permeability and leukocyte extravasation. These biological effects were associated with an improvement in graft function and survival [71]. This nonviral gene therapeutic approach led to persistent and uniform transgene expression in the rat lung tissue without significant toxicity and inflammation.
One antioxidant enzyme that has been under intense investigation is HO-1. Recent experimental data suggested that the stress-inducible HO-1 may play an important role in host’s defense against oxidant-induced lung injury [72]. Specifically, HO-1 is involved in the resolution of inflammation by modulating apoptotic cell death or cytokine expression. Overexpression of exogenous HO-1 gene provides a therapeutic effect in a murine model of acute lung injury caused by type A influenza virus. HO-1 overexpression resulted in suppression of pathological changes, intrapulmonary hemorrhage as well as inflammatory cells in the lung associated with enhanced survival of animals. HO-1 gene transfer reduced cellular apoptosis. Since oxidative stress and lung injury are involved in many lung disorders, such as pneumonia induced by a variety of microorganisms and pulmonary fibrosis, HO-1 may be clinically useful for gene therapy targeting a wide variety of lung disorders including acute pneumonia and pulmonary fibrosis [72].
Pulmonary toxicity is a major complication of irradiation to the lung or total body irradiation used in patients prior to bone marrow transplantation. Antioxidant enzymes have been shown to be beneficial in irradiation-induced lung injury [73,74]. Intratracheal injection of Mn-SOD plasmid/liposome prior to irradiation in mice resulted in increased survival compared to irradiated controls. The treatment significantly reduced late irradiation damage. These results support the potential benefit of intrapulmonary Mn-SOD gene delivery in lung cancer patients for protection of normal lung tissue from irradiation while allowing effective tumor control [73].
5.0 Antioxidant gene therapy for ischemic liver disorders
Due to the dual blood supply system (arterial and venous), the liver is less vulnerable to suffer ischemic injury caused by portal vein thrombosis (PVT) or superior mesenteric artery embolism or thrombosis. For PVT, the main clinical features are the manifestations of the primary disease leading to PVT plus progressive portal hypertension and its complications. Patients with myeloproliferative disorder, cirrhosis, Budd-Chiari syndrome, post-living donor, or liver transplant recipients have a higher risk to spontaneously develop PVT. Primary hepatocellular carcinoma (HCC) and pancreatic carcinoma may cause occlusion of the portal vein due to indirect invasion and local compression. Superior mesenteric artery embolus or thrombus predominantly manifests the symptoms of intestinal ischemia. Selective hepatic artery branch embolization in combination with chemotherapeutic agents as an adjuvant treatment for unresectable liver cancer can also lead to ischemic liver injury [75], and, I/R-associated donor damage is a major challenge in liver transplant, especially when marginal liver grafts are used for small-for-size or living donor transplant. In these cases, pronounced oxidant stress arises from fatty or/and fibrotic changes in marginal grafts, the organ procurement, and transplant procedure.
5.1 Ischemia/reperfusion-induced liver injury and oxidant stress
Hepatic I/R injury occurs when there is a transient blockage of blood supply to the liver and subsequent re-establishment of the blood supply. The severity of the damage depends on the duration of ischemia, but the injury process is more extensive during the reperfusion period than the ischemia [76]. The injury is characterized by the production of toxic ROS, disturbance of the microcirculation, and activation of the coagulation system. The injury is classified into three types: warm, cold ischemic and rewarming, depending on whether the ischemic organ is located in situ (warm), or undergoing cold ischemia preservation (cold). The rewarming ischemia typically occurs during manipulation of the graft when the cold liver is subjected to room or body temperature while performing the vascular reconstruction [77].
The pathogenesis of hepatic I/R injury has been studied for nearly three decades, and many crucial elements of the pathogenesis have been identified [77,78]. Development of hepatic I/R injury can be divided into initial and late phases [79]. The initial phase (less than 2 hours after reperfusion) is characterized by oxidative stress, where production and release of ROS appear to directly result in hepatocellular injury. Our recent study showed that there was a significant increase in O2−. and H2O2 levels in liver homogenates during the early stage of the reperfusion [9]. The late phase of liver injury from 6 to 48 hours after hepatic reperfusion is an inflammatory disorder thought to be mediated by recruited neutrophils. The cells release proteolytic enzymes and ROS, which contribute to the damage of hepatocytes and sinusoidal endothelial cells (SEC). The primary ROS generated by neutrophils include O2−., HO−., H2O2, and their derivatives, such as ONOO−.. Activated neutrophils also release elastase, cathepsin G, heparanase, collagenase and hydrolytic enzymes that appear to be directly cytotoxic to hepatocytes [77,78].
Our recent investigation with a sensitive chemiluminescent probe, MCLA (2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo [1,2-α] pyrazin-3-one), for O2−. and singlet oxygen, demonstrated that there is enhanced chemiluminescence of O2−. during the early stage of the reperfusion, as early as 5 min after the beginning of the reperfusion, and that EC-SOD gene delivery almost completely eliminated the oxidant stress and attenuated the liver injury caused by the I/R procedure [9]. O2−. is a central and initial species that is readily converted to other species when SOD activity is inadequate [8]. A significant amount of O2−. is thought to be generated by infiltrative neutrophils, activated Kupffer cells, and damaged hepatocytes during the I/R procedure [80]. The release of O2−. in hepatic I/R injury was demonstrated based on the fact that allopurinol, a specific inhibitor of xanthine oxidase (XO), attenuated hepatic I/R injury [81]. Recently, attention has been paid to Nox, which represents a major source of ROS in the microcirculation. The subunits of the membrane-bound enzyme catalyze the reduction of molecular O2 to O2−., and ultimately H2O2, using NADPH as the electron donor [82]. The structure and function of Nox are well characterized. They are composed of the cytoplasmic subunits p40phox, p47phox and p67phox and the GTP-binding protein Rac as well as the membrane-bound cytochrome-b558 complex (consisting of p22phox and gp91phox). The cascade of events required for Nox activation is thought to be initiated by the phosphorylation of p47phox, enabling it to bind to gp91phox. This activation results in the well-characterized generation of O2−.. Our recent study suggests that Nox activation is a major source of increased O2−. generation during hepatic I/R-induced injury because the use of a Nox-specific inhibitor, apocynin, decreased MCLA chemiluminescent signal intensity and partially prevented the injury in a warm hepatic I/R model [83]. A study with Nox knock-out mice suggests that Nox-derived O2−. from neutrophils or Kupffer cells may contributes to elevated O2−. levels [84]. O2−. originating from a variety of cell types may be released into the extracellular space when cells are injured, and cause toxicity to other surrounding cell types. Accumulated O2−. in the extracellular space and intracellular subcellular compartments may be converted to more toxic H2O2, HO−., or ONOO−. in the presence of H+, H2O2, and nitric oxide (NO) [8]. These O2−.-derived ROS participate in the inflammatory process, and are critical components in the mediation of apoptotic and/or necrotic cell death of parenchymal cells and SEC [77].
It has been determined that both necrosis and apoptosis occur in hepatic I/R injury, and that during the extended ischemic phase, necrosis is a dominant pathway of cell death; whereas, during the late phase of reperfusion apoptosis accounts for the majority of the cell death. Thus, the entire I/R procedure is an oncotic process [85,86]. Growing evidence supports the notion that oxidant stress is the major initiator in eliciting signaling pathways that lead to the onset of necrosis/apoptosis during the hepatic I/R procedure, especially in the early stage of the process. This establishes the rationale for the use of antioxidants, free radical scavengers, and antioxidant and anti-apoptotic gene transfer for the prevention of hepatic I/R-associated injury, as well as improvement of donor organ quality, function and survival after transplant.
5.2 Antioxidant gene therapy for hepatic ischemia/reperfusion injury
Providing more SOD activity by delivering the enzyme or SOD mimetics has been shown to be effective in preventing a variety of forms of liver injury [87]. However, the half-life of SOD is only six minutes [87]. Therefore, gene delivery of SOD, catalase or in combination may offer better protection against ROS overload, minimize the potential toxicity of SOD mimetics [8], and overcome the short half-life of the enzyme in the body. Adenoviral vectors of Cu/Zn-SOD, Mn-SOD, and EC-SOD have been shown to protect against warm I/R injury in mouse liver [88,89]. In these studies, adenoviral EC-SOD vectors were found to be somewhat less effective than other two SOD isoform vectors [90]. It seems that sufficient gene expression is critical because clear protection was only attained when the adenoviral titer was increased, and a 3-fold increase in liver SOD activity was reached [89]. Moreover, it is known that adenoviral vectors possess a number of disadvantages [91]. For long-term antioxidant gene transfer, we have developed a recombinant lentiviral vector encoding Cu/Zn-SOD. The portal vein injection of lentiviral vector led to sustained SOD activity in mouse liver for one month (the length of the experiment), and the overexpression of Cu/Zn-SOD prevented carbon tetrachloride (CCl4)-induced liver injury in mice [92].
We have significantly improved a non-viral gene delivery approach by polymerizing a cationic lipid to form polymerized cationic lipid (PCL) [93,94]. Polylipid nanoparticles (PLNP) are co-formulated from PCL and cholesterol, and are small in size (approximately 150 nm in diameter). These PLNP are non-cytotoxic in vitro, and not toxic to the liver in vivo. They are also very stable in serum-containing medium [93]. These serum-resistant PLNP have the least serum-binding activity in comparison with other commercially available cationic lipids when tested in vivo [93]. PLNP gave rise to substantial transgene expression in the liver when PLNP-DNA complexes (polyplexes) were administered through the portal vein, and when thyroid hormone (T3) was employed as a stimulus for hepatocyte regeneration [93]. In our studies, PLNP-mediated EC-SOD or catalase gene delivery led to an approximately 10-fold increase in liver SOD and catalase activity, and to a 50-fold increase in EC-SOD mRNA levels. The elevated SOD or catalase activity in the liver protected mice from hepatic I/R injury [9]. Our findings demonstrate that mice receiving either EC-SOD and/or catalase gene delivery markedly reduced O2−. and H2O2 levels, lowered serum levels of ALT, restored liver GSH content and reversed liver MDA content. These findings coincided with a striking increase in SOD or catalase activity, and their overexpression at the mRNA and protein levels [9]. This approach is being formulated for the next stages of organ transplantation experiments and large animal studies [9,95].
In addition to SOD or catalase, other antioxidant enzyme genes have been also used in the prevention of hepatic I/R injury. In an early study, treating genetically obese Zucker rats with the HO-1 inducer, cobalt protoporphyrin (CoPP), or with adenoviral HO-1 (Ad-HO-1) vector significantly improved portal venous blood flow, increased bile production, and decreased hepatocyte injury. Following cold ischemia/isotransplantation, HO-1 overexpression induced by CoPP or Ad-HO-1 therapy significantly extended animal survival. This effect correlated with preserved hepatic architecture, improved liver function, and suppressed infiltration by T cells and macrophages [20]. The protective role of HO-1 gene upregulation was further documented in different models of immune-mediated acute liver injury [19]. In a separate study, prior administration of an adenoviral vector encoding inducible HO-1 prevented remote liver injury during systemic inflammatory responses to limb ischemia [18]. Thus, there exists growing evidence that induction of endogenous HO-1 expression or overexpressing HO-1 by gene transfer is beneficial in preventing hepatic I/R injury in which oxidant stress is critical for its pathogenesis.
Instead of direct delivery of antioxidant genes, inhibiting Nox activation by targeting upstreaming signaling molecules, such as Rho-kinase, has been investigated in a rat model of hepatic I/R injury [96]. An adenoviral vector was used to deliver the dominant-negative Rho-kinase gene in rats with Kupffer cell depletion. The overexpression of dominant-negative Rho-kinase interrupted the function of native Rho-kinase activity and down-stream Nox activation in hepatocytes, reduced ROS production, suppressed the release of pro-inflammatory cytokines, and ameliorated the lethal liver injury with a significant prolongation of survival. These results suggest that the Rho-kinase-mediated pathway plays a major role in ROS production through Nox in hepatocytes during an early phase of reperfusion, and they suggest a new therapeutic target for the prevention of primary graft failure in liver transplantation [96].
In summary, most gene transfer studies are performed prior to donor organ harvest, and the level and duration of transgene expression are critical for the function of implanted grafts. To date, no study is available to address whether an antioxidant gene can be delivered during the window of donor organ procurement and implantation, or after grafts are implanted. The delivery of an antioxidant gene during the transplant window or post-transplant would be helpful for addressing oxidant stress during the reperfusion period and graft failure after transplant. Neither viral nor non-vector systems are fully optimized for clinical use yet. For example, in the context of our nonviral PLNP system, more studies are needed to critically analyze the best administration routes, dose and time points of PLNP delivery, as well as to evaluate the efficacy, species differences in immune responses, and adverse effects in large animals. All these are necessary to translate the approach to clinical use.
6.0. Ischemic intestinal damage and antioxidant therapy
Intestinal ischemia occurs when the main trunk or branches of the superior mesenteric artery (SMA) is blocked by embolus or thrombosis as part of systemic thrombosis, temporary occlusion of the mesenteric artery during abdomen surgery. The incidence of intestinal ischemia has increased steadily over the past 25 years due to the advanced mean age of the population, increasing number of critically ill patients, and a greater recognition of the condition [97]. SMA embolism accounts for 50% of acute mesenteric arterial ischemia (AMI) with sudden severe abdominal pain. Emboli most commonly originate from thrombi found in the left side of heart, and are dislodged secondary to a cardiac arrhythmia, less commonly from atherosclerotic plague in the artery or a thrombosed aortic aneurysm. SMA thrombosis accounts for 15% of AMI, and may present acutely or as a slowly evolving chronic disorder [97]. The key treatment for AMI is surgical revascularization, often with resection of necrotic bowel; and angiographic techniques play an adjunctive but critical role. Adjuvant drug therapies include vasodilators, thrombolytics, and anticoagulant agents [97]. Increasing attention has been paid to antioxidants based on enhanced oxidant stress in this I/R process. Some antioxidants are classic, for example ascorbic acid, vitamin E and their analogues [98] or others having been clinically approved for other conditions (mannitol, N-acetyl cysteine, pentoxifylline) [99,100]. Another class of agents includes newly synthesized or extracted natural compounds, such as compound IA (an antioxidant, non-steroidal anti-inflammatory agent) [101], green tee polyphenol extract [102], mesna [103], tempol [104], etc. However, most of these agents have been tested only in animal experiments, and whether they will be evaluated in clinical trials remains unclear [105]. To date, there is no report available concerning the use of antioxidant gene transfer as a therapeutic approach for AMI or chronic non-occlusive superior mesenteric ischemia. However, HSV-mediated Mn-SOD gene delivery was used to prevent enteritis caused by radiation in mice [106].
6.1 Rationale for the use of antioxidants as therapeutics for superior mesenteric ischemia
Like ischemic conditions in many other organs, the damage to the intestinal tract caused by reperfusion following an ischemic insult is an example of oxidative injury, and the evidence exists that oxidative stress plays a critical role in this process [107]. XO is one of major sources of abundant O2−., as evidenced by the fact that that increased permeability secondary to ischemia can be mimicked by local intra-arterial injection of hypoxanthine and XO [108], and that enhanced leukocyte-endothelium interactions following colonic I/R in mice were largely abrogated by pretreatment with SOD or allopurinol [109]. Allopurinol is an inhibitor of XO, and has been shown to be protective against I/R-induced injury in the liver [83]. Our recent study demonstrated that Nox is critical for hepatic I/R-induced injury [83]; whereas, no similar report has been seen with intestinal ischemic damage. Further evidence supporting the role of oxidant stress in the pathogenesis of intestinal ischemic injury is that GSH levels were decreased following reperfusion in rats [103]. These changes occurred concurrently with increased MDA levels in animals and patients with intestinal ischemia [109,110]. Moreover, a preconditioning procedure to the small intestine prevented subsequent standard I/R insult to the same segment of the gut. N-ω-nitro-L-arginine, a blocker of NO synthase, inhibited the role of pre-ischemic conditioning [111], suggesting that NO synthesis is involved in the preischemic conditioning-associated protection. The role of NO production in intestinal ischemic injury is dichotomous. NO production may be beneficial to the vasodilation of ischemic tissue; on the other hand, excessive NO accumulation is cytotoxic and proapoptotic for vascular endothelial cells and mucosal epithelial cells [112]. The accumulation of neutrophils in the ischemic area is thought to be a major element in causing tissue damage in several ways, by releasing proteolytic enzymes and ROS, and by causing the physical impairment of the microcirculation and thereby extending the ischemic duration of the involved tissue [107]. In fact, neutrophils might be the major sources of O2−. via the Nox pathway as proved in other systems [113].
Other mediators during intestinal ischemic injury include endothelins (ETs) [114], inducible heat shock proteins (HSPs) [107], and HO-1. ETs are potent and long-lasting vasconstrictive peptides. Together with increased neutrophil infiltration, and microvascular and mucosal dysfunction, ETs contribute to the pathogenesis of I/R-induced intestinal damage. Endothelial receptor antagonists, BQ485+BQ788, improved microscopic damage, reversed increased blood flow resistance, and reduced infiltration of neutrophil infiltration as evidenced by reduced myeloperoxidase activity in ischemic intestinal tissue [114]. Inducible HSPs are intracellular stress proteins (HSP-70 and HSP-72 and HSP-73) that have been shown to accumulate in the ischemic area [107], and increased HSP levels may be associated with protection by pre-ischemic conditioning. As noted earlier, HO-1 is a stress-inducible protein capable of modulating inflammation, oxidative stress, and cell death. HO-1 was found to be highly expressed in the mucosa of ischemic colitis [115], and CoPP-induced upregulation of HO-1 before a warm I/R insult resulted in significant reduction of intestinal tissue injury [116]. These factors together with ROS, neutrophil accumulation and other factors contribute to the further progression of tissue damage in a complicated interplay [107], and are targets of novel therapeutics.
6.2 Antioxidant treatments of intestinal ischemia/reperfusion injury
The main objective of the treatment is the restoration of blood flow to normal levels in ischemic vascular beds. Importantly, if bowel is rendered ischemic for more prolonged periods, then antioxidants and other treatments have no effect, indicating that the oxidant damage is only operant in the initial reversible phase of tissue damage. There appears to be a critical time point of 6–8 hours after infarction [97], and beyond this point, the damage to the intestinal tract will be irreversible. Thus, early diagnosis of acute superior mesenteric embolus or thrombosis at a time point where the intestine has not lost its viability is the key for the implementation of treatments and for improving the survival and prognosis of patients. The guidelines for proper diagnosis and surgical and pharmacologic managements of AMI are covered elsewhere [97,105]. The following is the summary of recent attempts in the use of antioxidant and biological therapy for minimizing intestinal I/R injury, mostly in animal experiments.
6.2.1 Antioxidant enzymes and mimetics
The usefulness of SOD alone in the prevention of intestinal I/R injury has been documented in animal experiments, and co-use with catalase may achieve better protection in animal studies. However, SOD has a very short half-life in its activity, and its clinical application is limited. SOD mimetics exert similar activity, are permeable for the cell membrane, and have a longer bioactivity. M40401, a new Mn-based SOD mimetic, was tested in a rat model of intestinal I/R injury. M40401 significantly improved animal survival after splanchnic artery occlusion, decreased the extent of tissue damage in ileum sections, and MDA levels and MPO activity, as well as neutrophil infiltration. The prior administration of the mimetic exhibited potent SOD activity as indicated by much lower ONOO−. levels in the tissue. Reduced neutrophil infiltration will also decrease the source of O2−. from these cells. Therefore, it appears that an SOD mimetic has its potential use in the treatment of intestinal I/R injury. Viral and non-viral delivery of SOD isoform and catalase genes has been used in I/R-induced injury in other organs [9,57]. It is intriguing to investigate whether antioxidative gene delivery would achieve greater protection against injury when compared to administration of SOD or its mimetic, because the production of the transgene should extend the protective effects; Mn-SOD gene delivery is beneficial for radiation-caused enteritis [106]; and oral gene delivery approach has been developed [117].
6.2.2 Erythropoiethin
Erythropoiethin (EPO) is a glycoprotein cytokine produced primarily by the kidneys in regulation of red blood cell production, and is commonly used in the clinics for patients after chemotherapy or anemia. Besides its angiogenic action, anti-inflammatory and antiapoptotic property, EPO has been shown to be antioxidative and cytoprotective. These features are mediated through its receptor, consisting of a heteromeric complex containing an EPO receptor and a β common receptor subunit [118]. A recent study showed that before and after an intestinal I/R insult, EPO administration partially reversed the tissue damage, restored tissue catalase activity, reduced MDA levels, neutrophil MPO activity and apoptotic cell count, as well as minimized eNOS expression. Thus, EPO is effective in attenuating intestinal I/R-induced injury in rats, and the protective effects are associated with its antioxidative and cytoprotective features [119]. This study offers a possibility that exogenous EPO delivery might be replaced by endogenous production via a mode of gene transfer approach to the gut.
In summary, occlusive acute mesenteric artery embolism or thrombosis-induced ischemia requires an immediate surgical attention when there are peritoneal signs. Indications for pharmacological treatments are those who have an early diagnosis and do not need surgical interventions. The treatments mainly include thrombolytics and vasodilators. For chronic mesenteric ischemia, both surgical and pharmacotherapy can be considered [120]. Vasoconstrictive agents may potentiate the ischemia and cause irreversible damage to the ischemic tissue, and should be avoided. Efficacy of antioxidant or biological treatments has not been verified in randomized clinical trials. Thus, whether these new strategies will be used in clinical medicine depends on the results of clinical studies. However, some of the antioxidant therapeutic options discussed in this section suggest new directions for clinical investigation of these modalities.
7.0 Antioxidant Gene Transfer for Ischemic Cerebral Diseases
7.1 Scales of the clinical problems
CNS ischemia occurs due to an interruption of blood flow, most often secondary to cardiac arrest, embolus, thrombus, or hypotension. Predictable exposure to focal brain ischemia may occur during neurosurgical resection, carotid endarterectomy, cardiac artery bypass (CABG) or total circulatory arrest procedures [121]. Risks of spinal cord ischemia leading to paraplegia after thoracic aneurysm surgery can be as high as 40% [122]. Currently, there are no effective therapeutic interventions. Therefore, the ability to successfully deliver and express exogenous antioxidant gene sequences to the CNS has widespread clinical application after injury or in neurodegenerative disease.
When the balance between free radical production during normal cellular respiration and free radical degradation is disrupted due to ischemia or reperfusion, tissue damage can occur due to increased production of ROS [123,124]. There are multiple pathways of cellular damage that can result from increased ROS, including signaling cascades, disruption of lipid membranes, increased excitatory amino acids (EAA), initiation of apoptosis, protein modification, and DNA damage [125]. As described in the preceding sections, the delivery of anti-oxidant gene sequences such as SOD, Gpx, or HO-1 has been used to attenuate post-ischemic injury.
7.2 Specific targets in the CNS
A number of targets for antioxidant gene delivery have been identified in the CNS. ROS are thought to play a role in Parkinson’s disease [126,127], and amyotrophic lateral sclerosis (ALS) [128–130], as well as in ischemia. HO-1 is elevated in the oxidative stress that occurs after traumatic brain injury (TBI) in humans [131]. Growing evidence suggests that HO-1 is not only important in the degradation of heme, but also possesses antioxidant properties, regulates vascular tone, and prevents apoptosis in endothelial cells [132]. Overexpression of this protective enzyme is a strategy to increase ROS conversion, particularly after hemorrhage [132], which should be especially useful after stroke.
Several animal models have successfully targeted SOD. For example, intraperitoneal injection of Tat-SOD fusion protein in a gerbil model protected against transient forebrain ischemia [133]. Neuronal nitric oxide synthase (nNOS) mediated nitration and inactivation of MnSOD is a potential target due to enhanced peroxynitrite production [134]. Mn-SOD nitration mediated by adriamycin has also been linked to CNS toxicity [135], and can in part be limited by elevation of brain GSH [136]. Overexpression of Cu/Zn-SOD to block mitochondrial caspase activation also protected neurons in a global ischemia model [137].
There have been several animal models that target post-injury apoptosis through antioxidant gene over-expression or delivery. As mentioned earlier, HSV-mediated Gpx overexpression in the striatum of rat brain was able to prevent subsequent ischemic damage, to improve neural survival, and to reduce neuronal oxidant stress and apoptotic death [10]. In another study, catalase overexpression protected neurons in a stroke model [138] by inhibiting cytochrome C release and by increasing Bcl-2, an anti-apoptotic protein. Inhibition of apoptosis is involved in many of these antioxidant strategies, including delivery to ‘sensitive to apoptosis gene’ (SAG). An Ad-CMV-SAG expression in mouse brain protected from ischemic injury by decreased ROS and apoptosis [139]. Finally, neuronal apoptosis inhibitory protein (NAIP) prevented apoptosis in the brain in response to multiple stimuli including ischemia [140], and it is clear that there is a synergy between reducing apoptosis through antioxidant targeting.
Cerebral I/R leads to upregulation of the 12/15-lipoxygenase, which leads to edema and microvascular injury [141]. Treatment with lipoxygenase inhibitors, such as siRNA, blocked injury and reduced edema, and offers another target for gene expression. Another strategy that has been implemented with both adenoviral and lentiviral vectors is the use of the Nrf2 transcription factor to drive the antioxidant response element (ARE) in NRF2-expressing astrocytes [142,143]. Oxidative injury in rodent models of focal ischemia has been shown in the endoplasmic reticulum [144]. Oxidative ischemic stress results in TNK3 activation through kainate receptor glutamate receptor 6 (GluR6) binding to PSD95, via MLK3 signaling. Delivery of the antioxidant N-acetylcysteine blocked this activation [145].
7.3 Non-viral delivery of HSP mRNA or cDNA for CNS ischemia
In our own work, we hypothesize that cellular survival can be improved by expressing therapeutic anti-oxidant genes in the CNS using non-viral delivery of DNA or mRNA vectors. The long-range goal of such a therapy is to prevent or minimize CNS damage by the introduction of intracellular nucleic acids that encode for anti-oxidant proteins but avoids the risks of viral vectors. Delivery and rapid expression of appropriate anti-oxidant gene sequences in the CNS can minimize ischemic CNS and spinal cord injury, provided that delivery and distribution can achieve widespread gene expression in the CNS. To develop clinically applicable approaches for neural gene transfer, we take advantage of cerebral spinal fluid (CSF), which is an effective way to express foreign genes in the brain [146–148]. This technique is intended for those clinical applications in which the risks of the CNS injury are sufficiently high so as to justify the potential relatively minor risks of intrathecal CNS delivery, such as after tissue plasminogen activator treatment to minimize the reperfusion injury. mRNA offers advantages over DNA vectors for many clinical applications, because transfected mRNA is available for translation once delivered into cytoplasm and is therefore expressed much more rapidly than DNA, whereas DNA must be transported into the nucleus for transcription before transport back to the cytoplasm [149–151]. The short duration of expression with mRNA transfection is also an advantage in some clinical situations.
7.3.1 Heat shock proteins in antioxidant strategies
The heat shock proteins (HSPs), in particular HSP70, appear to be involved in the regulation of many stress responses, from cellular to the entire organism. For example, HSP70 helps regulate cellular redox status in ischemia [152], possibly through a mechanism whereby nuclear HSP70 blocks oxidative stress induced high mobility group box 1 protein (HMGB1) cytoplasmic translocation [153]. The Cu/Zn-SOD mutation is responsible for approximately 10% of familial ALS, and overexpression of Hsp70 or Hsp27 prevents apoptosis in a mutant Cu/Zn-SOD model [154]. Gene transfer therapy with an HSV vector expressing HSP70 improved neuron survival after focal cerebral ischemia and kainate-induced excitotoxicity [155,156], and this was likely due at least in part to the role in preventing apoptosis caused by the oxidative stress of excitotoxicity. In a similar example, hippocampal neurons transfected with the HSP70 gene using an HSV vector improved neuron survival in a rat model of middle cerebral artery occlusion [157]. Recent reports also demonstrate a role for HSP70 after CNS injury. A gutted HSV vector [158] provided some of the first in vivo evidence for cellular protection using HSP70 after injury, and this is thought in part to be due to the antioxidant interaction of HSP70.
In summary, the delivery of antioxidant genes to CNS by viral or non-viral vector is possible. Non-viral vectors may offer advantages over viral methods in both safety and ease of use. The CSF provides an efficient access to the brain and spinal cord. It seems that the far-reaching and efficient distribution, uptake and expression of our RNA and DNA vectors are due to non-specific bulk transport, facilitated by cardiac oscillations along intra-parenchymal peri-vascular channels and across less-than-tight junctions in the brain [151,159].
8.0 Prospects and Conclusions
Ischemic diseases in cardiovascular and CNS account for the majority of morbidity and mortality worldwide, and the incidence is increasing due to an aging population. Ischemic injury occurs when there is reduced blood supply or complete occlusion of artery. The causes for ischemic insults vary from organ to organ, and rupture of atherosclerotic plagues with resultant formation of thrombi represents a major cause for acute ischemic injury in heart, brain, lung, intestinal tract and other organs. Intermittent constriction or compression from outside of vessels also causes a reduced or cessation of blood supply. Lung, heart and liver transplantation remains the only effective therapy for end-stage lung, heart or liver diseases. Due to the scarcity of donor organs, majority of patients who qualify for transplantation will not be transplanted, and eventually die on the waiting lists. For the same reason, marginal or small size grafts are used for transplant. Recipients with marginal or small size grafts have a higher possibility of developing graft failure and severe I/R-associated injury.
Both ischemic damage and I/R-associated donor injury share similar pathologic pathways, i.e. enhanced oxidant stress and ROS-triggered signaling mechanisms leading to cell death via apoptosis and/or necrosis. Indeed, growing evidence has demonstrated that therapy using antioxidants, free radical scavengers or their mimetics, as well as antioxidant gene transfer can be beneficial by reducing oxidant stress, blocking the activation of signaling mechanisms leading to cellular apoptosis, attenuating tissue damage, and promoting tissue recovery. Thus, the antioxidant therapy at an early stage is considered as an adjuvant regimen in a variety of ischemic disorders.
Gene therapy as a therapeutic paradigm holds tremendous promise for different types of genetic deficiencies, cancer and varieties of ailments. With improvements in vector systems and gene transfer approaches, successful preclinical or clinical trails are encouraging in exploring this treatment in ischemic disorders or I/R-induced injury in organ transplant. Overexpression of SOD gene has been shown to be beneficial for the recovery of cardiac ischemic attack and prolongs animal survival in preclinical experiments. Induction of endogenous HO-1 expression or HO-1 gene transfer prevents experimental vascular injury. Anti-oxidant enzyme or its gene transfer benefits not only for a variety of lung disorders, including COPD, irradiation lung damage, but also for lung graft function and survival in recipients. Antioxidant gene transfer resulted in much reduced hepatic I/R injury and improved function and survival of fatty liver grafts in experiments with rodents. Although no gene transfer study has been reported with ischemic intestinal attack so far, antioxidants are shown to be effective in attenuating the injury. Gpx gene delivery has been shown to prevent ischemic stroke in rat, even with post-ischemic gene transfer, and the neuroprotective mechanism involves attenuation of apoptosis-related events [10]. Even though the results of these translational studies are encouraging, several critical issues remain unanswered, and further investigations to examine the usefulness of various antioxidant gene transfer approaches are warranted. For example, large animal studies are required to evaluate the beneficial effects of this new treatment paradigm. The most advantageous type of vectors to be used for certain tissue or organ as well as the particular enzymes or combination thereof is likely to be additional keys to successful treatment. Both timing for gene delivery and long-term follow up are critical. Moreover, safety concerns are paramount; hence, side effect profiles need to be carefully determined. The possibilities of these innovative gene transfer approaches are far reaching once they prove to be feasible and safe for clinical use. Millions of patients with ischemic disorders may benefit from this new treatment for their illness, and more donor organs will function and survive in recipients with less I/R injury.
Acknowledgments
The work described in the review article is partially supported by grants from the NIH and the Technology Transfer Grant by the UC Davis Medical Center to J.W. (DK069939), J.G.H. (NS1960 & NS046591), and N.C. (HL75274, HL85727, HL85844 & VA Merit Review Award).
Abbreviations in this manuscript
- AAV
adeno-associated virus
- ALT
alanine aminotransferase
- CSF
cerebral spinal fluid
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- CoPP
cobalt protoporphyrin
- DVT
deep vein thrombosis
- EC-SOD
extracellular superoxide dismutase
- Gpx
glutathione peroxidase
- GSH
reduced form of glutathione
- HO-1
heme oxygenase-1
- HSV
herpes simplex virus
- I/R
ischemia/reperfusion
- MDA
malondialdehyde
- Nox
NADPH oxidase
- PLNP
polylipid nanoparticles
- PVT
portal vein thrombosis
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SEC
sinusoidal endothelial cells
- XO
xanthine oxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10.0 References
- 1.Karlsson K, Sandstrom J, Edlund A, Marklund SL. Turnover of extracellular-superoxide dismutase in tissues. Lab Invest. 1994;70:705–10. [PubMed] [Google Scholar]
- 2.Venugopal SK, Wu J, Catana AM, Eisenbud L, He SQ, Duan YY, Follenzi A, Zern MA. Lentivirus-mediated superoxide dismutase1 gene delivery protects against oxidative stress-induced liver injury in mice. Liver Int. 2007;27:1311–22. doi: 10.1111/j.1478-3231.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Gu J, Zhao L, He L, Qian W, Wang J, Wang Y, Qian Q, Qian C, Wu J, Liu XY. Complete elimination of colorectal tumor xenograft by combined manganese superoxide dismutase with tumor necrosis factor-related apoptosis-inducing ligand gene virotherapy. Cancer Res. 2006;66:4291–8. doi: 10.1158/0008-5472.CAN-05-1834. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Liu L, Yen RD, Catana A, Nantz MH, Zern MA. Liposome-mediated extracellular superoxide dismutase gene delivery protects against acute liver injury in mice. Hepatology. 2004;40:195–204. doi: 10.1002/hep.20288. [DOI] [PubMed] [Google Scholar]
- 5.Marklund SL. Extracellular superoxide dismutase. Methods Enzymol. 2002;349:74–80. doi: 10.1016/s0076-6879(02)49322-6. [DOI] [PubMed] [Google Scholar]
- 6.Winterbourn CC, Stern A. Human red cells scavenge extracellular hydrogen peroxide and inhibit formation of hypochlorous acid and hydroxyl radical. J Clin Invest. 1987;80:1486–91. doi: 10.1172/JCI113230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Danielsson A, Zern MA. Toxicity of hepatotoxins: new insights into mechanisms and therapy. Expert Opin Investig Drugs. 1999;8:585–607. doi: 10.1517/13543784.8.5.585. [DOI] [PubMed] [Google Scholar]
- 8.Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–90. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- 9.He SQ, Zhang YH, Venugopal SK, Dicus CW, Perez RV, Ramsamooj R, Nantz MH, Zern MA, Wu J. Delivery of antioxidative enzyme genes protects against ischemia/reperfusion-induced liver injury in mice. Liver Transpl. 2006;12:1869–79. doi: 10.1002/lt.21001. [DOI] [PubMed] [Google Scholar]
- 10.Hoehn B, Yenari MA, Sapolsky RM, Steinberg GK. Glutathione peroxidase overexpression inhibits cytochrome C release and proapoptotic mediators to protect neurons from experimental stroke. Stroke. 2003;34:2489–94. doi: 10.1161/01.STR.0000091268.25816.19. [DOI] [PubMed] [Google Scholar]
- 11.Zhu HL, Stewart AS, Taylor MD, Vijayasarathy C, Gardner TJ, Sweeney HL. Blocking free radical production via adenoviral gene transfer decreases cardiac ischemia-reperfusion injury. Mol Ther. 2000;2:470–5. doi: 10.1006/mthe.2000.0193. [DOI] [PubMed] [Google Scholar]
- 12.Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:1767–812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 13.Hegazy KA, Dunn MW, Sharma SC. Functional human heme oxygenase has a neuroprotective effect on adult rat ganglion cells after pressure-induced ischemia. Neuroreport. 2000;11:1185–9. doi: 10.1097/00001756-200004270-00008. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Simpson JA, Brunt KR, Ward CA, Hall SR, Kinobe RT, Barrette V, Tse MY, Pang SC, Pachori AS, Dzau VJ, Ogunyankin KO, Melo LG. Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H48–59. doi: 10.1152/ajpheart.00741.2006. [DOI] [PubMed] [Google Scholar]
- 15.Pachori AS, Melo LG, Zhang L, Solomon SD, Dzau VJ. Chronic recurrent myocardial ischemic injury is significantly attenuated by pre-emptive adeno-associated virus heme oxygenase-1 gene delivery. J Am Coll Cardiol. 2006;47:635–43. doi: 10.1016/j.jacc.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Blydt-Hansen TD, Katori M, Lassman C, Ke B, Coito AJ, Iyer S, Buelow R, Ettenger R, Busuttil RW, Kupiec-Weglinski JW. Gene transfer-induced local heme oxygenase-1 overexpression protects rat kidney transplants from ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:745–54. doi: 10.1097/01.asn.0000050760.87113.25. [DOI] [PubMed] [Google Scholar]
- 17.Pachori AS, Melo LG, Hart ML, Noiseux N, Zhang L, Morello F, Solomon SD, Stahl GL, Pratt RE, Dzau VJ. Hypoxia-regulated therapeutic gene as a preemptive treatment strategy against ischemia/reperfusion tissue injury. Proc Natl Acad Sci U S A. 2004;101:12282–7. doi: 10.1073/pnas.0404616101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarter SD, Badhwar A, Scott JR, Akyea TG, Bihari A, Dungey AA, Harris KA, Potter RF. Remote liver injury is attenuated by adenovirus-mediated gene transfer of heme oxygenase-1 during the systemic inflammatory response syndrome. Microcirculation. 2004;11:587–95. doi: 10.1080/10739680490503384. [DOI] [PubMed] [Google Scholar]
- 19.Sass G, Soares MC, Yamashita K, Seyfried S, Zimmermann WH, Eschenhagen T, Kaczmarek E, Ritter T, Volk HD, Tiegs G. Heme oxygenase-1 and its reaction product, carbon monoxide, prevent inflammation-related apoptotic liver damage in mice. Hepatology. 2003;38:909–18. doi: 10.1053/jhep.2003.50386. [DOI] [PubMed] [Google Scholar]
- 20.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631–9. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pachori AS, Melo LG, Zhang L, Solomon SD, Dzau VJ. Chronic recurrent myocardial ischemic injury is significantly attenuated by pre-emptive adeno-associated virus heme oxygenase-1 gene delivery. Journal of the American College of Cardiology. 2006;47:635–43. doi: 10.1016/j.jacc.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 22.Yen RD, Zern MA, Wu J. Molecular therapy for hepatic fibrosis. In: Weber D, editor. Trends in Chemotherapy Research. Nova Science Publishers, Inc; Hauppauge, N.Y: 2006. pp. 1–23. [Google Scholar]
- 23.Brunetti-Pierri N, Ng P. Progress and prospects: gene therapy for genetic diseases with helper-dependent adenoviral vectors. Gene Ther. 2008;15:553–60. doi: 10.1038/gt.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartman ZC, Appledorn DM, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz BR, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther. 2008;16:1189–99. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehse B, Roeder I. Insertional mutagenesis and clonal dominance: biological and statistical considerations. Gene Ther. 2008;15:143–53. doi: 10.1038/sj.gt.3303052. [DOI] [PubMed] [Google Scholar]
- 27.Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36:184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 28.Hurttila H, Koponen JK, Kansanen E, Jyrkkanen HK, Kivela A, Kylatie R, Yla-Herttuala S, Levonen AL. Oxidative stress-inducible lentiviral vectors for gene therapy. Gene Ther. 2008 doi: 10.1038/gt.2008.75. [DOI] [PubMed] [Google Scholar]
- 29.Lachmann R. Herpes simplex virus-based vectors. International Journal of Experimental Pathology. 2004;85:177–90. doi: 10.1111/j.0959-9673.2004.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–66. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 31.Suda T, Liu D. Hydrodynamic gene delivery: its principles and applications. Mol Ther. 2007;15:2063–9. doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 32.Alino SF, Herrero MJ, Noguera I, Dasi F, Sanchez M. Pig liver gene therapy by noninvasive interventionist catheterism. Gene Ther. 2007;14:334–43. doi: 10.1038/sj.gt.3302873. [DOI] [PubMed] [Google Scholar]
- 33.Khorsandi SE, Bachellier P, Weber JC, Greget M, Jaeck D, Zacharoulis D, Rountas C, Helmy S, Helmy A, Al-Waracky M, Salama H, Jiao L, Nicholls J, Davies AJ, Levicar N, Jensen S, Habib N. Minimally invasive and selective hydrodynamic gene therapy of liver segments in the pig and human. Cancer Gene Therapy. 2008;15:225–30. doi: 10.1038/sj.cgt.7701119. [DOI] [PubMed] [Google Scholar]
- 34.Brunetti-Pierri N, Stapleton GE, Palmer DJ, Zuo Y, Mane VP, Finegold MJ, Beaudet AL, Leland MM, Mullins CE, Ng P. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol Ther. 2007;15:732–40. doi: 10.1038/sj.mt.6300102. [DOI] [PubMed] [Google Scholar]
- 35.Wolff JA, Rozema DB. Breaking the bonds: non-viral vectors become chemically dynamic. Mol Ther. 2008;16:8–15. doi: 10.1038/sj.mt.6300326. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Nantz MH, Zern MA. Targeting hepatocytes for drug and gene delivery: emerging novel approaches and applications. Front Biosci. 2002;7:d717–25. doi: 10.2741/A806. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Szoka FC., Jr Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–49. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 38.Abrescia P, Golino P. Free radicals and antioxidants in cardiovascular diseases. Expert Rev Cardiovasc Ther. 2005;3:159–71. doi: 10.1586/14779072.3.1.159. [DOI] [PubMed] [Google Scholar]
- 39.Cantor EJ, Mancini EV, Seth R, Yao XH, Netticadan T. Oxidative stress and heart disease: cardiac dysfunction, nutrition, and gene therapy. Curr Hypertens Rep. 2003;5:215–20. doi: 10.1007/s11906-003-0023-z. [DOI] [PubMed] [Google Scholar]
- 40.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 41.Higashi Y, Nishioka K, Umemura T, Chayama K, Yoshizumi M. Oxidative stress, endothelial function and angiogenesis induced by cell therapy and gene therapy. Curr Pharm Biotechnol. 2006;7:109–16. doi: 10.2174/138920106776597658. [DOI] [PubMed] [Google Scholar]
- 42.Levonen AL, Vahakangas E, Koponen JK, Yla-Herttuala S. Antioxidant gene therapy for cardiovascular disease: current status and future perspectives. Circulation. 2008;117:2142–50. doi: 10.1161/CIRCULATIONAHA.107.718585. [DOI] [PubMed] [Google Scholar]
- 43.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–21. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Chiamvimonvat N, O’Rourke B, Kamp TJ, Kallen RG, Hofmann F, Flockerzi V, Marban E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ Res. 1995;76:325–34. doi: 10.1161/01.res.76.3.325. [DOI] [PubMed] [Google Scholar]
- 45.Hu H, Chiamvimonvat N, Yamagishi T, Marban E. Direct inhibition of expressed cardiac L-type Ca2+ channels by S-nitrosothiol nitric oxide donors. Circ Res. 1997;81:742–52. doi: 10.1161/01.res.81.5.742. [DOI] [PubMed] [Google Scholar]
- 46.Gill JS, McKenna WJ, Camm AJ. Free radicals irreversibly decrease Ca2+ currents in isolated guinea-pig ventricular myocytes. Eur J Pharmacol. 1995;292:337–40. doi: 10.1016/0926-6917(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 47.Sabaawy HE, Zhang F, Nguyen X, ElHosseiny A, Nasjletti A, Schwartzman M, Dennery P, Kappas A, Abraham NG. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–5. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 48.Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A, Griese DP, Dell’Acqua G, Mann MJ, Oyama J, Yet SF, Layne MD, Perrella MA, Dzau VJ. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105:602–7. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Pachori AS, Ward CA, Davis JP, Gnecchi M, Kong D, Zhang L, Murduck J, Yet SF, Perrella MA, Pratt RE, Dzau VJ, Melo LG. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. Faseb J. 2006;20:207–16. doi: 10.1096/fj.05-4435com. [DOI] [PubMed] [Google Scholar]
- 50.Tulis DA, Durante W, Liu X, Evans AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001;104:2710–5. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- 51.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–8. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 52.Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–25. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- 53.Chu Y, Iida S, Lund DD, Weiss RM, DiBona GF, Watanabe Y, Faraci FM, Heistad DD. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circ Res. 2003;92:461–8. doi: 10.1161/01.RES.0000057755.02845.F9. [DOI] [PubMed] [Google Scholar]
- 54.Laukkanen MO, Kivela A, Rissanen T, Rutanen J, Karkkainen MK, Leppanen O, Brasen JH, Yla-Herttuala S. Adenovirus-mediated extracellular superoxide dismutase gene therapy reduces neointima formation in balloon-denuded rabbit aorta. Circulation. 2002;106:1999–2003. doi: 10.1161/01.cir.0000031331.05368.9d. [DOI] [PubMed] [Google Scholar]
- 55.Brasen JH, Leppanen O, Inkala M, Heikura T, Levin M, Ahrens F, Rutanen J, Pietsch H, Bergqvist D, Levonen AL, Basu S, Zeller T, Kloppel G, Laukkanen MO, Yla-Herttuala S. Extracellular superoxide dismutase accelerates endothelial recovery and inhibits in-stent restenosis in stented atherosclerotic Watanabe heritable hyperlipidemic rabbit aorta. J Am Coll Cardiol. 2007;50:2249–53. doi: 10.1016/j.jacc.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 56.Li Q, Bolli R, Qiu Y, Tang XL, Guo Y, French BA. Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation. 2001;103:1893–8. doi: 10.1161/01.cir.103.14.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agrawal RS, Muangman S, Layne MD, Melo L, Perrella MA, Lee RT, Zhang L, Lopez-Ilasaca M, Dzau VJ. Pre-emptive gene therapy using recombinant adeno-associated virus delivery of extracellular superoxide dismutase protects heart against ischemic reperfusion injury, improves ventricular function and prolongs survival. Gene Ther. 2004;11:962–9. doi: 10.1038/sj.gt.3302250. [DOI] [PubMed] [Google Scholar]
- 58.Yang J, Marden JJ, Fan C, Sanlioglu S, Weiss RM, Ritchie TC, Davisson RL, Engelhardt JF. Genetic redox preconditioning differentially modulates AP-1 and NF kappa B responses following cardiac ischemia/reperfusion injury and protects against necrosis and apoptosis. Mol Ther. 2003;7:341–53. doi: 10.1016/s1525-0016(02)00061-8. [DOI] [PubMed] [Google Scholar]
- 59.Woo YJ, Zhang JC, Vijayasarathy C, Zwacka RM, Englehardt JF, Gardner TJ, Sweeney HL. Recombinant adenovirus-mediated cardiac gene transfer of superoxide dismutase and catalase attenuates postischemic contractile dysfunction. Circulation. 1998;98:II255–60. discussion II260-1. [PubMed] [Google Scholar]
- 60.Berg K, Wiseth R, Bjerve K, Brurok H, Gunnes S, Skarra S, Jynge P, Basu S. Oxidative stress and myocardial damage during elective percutaneous coronary interventions and coronary angiography. A comparison of blood-borne isoprostane and troponin release. Free Radic Res. 2004;38:517–25. doi: 10.1080/10715760410001688339. [DOI] [PubMed] [Google Scholar]
- 61.Tsimikas S, Lau HK, Han KR, Shortal B, Miller ER, Segev A, Curtiss LK, Witztum JL, Strauss BH. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164–70. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- 62.Leite PF, Danilovic A, Moriel P, Dantas K, Marklund S, Dantas AP, Laurindo FR. Sustained decrease in superoxide dismutase activity underlies constrictive remodeling after balloon injury in rabbits. Arterioscler Thromb Vasc Biol. 2003;23:2197–202. doi: 10.1161/01.ATV.0000093980.46838.41. [DOI] [PubMed] [Google Scholar]
- 63.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, van Domburg RT, Hess OM, Boersma E, Meier B, Windecker S, Serruys PW. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667–78. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 64.Pfisterer ME. Late stent thrombosis after drug-eluting stent implantation for acute myocardial infarction: a new red flag is raised. Circulation. 2008;118:1117–9. doi: 10.1161/CIRCULATIONAHA.108.803627. [DOI] [PubMed] [Google Scholar]
- 65.Mutlu GM, Ahkmedov AT, Lum H, Factor P. Potential genetic therapies for acute lung injury. Curr Gene Ther. 2004;4:487–95. doi: 10.2174/1566523043346057. [DOI] [PubMed] [Google Scholar]
- 66.Wilkes DS, Egan TM, Reynolds HY. Lung transplantation: opportunities for research and clinical advancement. Am J Respir Crit Care Med. 2005;172:944–55. doi: 10.1164/rccm.200501-098WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol. 2003;21:392–8. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 68.Fiser SM, Tribble CG, Long SM, Kaza AK, Cope JT, Laubach VE, Kern JA, Kron IL. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121:1069–75. doi: 10.1067/mtc.2001.113603. [DOI] [PubMed] [Google Scholar]
- 69.Nowak K, Weih S, Metzger R, Albrecht RF, 2nd, Post S, Hohenberger P, Gebhard MM, Danilov SM. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol. 2007;293:L162–9. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 70.Liu H, Liu L, Fletcher BS, Visner GA. Sleeping Beauty-based gene therapy with indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. Faseb J. 2006;20:2384–6. doi: 10.1096/fj.06-6228fje. [DOI] [PubMed] [Google Scholar]
- 71.Liu H, Liu L, Visner GA. Nonviral gene delivery with indoleamine 2,3-dioxygenase targeting pulmonary endothelium protects against ischemia-reperfusion injury. Am J Transplant. 2007;7:2291–300. doi: 10.1111/j.1600-6143.2007.01942.x. [DOI] [PubMed] [Google Scholar]
- 72.Otterbein LE, Lee PJ, Chin BY, Petrache I, Camhi SL, Alam J, Choi AM. Protective effects of heme oxygenase-1 in acute lung injury. Chest. 1999;116:61S–63S. doi: 10.1378/chest.116.suppl_1.61s-a. [DOI] [PubMed] [Google Scholar]
- 73.Guo H, Epperly MW, Bernarding M, Nie S, Gretton J, Jefferson M, Greenberger JS. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) intratracheal gene therapy reduction of irradiation-induced inflammatory cytokines does not protect orthotopic Lewis lung carcinomas. In Vivo. 2003;17:13–21. [PubMed] [Google Scholar]
- 74.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JE, Bar-Sagi D, Archer H, Carlos T, Guo H, Greenberger JS. Pulmonary irradiation-induced expression of VCAM-I and ICAM-I is decreased by manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) gene therapy. Biol Blood Marrow Transplant. 2002;8:175–87. doi: 10.1053/bbmt.2002.v8.pm12014807. [DOI] [PubMed] [Google Scholar]
- 75.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, Yamaoka Y. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 76.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–36. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 77.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–57. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 78.Vardanian AJ, Busuttil RW, Kupiec-Weglinski JW. Molecular mediators of liver ischemia and reperfusion injury: a brief review. Mol Med. 2008;14:337–45. doi: 10.2119/2007-00134.Vardanian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl. 2005;11:1031–47. doi: 10.1002/lt.20504. [DOI] [PubMed] [Google Scholar]
- 80.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 81.Karwinski W, Soreide O. Allopurinol improves scavenging ability of the liver after ischemia/reperfusion injury. Liver. 1997;17:139–43. doi: 10.1111/j.1600-0676.1997.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 82.Dorman RB, Wunder C, Saba H, Shoemaker JL, MacMillan-Crow LA, Brock RW. NAD(P)H oxidase contributes to the progression of remote hepatic parenchymal injury and endothelial dysfunction, but not microvascular perfusion deficits. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1025–32. doi: 10.1152/ajpgi.00246.2005. [DOI] [PubMed] [Google Scholar]
- 83.Liu PG, He SQ, Zhang YH, Wu J. Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World J Gastroenterol. 2008;14:2832–7. doi: 10.3748/wjg.14.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harada H, Hines IN, Flores S, Gao B, McCord J, Scheerens H, Grisham MB. Role of NADPH oxidase-derived superoxide in reduced size liver ischemia and reperfusion injury. Arch Biochem Biophys. 2004;423:103–8. doi: 10.1016/j.abb.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 85.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 86.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen WD, Kim DH, Alam HB, Provido HS, Kirkpatrick JR. Polyethylene glycol-superoxide dismutase inhibits lipid peroxidation in hepatic ischemia/reperfusion injury. Crit Care. 1999;3:127–30. doi: 10.1186/cc358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lehmann TG, Wheeler MD, Schoonhoven R, Bunzendahl H, Samulski RJ, Thurman RG. Delivery of Cu/Zn-superoxide dismutase genes with a viral vector minimizes liver injury and improves survival after liver transplantation in the rat. Transplantation. 2000;69:1051–7. doi: 10.1097/00007890-200003270-00007. [DOI] [PubMed] [Google Scholar]
- 89.Wheeler MD, Katuna M, Smutney OM, Froh M, Dikalova A, Mason RP, Samulski RJ, Thurman RG. Comparison of the effect of adenoviral delivery of three superoxide dismutase genes against hepatic ischemia-reperfusion injury. Hum Gene Ther. 2001;12:2167–77. doi: 10.1089/10430340152710513. [DOI] [PubMed] [Google Scholar]
- 90.Lehmann TG, Wheeler MD, Froh M, Schwabe RF, Bunzendahl H, Samulski RJ, Lemasters JJ, Brenner DA, Thurman RG. Effects of three superoxide dismutase genes delivered with an adenovirus on graft function after transplantation of fatty livers in the rat. Transplantation. 2003;76:28–37. doi: 10.1097/01.TP.0000065299.29900.17. [DOI] [PubMed] [Google Scholar]
- 91.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–40. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- 92.Venugopal SK, Wu J, Catana AM, Eisenbud L, He SQ, Duan YY, Bishop C, Follenzi A, Gupta S, Zern MA. Lentivirus-mediated copper-zinc superoxide dismutase gene delivery protects the liver in mice against oxidative stress. Liver International. 2007;27:1311–1322. doi: 10.1111/j.1478-3231.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- 93.Liu L, Zern MA, Lizarzaburu ME, Nantz MH, Wu J. Poly(cationic lipid)-mediated in vivo gene delivery to mouse liver. Gene Ther. 2003;10:180–7. doi: 10.1038/sj.gt.3301861. [DOI] [PubMed] [Google Scholar]
- 94.Wu J, Lizarzaburu ME, Kurth MJ, Liu L, Wege H, Zern MA, Nantz MH. Cationic lipid polymerization as a novel approach for constructing new DNA delivery agents. Bioconjug Chem. 2001;12:251–7. doi: 10.1021/bc000097e. [DOI] [PubMed] [Google Scholar]
- 95.Luedde T, Trautwein C. The role of oxidative stress and antioxidant treatment in liver surgery and transplantation. Liver Transpl. 2006;12:1733–5. doi: 10.1002/lt.20990. [DOI] [PubMed] [Google Scholar]
- 96.Shiotani S, Shimada M, Taketomi A, Soejima Y, Yoshizumi T, Hashimoto K, Shimokawa H, Maehara Y. Rho-kinase as a novel gene therapeutic target in treatment of cold ischemia/reperfusion-induced acute lethal liver injury: effect on hepatocellular NADPH oxidase system. Gene Ther. 2007;14:1425–33. doi: 10.1038/sj.gt.3303000. [DOI] [PubMed] [Google Scholar]
- 97.Frishman WH, Novak S, Brandt LJ, Spiegel A, Gutwein A, Kohi M, Rozenblit G. Pharmacologic management of mesenteric occlusive disease. Cardiol Rev. 2008;16:59–68. doi: 10.1097/CRD.0b013e31815a6600. [DOI] [PubMed] [Google Scholar]
- 98.Hsiao G, Yen MH, Lee YM, Sheu JR. Antithrombotic effect of PMC, a potent alpha-tocopherol analogue on platelet plug formation in vivo. Br J Haematol. 2002;117:699–704. doi: 10.1046/j.1365-2141.2002.03492.x. [DOI] [PubMed] [Google Scholar]
- 99.Byrka-Owczarek K, Steplewska-Mazur K, Krason M, Bohosiewicz J, Koszutski T, Wojtynek G. The evaluation of the protective action of antioxidants on small intestine of rabbits experimentally injured by ischemia and reperfusion. J Pediatr Surg. 2004;39:1226–9. doi: 10.1016/j.jpedsurg.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 100.Tireli GA, Salman T, Ozbey H, Abbasoglu L, Toker G, Celik A. The effect of pentoxifylline on intestinal anastomotic healing after ischemia. Pediatr Surg Int. 2003;19:88–90. doi: 10.1007/s00383-002-0741-3. [DOI] [PubMed] [Google Scholar]
- 101.Poussios D, Andreadou I, Papalois A, Rekka E, Gavalakis N, Aroni K, Kourounakis PN, Fotiadis C, Sechas MN. Protective effect of a novel antioxidant non-steroidal anti-inflammatory agent (compound IA) on intestinal viability after acute mesenteric ischemia and reperfusion. Eur J Pharmacol. 2003;465:275–80. doi: 10.1016/s0014-2999(03)01488-2. [DOI] [PubMed] [Google Scholar]
- 102.Muia C, Mazzon E, Di Paola R, Genovese T, Menegazzi M, Caputi AP, Suzuki H, Cuzzocrea S. Green tea polyphenol extract attenuates ischemia/reperfusion injury of the gut. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:364–74. doi: 10.1007/s00210-005-1076-0. [DOI] [PubMed] [Google Scholar]
- 103.Ypsilantis P, Lambropoulou M, Tentes I, Kortsaris A, Papadopoulos N, Simopoulos C. Mesna protects intestinal mucosa from ischemia/reperfusion injury. J Surg Res. 2006;134:278–84. doi: 10.1016/j.jss.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 104.Aydin C, Teke Z, Aytekin F, Yenisey C, Kabay B, Simsek NG, Tekin K. Tempol prevents harmful effects of remote ischemia reperfusion injury on healing of experimental colonic anastomoses. Int J Colorectal Dis. 2007;22:325–31. doi: 10.1007/s00384-006-0149-y. [DOI] [PubMed] [Google Scholar]
- 105.Kozuch PL, Brandt LJ. Review article: diagnosis and management of mesenteric ischaemia with an emphasis on pharmacotherapy. Aliment Pharmacol Ther. 2005;21:201–15. doi: 10.1111/j.1365-2036.2005.02269.x. [DOI] [PubMed] [Google Scholar]
- 106.Guo HL, Wolfe D, Epperly MW, Huang S, Liu K, Glorioso JC, Greenberger J, Blumberg D. Gene transfer of human manganese superoxide dismutase protects small intestinal villi from radiation injury. Journal of Gastrointestinal Surgery. 2003;7:229–35. doi: 10.1016/s1091-255x(02)00186-5. discussion 235–6. [DOI] [PubMed] [Google Scholar]
- 107.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–77. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 108.Grogaard B, Parks DA, Granger DN, McCord JM, Forsberg JO. Effects of ischemia and oxygen radicals on mucosal albumin clearance in intestine. Am J Physiol. 1982;242:G448–54. doi: 10.1152/ajpgi.1982.242.5.G448. [DOI] [PubMed] [Google Scholar]
- 109.Riaz AA, Wan MX, Schafer T, Dawson P, Menger MD, Jeppsson B, Thorlacius H. Allopurinol and superoxide dismutase protect against leucocyte-endothelium interactions in a novel model of colonic ischaemia-reperfusion. Br J Surg. 2002;89:1572–80. doi: 10.1046/j.1365-2168.2002.02279.x. [DOI] [PubMed] [Google Scholar]
- 110.Otamiri TA, Tagesson C, Sjodahl R. Increased plasma malondialdehyde in patients with small intestinal strangulation obstruction. Acta Chir Scand. 1988;154:283–5. [PubMed] [Google Scholar]
- 111.Vlasov TD, Smirnov DA, Nutfullina GM. Preconditioning of the small intestine to ischemia in rats. Neurosci Behav Physiol. 2002;32:449–53. doi: 10.1023/a:1015896614819. [DOI] [PubMed] [Google Scholar]
- 112.Wu B, Iwakiri R, Tsunada S, Utsumi H, Kojima M, Fujise T, Ootani A, Fujimoto K. iNOS enhances rat intestinal apoptosis after ischemia-reperfusion. Free Radic Biol Med. 2002;33:649–58. doi: 10.1016/s0891-5849(02)00917-6. [DOI] [PubMed] [Google Scholar]
- 113.Abdelrahman M, Mazzon E, Bauer M, Bauer I, Delbosc S, Cristol JP, Patel NS, Cuzzocrea S, Thiemermann C. Inhibitors of NADPH oxidase reduce the organ injury in hemorrhagic shock. Shock. 2005;23:107–14. doi: 10.1097/01.shk.0000151028.15377.f7. [DOI] [PubMed] [Google Scholar]
- 114.Oktar BK, Gulpinar MA, Bozkurt A, Ghandour S, Cetinel S, Moini H, Yegen BC, Bilsel S, Granger DN, Kurtel H. Endothelin receptor blockers reduce I/R-induced intestinal mucosal injury: role of blood flow. Am J Physiol Gastrointest Liver Physiol. 2002;282:G647–55. doi: 10.1152/ajpgi.2002.282.4.G647. [DOI] [PubMed] [Google Scholar]
- 115.Yun KJ, Choi SC, Oh JM. [Expression of heme oxygenase-1 in ischemic colitis] Korean J Gastroenterol. 2005;45:335–9. [PubMed] [Google Scholar]
- 116.Wasserberg N, Pileggi A, Salgar SK, Ruiz P, Ricordi C, Inverardi L, Tzakis AG. Heme oxygenase-1 upregulation protects against intestinal ischemia/reperfusion injury: a laboratory based study. Int J Surg. 2007;5:216–24. doi: 10.1016/j.ijsu.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 117.Somavarapu S, Bramwell VW, Alpar HO. Oral plasmid DNA delivery systems for genetic immunisation. Journal of Drug Targeting. 2003;11:547–53. doi: 10.1080/10611860410001683022. [DOI] [PubMed] [Google Scholar]
- 118.Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie QW, Smart J, Su-Rick CJ, Pobre E, Diaz D, Gomez D, Hand C, Coleman T, Cerami A. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–12. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guneli E, Cavdar Z, Islekel H, Sarioglu S, Erbayraktar S, Kiray M, Sokmen S, Yilmaz O, Gokmen N. Erythropoietin protects the intestine against ischemia/reperfusion injury in rats. Mol Med. 2007;13:509–17. doi: 10.2119/2007-00032.Guneli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sreenarasimhaiah J. Chronic mesenteric ischemia. Curr Treat Options Gastroenterol. 2007;10:3–9. doi: 10.1007/s11938-007-0051-x. [DOI] [PubMed] [Google Scholar]
- 121.Bokesch PM, Marchand J, Seirafi PA, Deiss JM, Warner KG, Bronson RT, Kream RM. Immediate-early gene expression in ovine brain after cardiopulmonary bypass and hypothermic circulatory arrest. Anesthesiology. 1996;85:1439–46. doi: 10.1097/00000542-199612000-00026. [DOI] [PubMed] [Google Scholar]
- 122.McCullough JL, Hollier LH, Nugent M. Paraplegia after thoracic aortic occlusion: influence of cerebrospinal fluid drainage. Experimental and early clinical results. J Vasc Surg. 1988;7:153–60. [PubMed] [Google Scholar]
- 123.Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54:271–84. doi: 10.1124/pr.54.2.271. [DOI] [PubMed] [Google Scholar]
- 124.Schaller B. Prospects for the future: the role of free radicals in the treatment of stroke. Free Radic Biol Med. 2005;38:411–25. doi: 10.1016/j.freeradbiomed.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 125.Shi H, Liu KJ. Cerebral tissue oxygenation and oxidative brain injury during ischemia and reperfusion. Front Biosci. 2007;12:1318–28. doi: 10.2741/2150. [DOI] [PubMed] [Google Scholar]
- 126.Koller WC, Cersosimo MG. Neuroprotection in Parkinson’s disease: an elusive goal. Curr Neurol Neurosci Rep. 2004;4:277–83. doi: 10.1007/s11910-004-0052-2. [DOI] [PubMed] [Google Scholar]
- 127.LeWitt PA, Taylor DC. Protection against Parkinson’s disease progression: clinical experience. Neurotherapeutics. 2008;5:210–25. doi: 10.1016/j.nurt.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rizzardini M, Mangolini A, Lupi M, Ubezio P, Bendotti C, Cantoni L. Low levels of ALS-linked Cu/Zn superoxide dismutase increase the production of reactive oxygen species and cause mitochondrial damage and death in motor neuron-like cells. J Neurol Sci. 2005;232:95–103. doi: 10.1016/j.jns.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 129.Liu R, Li B, Flanagan SW, Oberley LW, Gozal D, Qiu M. Increased mitochondrial antioxidative activity or decreased oxygen free radical propagation prevent mutant SOD1-mediated motor neuron cell death and increase amyotrophic lateral sclerosis-like transgenic mouse survival. J Neurochem. 2002;80:488–500. doi: 10.1046/j.0022-3042.2001.00720.x. [DOI] [PubMed] [Google Scholar]
- 130.An JJ, Lee YP, Kim SY, Lee SH, Kim DW, Lee MJ, Jeong MS, Jang SH, Kang JH, Kwon HY, Kang TC, Won MH, Cho SW, Kwon OS, Lee KS, Park J, Eum WS, Choi SY. Transduction of familial amyotrophic lateral sclerosis-related mutant PEP-1-SOD proteins into neuronal cells. Mol Cells. 2008;25:55–63. [PubMed] [Google Scholar]
- 131.Cousar JL, Lai Y, Marco CD, Bayir H, Adelson PD, Janesko-Feldman KL, Kochanek PM, Clark RS. Heme oxygenase 1 in cerebrospinal fluid from infants and children after severe traumatic brain injury. Dev Neurosci. 2006;28:342–7. doi: 10.1159/000094160. [DOI] [PubMed] [Google Scholar]
- 132.Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, Dulak J. Heme Oxygenase-1 and the Vascular Bed: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 133.Kim DW, Eum WS, Jang SH, Kim SY, Choi HS, Choi SH, An JJ, Lee SH, Lee KS, Han K, Kang TC, Won MH, Kang JH, Kwon OS, Cho SW, Kim TY, Park J, Choi SY. Transduced Tat-SOD fusion protein protects against ischemic brain injury. Mol Cells. 2005;19:88–96. [PubMed] [Google Scholar]
- 134.Bayir H, Kagan VE, Clark RS, Janesko-Feldman K, Rafikov R, Huang Z, Zhang X, Vagni V, Billiar TR, Kochanek PM. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem. 2007;101:168–81. doi: 10.1111/j.1471-4159.2006.04353.x. [DOI] [PubMed] [Google Scholar]
- 135.Tangpong J, Cole MP, Sultana R, Estus S, Vore M, St Clair W, Ratanachaiyavong S, St Clair DK, Butterfield DA. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J Neurochem. 2007;100:191–201. doi: 10.1111/j.1471-4159.2006.04179.x. [DOI] [PubMed] [Google Scholar]
- 136.Joshi G, Hardas S, Sultana R, St Clair DK, Vore M, Butterfield DA. Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: Implication for chemobrain. J Neurosci Res. 2007;85:497–503. doi: 10.1002/jnr.21158. [DOI] [PubMed] [Google Scholar]
- 137.Sugawara T, Noshita N, Lewen A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci. 2002;22:209–17. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gu W, Zhao H, Yenari MA, Sapolsky RM, Steinberg GK. Catalase over-expression protects striatal neurons from transient focal cerebral ischemia. Neuroreport. 2004;15:413–6. doi: 10.1097/00001756-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 139.Yang GY, Pang L, Ge HL, Tan M, Ye W, Liu XH, Huang FP, Wu DC, Che XM, Song Y, Wen R, Sun Y. Attenuation of ischemia-induced mouse brain injury by SAG, a redox-inducible antioxidant protein. J Cereb Blood Flow Metab. 2001;21:722–33. doi: 10.1097/00004647-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 140.Xu DG, Crocker SJ, Doucet JP, St-Jean M, Tamai K, Hakim AM, Ikeda JE, Liston P, Thompson CS, Korneluk RG, MacKenzie A, Robertson GS. Elevation of neuronal expression of NAIP reduces ischemic damage in the rat hippocampus. Nat Med. 1997;3:997–1004. doi: 10.1038/nm0997-997. [DOI] [PubMed] [Google Scholar]
- 141.Jin G, Arai K, Murata Y, Wang S, Stins MF, Lo EH, van Leyen K. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39:2538–43. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A. 2005;102:244–9. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li J, Calkins MJ, Johnson DA, Johnson JA. Role of Nrf2-dependent ARE-driven antioxidant pathway in neuroprotection. Methods Mol Biol. 2007;399:67–78. doi: 10.1007/978-1-59745-504-6_6. [DOI] [PubMed] [Google Scholar]
- 144.Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Chan PH. Oxidative injury to the endoplasmic reticulum in mouse brains after transient focal ischemia. Neurobiol Dis. 2004;15:229–39. doi: 10.1016/j.nbd.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 145.Zhang QG, Tian H, Li HC, Zhang GY. Antioxidant N-acetylcysteine inhibits the activation of JNK3 mediated by the GluR6-PSD95-MLK3 signaling module during cerebral ischemia in rat hippocampus. Neurosci Lett. 2006;408:159–64. doi: 10.1016/j.neulet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 146.Anderson DM, Hall LL, Ayyalapu AR, Irion VR, Nantz MH, Hecker JG. Stability of mRNA/cationic lipid lipoplexes in human and rat cerebrospinal fluid: methods and evidence for nonviral mRNA gene delivery to the central nervous system. Hum Gene Ther. 2003;14:191–202. doi: 10.1089/10430340360535751. [DOI] [PubMed] [Google Scholar]
- 147.Haucke ES, Zou S, Scarfo KA, Nantz MH, Hecker JG. Whole animal in vivo imaging after transient non-viral lipid-mediated gene transfer to the rat central nervous system. Molecular Therapy. 2008 doi: 10.1038/mt.2008.183. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hecker JG, Hall LL, Irion VR. Nonviral gene delivery to the lateral ventricles in rat brain: initial evidence for widespread distribution and expression in the central nervous system. Mol Ther. 2001;3:375–84. doi: 10.1006/mthe.2001.0272. [DOI] [PubMed] [Google Scholar]
- 149.Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci U S A. 1989;86:6077–81. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hecker RB, Hays JV, Champ JD, Rubal BJ. Myocardial ischemia and stunning induced by topical intranasal phenylephrine pledgets. Mil Med. 1997;162:832–5. [PubMed] [Google Scholar]
- 151.Mamot C, Nguyen JB, Pourdehnad M, Hadaczek P, Saito R, Bringas JR, Drummond DC, Hong K, Kirpotin DB, McKnight T, Berger MS, Park JW, Bankiewicz KS. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J Neurooncol. 2004;68:1–9. doi: 10.1023/b:neon.0000024743.56415.4b. [DOI] [PubMed] [Google Scholar]
- 152.Guo S, Wharton W, Moseley P, Shi H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones. 2007;12:245–54. doi: 10.1379/CSC-265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, Wang K, Wang H, Xiao X. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol. 2007;178:7376–84. doi: 10.4049/jimmunol.178.11.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Patel YJ, Payne Smith MD, de Belleroche J, Latchman DS. Hsp27 and Hsp70 administered in combination have a potent protective effect against FALS-associated SOD1-mutant-induced cell death in mammalian neuronal cells. Brain Res Mol Brain Res. 2005;134:256–74. doi: 10.1016/j.molbrainres.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 155.Yenari MA, Dumas TC, Sapolsky RM, Steinberg GK. Gene therapy for treatment of cerebral ischemia using defective herpes simplex viral vectors. Ann N Y Acad Sci. 2001;939:340–57. doi: 10.1111/j.1749-6632.2001.tb03643.x. [DOI] [PubMed] [Google Scholar]
- 156.Yenari MA, Fink SL, Sun GH, Chang LK, Patel MK, Kunis DM, Onley D, Ho DY, Sapolsky RM, Steinberg GK. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann Neurol. 1998;44:584–91. doi: 10.1002/ana.410440403. [DOI] [PubMed] [Google Scholar]
- 157.Fink SL, Chang LK, Ho DY, Sapolsky RM. Defective herpes simplex virus vectors expressing the rat brain stress-inducible heat shock protein 72 protect cultured neurons from severe heat shock. J Neurochem. 1997;68:961–9. doi: 10.1046/j.1471-4159.1997.68030961.x. [DOI] [PubMed] [Google Scholar]
- 158.Hoehn B, Ringer TM, Xu L, Giffard RG, Sapolsky RM, Steinberg GK, Yenari MA. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J Cereb Blood Flow Metab. 2001;21:1303–9. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 159.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 160.Berges BK, Wolfe JH, Fraser NW. Transduction of brain by herpes simplex virus vectors. Mol Ther. 2007;15:20–9. doi: 10.1038/sj.mt.6300018. [DOI] [PubMed] [Google Scholar]