Lyme disease, caused by the bacterium Borrelia burgdorferi and transmitted by tick vectors, is the most commonly reported vector-borne disease in the temperate zone.1 More than 20 000 cases are recorded annually in the United States.2 In about 80% of cases, early Lyme disease is characterized by a skin lesion, erythema migrans, which expands to a diameter of more than 5 cm from the site of the tick bite.3 If left untreated, the disease can progress to early disseminated Lyme disease with neurologic (facial palsy, meningitis and meningoradiculoneuritis, also known as Bannwarth syndrome) and cardiac (usually atrioventricular block, sometimes with myopericarditis) involvement, and then to late disseminated Lyme disease with neurologic manifestations (peripheral neuropathy or encephalomyelitis) and Lyme arthritis.3,4
B. burgdorferi is transmitted by ticks, which feed on wildlife reservoir hosts of the pathogen, particularly rodents and birds. Ixodes scapularis, the blacklegged tick (Figure 1), is the main vector in eastern and central North America. Ixodes pacificus, the western blacklegged tick, is the main vector west of the Rocky Mountains. Both tick species are indiscriminate in their choice of host and will feed on humans; as such, they can transmit pathogens from wildlife to humans.
Figure 1.
The life stages of the blacklegged tick, Ixodes scapularis, which transmits Lyme disease. Nymphs (left-hand photograph) and adult females (right-hand photograph) are shown in various stages of engorgement from unfed (at the right of each picture) to fully engorged (at the left of each picture). The western blacklegged tick, Ixodes pacificus, is morphologically very similar to I. scapularis.
Recent studies have suggested that the risk of exposure to Lyme disease is emerging in Canada because the range of I. scapularis is expanding, a process that is predicted to accelerate with climate change. Here we review the available and emerging surveillance information and discuss its relevance to the early diagnosis and prevention of Lyme disease. We based this review on a search of the MEDLINE database using the key words “Lyme,” “Ixodes” and “Canada.”
Diagnosis of Lyme disease
Within several weeks after the tick bite and manifestation of the erythema migrans lesion, the lesion disappears and the bacterium disseminates hematogenously to other tissues, including additional skin sites (producing secondary erythema migrans lesions in some cases), the nervous system, the heart and the joints.3 Bacterial culture or use of polymerase chain reaction to amplify target sequences in clinical material (such as blood, skin, synovial fluid or cerebrospinal fluid) are diagnostic methods with low to moderate sensitivity.2,5 Public health laboratories in Canada,6,7 the United States2,8 and some European countries advocate a two-tiered serologic testing process, consisting of enzyme-linked immunosorbent assay followed by Western blot, to assist in diagnosis. Serologic testing is insensitive in very early Lyme disease, but the 2-tiered method is much more sensitive in detecting cases of disseminated Lyme disease.2,5,7,9 When used alone, enzyme-linked immunosorbent assay has limited specificity, but the specificity is improved by Western blotting, provided that the bands are interpreted according to criteria for positivity set by the US Centers for Disease Control and Prevention.8 Concerns about test specificity mean that interpretation of the 2-tiered test is informed by whether the patient has had contact with an endemic area, which is defined by ecological criteria.10 The diagnosis should be guided by the patient’s clinical situation, with the results of laboratory tests providing supportive evidence of infection.
Geographic distribution of risk and endemic areas
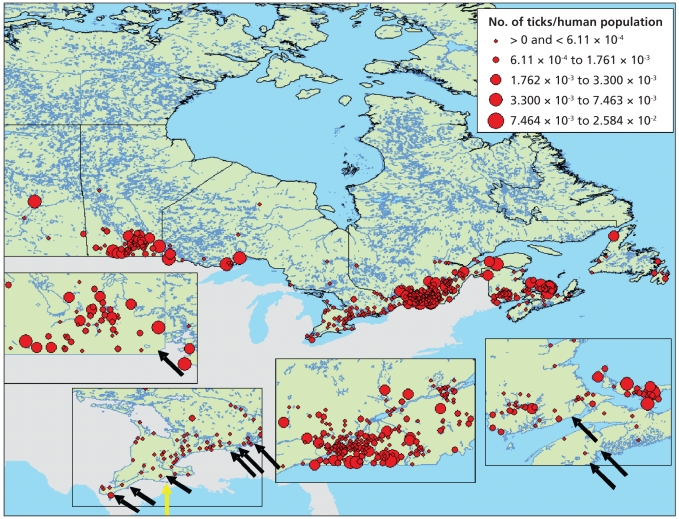
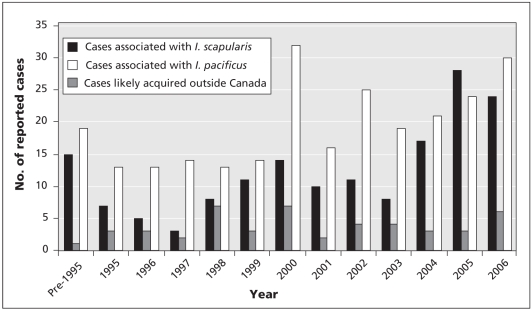
Endemic areas are localities where B. burgdorferi is demonstrably being transmitted by an established population of vector ticks.6 The number of documented endemic areas in Canada has increased recently. In the early 1990s, only 1 geographically discrete population of I. scapularis was known, at Long Point on the Ontario shore of Lake Erie.11 Since 1997, detection of human cases by vigilant clinicians and passive surveillance for ticks12 has led to the identification of populations of I. scapularis in southern Ontario, Nova Scotia, southeastern Manitoba and New Brunswick (Figure 2).13 In addition, active surveillance driven by risk maps (Box 1) has identified the possibility of additional emerging populations in southern Quebec, in the absence of a clear signal from passive surveillance.14 Data on human cases from provinces where Lyme disease is reportable suggest a corresponding recent increase in the number of cases acquired by Canadians without any history of travel abroad (Figure 3).15 Studies indicate that ambient temperature constrains the establishment of I. scapularis in Canada to the warmer regions of southeastern Manitoba, southern Ontario and Quebec and some regions of the Maritimes. As such, projected increases in temperature with climate change are expected to permit and accelerate the expansion of I. scapularis into Canada.13,16
Figure 2.
The distribution of Ixodes scapularis, reflecting information submitted to provincial and federal public health agencies from January 1990 to December 2003 and to the Lyme Disease Association of Ontario for 1993 to 1999 (red circles are centred on the centroid of the census subdivision from which the reports were submitted). Census subdivisions where resident populations of I. scapularis are currently known to occur are indicated by arrows, and the census subdivision containing the only I. scapularis population that was known in 1991 is indicated by the yellow arrow. Reproduced, with permission, from the Entomological Society of America.12
Box 1.
Risk maps for the occurrence of the Lyme disease vector Ixodes scapularis
The 2 most influential factors for the establishment of the tick in Canada are warmer temperatures and dispersion of the ticks on animal hosts.
Risk maps employ a risk algorithm comprising these influential factors.
The risk maps provide a model against which different surveillance methods can be compared and hypotheses on the likelihood of expanding geographic range tested.
Initial field validation suggests that the risk algorithm is a useful tool to identify areas where I. scalpularis ticks are becoming established.
Figure 3.
Annual number of cases of Lyme disease reported voluntarily by the provinces and territories since the late 1980s. Cases of Lyme disease in British Columbia were probably transmitted by Ixodes pacificus, whereas cases from all other provinces with cases that were potentially locally acquired (i.e., Manitoba, Ontario, Quebec, New Brunswick, Nova Scotia and Newfoundland and Labrador) were probably associated with Ixodes scapularis. Cases affecting patients with a history of travel to an endemic area outside Canada during the period when they likely acquired the infection are considered travel-related or nonendemic. Reproduced with permission from the Minister of Public Works and Government Services Canada, 2008.15
Surveillance by the British Columbia Centre for Disease Control suggests that established populations of I. pacificus and areas where B. burgdorferi is endemic are widely distributed in southern British Columbia. The prevalence of B. burgdorferi infection in host-seeking I. pacificus ticks is usually lower (typically less than 10%17,18) than that in I. scapularis (typically greater than 25% in the northeastern United States and southeastern Canada1,19). Therefore, the risk of Lyme disease in endemic areas where I. pacificus is the vector is generally lower than where I. scapularis is the vector.
Overall, there is a low risk of exposure to Lyme disease from tick vectors in the nonendemic areas of all 10 provinces. This risk is due to ticks dispersed from endemic areas by migratory birds (Figure 2).12,20,21 About 10% of these bird-transported ticks are infected with B. burgdorferi,12 and small numbers of cases of human Lyme disease occur outside endemic areas.22
Conclusions and recommendations
The number of known endemic areas of Lyme disease in Canada is increasing because the range of I. scapularis is expanding in the eastern and central provinces. National surveillance must be able to identify this changing pattern. Lyme disease is potentially preventable if people wear appropriate clothing and use N,N-diethyl-meta-toluamide (DEET) repellents. Removal of infected ticks from a person within 24 hours of attachment usually prevents transmission of B. burgdorferi,23 and early Lyme disease is usually easily treated with antibiotics.4,24 However, prompt treatment requires recognition of vector ticks and erythema migrans lesions by affected members of the public and prompt diagnosis by clinicians.24 If Lyme disease is not recognized during the early stages, patients may suffer seriously debilitating disease, which may be more difficult to treat.4 Therefore, an important function of surveillance is to inform both the public and clinicians about the local risk level and the need for prudent administration of regimens appropriate for prevention and early diagnosis of Lyme disease.
As of 2009, physicians will be required to report clinically confirmed and suspected cases of Lyme disease to the national surveillance authority. Such reporting will assist in identifying the burden associated with this disease. We also advocate using environmental indicators of the risk of Lyme disease (i.e., the occurrence of ticks or environmental data for risk maps) for surveillance, to both support the diagnosis of the disease and to pre-empt its occurrence in humans. Researchers are now using the gold-standard surveillance method of intensive field study to validate risk maps for Lyme disease vectors14 as public health tools, so that these maps can be used to identify new endemic areas (Box 1). These studies and the risk maps can then be used to evaluate, select and target surveillance methods, such as passive surveillance for tick vectors (already in use but with effort that varies geographically12) and surveillance for the presence of Lyme disease vectors on, and evidence of B. burgdorferi infection in, sentinel animals.25,26 With these methods, it may be possible to predict and then confirm how ticks are spreading in Canada and how endemic areas are expanding, which should in turn allow the targeting of public and physician awareness programs to the areas of greatest risk, for maximum effect.
We conclude that Lyme disease is emerging in Canada, that effective, enhanced surveillance involving federal and provincial agencies needs to be instigated and that clinician awareness of Lyme disease will be crucial to minimizing its impact.
Key points
Expansion of the geographic range of the tick vector Ixodes scapularis in Canada is leading to increasing numbers of endemic areas for Lyme disease.
Because the specificity of serologic tests for Lyme disease may not be high, epidemiologic findings about the likelihood of exposure to ticks that transmit Lyme disease inform the serologic diagnosis, rather than the other way around.
Current passive surveillance for tick vectors has identified new endemic areas, but additional methods are needed to precisely identify where Lyme disease is emerging in Canada.
Enhanced surveillance tools such as risk maps and risk algorithms help in identifying areas where I. scapularis ticks are becoming established
Clinicians’ vigilance for human cases, particularly in non-endemic areas, will greatly assist these surveillance efforts.
Footnotes
Competing interests: None declared.
Contributors: Dr. Ogden was the lead author and provided information on Lyme disease ecology and surveillance. Dr. Lindsay provided Figure 1, contributed to and critically assessed the article content, and provided information on surveillance and Lyme disease vectors in Canada. Dr. Morshed contributed to and critically assessed the article content and provided information on the ecology of Lyme disease in British Columbia. Dr. Sockett contributed to and critically assessed the article content and provided information on surveillance and public health policy. Dr. Artsob contributed to and critically assessed the article content and provided information on the laboratory diagnosis of Lyme disease. Each of the authors approved the final version submitted for publication.
This article has been peer reviewed.
REFERENCES
- 1.Kurtenbach K, Hanincova K, Tsao J, et al. Key processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol. 2006;4:660–9. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Lyme disease — United States, 2003–2005. MMWR Morb Mortal Wkly Rep. 2007;56:573–6. [PubMed] [Google Scholar]
- 3.Aguero-Rosenfeld ME, Wang G, Schwartz I, et al. Diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 5.Nowakowski J, Schwartz I, Liveris D, et al. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin Infect Dis. 2001;33:2023–7. doi: 10.1086/324490. [DOI] [PubMed] [Google Scholar]
- 6.Consensus conference on Lyme disease. CMAJ. 1991;144:1627–32. [PMC free article] [PubMed] [Google Scholar]
- 7.Canadian Public Health Laboratory Network. The laboratory diagnosis of Lyme borreliosis: guidelines from the Canadian Public Health Laboratory Network. Can J Infect Dis Med Microbiol. 2007;18:145–8. doi: 10.1155/2007/495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–1. [PubMed] [Google Scholar]
- 9.Dressler F, Whalen JA, Reinhardt BN, et al. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 10.Tugwell P, Dennis DT, Weinstein A, et al. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1109–23. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 11.Barker IK, Surgeoner GA, Artsob H, et al. Distribution of the Lyme disease vector, Ixodes dammini (Acari: Ixodidae) and isolation of Borrelia burgdorferi in Ontario, Canada. J Med Entomol. 1992;29:1011–22. doi: 10.1093/jmedent/29.6.1011. [DOI] [PubMed] [Google Scholar]
- 12.Ogden NH, Trudel L, Artsob H, et al. Ixodes scapularis ticks collected by passive surveillance in Canada: analysis of geographic distribution and infection with the Lyme borreliosis agent Borrelia burgdorferi sensu lato. J Med Entomol. 2006;43:600–9. doi: 10.1603/0022-2585(2006)43[600:ISTCBP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Ogden NH, Bigras-Poulin M, O’Callaghan CJ, et al. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int J Parasitol. 2005;35:375–89. doi: 10.1016/j.ijpara.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Ogden NH, St-Onge L, Barker IK, et al. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. Int J Health Geogr. 2008;7:24. doi: 10.1186/1476-072X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogden NH, Lindsay LR, Morshed M, et al. The rising challenge of Lyme borreliosis in Canada. [(accessed 2009 May 12)];Can Commun Dis Rep. 2008 34:1–19. Available: www.phac-aspc.gc.ca/publicat/ccdr-rmtc/08vol34/dr-rm3401a-eng.php. [PubMed]
- 16.Ogden NH, Maarouf A, Barker IK, et al. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int J Parasitol. 2006;36:63–70. doi: 10.1016/j.ijpara.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Holden K, Boothby JT, Kasten RW, et al. Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus ticks from California, USA. Vector Borne Zoonotic Dis. 2006;6:99–102. doi: 10.1089/vbz.2006.6.99. [DOI] [PubMed] [Google Scholar]
- 18.Lane RS, Foley JE, Eisen L, et al. Acarologic risk of exposure to emerging tick-borne bacterial pathogens in a semirural community in northern California. Vector Borne Zoonotic Dis. 2001;1:197–210. doi: 10.1089/153036601753552567. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay LR, Barker IK, Surgeoner GA, et al. Duration of Borrelia burgdorferi infectivity in white-footed mice for the tick vector Ixodes scapularis under laboratory and field conditions in Ontario. J Wildl Dis. 1997;33:766–75. doi: 10.7589/0090-3558-33.4.766. [DOI] [PubMed] [Google Scholar]
- 20.Ogden NH, Lindsay LR, Hanincová K, et al. Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks, and Borrelia burgdorferi and Anaplasma phagocytophilum in Canada. Appl Environ Microbiol. 2008;74:1780–90. doi: 10.1128/AEM.01982-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morshed MG, Scott JD, Fernando K, et al. Migratory songbirds disperse ticks across Canada, and first isolation of the Lyme disease spirochete, Borrelia burgdorferi, from the avian tick, Ixodes auritulus. J Parasitol. 2005;91:780–90. doi: 10.1645/GE-3437.1. [DOI] [PubMed] [Google Scholar]
- 22.Vrbova L, Middleton D. Descriptive epidemiology of Lyme disease in Ontario: 1999–2004. Can Commun Dis Rep. 2006;32:247–57. [PubMed] [Google Scholar]
- 23.Schwan TG, Piesman J. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg Infect Dis. 2002;8:115–21. doi: 10.3201/eid0802.010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wormser GP. Prevention of Lyme borreliosis. Wien Klin Wochenschr. 2005;117:385–91. doi: 10.1007/s00508-005-0362-7. [DOI] [PubMed] [Google Scholar]
- 25.Duncan AW, Correa MT, Levine JF, et al. The dog as a sentinel for human infection: prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic states. Vector Borne Zoonotic Dis. 2004;4:221–9. doi: 10.1089/vbz.2004.4.221. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JL, Ginsberg HS, Zhioua E, et al. Passive tick surveillance, dog seropositivity, and incidence of human Lyme disease. Vector Borne Zoonotic Dis. 2004;4:137–42. doi: 10.1089/1530366041210710. [DOI] [PubMed] [Google Scholar]