Abstract
Abstinence symptoms in cannabis-dependent individuals are believed to contribute to the maintenance of regular marijuana use. However, there are currently no medications approved by the FDA to treat cannabis-related disorders. The only treatment currently shown consistently to alleviate cannabinoid withdrawal in both animals and humans is substitution therapy using the psychoactive constituent of marijuana, Δ9-tetrahydrocannabinol (THC). However, new genetic and pharmacological tools are available to increase endocannabinoid levels by targeting fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL), the enzymes responsible for the degradation of the endogenous cannabinoid ligands anandamide and 2-arachidonoylglycerol, respectively. In the present study, we investigated whether increasing endogenous cannabinoids levels, through the use of FAAH (−/−) mice as well as the FAAH inhibitor URB597 or the MAGL inhibitor JZL184, would reduce the intensity of withdrawal signs precipitated by the CB1 receptor antagonist rimonabant in THC-dependent mice. Strikingly, acute administration of either URB597 or JZL184 significantly attenuated rimonabant-precipitated withdrawal signs in THC-dependent mice. In contrast, FAAH (−/−) mice showed identical withdrawal responses as wild-type mice under a variety of conditions, suggesting that the absence of this enzyme across the development of dependence and during rimonabant challenge does not affect withdrawal responses. Of importance, subchronic administration of URB597 did not lead to cannabinoid dependence and neither URB597 nor JZL184 impaired rotarod motor coordination. These results support the concept of targeting endocannabinoid metabolizing enzymes as a promising treatment for cannabis withdrawal.
Key words: anandamide, cannabis dependence, fatty acid amide hydrolase (FAAH), monoacylglycerol lipase (MAGL), 2-arachindonoylglycerol (2-AG)
INTRODUCTION
Cannabis sativa is by far the most commonly used illicit drug in the USA, representing 73% of all illicit drug use and more than half of these individuals use marijuana exclusively. Of over 14 million people who use marijuana in the USA, almost four million are classified as being dependent or abusing (1). While it is common public perception that marijuana poses reduced physical dependency risk compared to other drugs of abuse, repeated marijuana smoking has been demonstrated to produce a distinct abstinence syndrome in clinical settings (2,3). The symptoms of this syndrome include anxiety, irritability, stomach pains, disrupted sleep, and general physical discomfort. Marijuana withdrawal has been compared to that of tobacco and is reported to increase craving and desire to resume use (4,5). A similar abstinence syndrome has also been shown upon cessation of repeated oral Δ9-tetrahydrocannbinol (THC), the primary psychoactive component of marijuana in human studies (6). Any abstinence syndrome may increase the desire to continue drug use and represents a complication in treating dependence.
Despite representing more than half of all classified drug abusers and an average of one million people receiving treatment each year for marijuana dependence, there are currently no approved pharmacological treatments available for cannabis dependence. THC is the most reliable and effective pharmacological agent identified that reduces cannabis withdrawal signs in both preclinical (7–9) and clinical (10,11) studies. In fact, many common treatments employed for tobacco cessation and other drugs of abuse actually worsened marijuana withdrawal symptoms (10,12). Thus, there is a need to examine marijuana withdrawal treatment as a unique and separate area of research.
Rodent models of precipitated cannabinoid withdrawal have been well characterized since the introduction of the selective CB1 receptor antagonist, rimonabant (13,14). Mice exposed to either repeated marijuana smoke or injections of THC display similar physical withdrawal symptoms (8), with the most common signs being paw tremors and head twitches (15,16). These withdrawal behaviors have been correlated with increased adenylyl cyclase activity in cerebellum (17) in marked contrast to acute cannabinoid actions that inhibit adenylyl cyclase activity (18). In addition, repeated THC administration results in significant desensitization and downregulation of CB1 receptors, consistent with behavioral tolerance seen in vivo (19). The observations that nonhuman primates self-administer THC (20) and that THC elicits a discriminative cue in animals (21) increase our understanding of cannabinoid dependence.
The endogenous cannabinoid system has become a rapidly developing area of research in recent years. This system consists of two receptor subtypes (CB1 and CB2) and several endogenous lipid-based signaling molecules that bind to these receptors (endocannabinoids). The two best characterized endogenous ligands, anandamide (AEA) and 2-arachindonoylglycerol (2-AG), are formed from membrane phospholipid precursors on-demand and are then rapidly eliminated by enzymatic degradation (for review, see (22)). The primary enzyme responsible for AEA degradation is fatty acid amid hydrolase (FAAH), which upon genetic or pharmacological inactivation leads to up to 10-fold increases in brain AEA levels (23,24). FAAH (−/−) mice display wild-type behavioral responses in most tests, with mild to moderate hypoalgesic and anxiolytic-like phenotypes (25,26). Inhibitors of FAAH, such as URB597, have been characterized in the literature (24,27) and show promising therapeutic efficacy in a variety of pathologies (for review, see (28)), with little evidence of cannabimimetic effects or abuse liability (29–31). The enzymatic degradation of 2-AG is primarily due to the activity of monoacylglycerol lipase (MAGL), which accounts for approximately 85% of 2-AG degradation in brain. Other enzymes identified as responsible for 2-AG degradation include ABHD12 and ABHD6, which have yet to be fully characterized (32). JZL184 is the first selective inhibitor of MAGL and when administered systemically led to 8-fold increases in brain 2-AG levels, hypoalgesia, and a subset of other cannabinoid effects (33).
In the present study, we employed FAAH (−/−) mice as well as URB597 (34) and JZL184 (33) to investigate whether increasing endogenous cannabinoid levels can ameliorate cannabinoid withdrawal responses. URB597 does not elicit rewarding effects in the conditioned place preference test, does not produce generalization to the discriminative effects of THC in rats (35), and is not self-administered by monkeys (29). These findings suggest that indirect cannabinoid agonists possess less dependence liability than THC. In the first series of experiments, we examined whether FAAH (−/−) mice would display a decrease in the severity of THC withdrawal responses. In the second set of experiments, we investigated whether acute administration of either URB597 or JZL184 would suppress the somatic signs of THC withdrawal. In the final experiments, the physical liability of URB597 and overall motor suppressant effects of both inhibitors were examined to determine any side effects undesirable for therapeutic use.
METHODS
Subjects
The subjects were adult male C57BL/6J mice that were purchased from the Jackson Laboratory (Bar Harbor, ME). Also serving as subjects were adult, male and female FAAH (−/−) and (+/+) mice that were obtained from the Center Transgenic Colony at Virginia Commonwealth University (Richmond, VA) backcrossed onto a C57BL/6J (at least 13 generations) background. Mice were kept on a 12-h light/dark cycle, with all experiments performed during the light cycle. Mice were housed 4–6/cage in a temperature-controlled (20–22°C) environment, with food and water available ad libitum except during testing. All experiments were performed with the approval of the Institutional Animal Care and Use Committee at Virginia Commonwealth University in accordance with the Guide for the Care and Use of Laboratory Animals.
Drugs
THC and rimonabant were obtained from the National Institute on Drug Abuse (Bethesda, MD), while URB597 was purchased from Cayman Chemical (Ann Arbor, MI). JZL184 was synthesized as described previously (33). All other chemical components for vehicles were obtained from Sigma–Aldrich (St. Louis, MO) unless otherwise stated. THC and rimonabant were dissolved in a vehicle mixture of ethanol/alkamuls-620 (Rhone-Poulenc, Princeton, NJ)/saline in a ratio of 1:1:18. URB597 was dissolved in a vehicle containing Tween 80/DMSO/saline in a ratio of 1:2:7. JZL184 was dissolved in a vehicle of PEG 200/Tween 80 in a ratio of 4:1. THC, rimonabant, and URB597 were diluted to an injection volume of 10 μL/g body mass, while JZL184 was injected at a volume of 4 μL/g body mass to limit vehicle effects. URB597 was administered 1 h before testing to coincide with previous findings of peak anandamide elevations at this time point (24). Similarly, JZL184 was administered 2 h before testing to coincide with peak levels of 2-AG elevations following systemic administration (33).
Rimonabant-Precipitated THC Withdrawal
Mice were given subcutaneous injections of THC to induce dependence under either a high- or low-dosing regimen. In the high-dose regimen, mice were given two daily injections of THC (50 mg/kg, s.c.) for five and a half days, with each injection separated by approximately 12 h. In the low-dose regimen, each mouse was given a single daily injection of THC (10 mg/kg, s.c.) for 6 days. In both conditions, the mice were given an i.p. injection of rimonabant 30 min after THC. All mice were then monitored and scored as described below for 1 h following rimonabant injection.
Behavioral Evaluation of Somatic Withdrawal Signs
Animals were pretreated with test drugs at times described above, followed by the described doses of THC just prior to placement in observation chambers. All animals were placed into white (for contrast) acrylic chambers (20 × 20 × 20 cm), with a clear acrylic front panel and a mirrored back panel, for a 30-min period for acclimation to the test chamber. The chambers were enclosed in sound-attenuating cabinets, designed and custom built at Virginia Commonwealth University, that contained an indirect filtered LED light source and fans for air circulation and white noise. At the 30-min time point, the animals were briefly removed from the chambers, given an i.p. injection of rimonabant, and immediately returned to the chambers for a 1-h observation period. The chambers were wiped clean with water just before the mice were returned for the observation period. Behavior was recorded through the clear front panel using a series of Fire-i™ digital cameras (Unibrain, San Ramon, CA), and the videos were processed and saved using ANY-maze™ video tracking software (Stoelting Co., Wood Dale, IL). Chambers were fully sanitized at the end of each testing day using ammonia-based cleansers and soap then left to air dry at least 2 days to dissipate any odors.
The videos were subsequently placed in randomized order in a separate ANY-maze™ protocol for a trained observer to score using a keyboard-based behavioral tracking system blinded to treatment group. ANY-maze™ software was used to track key presses assigned to somatic withdrawal behaviors for both time pressed and/or number of occurrences. Videos were scored using time sampling, examining periods of 5-min intervals, and then moving 5 min ahead on the video starting at minute 5 post-rimonabant injection (i.e., 5–10 min, 15–20 min, etc.). At the end of the hour video, each animal had a similar sampled 30-min period observed and scored from their recordings.
While a number of behavioral endpoints were observed that have been previously described in the literature as common in mice going through cannabinoid withdrawal (i.e., ptosis, retropulsion, piloerection, etc.), behaviors scored and presented are the most common, quantifiable, and with the highest inter-rater reliability (15). The primary behavior observed was front paw tremors that included a range of behavior from single-paw twitches to full fluttering or shaking of both paws simultaneously. These motions of the paws are not typical of normal behavior. Also recorded were head twitches, which generally manifest as rotational shakes of the head, similar to what is described as “wet dog shakes” in rats. The third behavior that was quantified was hind leg scratching that involved any repetitive scratching motion of the head or torso by either hind leg. All behaviors were counted as new incidences if either separated by at least 1 s and/or interceded by another distinct behavior (i.e., crawling, climbing, grooming).
Extraction and Quantification of Endocannabinoids by LC/MS
To quantify AEA and 2-AG levels in brain, both FAAH (+/+) and (−/−) mice were treated with the high THC-dosing regimen and challenged with rimonabant (10 mg/kg, i.p.), as described above. Thirty minutes into the withdrawal period, the subjects were decapitated and their brains were removed. The cerebellum and forebrain/midbrain regions were dissected. These global regions were selected based on studies showing that the cerebellum may contribute to precipitated cannabinoid somatic withdrawal signs in mice (17), while midbrain and cortical regions show a great deal of CB1 receptor downregulation and desensitization following subchronic THC administration (19,36). Both sections were snap frozen in liquid nitrogen and stored at −80°C.
On the day of processing, tissues were weighed and homogenized with 1.4 ml chloroform/methanol (2:1 v/v containing 0.0348 mg PMFS/ml) after the addition of internal standards to each sample (2 pmol AEA-d8 and 1 nmol 2-AG-d8). Homogenates were then mixed with 0.3 ml of 0.73% w/v NaCl, vortexed, and then centrifuged for 10 min at 4,000 rpm (4°C). The aqueous phase plus debris were collected and extracted two more times with 0.8 ml chloroform. The organic phases from the three extractions were pooled and the organic solvents were evaporated under nitrogen gas. Dried samples were reconstituted with 0.1 ml chloroform and mixed with 1 ml ice-cold acetone. The mixtures were then centrifuged for 5 min at 3,000 rpm and 4°C to precipitate the proteins. The upper layer of each sample was collected and evaporated under nitrogen. Dried samples were reconstituted with 0.1 ml methanol and placed in autosample vials for analysis.
LC/MS/MS was used to quantify AEA and 2-AG. The mobile phase consisted of (10:90) water/methanol with 0.1% ammonium acetate and 0.1% formic acid. The column used was a Discovery HS C18, 4.6 × 15 cm, 3 μm (Supelco, PA). The mass spectrometer was run in Electrospray Ionization, in positive mode. Ions were analyzed in multiple-reaction monitoring mode, and the following transitions were monitored: (348 > 62) and (348 > 91) for AEA; (356 > 62) for AEA-d8; (379 > 287) and (279 > 269) for 2-AG; and (387 > 96) for 2AG-d8. A calibration curve was constructed for each assay based on linear regression using the peak area ratios of the calibrators. The extracted standard curves ranged from 0.03 to 40 pmol for AEA and from 0.05 to 64 nmol for 2-AG.
Rotarod Motor Coordination Testing
Mice were trained for at least 3 days before testing to remain on a rotating 1.25-in. rotarod (IITC Life Sciences, Woodland Hills, CA) until able to stay on a rotarod maintained at 16 rpm. On drug-test days, the rotarod was set to accelerate from 1 to 16 rpm over the course of 60 s. The data shown reflect the rpm speed at which the animal fell off, 16 rpm representing animals that remained on the rotarod during testing.
On test days, a baseline test was given prior to drug administration. THC (40 mg/kg) was administered at a dose that demonstrated significant motor impairment in preliminary testing and then was tested for CB1-receptor specificity by treating animals with rimonabant (3 mg/kg) 10 min before THC administration. For the enzyme inhibitor tests, URB597 (10 mg/kg) and JZL184 (16 mg/kg) were given at the same doses as those used in withdrawal experiments. All drugs were tested at time points before, during, and after times observed during the withdrawal tests.
Data Presentation and Analysis
All data are reported as mean ± SEM. The somatic withdrawal behaviors were the scored observations of a 30-min sample observation period from the 1 h recording. Noncontinuous behaviors, such as head twitches and paw tremors, are presented as number of incidences observed. The continuous behavior of hind leg scratching is presented as total time observed scratching. All endocannabinoid levels are reported as mole per gram tissue. Rotarod data are expressed as the average RPM value at which the animal fell off the apparatus. Experiments with only two treatment groups were analyzed for statistical significance using the Student’s t test. Experiments with more than two groups were analyzed using two-way analysis of variance (ANOVA), rotarod testing analyzed using repeated measures ANOVA. Significant ANOVAs were followed by Tukey’s post hoc test, while Dunnett’s post hoc test was used for the rimonabant dose-response experiment. Resulting p values of less than 0.05 were considered significant. ED50 values (with 95% confidence intervals) were calculated using the least-squares linear regression method.
RESULTS
Rimonabant Precipitates Similar Somatic Withdrawal Signs in FAAH (−/−) and (+/+) Given Repeated Injections of THC
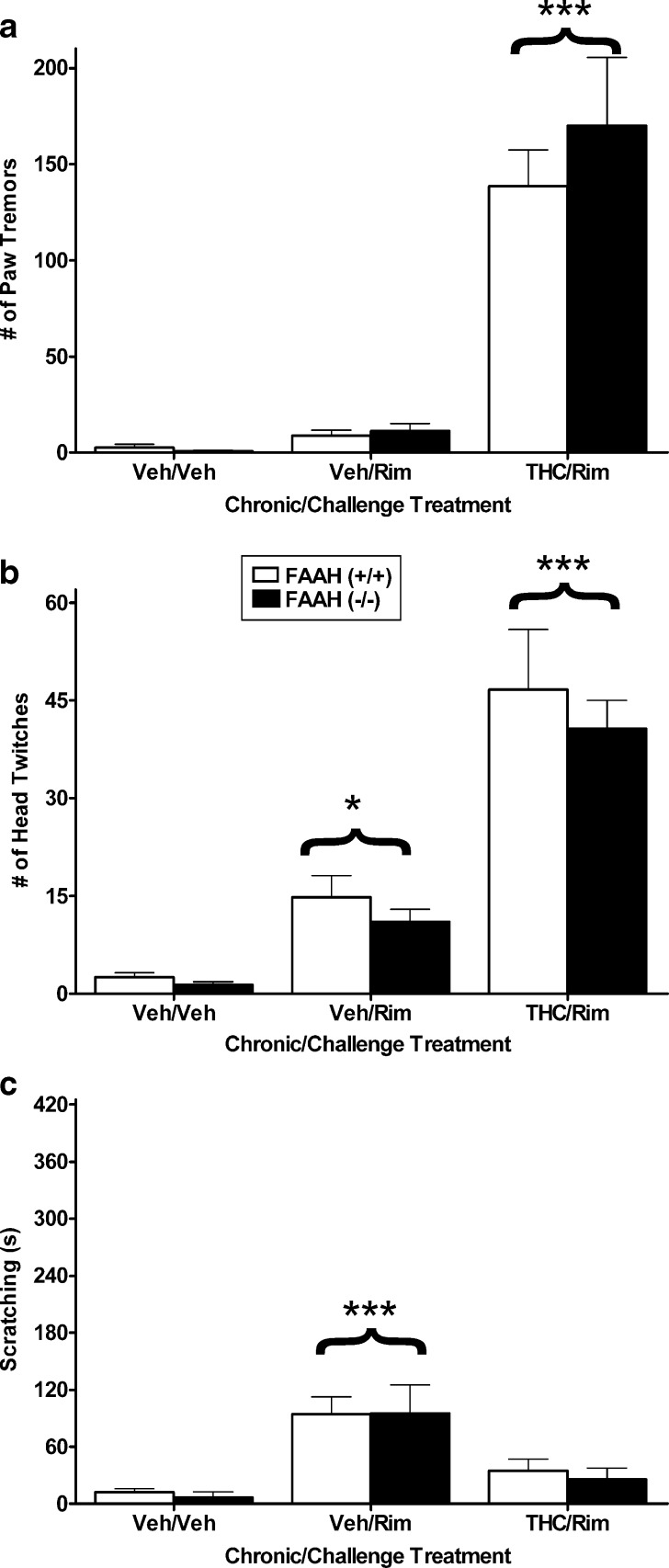
The purpose of this series of experiments was to determine whether THC dependence would be reduced in FAAH (−/−) mice compared to FAAH (+/+) mice. In the first experiment, FAAH (−/−) and (+/+) mice were treated in the high THC-dosing regimen or given vehicle for 5.5 days. On the sixth day, the vehicle-treated mice were given an acute injection of vehicle or rimonabant (10 mg/kg), while all the THC-treated mice were given an acute injection of rimonabant (10 mg/kg). Previous research from our laboratory indicated that mice treated repeatedly with THC and challenged with vehicle do not exhibit any withdrawal symptoms (7,8). Front paw tremors or fluttering were the primary somatic signs observed. As seen in Fig. 1a, a significant main effect of treatment on the number of paw tremors was observed [F(2,30) = 54.0, p < 0.001] in which rimonabant precipitated increases in paw flutters only in groups that received repeated THC compared to the other groups (p < 0.001). However, there was no significant effect for either genotype (p = 0.44) or interaction between genotype and treatment (p = 0.55). Figure 1b shows similar results for head twitches, with a significant effect of treatment [F(2,30) = 47.0, p < 0.001] but no significant differences for the main effect of genotype or the genotype by treatment interaction. Rimonabant precipitated significantly more head twitches in THC-dependent mice than in each of the other groups (p < 0.001). However, mice treated repeatedly with vehicle and challenged with rimonabant showed a small, but significant, increase in head twitching compared to vehicle control mice (p < 0.05). A significant treatment effect was found for hind-leg scratching behavior [F(2,30) = 14.9, p < 0.001; Fig. 1c], and again, there was no influence of genotype. Rimonabant increased scratching in mice given repeated vehicle injections compared to the other two groups (p < 0.001).
Fig. 1.
FAAH (−/−) and (+/+) mice show similar somatic withdrawal signs following a high THC (50 mg/kg twice daily for 5.5. days) dosing regimen. Rimonabant precipitated significant increases in paw tremors a and head twitches b in mice treated repeatedly with THC, regardless of genotype. No significant genotype differences were found. c Rimonabant elicited a significant increase in scratching behavior in mice treated repeatedly with vehicle, regardless of phenotype. n = 6 mice per group. *p < 0.05 versus vehicle control, ***p < 0.001 versus all other groups
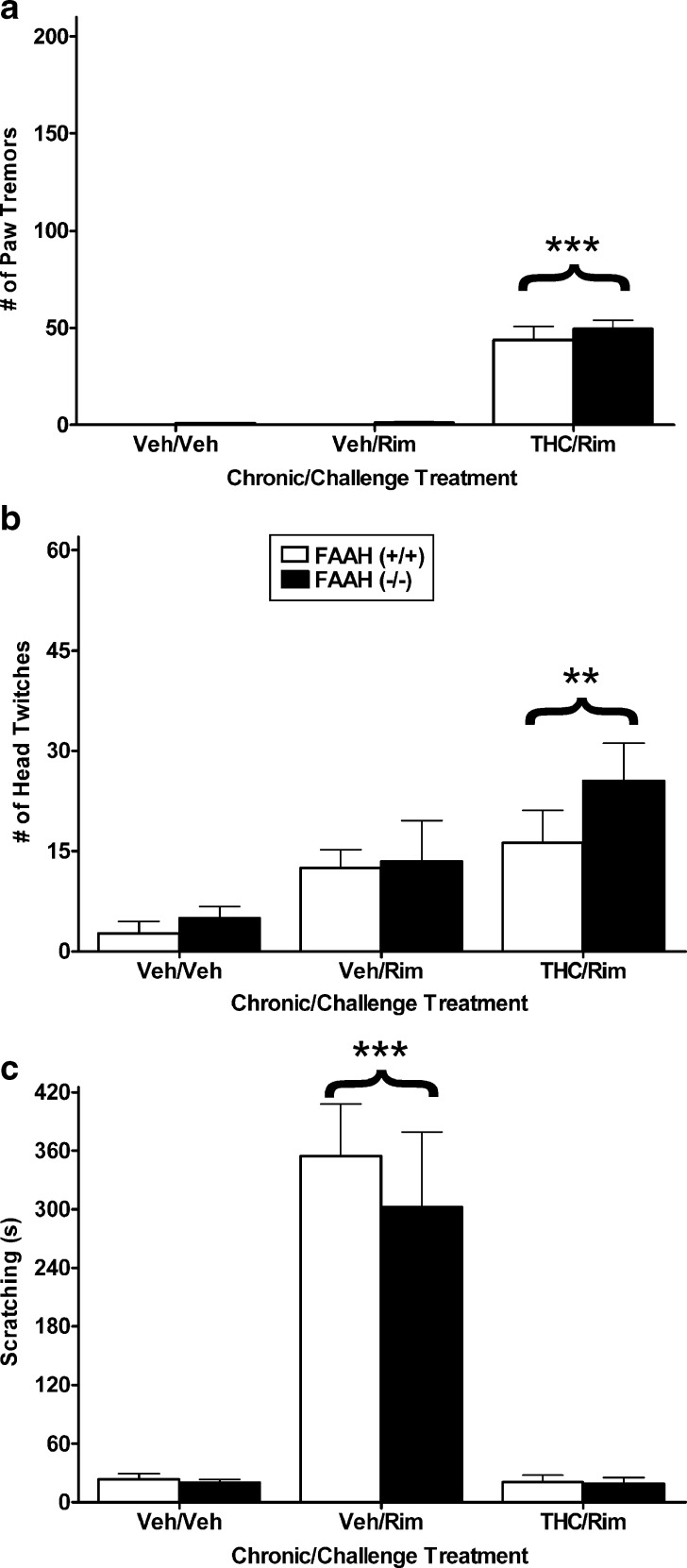
Although FAAH (−/−) mice did not display significant decreases in withdrawal behavior, it is possible that ceiling effects caused by the high THC-dosing regimen obscured subtle genotype differences. Thus, a follow-up experiment was conducted using a mild THC-dosing regimen to examine whether severity of rimonabant precipitated withdrawal is altered in FAAH (−/−) mice. Rimonabant precipitated paw tremors [F(2, 26) = 97.5, p < 0.001; Fig. 2a] and head twitching [F(2, 26) = 5.9, p < 0.05; Fig. 2b] in mice treated repeatedly with THC compared to the other two groups of mice. However, there was no effect of genotype on either of these withdrawal responses. As seen in Fig. 2c, vehicle-treated mice receiving rimonabant alone, regardless of genotype, spent significantly more time scratching than the other two treatment groups [F(2, 26) = 34.9, p < 0.001]. Because we and others have found that rimonabant induces scratching behavior in drug naïve mice (8,37), this behavior is not considered a withdrawal response and is not reported in subsequent experiments.
Fig. 2.
FAAH (−/−) and (+/+) mice show similar somatic withdrawal signs following a low THC (10 mg/kg, once daily for 6 days)-dosing regimen. Rimonabant precipitated a significant increase in paw tremors a and head twitches b in mice treated repeatedly with THC, regardless of genotype. No significant genotype differences were found. c Rimonabant only elevated scratching in mice receiving repeated injections of vehicle. n = 6 mice per group. **p < 0.01 versus vehicle control, ***p < 0.001 versus all other groups
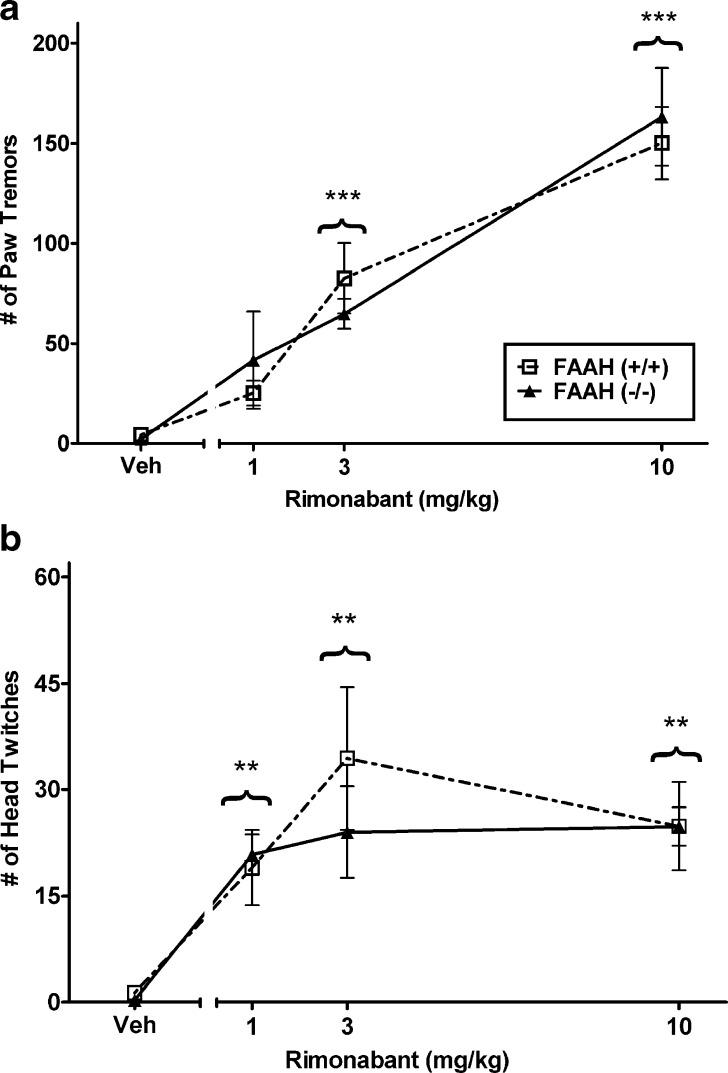
In the next experiment, we examined whether rimonabant would be more potent in precipitating withdrawal in FAAH (−/−) mice than in FAAH (+/+) mice. Both genotypes were subjected to the high THC-dosing regimen (i.e., 50 mg/kg twice a day for 5.5 days) and the dose-response relationship of rimonabant in precipitating paw tremors and head shakes was determined. Rimonabant elicited a significant dose-responsive effect on paw tremors [F(3, 37) = 38.4, p < 0.001; Fig. 3a], with both 3 and 10 mg/kg of rimonabant precipitating tremors significantly above those of mice given an acute injection of vehicle (p < 0.001). The ED50 values in FAAH (+/+) and (−/−) mice were 2.9 mg/kg (95% C.I. 2.1 to 4.0 mg/kg) and 2.9 mg/kg (95% C.I. 1.7 to 4.7 mg/kg), respectively. The observation that rimonabant was equipotent in precipitating withdrawal in both genotypes further demonstrates that deletion of FAAH does not affect withdrawal responses in THC-dependent mice. Rimonabant challenge also precipitated a significant increase in head twitching [F(3, 37) = 12.4, p < 0.001; Fig. 3b]. Each dose of rimonabant increased this effect compared to vehicle (p < 0.01).
Fig. 3.
Rimonabant (1, 3, and 10 mg/kg) dose-dependently increased the incidence of paw tremors a in mice treated with a high THC-dosing regimen. Rimonabant was equipotent in eliciting paw tremors between FAAH (+/+) mice and FAAH (−/−) mice. b Rimonabant also precipitated a significant increase in head twitching compared to vehicle in THC-dependent mice. n = 6 mice per group. **p < 0.01, ***p < 0.001 versus vehicle determined by Dunnett’s post hoc
Quantification AEA and 2-AG Brain Levels in THC-Dependent FAAH (+/+) and (−/−) Mice Undergoing Withdrawal
To examine whether rimonabant (10 mg/kg) altered endogenous cannabinoid levels in FAAH (+/+) or (−/−) mice treated with repeated injections of either vehicle or THC (50 mg/kg), cerebellum and combined forebrain/midbrain were obtained from these mice. As summarized in Table I, equivalent AEA levels were found in cerebellum in THC-dependent and nondependent mice, though AEA levels were approximately 10-fold higher in FAAH (−/−) mice than in FAAH (+/+) mice [F(1, 28) = 242.4, p < 0.001]. Similarly, 2-AG levels did not differ between THC-dependent and nondependent mice. However, FAAH (−/−) mice showed significant decreases in cerebellar 2-AG content regardless of subchronic treatment [F(1, 28) = 5.5, p < 0.05]. In the forebrain/midbrain, FAAH (−/−) mice also displayed significant increases in AEA, regardless of subchronic treatment [F(1, 29) = 313.9, p < 0.001]. However, a significant interaction was found between genotype and subchronic treatment [F(1, 29) = 5.6, p < 0.05]. A small but significant reduction of AEA levels was found in the forebrain/midbrain of THC-treated FAAH (−/−) mice compared to vehicle-treated FAAH (−/−) mice (Tukey’s post hoc; p < 0.05). Finally, there were no differences in 2-AG levels in forebrain/midbrain tissues based on genotype or subchronic THC treatment.
Table I.
Brain Endocannabinoid Levels in FAAH (−/−) and (+/+) Mice Given Repeated Injections of Vehicle or THC (50 mg/kg) Twice a Day for 5.5 Days
| Tissue | FAAH genotype | Subchronic treatment | AEA (pм/g tissue) | 2-AG (nм/g tissue) |
|---|---|---|---|---|
| Cerebellum | +/+ | Vehicle | 2.7 ± 0.35 | 14.1 ± 0.69 |
| +/+ | THC | 3.0 ± 0.60 | 12.4 ± 1.11 | |
| −/− | Vehicle | 29.7 ± 1.97 | 11.8 ± 0.69 | |
| −/− | THC | 32.2 ± 2.95 | 10.3 ± 1.11 | |
| Forebrain/Midbrain | +/+ | Vehicle | 3.4 ± 0.53 | 12.6 ± 0.97 |
| +/+ | THC | 2.7 ± 0.38 | 11.3 ± 0.56 | |
| −/− | Vehicle | 27.6 ± 1.58 | 10.1 ± .079 | |
| −/− | THC | 21.1 ± 1.85* | 12.1 ± 1.50 |
All mice were given an i.p. injection of rimonabant (10 mg/kg), and brain tissue was obtained 30 min later. N = 8 mice/group. Values are expressed as mean ± SEM
*p < 0.05 vs. FAAH (−/−) mice treated given subchronic vehicle administration
Acute Administration of the FAAH Inhibitor, URB597, Reduces the Severity of Rimonabant-Precipitated Withdrawal in THC-Dependent Mice
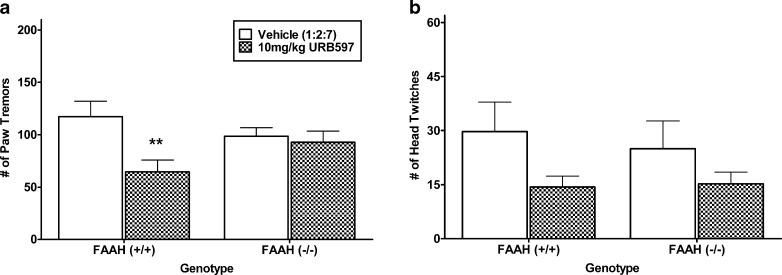
FAAH (−/−) mice possess constitutively elevated levels of AEA that would have occurred presumably across the development of dependence and during rimonabant challenge. Thus, in the next experiment we investigated whether acute blockade of FAAH using the irreversible FAAH inhibitor, URB597, would reduce the severity of rimonabant-precipitated withdrawal responses in mice subjected to the high THC-dosing regimen. The experiment was conducted in both FAAH (+/+) and (−/−) mice to determine the specificity of any URB597 effects to its actions on FAAH activity.
As seen in Fig. 4a, URB597 reduced paw tremors during THC withdrawal by approximately 40% in FAAH (+/+) mice but had no effect in FAAH (−/−) animals, as reflected by an interaction between URB597 treatment and FAAH genotype [F(1, 27) = 4.4, p < 0.05]. ANOVA revealed a main effect of URB597 on head twitches during THC withdrawal [F(1, 27) = 4.5, p < 0.05]. However, there was no main effect of FAAH genotype and no interaction between FAAH genotype and URB597 treatment. Planned comparisons did not reveal significant differences between URB597 and vehicle for each respective genotype (Fig. 4b).
Fig. 4.
Assessment of the irreversible FAAH inhibitor, URB597 (10 mg/kg), on the incidence of rimonabant-precipitated withdrawal behavior in FAAH (+/+) and (−/−) mice that were treated subchronically with a high THC-dosing regimen. a URB597 reduced the incidence of rimonabant-precipitated paw tremors in FAAH (+/+) THC-dependent mice but was without effect in FAAH (−/−) mice. b URB597 reduced the incidence of rimonabant-precipitated head twitches, regardless of genotype. n = 8 mice per group. **p < 0.01 versus FAAH (+/+) vehicle control group
Acute Administration of the MAGL Inhibitor, JZL184, Reduces the Severity of Rimonabant-Precipitated Withdrawal in THC-Dependent Mice
There are currently no available MAGL knockout mice to examine the impact of deleting this enzyme and concomitant elevations in 2-AG levels on THC withdrawal. However, the first selective MAGL inhibitor reported, JZL184, shows a partial set of CB1-receptor-mediated behavioral effects in the cannabimimetic tetrad test (hypomotility, hypothermia, and analgesia) (33). To examine if acute elevation of 2-AG levels can reduce somatic signs of rimonabant precipitated withdrawal in THC-dependent mice, vehicle or JZL184 (16 mg/kg) was administered 2 h before rimonabant injection. The high THC-dosing regimen was used. Testing was performed in both FAAH (+/+) and (−/−) mice to examine the specificity of drug effects to FAAH and to ascertain whether simultaneous elevation of AEA and 2-AG levels causes differential responses.
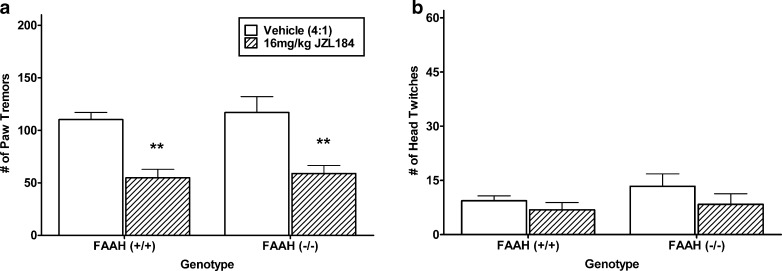
As seen in Fig. 5a, JZL184 reduced the incidence of paw tremor activity during THC withdrawal by approximately 50% [F(1, 21) = 33.3, p < 0.001]. Unlike the reduction seen in URB597, JZL184 was equally effective in reducing tremors in both FAAH (+/+) and (−/−) mice. Also in contrast to URB597, there were no effects of JZL184 treatment on the occurrence of head twitching during withdrawal (Fig. 5b). However, it should be noted that the signal of rimonabant-precipitated head shakes in THC-dependent mice was considerably lower than the signal in the previous experiments (see Figs. 1b, 3b, and 4b). This reduced signal of head twitches may have been the consequence of the PEG-Tween 80 vehicle used for JZL184.
Fig. 5.
Assessment of the irreversible MAGL inhibitor JZL184 (16 mg/kg), on the incidence of rimonabant-precipitated withdrawal behavior in FAAH (+/+) and (−/−) mice that were treated subchronically with a high THC-dosing regimen. a JZL184 reduced the incidence of rimonabant-precipitated paw tremors in both FAAH (+/+) and (−/−) mice. b No significant effect was found on head twitches. n = 6–7 mice per group. **p < 0.01 versus respective genotype vehicle control
Rimonabant Precipitated Withdrawal of Repeated URB597
The purpose of this experiment was to examine whether repeated administration of FAAH inhibitors produces a cannabimimetic physical dependence. Mice were treated with URB597 (10 mg/kg) or vehicle twice daily for 5.5 days and were challenged with rimonabant 1 h after their final injection. No irregular behaviors were observed or noted during the recording or scoring of the videos, and all the same somatic signs tracked during THC withdrawal were quantified. Rimonabant produced no significant differences between mice that were given repeated injections of URB597 and vehicle on paw tremors [t(14) = 0.8, p = 0.44] as well as head twitching [t(14) = 0.4, p = 0.68]. These results are summarized in Table II.
Table II.
Rimonabant (10 mg/kg) does not Precipitate THC-like Withdrawal Responses in Mice Given Repeated Injections of URB597
| Subchronic treatment | Paw flutters | Head twitches |
|---|---|---|
| Vehicle | 9.4 ± 2.2 | 14.4 ± 2.6 |
| URB597 | 12.1 ± 2.7 | 16.4 ± 4.0 |
All mice were given two daily s.c. injections of vehicle or URB597 (10 mg/kg) for 5 days. On day 6, each subject received their respective injection of vehicle or URB597, followed by an i.p. injection of rimonabant (10 mg/kg) 60 min later. N = 8 mice/group. Values are expressed as mean ± SEM
Rotarod Motor Coordination Tests
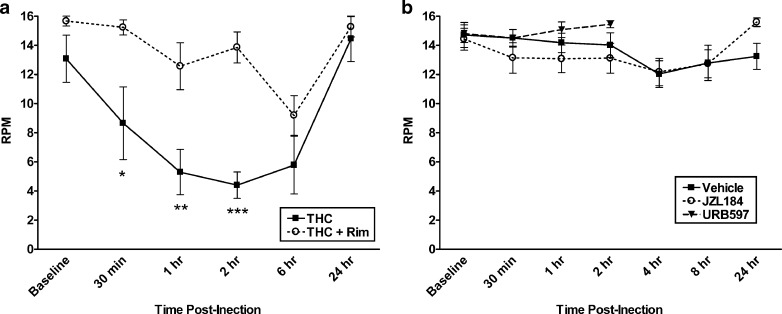
While URB597 does not appear to affect locomotor activity (28), JZL184 has been reported to suppress spontaneous activity (33); however, neither compound has been examined in the rotarod test, an assay used to assess motor coordination. In order to evaluate whether URB597 or JZL184 elicits motor deficits that may interfere with the expression of somatic withdrawal signs, both endocannabinoid modulators were evaluated in this assay. In an initial experiment, we examined the effects of THC (40 mg/kg) vs. rimonabant (3 mg/kg) on performance in the rotarod test. As shown in Fig. 6a, THC significantly impaired performance in the rotarod test, with a significant interaction between THC treatment and time [F(6, 60) = 2.4, p < 0.05]. THC reduced performance from baseline beginning at 30 min post-injection and continued to impair performance up to 6 h (Dunnett’s post hoc; p < 0.05). Rimonabant significantly blocked THC-induced rotarod impairment for up to 2 h (Dunnett’s post hoc; p < 0.05).
Fig. 6.
THC, but neither URB597 nor JZL184, impaired motor performance in the rotarod task. a THC (40 mg/kg) impairs rotarod performance, expressed as RPM at which point mice fell off the rod. Rimonabant (3 mg/kg) pretreatment blocked THC-induced rotarod impairment. b Neither URB597 (10 mg/kg) nor JZL184 (16 mg/kg) adversely affected motor coordination in the rotarod test. Doses of each inhibitor used in rotarod test were found effective in suppressing somatic withdrawal signs. n = 6–12 mice per group. *p < 0.05, **p < 0.01, ***p < .001 versus baseline time point and rimonabant control group determined by Dunnett’s post hoc
The final experiment examined whether URB597 (10 mg/kg) or JZL184 (16 mg/kg) would impair performance in the rotarod test (Fig. 6b). URB597 showed no significant impairment of rotarod performance compared to baseline or vehicle-treated control mice for up to 2 h post-treatment, which includes the time period that was used for observation in the withdrawal tests, as well as the peak of AEA enhancement (24). JZL184, which elevates 2-AG brain levels up to 8 h post-injection (33), also showed no evidence of motor impairment compared to baseline or vehicle controls for a 24-h period following treatment.
DISCUSSION
In the present study, we investigated the role of endocannabinoid degradative enzymes in THC dependence. Specifically, we examined the impact of increasing AEA or 2-AG levels on somatic withdrawal signs precipitated by the CB1 receptor antagonist rimonabant in THC-dependent mice. Despite persistent elevation of AEA above that of wild-type mice, FAAH (−/−) mice showed no alterations in rimonabant-precipitated withdrawal responses across a variety of experimental conditions. Thus, the constitutive absence of this enzyme across ontogeny does not affect the development of physical dependence to THC. Strikingly, the FAAH inhibitor URB597 and the MAGL inhibitor JZL184 ameliorated withdrawal responses in THC-dependent mice when administered acutely. In FAAH (−/−) mice, URB597 no longer reduced precipitated paw tremors, while JZL184 maintained its efficacy. This pattern of findings is consistent with the notion that these drugs produce their effects through the inhibition of distinct enzymes. Unlike direct-acting cannabinoid agonists that possess dependence liability, repeated administration of URB597 alone did not lead to physical dependence. These findings indicate that increasing endogenous cannabinoid signaling may represent a novel strategy to treat cannabis dependence.
The characteristic pattern of behavior associated with rimonabant-precipitated somatic withdrawal signs in THC-dependent mice reported here is similar to that previously characterized in the literature (7,15). Tremors in the front paws continue to be the most consistent, quantifiable, and consistently dose-responsive. Paw tremors were dose-responsive to both the dose of THC that was subchronically administered and dose of rimonabant used to precipitate withdrawal, making it the principal behavior in defining THC dependence in mice. Rimonabant elicited head twitches in nondependent mice, but this effect was augmented in THC-dependent mice. On the other hand, scratching behavior appears to be an intrinsic effect of rimonabant. Previous research has demonstrated that rimonabant induces scratching in a dose-responsive manner and is blocked by cannabinoid agonists (38). In fact, our research has shown that the endocannabinoid system may play a modulatory role in scratching behavior (39).
Given the observation that URB597 reduced rimonabant-precipitated withdrawal signs in THC-dependent mice, the lack of a FAAH (−/−) phenotype in this withdrawal model was somewhat surprising. Though one might expect that enhanced endocannabinoid signaling might provide a protective mechanism against cannabinoid withdrawal, especially as AEA is discretely produced on-demand under conditions of stress (40), it is also possible that elevated endocannabinoids during the development of dependence may have enhanced the severity of precipitated withdrawal. Despite having consistently elevated AEA levels nearly 10-fold above that of the wild-type animals, FAAH-deficient mice have previously been demonstrated to display similar responses to acute THC in a battery of cannabinoid sensitive behaviors as wild-type animals (23). FAAH (−/−) and (+/+) mice also have identical levels of CB1 receptors in brain and possess similar binding affinities to [3H]CP-55,940, suggesting no abnormalities in receptor number or function (25). However, the possibility exists that other compensatory actions occurred due to genetic deletion of this gene across ontogeny. Follow-up studies into AEA and 2-AG content in the brains of FAAH (−/−) mice showed lower 2-AG content in the cerebellum compared to wild-type mice, which may be a compensatory mechanism for consistent AEA elevations. AEA content in midbrain/forebrain regions was reduced approximately 23% in THC-treated FAAH (−/−) mice compared to vehicle-treated FAAH (−/−) mice, suggesting the possibility that THC dependence or withdrawal may elicit negative feedback on AEA biosynthesis. Reductions in AEA content of midbrain/forebrain regions have been previously reported in rats during THC withdrawal; similarly, no alterations were seen in cerebellum (36). Subchronic THC elicits less CB1 receptor desensitization and downregulation in the cerebellum than in midbrain areas, such as thalamus and hippocampus (19). Although the reduced AEA levels detected in midbrain/forebrain region of the THC-dependent FAAH (−/−) mice might contribute to the normalized rimonabant-precipitated somatic withdrawal responses in the THC-dependent animals, it should be noted that AEA levels were still elevated approximately 8-fold in the FAAH (−/−) mice compared to the wild-type mice. At any rate, genetic deletion of FAAH does not appear to influence rimonabant-precipitated withdrawal responses in THC-dependent mice across a variety of different conditions.
Because FAAH (−/−) mice possess constitutively elevated levels of AEA throughout the development of dependence, we examined the effect of acute FAAH inhibition through pharmacological means. The FAAH inhibitor, URB597, was administered just before rimonabant challenge in order to elevate AEA levels during the withdrawal period. The short-term elevation of AEA during this period significantly attenuated the severity of the withdrawal behavior, as seen primarily in the reduced amount of paw tremors (Fig. 4a). This reduction in paw tremors by URB597 was completely absent in FAAH (−/−) mice, showing that the effect seen was specific to the actions of URB597 on FAAH activity. Conversely, it appears that the mechanism by which URB597 altered expression of head twitching was FAAH-independent. Although FAAH blockade leads to increased levels of AEA, this enzyme also regulates the catabolism of noncannabinoid fatty acid amides, including N-palmitoyl ethanolamine (PEA), N-oleoyl ethanolamine (OEA), oleamide, and N-acyl taurines (23,41). Thus, it is unclear whether the beneficial effects of URB597 in reducing THC withdrawal responses is related to elevated levels of AEA and/or the other substrates of FAAH. At any rate, the present results suggest that URB597 or other FAAH inhibitors may be a promising pharmacotherapeutic approach to alleviate THC withdrawal responses.
With recent advances allowing systemic examination of MAGL inhibition and consequential 2-AG elevations, we examined whether elevating 2-AG could similarly reduce somatic THC withdrawal symptoms. While less is known about the behavioral consequences of 2-AG inhibition, the concentration of this endocannabinoid is more than 100-fold greater than that of AEA in the brain. However, it is possible much of the 2-AG in the body does not play a role in cannabinoid signaling (42). Acute administration of the MAGL inhibitor, JZL184, significantly reduced paw tremor incidence. One might predict an increased efficacy of JZL184 in reducing withdrawal symptoms in FAAH (−/−) mice, since both major endogenous cannabinoids, AEA and 2-AG, are elevated above that of wild-type mice. However, this was not the case. The observation that JZL184 was equally efficacious in FAAH (+/+) and (−/−) mice indicates that the mechanism of JZL184 was independent of FAAH activity. However, given the limits with knockout animals, full characterization of dual inhibition of both enzymes on cannabinoid withdrawal is warranted in future studies.
The lack of rimonabant-precipitated cannabinoid withdrawal signs by repeated URB597 injection is an important observation for the clinical development of this drug and has important implications for the development of other FAAH inhibitors. Cannabis withdrawal is not recognized in the current Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) but is currently under debate for inclusion in the next edition (43). Without widespread medical consensus as to the severity (or even existence) of such a condition, possible treatment options must present minimal risk in contributing to any further dependence problems. The present findings examining endocannabinoid attenuation of withdrawal adds to a growing body of literature demonstrating that URB597 lacks the rewarding properties that is typical of exogenous cannabinoids. URB597 fails to elicit conditioned place preferences (35) and also fails to generalize in rats trained to discriminate the drug effects of THC (31,35). In addition, URB597 does not increase dopamine release in the shell of the nucleus accumbens (30), a common hallmark of almost all substances of abuse. Moreover, it has recently been shown that monkeys previously trained to administer other drugs of abuse, including THC, will not self-administer URB597. Finally, URB597 also lacks the ability to prime reinstatement, and fails to increase self-administration, in monkeys receiving either THC or cocaine (29). The aforementioned study suggests that not only do FAAH inhibitors lack rewarding properties but they also do not enhance the dependence liability of common drugs of abuse.
The lack of effects of URB597 in the rotarod test complement the results of other studies showing that genetic deletion or pharmacological inhibition of FAAH does not elicit any apparent untoward motor effects. In contrast, THC elicited motor incoordination that persisted for up to 6 h. This impairment was reversed by rimonabant, demonstrating a CB1 receptor mechanism of action. While mice receiving JZL184 display a decrease in spontaneous activity and exhibit a flattened posture reminiscent of mice receiving THC, these mice were able to perform normally in the rotarod test throughout the full time course of demonstrated 2-AG elevations (33). These findings suggest that endocannabinoid elevation, through blockade of enzymatic degradation, is not sufficient to cause dependence or motor incoordination typical of high doses of THC and other exogenous cannabinoid receptor agonists.
CONCLUSION
Acute administration of the selective FAAH inhibitor, URB597, or the selective MAGL inhibitor, JZL184, significantly reduced somatic withdrawal symptoms precipitated by the CB1 receptor antagonist rimonabant in THC-dependent mice. These findings suggest that inhibitors of endocannabinoid metabolizing enzymes may offer an effective pharmacotherapy to treat cannabis withdrawal. Neither FAAH nor MAGL inhibition impaired gross motor function and repeated administration of URB597 did not lead to cannabinoid physical dependence. Collectively, these data suggest that endocannabinoid modulation represents a promising avenue of treatment for a challenging yet still controversial syndrome.
Acknowledgements
The authors would like to acknowledge the technical assistance of Noor S. Shubar Ali, Deborah Karp, and Megan O’Brien with rotarod testing. The work was supported by National Institute on Drug Abuse grants P01DA017259, R01DA15197, R01DA03672, R01DA02396, R01DA015683, P50DA005274, P01DA009789 and T32DA007027. Additional support was provided by Scholar Rescue Funds of The Institute of International Education, New York.
Abbreviations
- FAAH
Fatty acid amide hydrolase
- Rimonabant
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide HCl
- THC
{Delta}9-tetrahydrocannabinol
- URB597
Cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester
- C57
C57BL/6J mouse strain
- JZL184
4-Nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate
- MAGL
Monoacylglycerol lipase
- AEA
Anandamide
- 2-AG
2-Arachindonoylglycerol
References
- 1.Substance Abuse and Mental Health Services Administration: Office of Applied Studies. Results from the 2007 National Survey on Drug Use and Health: national findings. Rockville, MD: Dept. of Health and Human Services, Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2008.
- 2.Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393–402. doi: 10.1037/0021-843X.112.3.393. [DOI] [PubMed] [Google Scholar]
- 3.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999;141(4):395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 4.Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat. 2008;35(4):362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92(1–3):48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology (Berl) 1999;141(4):385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- 7.Lichtman AH, Fisher J, Martin BR. Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav. 2001;69(1–2):181–188. doi: 10.1016/S0091-3057(01)00514-7. [DOI] [PubMed] [Google Scholar]
- 8.Wilson DM, Varvel SA, Harloe JP, Martin BR, Lichtman AH. SR 141716 (Rimonabant) precipitates withdrawal in marijuana-dependent mice. Pharmacol Biochem Behav. 2006;85(1):105–113. doi: 10.1016/j.pbb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Beardsley PM, Balster RL, Harris LS. Dependence on tetrahydrocannabinol in rhesus monkeys. J Pharmacol Exp Ther. 1986;239(2):311–319. [PubMed] [Google Scholar]
- 10.Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29(1):158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- 11.Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86(1):22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW. Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology (Berl) 2001;155(2):171–179. doi: 10.1007/s002130000657. [DOI] [PubMed] [Google Scholar]
- 13.Aceto MD, Scates SM, Lowe JA, Martin BR. Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR 141716A. Eur J Pharmacol. 1995;282(1–3):R1–R2. doi: 10.1016/0014-2999(95)00447-S. [DOI] [PubMed] [Google Scholar]
- 14.Tsou K, Patrick SL, Walker JM. Physical withdrawal in rats tolerant to delta 9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. Eur J Pharmacol. 1995;280(3):R13–R15. doi: 10.1016/0014-2999(95)00360-W. [DOI] [PubMed] [Google Scholar]
- 15.Cook SA, Lowe JA, Martin BR. CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1998;285(3):1150–1156. [PubMed] [Google Scholar]
- 16.Hutcheson DM, Tzavara ET, Smadja C, Valjent E, Roques BP, Hanoune J, et al. Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9-tetrahydrocannabinol. Br J Pharmacol. 1998;125(7):1567–1577. doi: 10.1038/sj.bjp.0702228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzavara ET, Valjent E, Firmo C, Mas M, Beslot F, Defer N, et al. Cannabinoid withdrawal is dependent upon PKA activation in the cerebellum. Eur J Neurosci. 2000;12(3):1038–1046. doi: 10.1046/j.1460-9568.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- 18.Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986;29(3):307–313. [PubMed] [Google Scholar]
- 19.Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-b enzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212–2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther. 2002;303(1):36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- 20.Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169(2):135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- 21.Jarbe TU, Henriksson BG. Discriminative response control produced with hashish, tetrahydrocannabinols (delta 8-THC and delta 9-THC), and other drugs. Psychopharmacologia. 1974;40(1):1–16. doi: 10.1007/BF00429443. [DOI] [PubMed] [Google Scholar]
- 22.Clapper JR, Mangieri R, Piomelli D. The endocannabinoid system as a target for the treatment of cannabis dependence. Neuropharmacology. 2009;56(S1):235–243. doi: 10.1016/j.neuropharm.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98(16):9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313(1):352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- 25.Lichtman AH, Hawkins EG, Griffin G, Cravatt BF. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther. 2002;302(1):73–79. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- 26.Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109(3):319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Boger DL, Miyauchi H, Du W, Hardouin C, Fecik RA, Cheng H, et al. Discovery of a potent, selective, and efficacious class of reversible alpha-ketoheterocycle inhibitors of fatty acid amide hydrolase effective as analgesics. J Med Chem. 2005;48(6):1849–1856. doi: 10.1021/jm049614v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, et al. Pharmacological profile of the selective FAAH inhibitor KDS-4103 (URB597) CNS Drug Rev. 2006;12(1):21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, et al. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64(11):930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98(2):408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- 31.Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, et al. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharmacol Exp Ther. 2007;321(1):370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- 32.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14(12):1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5(1):37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9(1):76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 35.Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102(51):18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez S, Fernandez-Ruiz J, Di Marzo V, Hernandez M, Arevalo C, Nicanor C, et al. Behavioral and molecular changes elicited by acute administration of SR141716 to Delta9-tetrahydrocannabinol-tolerant rats: an experimental model of cannabinoid abstinence. Drug Alcohol Depend. 2004;74(2):159–170. doi: 10.1016/j.drugalcdep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Darmani NA, Pandya DK. Involvement of other neurotransmitters in behaviors induced by the cannabinoid CB1 receptor antagonist SR 141716A in naive mice. J Neural Transm. 2000;107(8–9):931–945. doi: 10.1007/s007020070043. [DOI] [PubMed] [Google Scholar]
- 38.Janoyan JJ, Crim JL, Darmani NA. Reversal of SR 141716A-induced head-twitch and ear-scratch responses in mice by delta 9-THC and other cannabinoids. Pharmacol Biochem Behav. 2002;71(1–2):155–162. doi: 10.1016/S0091-3057(01)00647-5. [DOI] [PubMed] [Google Scholar]
- 39.Schlosburg JE, Boger DL, Lichtman AH. Endocannabinoid modulation of scratching response in an acute allergenic model: a new prospective neural therapeutic target for pruritus. J Pharmacol Exp Ther. 2009;329(1):314–323. doi: 10.1124/jpet.108.150136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435(7045):1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 41.Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry. 2006;45(30):9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- 42.Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, Fernandez-Ruiz JJ, et al. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem Biophys Res Commun. 1999;256(2):377–380. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- 43.Crowley TJ. Adolescents and substance-related disorders: research agenda to guide decisions on Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-V) Addiction. 2006;101(Suppl 1):115–124. doi: 10.1111/j.1360-0443.2006.01594.x. [DOI] [PubMed] [Google Scholar]