Abstract
The purpose of this study was to evaluate the relationship of respiratory quotient (RQ), a surrogate marker of substrate oxidation, as well as body composition and dietary intake to resting energy expenditure (REE) among HIV-infected patients in the current era of HAART and among non HIV-infected control subjects. Resting energy expenditure (REE) is increased in HIV-infected patients, but little is known regarding the potential contribution of altered substrate metabolism, body composition and dietary intake to increased energy expenditure in this population. RQ, REE, body composition and dietary intake parameters were assessed in 283 HIV-infected patients and 146 community-derived HIV-negative controls that were evaluated for metabolic studies between 1998 and 2005. RQ was lower (0.83±0.00 vs. 0.85±0.01, P=0.005) whereas REE adjusted for fat free mass (FFM) was higher (31.8±0.3 vs. 29.8±0.3 kcal/d/kg, P=<0.0001) in HIV-infected compared to control subjects. In multivariate modeling among HIV-infected patients, including age, gender and parameters of immune function, FFM (beta=24.811334, P <0.0001), visceral adiposity (beta=0.7182746, P=0.008), and total body fat (beta=8.0506839, P=0.041) were positively associated with REE, whereas RQ was negatively associated with REE (beta= −528.4808, P=0.024). Overall r2=0.705, P<0.0001 for the model. In control subjects, by contrast, only visceral adiposity (beta = 1.0612073, P=0.004), total body fat (beta = 15.805547, P=0.010), and FFM (beta = 22.613005, P <0.0001) were significant predictors of REE, and there was no relationship with RQ. Overall r2=0.825, P<0.0001 for the model. These data suggest that alterations in substrate metabolism may contribute to increased REE in HIV-infected patients compared to control subjects.
Keywords: HIV, resting energy expenditure, lipid oxidation, substrate metabolism, RQ
1. Introduction
Little is known regarding factors that contribute to increased resting energy expenditure (REE) among individuals with HIV. Several studies have described elevated REE in HIV-infected individuals in the pre-HAART era, and suggest that REE may contribute to wasting (1), and may be increased in association with viral load and CD4 count (2). Recent studies have evaluated REE in HIV-infected patients with lipodystrophy. These studies have reported conflicting results with regards to the effects of HIV on REE (3, 4). In a recent meta-analysis, the authors concluded that REE, when adjusted for fat free mass (FFM), was higher in HIV-positive patients when compared to the healthy controls (5). The purpose of this study is to evaluate the relationship of respiratory quotient (RQ), a surrogate marker of substrate oxidation, as well as body composition, metabolic parameters and dietary intake to REE among HIV-infected patients in the current era of HAART, in comparison to non HIV-infected control subjects.
2. Subjects and Controls
Data were prospectively collected from 1998–2005 in 283 HIV-infected patients participating in metabolic studies at the Massachusetts General Hospital (MGH) and Massachusetts Institute of Technology (MIT), and 146 HIV-negative subjects simultaneously recruited from the community as controls for these studies (6–20). HIV-infected patients with wasting (BMI <20 kg/m2) were not included in the analysis. HIV-infected patients were recruited from newspaper advertisement, community and referral-based practices. The subjects were 18–60 years old with documentation of HIV status. For subjects receiving antiretroviral (ARV) therapy, a stable regimen for a minimum of 6 weeks prior to evaluation was required. Subjects were excluded if they had a history of diabetes mellitus; were receiving concurrent therapy with insulin, antidiabetic agents, glucocorticoids, growth hormone, supraphysiologic testosterone replacement, or anabolic steroids; were current substance abusers; had a major opportunistic infection within the 6 weeks prior to the study; had a thyroid disorder; or were pregnant or breast-feeding within the past year. The HIV-negative controls were recruited through hospital and local advertisements using similar exclusion criteria and tested negative for HIV disease by ELISA and Western Blot. For both HIV-infected and control groups, baseline data were obtained before any intervention. All participants provided informed consent. The studies were approved by the Institutional Review Boards at both the Massachusetts General Hospital and Massachusetts Institute of Technology. These data represent a subset of a larger dataset, which demonstrated differences in dietary fat intake (21) and relationship of VAT and SAT to BMI (22). Data on the relationship of RQ, body composition and dietary intake to REE have not previously been published from the dataset. Patients were not recruited based on lipodystrophy status, but lipodystrophy was characterized by investigators on the basis of evidence of fat accumulation in the trunk, breast, or neck and loss of fat in the face or extremities on physical examination as previously described (23).
All subjects were studied after an overnight fast of 12 hours. Each individual had a complete medical history and a physical examination. Triglycerides, cholesterol, HDL and glucose were measured using standard techniques (14). Complete blood count, CD4 count, and HIV viral load were obtained.
Dual-energy x-ray absorptiometry (Hologic QDR-4500A, Hologic Inc., Waltham, MA) was used to determine fat mass and fat-free mass (FFM). The DXA technique has a precision error of 3% for fat mass and 1.5% for FFM (24). Cross-sectional abdominal computed tomography (CT) scans were performed to assess subcutaneous and visceral adipose tissue area (SAT and VAT, respectively) (25). After and overnight fast, resting energy expenditure (REE) was measured by indirect calorimetry (Deltatrac or Vmax29, Sensormedics, Yorba Linda, CA, USA). Subjects sat quietly in a thermal neutral room for approximately 15 min before the study began. Oxygen consumption and carbon dioxide production were measured continuously. Measurements were recorded for 20 minutes and the final 15 minutes of recordings were analyzed. Respiratory quotient (RQ) is the ratio of CO2 production and O2 consumption (VCO2/VO2). The normal range of RQ in humans is 0.67–1.2 (26). Metabolic rates were further validated by calculating percent predicted basal metabolic rate. The basal metabolic rate was calculated using the Harris-Benedict equation (27).
Self-reported levels of physical activity were assessed with the Modifiable Activity Questionnaire (28). Activity questionnaires were available in 207 HIV-infected subjects and 36 control subjects. Physical activity level was calculated as the product of the duration and frequency of each activity (in hours per week), weighted by an estimate of the metabolic equivalent of that activity (MET) and summed for all activities performed, with the result expressed as the average MET-hours per week.
Dietary intake was obtained via 4-day food records (3 weekdays and 1 weekend day) in 282 HIV-infected subjects and 93 controls, and via multiple-pass 24-hour recall in an additional 53 controls. For the 4-day food records and 24-hour recall, participants were instructed by trained research dietitians to record completely all food and drink consumed. Dietary intake data were analyzed using Nutrition Data System for Research software versions 4.02_30 through 2004, developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN. Anthropometric determinations were made in triplicate using an inelastic tape measure for circumferences and a wall-mounted stadiometer (Holtain, Ltd, Crymych, United Kingdom) and digital scale for height and weight respectively. Waist and hip circumference were determined using standard methods. All measurements were completed by trained research dietitians.
2.1 Statistical Analysis
Comparison of demographic, body composition, energy and metabolic parameters was made by HIV status using the t-test for continuous variables and the Chi-square test for categorical variables. P values were also determined using ANOVA, controlling for age and BMI. Within each group, HIV and control, separate multivariate regression models were constructed using REE as the dependent variable and age, BMI, gender, carbohydrate and fat intake, VAT, FFM, RQ, triglyceride level and activity level as the independent variables for the HIV-infected patients. Immune function parameters were also included in the model for HIV-infected patients. Covariates for inclusion were based on results of univariate regression analysis. For multivariate and stepwise regression analyses, estimates equal change in REE (kcal/day) per 1 unit change in the independent variable. In addition, forward stepwise regression models were conducted, with a criteria of P<0.1 to enter the model in order to establish the hierarchy by which factors contributed to REE and the marginal contribution of each factor.
All values are expressed as mean ± SEM unless otherwise indicated. Statistical analyses were performed using SAS JMP software, version 5.0.1 (SAS Institute).
3. Results
Among the 283 HIV-infected and 146 non-HIV-infected subjects, there were no statistically significant differences in gender or race between the HIV-infected and control subjects. BMI was significantly lower in the HIV-infected population compared to the control population (26.9±0.4 vs. 29.1±0.5 kg/m2, P= 0.0004) and HIV-infected subjects were slightly older than HIV-uninfected controls (42±0 vs. 40±1 y, P=0.005) (Table 1).
Table 1.
Demographics in HIV-Infected and Non-HIV-Infected Subjects
| Variable | HIV+1 (N=283) | Control1 (N=146) | P- value2 |
|---|---|---|---|
| Demographics | |||
| Age (y) | 42 ± 0 | 40 ± 1 | 0.005 |
| BMI (kg/m2) | 26.9 ± 0.4 | 29.1 ± 0.5 | 0.0004 |
| Gender (%) | 0.81 | ||
| Male | 48.8 | 50.0 | |
| Female | 51.2 | 50.0 | |
| Race (%) | 0.44 | ||
| Caucasian | 51.2 | 59.6 | |
| African American | 33.2 | 27.4 | |
| Hispanic | 9.2 | 7.5 | |
| Other | 6.4 | 5.5 | |
| HIV Parameters | |||
| CD4 (#/mm3) | 442 ± 16 | - | - |
| Log10 Viral Load (copies/mL) | 2.7 ± 0.1 | - | - |
| Duration HIV (y) | 8.8 ± 0.3 | - | - |
| Currently taking PI (%) | 61.7 | - | - |
| Currently taking NRTI (%) | 92.5 | - | - |
| Currently taking NNRTI (%) | 37.9 | - | - |
Abbreviations: PI: Protease Inhibitor, NRTI: Nucleoside Reverse Transcriptase Inhibitor, NNRTI: Non-Nucleoside Reverse Transcriptase Inhibitor
Results expressed as mean ± SEM
p values for the differences between HIV-infected and control subjects derived from Chi-square testing for categorical variables and t-test for continuous variables.
The HIV-infected subjects demonstrated higher REE/FFM (31.8±0.3 vs. 29.8±0.3 kcal/d/kg, P<0.0001, HIV vs. control), lower RQ (0.83±0.00 vs. 0.85±0.01, P=0.005) and lower activity levels (55±5 vs. 106±22 MET-hours, P=0.001) compared to the control subjects. Percentage of predicted BMR was (108±1 vs. 98±1 % HIV vs. control, P< 0.0001). These differences remained significant in adjusted analyses accounting for age and BMI. REE was not different in unadjusted analyses, but was significantly different in the adjusted analysis (1730±22 vs. 1705±40 kcal/d, P=0.027) (Table 2).
Table 2.
Body Composition, Energy and Metabolic Parameters in HIV-Infected and Non-HIV-Infected Subjects
| Variable | HIV+1 (N=283) | Control1 (N=146) | P- value2 | P-value adjusted for Age & BMI |
|---|---|---|---|---|
| Body Composition Parameters | ||||
| Waist (cm) | 94.8 ± 0.8 | 97.2 ± 1.5 | 0.13 | 0.0003 |
| Hip (cm) | 100.4 ± 0.7 | 108.0 ± 1.2 | <0.0001 | <0.0001 |
| Waist-to-Hip Ratio | 0.94 ± 0.00 | 0.90 ± 0.01 | <0.0001 | <0.0001 |
| CT VAT (cm2) | 121.5 ± 4.1 | 130.8 ± 7.9 | 0.25 | 0.020 |
| CT SAT (cm2) | 231.3 ± 9.1 | 319.3 ± 17.3 | <0.0001 | 0.64 |
| Total Body Fat (kg) | 21.1 ± 0.6 | 26.4 ± 1.2 | <0.0001 | 0.011 |
| Fat Free Mass (kg) | 54.7 ± 0.7 | 56.5 ± 1.1 | 0.12 | 0.85 |
| Energy Parameters | ||||
| REE (kcal/d) | 1730 ± 22 | 1705 ± 40 | 0.56 | 0.027 |
| % of Predicted BMR 3 | 108 ± 1 | 98 ± 1 | <0.0001 | <0.0001 |
| REE/Fat Free Mass (kcal/d/kg) | 31.8 ± 0.3 | 29.8 ± 0.3 | <0.0001 | <0.0001 |
| RQ | 0.83 ± 0.00 | 0.85 ± 0.01 | 0.005 | 0.025 |
| Activity (MET-hours) 4 | 55 ± 5 | 106 ± 22 | 0.001 | 0.002 |
| Dietary Parameters | ||||
| Total Calories (kcal/d) | 2156 ± 46 | 2123 ± 60 | 0.66 | 0.85 |
| Total Dietary Carbohydrates (g/d) | 262 ± 6 | 257 ± 8 | 0.60 | 0.97 |
| % Dietary Carbohydrates | 48.9 ± 0.5 | 49.0 ±0.9 | 0.92 | 0.60 |
| Total Dietary Fat (g/d) | 85 ± 2 | 81 ± 3 | 0.28 | 0.27 |
| % Dietary Fat | 35.3 ± 0.4 | 34.0 ± 0.7 | 0.090 | 0.041 |
| Total Dietary SFA (g/d) | 29.9 ± 0.8 | 27.3 ± 1.2 | 0.068 | 0.044 |
| Total Dietary Protein (g/d) | 87 ± 2 | 88 ± 3 | 0.81 | 0.75 |
| Metabolic Parameters | ||||
| Triglycerides (mg/dL) | 203 ± 12 | 130 ± 11 | <0.0001 | 0.0006 |
| Serum Cholesterol (mg/dL) | 189 ± 3 | 178 ± 3 | 0.011 | 0.005 |
| High Density Lipoprotein (mg/dL) | 42 ± 1 | 48 ± 1 | <0.0001 | <0.0001 |
| Fasting Glucose (mg/dL) | 90 ± 1 | 89 ± 1 | 0.52 | 0.45 |
| FFA (mEq/liter) | 0.52 ± 0.03 | 0.52 ± 0.02 | 0.91 | 0.56 |
Abbreviations: CT: CAT Scan, VAT: Visceral Adipose Tissue, SAT: Subcutaneous Adipose Tissue, REE: Resting Energy Expenditure, BMR: Basal Metabolic Rate, RQ: Respiratory Quotient, SFA: Saturated Fatty Acids, FFA: Free Fatty Acid
Results expressed as mean ± SEM
p values for the differences between HIV-infected and control subjects determined by t-test and mixed effects ANOVA controlling for age and BMI
Determined by the Modifiable Activity Questionnaire and recorded as Metabolic Equivalents (MET), n=36
Hip circumference (100.4±0.7 vs. 108.0±1.2 cm, P<0.0001), total body fat (21.1±0.6 vs. 26.4±1.2 kg, P<0.0001), and HDL cholesterol (42±1 vs. 48±1 mg/dL, P<0.0001) were significantly lower in the HIV-infected subjects when compared with the HIV-uninfected subjects, while triglycerides (203±12 vs. 130±11 mg/dL, P<0.0001) and total cholesterol (189±3 vs. 178±3 mg/dL, P=0.011) were higher among HIV-infected subjects (Table 2). There were no statistically significant differences in waist circumference, FFM, and total caloric, carbohydrate, fat and protein intake among the HIV-infected and non-HIV infected subjects, though % fat intake and saturated fat intake were higher in the HIV-group, after adjusting for age and BMI, as previously reported (22) (Table 2).
VAT area was not different between the groups in unadjusted analyses, but was lower in the HIV-group after adjusting for age and BMI (121.5±4.1 vs. 130.8±7.9 cm2, P=0.020). In contrast, SAT was lower in HIV vs. control in unadjusted analyses, but not after adjusting for age and BMI (Table 2).
Among the HIV-infected subjects, 192 were classified with lipodystrophy and 91 were classified without lipodystrophy. There was no difference in age, race, viral load, or REE based on lipodystrophy status. BMI (27.4±0.4 vs. 25.6±0.6 kg/m2, P=0.007), CD4 (474±19 vs. 372±28 #/mm2, P=0.003), WHR (0.97±0.01 vs. 0.90±0.01, P=<0.0001) and RQ (0.83±0.01 vs. 0.81±0.01, P=0.034), were significantly higher in the HIV-infected subjects with lipodystrophy compared to those without lipodystrophy. REE/FFM (32.1±0.3 vs. 31.1±0.3 kcal/d/kg, P=0.063) tended to be higher in the lipodystrophic group, although this did not reach statistical significance. Subanalyses were also performed based on viral load. Of the patients who had viral loads done in the study, 72 had undetectable viral loads and 82 had detectable viral loads, REE was not different between these patients (1661±42.1 vs. 1637±39.4, P=0.680). Patients with HIV and undetectable viral loads did have increased REE compared to healthy controls (1768±28.9 vs. 1705±33.8, P=0.033).
The relationship of REE to metabolic and immune parameters in HIV and control subjects is shown in Table 3. BMI (r=0.30, P<0.0001), RQ (r=−0.12, P=0.047), dietary fat intake (r=0.28, P<0.0001), dietary carbohydrate intake (r=0.25, P<0.0001), VAT area (r=0.35, P<0.0001), FFM (r=0.77, P<0.0001), triglyceride (r=0.27, P<0.0001) and MET-hours (r=0.19, P=0.007) were related to REE in univariate regression analyses among the HIV group whereas BMI (r=0.68, P<0.0001), age (r=0.38, P<0.0001), dietary fat intake (r=0.42, P<0.0001), dietary carbohydrate intake (r=0.18, P=0.029), VAT area (r=0.69, P<0.0001), SAT area (r=0.52, P<0.0001), FFM (r=0.88, P<0.0001) and total body fat (r=0.57, P<0.0001) were related to REE in the control subjects. Total calories/day were related to REE in the HIV-infected subjects (r=0.33, P<0.0001) and control subjects (r=0.12, p<0.0001), however, after adjusting for fat and carbohydrate intake, these relationships were no longer significant (data not shown). In the lipodystrophic group, similar relationships between metabolic variables and REE were seen. BMI (r=0.32, P<0.0001), dietary fat intake (r=0.22, P=0.003), dietary carbohydrate intake (r=0.23, P=0.003), VAT area (r=0.36, P<0.0001), FFM (r=0.79, P<0.0001), and triglyceride (r=0.29, P<0.0001) were related to REE in univariate analysis. Use of PI (1769±28 vs. 1706±40 kcal/d, P=0.19), NRTI (1748±25 vs. 1706±62 kcal/d, P=0.64) and NNRTI (1723±35 vs. 1758±31 kcal/d, P=0.47) (current use vs. non-use respectively for each comparison) was not related to REE in the HIV group nor in the HIV group with lipodystrophy (data not shown).
Table 3.
Univariate Regression Analyses for REE (kcal/d)
| HIV-Infected Subjects | ||
|---|---|---|
| Parameter | r | P-value |
| BMI (kg/m2) | 0.30 | <0.0001 |
| Age (y) | −0.04 | 0.53 |
| Respiratory Quotient | −0.12 | 0.047 |
| Dietary Fat (g/d) | 0.28 | <0.0001 |
| Dietary Carbohydrate (g/d) | 0.25 | <0.0001 |
| CT VAT (cm2) | 0.35 | <0.0001 |
| CT SAT (cm2) | −0.0004 | 0.99 |
| Fat Free Mass (kg) | 0.77 | <0.0001 |
| Total Body Fat (kg) | 0.02 | 0.68 |
| CD4 (#/mm3) | 0.07 | 0.26 |
| Triglycerides (mg/dL) | 0.27 | <0.0001 |
| Activity (MET-hours) | 0.19 | 0.007 |
| Log10 Viral Load (copies/mL) | 0.007 | 0.93 |
| FFA (mEq/liter) | −0.03 | 0.78 |
|
| ||
|
Control Subjects | ||
| Parameter | r | P-value |
|
| ||
| BMI (kg/m2) | 0.68 | <0.0001 |
| Age (y) | 0.38 | <0.0001 |
| Respiratory Quotient | −0.04 | 0.61 |
| Dietary Fat (g/d) | 0.42 | <0.0001 |
| Dietary Carbohydrate (g/d) | 0.18 | 0.029 |
| CT VAT (cm2) | 0.69 | <0.0001 |
| CT SAT (cm2) | 0.52 | <0.0001 |
| Fat Free Mass (kg) | 0.88 | <0.0001 |
| Total Body Fat (kg) | 0.57 | <0.0001 |
| Activity (MET-hours) | −0.01 | 0.98 |
Relationships between covariates and Resting Energy Expenditure in univariate regression modeling among HIV infected subjects
Relationships between covariates and Resting Energy Expenditure in univariate regression modeling among control subjects
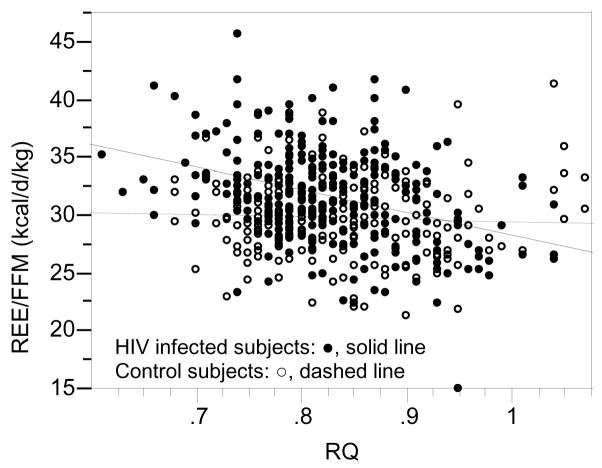
In univariate regression analysis REE/FFM, was significantly and negatively associated with RQ in HIV-infected subjects (REE/FFM (kcal/d/kg) = 47.790534-19.433101 (RQ), P<0.0001), while this relationship was not significant for control subjects (REE/FFM (kcal/d/kg) = 31.330088-1.7998838(RQ), P=0.61) (Figure 1).
Figure 1.
Relationship between Resting Energy Expenditure/Fat Free Mass and Respiratory Quotient
For HIV infected subjects (●, solid line; n=273) REE/FFM (kcal/d/kg) = 47.790534 – 19.433101 (RQ) (P<0.0001) and control subjects (○, dashed line; n=143) REE/FFM (kcal/d/kg) = 31.330088 – 1.7998838 (RQ) (P=0.61).
Multivariate regression analysis was performed for HIV-infected subjects, for REE (dependent variable) including BMI, age, gender, fat intake, total body fat and CD4 count, carbohydrate intake, visceral adiposity, RQ, FFM, triglyceride and activity levels as independent variables. Fat free mass (beta=24.811334, P <0.0001), visceral adiposity (beta=0.7182746, P=0.008), and total body fat (beta=8.0506839, P=0.041) were positively associated with REE, whereas RQ was negatively associated with REE (beta= −528.4808, P=0.024). Overall r2=0.705, P<0.0001 for the model (Table 4).
Table 4.
Multivariate Regression Analyses for REE (kcal/d)
| HIV-Infected Subjects, n=183 | |||
|---|---|---|---|
| Parameter | Estimate (beta) | SE | P value |
| BMI (kg/m2) | −16.33165 | 8.481426 | 0.056 |
| Age (y) | −0.866462 | 2.252714 | 0.70 |
| Gender | 41.237605 | 30.6016 | 0.18 |
| Respiratory Quotient | −528.4808 | 231.3074 | 0.024 |
| Dietary Fat (g) | 0.0782477 | 0.546601 | 0.89 |
| Dietary Carbohydrate (g) | 0.3525015 | 0.185404 | 0.059 |
| CT VAT (cm2) | 0.7182746 | 0.26755 | 0.008 |
| Fat Free Mass (kg) | 24.811334 | 2.944075 | <0.0001 |
| Total Body Fat (kg) | 8.0506839 | 3.911959 | 0.041 |
| CD4 (#/mm3) | −0.054781 | 0.060908 | 0.37 |
| Triglycerides (mg/dL) | 0.0773081 | 0.072956 | 0.29 |
| Activity (MET-hours) | 0.0114182 | 0.18809 | 0.95 |
|
| |||
|
Control Subjects, n=119 | |||
| Parameter | Estimate (beta) | SE | P value |
|
| |||
| BMI (kg/m2) | −5.85139 | 9.998695 | 0.56 |
| Age (y) | −1.653699 | 2.412292 | 0.50 |
| Gender | 0.7825825 | 30.59653 | 0.98 |
| Respiratory Quotient | −19.85478 | 215.2759 | 0.93 |
| Dietary Fat (g) | 0.7520528 | 0.501677 | 0.14 |
| Dietary Carbohydrate (g) | −0.201068 | 0.206064 | 0.33 |
| CT VAT (cm2) | 1.0612073 | 0.355541 | 0.004 |
| CT SAT (cm2) | −0.779323 | 0.41413 | 0.063 |
| Fat Free Mass (kg) | 22.613005 | 2.677683 | <0.0001 |
| Total Body Fat (kg) | 15.805547 | 6.011043 | 0.010 |
Relationships between covariates and Resting Energy Expenditure in regression modeling among HIV infected subjects (p<0.0001, r2 = 0.705). Variables in bold were significantly related to REE in the model.
Relationships between covariates and Resting Energy Expenditure in regression modeling among control subjects (p<0.0001, r2 = 0.825). Variables in bold were significantly related to REE in the model.
By contrast, among control subjects, visceral adiposity (beta = 1.0612073, P=0.004), total body fat (beta = 15.805547, P=0.010), and FFM (beta = 22.613005, P<0.0001) were significant predictors of REE, and there was no relationship with RQ. Overall r2=0.825, P<0.0001 for the model (Table 4).
In forward stepwise regression modeling in the HIV-infected subjects, with REE as the dependent variable, fat free mass, respiratory quotient, dietary carbohydrate and VAT were significant in stepwise modeling and accounted for 70% of the variance, with fat free mass (65%) contributing most in the model, and significant but smaller additional contributions from RQ, carbohydrate intake and VAT (Table 5). In the control subjects, FFM also contributed most to REE (72%), with an additional contribution of 8% from VAT, and smaller contributions from total body fat and SAT (Table 5).
Table 5.
Forward Stepwise Regression Analyses for REE (kcal/d)
| HIV-Infected Subjects, n=259 | |||
|---|---|---|---|
| Parameter | Estimate (beta) | R2 | P-value |
| Fat Free Mass (kg) | 25.2079461 | 0.649 | <0.0001 |
| Respiratory Quotient | −654.23617 | 0.665 | 0.004 |
| Dietary Carbohydrate (g) | 0.43184009 | 0.674 | 0.027 |
| CT VAT (cm2) | 0.68024196 | 0.689 | 0.004 |
|
Control Subjects, n=119 | |||
| Parameter | Estimate (beta) | R2 | P-value |
|
| |||
| Fat Free Mass (kg) | 22.6017946 | 0.722 | <0.0001 |
| CT VAT (cm2) | 0.99145624 | 0.806 | <0.0001 |
| Total Body Fat (kg) | 12.541516 | 0.812 | 0.048 |
| CT SAT (cm2) | −0.6914458 | 0.818 | 0.051 |
Relationships between covariates and Resting Energy Expenditure in stepwise regression modeling among HIV infected subjects.
Relationships between covariates and Resting Energy Expenditure in stepwise regression modeling among control subjects.
Among the HIV-infected patients, use of PI (0.82±0.01 vs. 0.84±0.01, P=0.26), NRTI (0.83±0.01 vs. 0.82±0.02, P=0.44) and NNRTI (0.83±0.01 vs. 0.83±0.01, P=0.44) (current use vs. non-use respectively for each comparison) was not related to RQ. On univariate regression, RQ was related to BMI (r=0.12, P=0.042), CD4 T-cell count (r=0.16, P=0.007), VAT area (r=0.13, P=0.029), FFM (r=0.14, P=0.019), MET-hours (r=−0.16, P=0.026) but none of these variables remained significant in multivariate regression analyses adjusting simultaneously for all covariates (data not shown). RQ was not related to dietary carbohydrate intake (r=−0.04, P=0.49), dietary fat intake (r=−0.04, P=0.50), HIV viral load (r=−0.11, P=0.18), SAT area (r=1.0, P=0.087), FFA (r=−0.08, P=0.44), or triglycerides (r=0.07, P=0.27).
Among control subjects, RQ was related to VAT area (r=0.30, P=0.0008), and triglycerides (r=0.18, P=0.033) in univariate regression analysis. RQ was not related to carbohydrate intake (r=0.02, P=0.84), dietary fat intake (r=0.04, P=0.63), FFA (r=−0.13, P=0.17), BMI (r=0.02, P=0.79), SAT area (r=0.17, P=0.066), FFM (r=0.02, P=0.79) or MET-hours (r=0.29, P=0.090) in the control subjects.
4. DISCUSSION
To our knowledge, this is the largest study to date investigating REE and RQ in HIV infected subjects and provides novel data regarding the relationship between RQ and REE in this population. Several studies examining REE in HIV-infected subjects have shown that FFM contributes to REE in this population (3, 29, 30), however no study thus far has shown that RQ is associated with REE among HIV-infected patients. The results of this study demonstrate lower RQ and higher REE adjusted for FFM in HIV-infected patients compared to the non-infected control subjects. While REE and REE adjusted for FFM were significantly associated with RQ in HIV-infected subjects, this was not the case in HIV-negative control subjects.
In general, the HIV infected subjects were infected with the HIV virus for more than 8 years, most were on HAART and had a mean CD4 count >400. These subjects demonstrated a number of the metabolic abnormalities consistent with the use of HAART, including increased triglyceride levels and reduced HDL. Subjects demonstrated a relative fat redistribution, but demonstrated lower levels of SAT, but not VAT, when compared to non BMI-matched controls, consistent with FRAM (31, 32).
REE/FFM, % predicted BMR, and BMI adjusted REE were all significantly higher in HIV than controls. Calculation of % predicted BMR was useful to characterize the differences observed between HIV and non HIV patients. Indeed, we observed a 10% difference with non HIV controls in % predicted BMR [108±1% vs. 98±1% P<0.0001) which is in the range of that seen in prior smaller studies (29, 33). The mechanism to explain elevated REE in the setting of HIV infection is still unclear. Shevitz et al. suggested that antiviral medications might directly stimulate metabolism or that metabolic demand might increase from a rejuvenated immune system in subjects on HAART (30). More recently, it has been postulated that mitochondrial dysfunction may contribute to increased REE and the effects of HAART or the HIV virus may lead to altered energy regulation (34).
In our study, we did not see a relationship between use of PI, NRTI and NNRTI and REE, nor between immune parameters and REE. In contrast, we demonstrated the expected strong relationship with FFM, and a smaller but significant contribution of lower RQ to increased REE, not seen in the control subjects. Dietary carbohydrate intake and VAT area also contributed to increased REE in regression modeling. The significant relationship we observed between VAT and REE corroborates data from Kosmiski et al. demonstrating a similar relationship in a smaller study of 32 patients (3). Among the HIV-infected patients, triglyceride levels and activity levels were related to REE on univariate regression analyses, but not on multivariate regression analyses. Activity levels in MET-hours were lower in the HIV-infected patients, suggesting that increased REE was not a function of increased activity levels.
In addition to an elevated REE, we also found a significantly lower RQ among the HIV infected subjects. In smaller studies among subjects with HIV associated lipodystrophy it has been suggested that elevated free fatty acids (FFA) may contribute to increased lipid oxidation and hypertriglyceridemia (35, 36). In our study, neither triglycerides nor FFA were associated with RQ. A relative increase in dietary fat intake might contribute to an increase in lipid oxidation in the HIV-infected patients as increased fat oxidation has been shown to be an adaptation to diets which are higher in fat (37, 38) among non HIV-infected patients. However, we did not see a relationship between dietary fat intake and RQ in the study, arguing against a significant effect of dietary fat intake on substrate oxidation in HIV-infected patients.
A novel finding in this study was that RQ was significantly associated with REE in HIV-infected patients. RQ is a surrogate marker of substrate oxidation and in the general population those with morbid obesity tend to have a lower RQ in addition to higher metabolic rates (39, 40). In this study, HIV-infected patients had a lower BMI than controls, arguing against this as an explanation for lower RQ. Furthermore, RQ remained lower in the HIV patients adjusting for BMI. There are conflicting results in the few studies that have evaluated RQ among HIV-infected patients and most of them have examined RQ in the context of smaller studies of lipodystrophy (3, 35). In a study with 43 subjects, Sutinen et al. observed that subjects on HAART with lipodystrophy have a lower RQ when compared to subjects on HAART without lipodystrophy (35), whereas Kosmiski et al. found no difference among patients with lipodystrophy (3). A lower RQ, may result in a greater post-prandial decrease in glycogen stores resulting in a suppression of satiety, an increase in appetite and therefore an increased food intake (41).
This study has some limitations. Causality cannot be determined from a cross–sectional study. Furthermore, we did not measure substrate oxidation directly, but were able to include data on intake of macronutrients, activity level and detailed measures of body composition and metabolic parameters. We demonstrate clear relationships between metabolic parameters, REE and RQ in a large study of well phenotyped HIV-infected patients compared to controls, which extends our knowledge of altered energy metabolism in the HIV population. It is possible that factors driving increased REE may also be driving increased fat oxidation, but further studies are needed to directly assess this relationship and fat oxidation rates. Finally, although RQ was statistically lower in the HIV group, the biological significance of this difference remains unclear.
In conclusion, we evaluated the relationship between REE and RQ in a large study of HIV-infected men and women compared with control subjects. Decreased RQ, is related to REE in HIV-infected patients and may play a role in metabolic abnormalities experienced by this population. Longitudinal studies are needed in order to follow these relationships over time in order to better establish the relationship between RQ, substrate oxidation and REE in HIV-infected patients.
Acknowledgments
We wish to thank the nutrition and nursing staff on the MGH and MIT GCRC for their dedicated patient care.
This work was supported in by NIH DKRO1-49302, NIH DK-02844, NIH T32HD-052691, NIH MO1-RR01066 and the Mary Fisher Clinical AIDS Research and Education Fund
Footnotes
The authors’ responsibilities were as follows— KF, SDL, CH, JH, JL and JL: responsible for subject recruitment and data acquisition; HK and JW: responsible for data entry; KF, LG, and SG: responsible for the data analysis and interpretation; KF and SG: responsible for the statistical analysis; KF and SG: responsible for the draft of the manuscript; KF, KF, SDL, CH, EA, JL, JH, SJ, HM, TS, JL, and SG: responsible for the critical revision of the manuscript and its important intellectual content; SG: responsible for obtaining funding; and SG: responsible for study supervision. None of the authors reported a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hommes MJ, Romijn JA, Godfried MH, et al. Increased resting energy expenditure in human immunodeficiency virus-infected men. Metabolism. 1990;39(11):1186–90. doi: 10.1016/0026-0495(90)90092-q. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan K, Tai VW, Schambelan M. Energy Expenditure in Human Immunodeficiency Virus Infection. N Engl J Med (letter) 1997;336:70–1. doi: 10.1056/NEJM199701023360115. [DOI] [PubMed] [Google Scholar]
- 3.Kosmiski LA, Kuritzkes DR, Lichtenstein KA, et al. Fat distribution and metabolic changes are strongly correlated and energy expenditure is increased in the HIV lipodystrophy syndrome. AIDS. 2001;15(15):1993–2000. doi: 10.1097/00002030-200110190-00012. [DOI] [PubMed] [Google Scholar]
- 4.van der Valk M, Reiss P, van Leth FC, et al. Highly active antiretroviral therapy-induced lipodystrophy has minor effects on human immunodeficiency virus-induced changes in lipolysis, but normalizes resting energy expenditure. J Clin Endocrinol Metab. 2002;87(11):5066–71. doi: 10.1210/jc.2002-020892. [DOI] [PubMed] [Google Scholar]
- 5.Batterham MJ. Investigating heterogeneity in studies of resting energy expenditure in persons with HIV/AIDS: a meta-analysis. Am J Clin Nutr. 2005;81(3):702–13. doi: 10.1093/ajcn/81.3.702. [DOI] [PubMed] [Google Scholar]
- 6.Dolan SE, Frontera W, Librizzi J, et al. The effects of a supervised home-based aerobic and progressive resistance training regimen in HIV-infected women: A randomized trial. Archives of Internal Medicine. 2006;166:1225–31. doi: 10.1001/archinte.166.11.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: A randomized controlled trial. JAMA. 2000;284(4):472–7. doi: 10.1001/jama.284.4.472. [DOI] [PubMed] [Google Scholar]
- 8.Koutkia P, Canavan B, Breu J, Grinspoon S. Effects of growth hormone-releasing hormone on bone turnover in human immunodeficiency virus-infected men with fat accumulation. J Clin Endocrinol Metab. 2005;90(4):2154–60. doi: 10.1210/jc.2004-1466. [DOI] [PubMed] [Google Scholar]
- 9.Rietschel P, Hadigan C, Corcoran C, et al. Assessment of Growth Hormone Dynamics in Human Immunodeficiency Virus- Related Lipodystrophy. J Clin Endocrinol Metab. 2001;86(2):504–10. doi: 10.1210/jcem.86.2.7175. [DOI] [PubMed] [Google Scholar]
- 10.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2006;91(8):2938–45. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadigan C, Kamin D, Liebau J, et al. Depot-specific regulation of glucose uptake and insulin sensitivity in HIV-lipodystrophy. Am J Physiol Endocrinol Metab. 2006;290(2):E289–98. doi: 10.1152/ajpendo.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med. 2006;166(8):902–8. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadigan C, Yawetz S, Thomas A, Havers F, Sax PE, Grinspoon S. Metabolic effects of rosiglitazone in HIV lipodystrophy: A randomized controlled trial. Ann Intern Med. 2004;140(10):786–94. doi: 10.7326/0003-4819-140-10-200405180-00008. [DOI] [PubMed] [Google Scholar]
- 14.Fitch KV, Anderson EJ, Hubbard JL, et al. Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome. AIDS. 2006;20(14):1843–50. doi: 10.1097/01.aids.0000244203.95758.db. [DOI] [PubMed] [Google Scholar]
- 15.Driscoll SD, Meininger GE, Lareau MT, et al. Effects of exercise training and metformin on body composition and cardiovascular indices in HIV infected patients. AIDS. 2004;18(3):465–73. doi: 10.1097/00002030-200402200-00013. [DOI] [PubMed] [Google Scholar]
- 16.Schurgin S, Canavan B, Koutkia P, Depaoli AM, Grinspoon S. Endocrine and metabolic effects of physiologic r-metHuLeptin administration during acute caloric deprivation in normal-weight women. J Clin Endocrinol Metab. 2004;89(11):5402–9. doi: 10.1210/jc.2004-1102. [DOI] [PubMed] [Google Scholar]
- 17.Meininger G, Hadigan C, Laposata M, et al. Elevated concentrations of free fatty acids are associated with increased insulin response to standard glucose challenge in human immunodeficiency virus-infected subjects with fat redistribution. Metabolism. 2002;51(2):260–6. doi: 10.1053/meta.2002.29999. [DOI] [PubMed] [Google Scholar]
- 18.Fleischman A, Johnsen S, Systrom DM, et al. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol Endocrinol Metab. 2007;292(6):E1666–73. doi: 10.1152/ajpendo.00550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S. Reduced bone density in HIV-infected women. AIDS. 2004;18(3):475–83. doi: 10.1097/00002030-200402200-00014. [DOI] [PubMed] [Google Scholar]
- 20.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among HIV-infected men receiving highly active antiretroviral therapy. Metabolism. 2002;51:1143–7. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 21.Joy T, Keogh HM, Hadigan C, et al. Relationship of Body Composition to BMI in HIV-infected Patients with Metabolic Abnormalities. JAIDS. 2007;47:174–84. doi: 10.1097/QAI.0b013e31815b0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joy T, Keough HM, Hadigan C, et al. Dietary Fat Intake and Relationship tp Serum Lipid Levels Among HIV-infected Subjects with Metabolic Abnormalities in the Era of HAART. AIDS. 2007;21:1591–600. doi: 10.1097/QAD.0b013e32823644ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadigan C, Rabe J, Meininger G, Aliabadi N, Breu J, Grinspoon S. Inhibition of lipolysis improves insulin sensitivity in protease inhibitor-treated HIV-infected men with fat redistribution. Am J Clin Nutr. 2003;77:490–4. doi: 10.1093/ajcn/77.2.490. [DOI] [PubMed] [Google Scholar]
- 24.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51(6):1106–12. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 25.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39(1):44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 26.Haugen HA, Chan LN, Li F. Indirect calorimetry: a practical guide for clinicians. Nutr Clin Pract. 2007;22(4):377–88. doi: 10.1177/0115426507022004377. [DOI] [PubMed] [Google Scholar]
- 27.Harris JA, Benedict FG. A Biometric Study of Basal Metabolism in Man. Carnegie Institute; Washington: 1919. [Google Scholar]
- 28.Kriska A, Knowler W, LaPorte R. Development of a questionnaire to examine the relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13:401–11. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 29.Grinspoon S, Corcoran C, Miller K, et al. Determinants of increased energy expenditure in HIV-infected women. Am J Clin Nutr. 1998;68(3):720–5. doi: 10.1093/ajcn/68.3.720. [DOI] [PubMed] [Google Scholar]
- 30.Shevitz AH, Knox TA, Spiegelman D, Roubenoff R, Gorbach SL, Skolnik PR. Elevated resting energy expenditure among HIV-seropositive persons receiving highly active antiretroviral therapy. AIDS. 1999;13(11):1351–7. doi: 10.1097/00002030-199907300-00012. [DOI] [PubMed] [Google Scholar]
- 31.Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42(5):562–71. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40(2):121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batterham MJ, Garsia R, Greenop P. Prevalence and predictors of HIV-associated weight loss in the era of highly active antiretroviral therapy. Int J STD AIDS. 2002;13(11):744–7. doi: 10.1258/095646202320753682. [DOI] [PubMed] [Google Scholar]
- 34.Chang E, Sekhar R, Patel S, Balasubramanyam A. Dysregulated energy expenditure in HIV-infected patients: a mechanistic review. Clin Infect Dis. 2007;44(11):1509–17. doi: 10.1086/517501. [DOI] [PubMed] [Google Scholar]
- 35.Sutinen J, Yki-Jarvinen H. Increased resting energy expenditure, fat oxidation, and food intake in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2007;292(3):E687–92. doi: 10.1152/ajpendo.00219.2006. [DOI] [PubMed] [Google Scholar]
- 36.Sekhar RV, Jahoor F, White AC, et al. Metabolic basis of HIV-lipodystrophy syndrome. Am J Physiol Endocrinol Metab. 2002;283(2):E332–7. doi: 10.1152/ajpendo.00058.2002. [DOI] [PubMed] [Google Scholar]
- 37.Smith SR, de Jonge L, Zachwieja JJ, et al. Fat and carbohydrate balances during adaptation to a high-fat. Am J Clin Nutr. 2000;71(2):450–7. doi: 10.1093/ajcn/71.2.450. [DOI] [PubMed] [Google Scholar]
- 38.Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr. 1997;66(2):276–82. doi: 10.1093/ajcn/66.2.276. [DOI] [PubMed] [Google Scholar]
- 39.Segal KR, Edano A, Blando L, Pi-Sunyer FX. Comparison of thermic effects of constant and relative caloric loads in lean and obese men. Am J Clin Nutr. 1990;51(1):14–21. doi: 10.1093/ajcn/51.1.14. [DOI] [PubMed] [Google Scholar]
- 40.Meylan M, Henny C, Temler E, Jequier E, Felber JP. Metabolic factors in the insulin resistance in human obesity. Metabolism. 1987;36(3):256–61. doi: 10.1016/0026-0495(87)90185-5. [DOI] [PubMed] [Google Scholar]
- 41.Valtuena S, Salas-Salvado J, Lorda PG. The respiratory quotient as a prognostic factor in weight-loss rebound. Int J Obes Relat Metab Disord. 1997;21(9):811–7. doi: 10.1038/sj.ijo.0800480. [DOI] [PubMed] [Google Scholar]