Abstract
AIM: To investigate the role of reactive oxygen species (ROS) in ethanol-mediated cell death of polarized hepatic (WIF-B) cells.
METHODS: In this work, WIF-B cultures were treated with pyrazole (inducer of cytochrome P4502E1, CYP2E1) and/or L-buthionine sulfoximine (BSO), a known inhibitor of hepatic glutathione (GSH), followed by evaluation of ROS production, antioxidant levels, and measures of cell injury (apoptosis and necrosis).
RESULTS: The results revealed that ethanol treatment alone caused a significant two-fold increase in the activation of caspase-3 as well as a similar doubling in ROS. When the activity of the CYP2E1 was increased by pyrazole pretreatment, an additional two-fold elevation in ROS was detected. However, the CYP2E1-related ROS elevation was not accompanied with a correlative increase in apoptotic cell injury, but rather was found to be associated with an increase in necrotic cell death. Interestingly, when the thiol status of the cells was manipulated using BSO, the ethanol-induced activation of caspase-3 was abrogated. Additionally, ethanol-treated cells displayed enhanced susceptibility to Fas-mediated apoptosis that was blocked by GSH depletion as a result of diminished caspase-8 activity.
CONCLUSION: Apoptotic cell death induced as a consequence of ethanol metabolism is not completely dependent upon ROS status but is dependent on sustained GSH levels.
Keywords: WIF-B cells, Alcohol, Fas/CD95, Glutathione, Caspase
INTRODUCTION
It has been documented that the clinical progression of alcoholic liver disease (ALD) is associated with an increase in hepatocellular damage that may involve the promotion of apoptotic mechanisms[1]. As part of the effort to clarify mechanisms associated with ethanol-mediated hepatocellular apoptosis, researchers have utilized a variety of model systems (e.g. isolated hepatocytes or liver tissue from animals). Unfortunately, limitations exist with such models as the cells/tissue do not survive well or readily lose liver-specific functions in culture, reducing the effectiveness of identifying molecules and pathways that may be involved in hepatotoxic events. The aim of this study was to evaluate alcohol-mediated cellular alterations associated with apoptotic mechanisms using a polarized hepatic (WIF-B) cell culture model.
The WIF-B cell is a cross between a human fibroblast (WI 38) and a Fao rat hepatoma cell[2]. This clone represents differentiated cells of hepatic origin that exhibit long-term viability in culture, develop a hepatocellular-polarized phenotype, and express genes coding for liver-specific proteins[3]. The use of WIF-B cells has recently emerged as an appropriate model for studying the effects of ethanol on cellular processes. Specifically, WIF-B cells endogenously express alcohol dehydrogenase (ADH) as well as CYP2E1 activity, allowing for the efficient metabolism of ethanol, and as a consequence, exhibit classic alcohol-mediated adverse effects such as triglyceride accumulation and apoptotic cell injury[4,5].
In general, the apoptotic cascade in hepatocytes can be triggered by signaling pathways that involve death receptor-mediated interactions and/or mitochondrial stress signals. These events result in the activation of cysteine proteases (caspases) which execute the proteolytic cleavage of proteins and the ultimate demise of the cell. In the case of ethanol-related cell death, several studies have demonstrated that the induction of caspases can be linked to the effects of various cytokines[6], the involvement of oxidative stress mechanisms[7], and glutathione depletion[8]. However, the contribution that ethanol and its metabolites make in enhancing reactive oxygen species (ROS) in WIF-B cells and what role oxidative stress plays in ethanol-mediated apoptosis in this model system is not known. Furthermore, discrepancies have been noted concerning the relationship that exists between antioxidant levels and hepatocellular damage associated with ALD, as GSH levels have been reported to be increased, decreased or unaltered following ethanol administration[9–11]. Additionally, it has been shown that the activation of apoptosis-executing caspases actually requires sustained glutathione levels as these proteases possess an essential cysteine thiol in the active site[12].
Therefore, this work examined the role that oxidative stress plays in ethanol-mediated apoptotic events in alcohol metabolizing cells which endogenously express ADH and CYP2E1, taking advantage of the ability to manipulate CYP2E1, ROS production, and antioxidant status in this culture model. Specifically, we examined hepatocellular death in ethanol-treated WIF-B cells by analyzing apoptosis along with correlative measurements of CYP2E1 expression, ROS production, and glutathione status.
MATERIALS AND METHODS
Materials
F-12 Coon’s modified culture medium, 4-methylpyrazole (pyr), diallyldisulfide (DAS), N-acetylcysteine (NAC), antimycin A (AA), and actinomycin D (Act D) were obtained from Sigma Chemical Co. (St. Louis, MO). Fetal Bovine Serum (FBS) was obtained from Gemini Bio-Products (Woodland, CA). All other materials were of reagent grade.
Cell culture and treatments
WIF-B cells were cultured in F-12 Coon’s modified media supplemented with 37.5 mL/L FBS as previously described[5]. Briefly, cells were cultured for 7-10 d to obtain maximal polarized phenotype followed by treatment with ethanol (25 and 50 mmol/L), 100 μmol/L DAS, or 5 mmol/L NAC for 48 h. CYP2E1 expression was induced by incubation with 0.25 mmol/L 4-methylpyrazole (pyr) for 4 d prior to ethanol and other treatments. GSH deficiency was induced in some cultures by adding 2 mmol/L buthionine-sulfoximine (BSO), an inhibitor of glutathione synthesis. Fas-mediated apoptosis was analyzed in cells treated with 25 mmol/L ethanol for 24 h prior to challenge with 0.5 g/L rabbit anti-rat Fas antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and 0.05 g/L actinomycin D.
The detection of oxidatively modified proteins
Protein oxidation was determined by detecting carbonyl groups within proteins using the Oxyblot® kit (Millipore, Temecula, CA) according to the manufacturer’s instructions. Following derivatization with dinitrophenylhydrozone (DNP), modified proteins were detected by Western Blotting analysis using the Odyssey® Infrared Imaging System (LI-COR Biosciences, Lincoln, NE). Resolved proteins (10% SDS-PAGE), on nitrocellulose membranes were probed with 1:150 anti-rabbit DNP and 1:5000 mouse anti-GAPDH (Millipore, Temecula, CA), followed by goat anti-mouse and anti-rabbit Infrared IRDye®-labeled secondary antibodies (LI-COR).
Assessment of intracellular oxidative stress
The level of H2O2 was determined using the redox-sensitive dye 2',7'-dichlorodihydrofluorescein diacetate, H2DCFDA (Invitrogen, Carlsbad, CA). Briefly, WIF-B cultures were incubated with 4 μmol/L H2DCFDA for 30 min in the dark at the end of the treatment period and the oxidized fluorescent product detected (excitation 488 nm and emission 525 nm).
Analysis of CYP2E1 activity
Utilizing the p-nitrophenol oxidation assay as described[13], the activity of CYP2E1 was measured in microsomal proteins (0.05 g) isolated as previously noted[4].
Caspase activation assays
The activity of caspase-3 was evaluated by measuring the cleavage of the fluorogenic substrate Ac-DEVD-AMC (BD PharMingen, San Diego, CA) as previously described[5]. The activation of caspase-8 was determined using a Colorimetric Assay (R&D Systems Inc, Minneapolis, MN) following the manufacturer’s instructions.
Flow cytometric determination of necrosis
The LIVE/DEAD® Fixable Cell Stain (Invitrogen, Carlsbad, CA) was used to determine necrosis based on the reaction of fluorescent reactive dye with intracellular amines following entry through the compromised membranes of necrotic cells. Following staining with the reactive dye, the cells were analyzed by flow cytometry using the FACSCalibur (Becton Dickinson) with a 585/42 band pass filter. The results are expressed as the percentage of non-viable (necrotic) cells (reflected by the shift in mean fluorescent intensity) measured in the total cell population.
Glutathione determination
The detection of intracellular levels of reduced glutathione (GSH) in WIF-B cells was assessed using a commercial kit (Chemicon APT 250; Millipore, Temecula, CA) that detected the fluorescent product produced (excitation 380 nm and emission 461 nm) following the incubation of cell lysates with a monochlorobimane dye that has a high affinity for glutathione.
DNA fragmentation assay
DNA integrity was analyzed using the ApoTargetTM DNA Ladder kit, as described by the manufacturer (Invitrogen, Carlsbad, CA). Detached cells were collected by centrifugation (400 r/min) followed by DNA extraction. DNA fragments were resolved and visualized following agarose gel electrophoresis (5 EV/cm) and ethidium bromide staining. As a positive control, DNA was extracted from WIF-B cells that were induced to undergo apoptosis by UV treatment (320 μW/cm2) as previously described[14].
Statistical analysis
Results refer to the average taken from three to seven experiments and are expressed as mean ± SE. Comparison of the values was performed using the Student t test with values, P < 0.05, considered significant.
RESULTS
Ethanol-mediated oxidative stress and its role in caspase activation
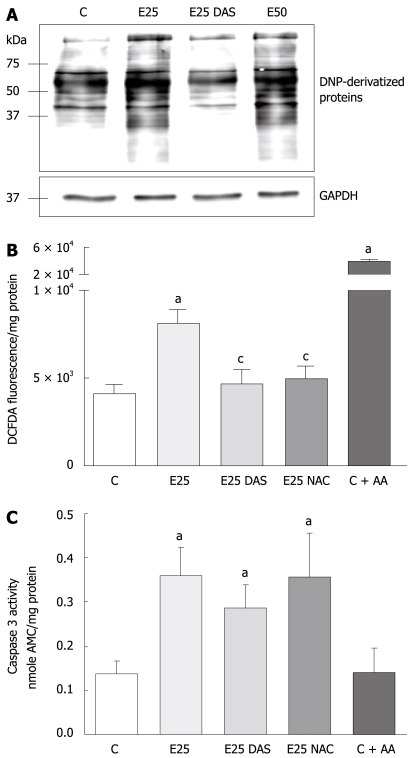
To evaluate the role of ethanol-mediated oxidative stress in the activation of apoptotic mechanisms in WIF-B cells, cultures were incubated with physiologically relevant concentrations (25 and 50 mmol/L) of ethanol in the presence or absence of DAS, and analyzed for the production of oxidative stress indices (protein carbonyl adduct formation and H2O2 generation) as well as caspase activation. It was determined that more cellular proteins were oxidatively modified as a result of ethanol treatment in comparison to control cells, and that the ethanol-mediated increase in carbonyl adducts was significantly reduced in the presence of DAS, an inhibitor of CYP2E1 (Figure 1A). In a similar fashion, using fluorescent spectrophotometry to detect the oxidized cleaved product, dichlorofluorescein (DCF), it was determined that ethanol treatment resulted in a two-fold increase in H2O2 production compared to untreated control cells (Figure 1B). Also, the presence of the antioxidant, NAC, provided significant protection against the ethanol-induced ROS elevation (Figure 1B). As a positive control for ROS production in these experiments, WIF-B cells were also incubated with AA, an inducer of H2O2 levels that resulted in a robust generation of DCF products as reflected by a 10-fold increase in ROS (Figure 1B). In contrast, when a measure of apoptosis (caspase activation) was conducted, it was determined that while ethanol treatment increased caspase-3 activity at a comparable two-fold elevation to that which was observed for ethanol-related ROS production, the introduction of DAS or NAC did not provide any protection against ethanol-mediated apoptosis. Correspondingly, in AA-treated cells that represented an overproduction of ROS, no induction of caspase activity could be detected (Figure 1C).
Figure 1.
Ethanol-induced protein oxidation, oxidative stress, and caspase activation in WIF-B cells. WIF-B cultures were cultured for 48 h in the absence or presence of ethanol, and DAS or NAC when indicated. As a positive control for H2O2 production, some cultures were treated with 2 μmol/L Antimycin A (C + AA) for 30 min prior to analysis. A: Protein oxidation analysis represented as DNP-specific proteins detected in WIF-B cell lysates using the OxyBlot protein oxidation analysis; B: H2O2 production was detected by dichlorofluorescein fluorescence quantification. Data from 5 independent experiments is represented as the amount of fluorescent (DCFDA) oxidized products detected in the treated cells per mg protein; C: The activation of the proapoptotic protease, caspase-3, was detected as described in “Material and Methods” for five independent experiments. aP < 0.05 vs control; cP < 0.05 vs ethanol alone-treated cells.
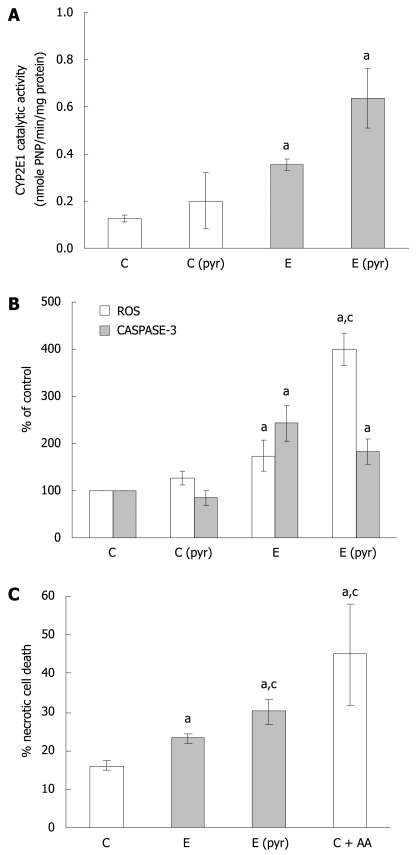
Role of CYP2E1-dependent mechanisms in ethanol-mediated cell death of WIF-B cells
In light of the observations demonstrating that ethanol treatment results in the induction of ROS as well as apoptosis, while inhibitors of ROS could not attenuate caspase-3 activation, further evaluation of CYP2E1-dependent mechanisms was performed. First, it was determined that ethanol administration alone enhances CYP2E1 catalytic activity (Figure 2A) as well as protein expression (data not shown) in WIF-B cultures. Also, pretreatment of the cells with pyrazole for 4 d prior to ethanol challenge resulted in a further enhancement of CYP2E1 (Figure 2A). Utilizing this ability to manipulate the CYP2E1-ethanol metabolizing system, we next compared the production of ROS with caspase-3 activation in pyrazole-pretreated WIF-B cells with or without ethanol. The results indicate that when CYP2E1 was enhanced by pyrazole, a correlative 2-fold enhancement in ROS generation was observed when compared to ethanol-alone treated cells (Figure 2B). However, when the activity of caspase-3 was evaluated under the same conditions, it was determined that the pyrazole induction of CYP2E1 was not associated with an enhancement of apoptosis over what was observed in ethanol-alone treated cells (Figure 2B). Despite the fact that the activation of caspase-3 was not affected by enhanced ROS, the pyrazole and ethanol-treated cells displayed similar damaging morphological changes such as the loss of cell-cell contacts. Indeed, following evaluation of cell viability by flow cytometry, it was determined that when CYP2E1 expression and ROS were enhanced, the presence of non-viable cells significantly increased, demonstrating enhancement of necrotic cell death (Figure 2C). Correspondingly, AA treated cells (which display high ROS levels yet no caspase activation) presented morphologically as necrotic ghost-like cells with cell viability that was markedly impaired.
Figure 2.
The relationship of CYP2E1 expression, oxidative stress, and WIF-B cell death following ethanol administration. WIF-B cultures were treated without (C) or with pyrazole (Cpyr) for 4 d followed by ethanol treatment for 48 h [E and E (pyr)]. A: CYP2E1 activity in isolated microsomes analyzed using the r-nitophenol oxidation assay in four independent experiments; B: H2O2 production representative of ROS generation detected by dichlorofluorescein production, and the activation of caspase-3 as a measure of apoptosis from seven independent experiments; C: Necrotic cell death evaluated by flow cytometric analysis in three independent experiments using the LIVE/DEAD cell stain as described in “Material and Methods”. aP < 0.05 vs control; cP < 0.05 vs ethanol alone-treated cells.
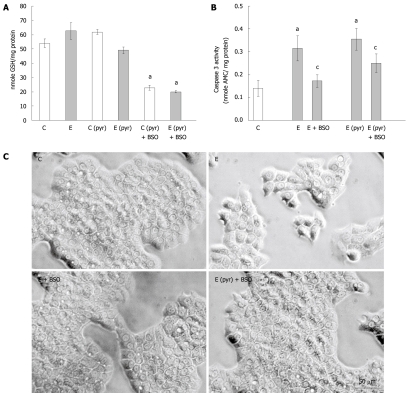
Regulation of ethanol-induced apoptosis by glutathione
Considering the above results indicating that oxidative stress levels did not correlate with apoptotic cell death, and the known role of glutathione in regulating redox balance, we next measured GSH levels and the activation of caspase-3 in the WIF-B cells following ethanol administration with or without pyrazole pretreatment and the inclusion of BSO. It was determined that ethanol treatment alone did not affect the content of reduced glutathione in the WIF-B cultures while the overexpression of CYP2E1 in pyrazole-treated cells resulted in a modest decline (20%) of GSH (Figure 3A). Also, as expected, the addition of BSO in the culture media significantly depleted GSH levels in the WIF-B cells (Figure 3A). Strikingly, this depletion of glutathione by BSO resulted in an abrogation of ethanol-mediated caspase-3 activation (Figure 3B) as well as the reversal of ethanol-induced morphological changes (Figure 3C).
Figure 3.
The effect of glutathione depletion on the induction of caspase activation in ethanol-treated WIF-B cells. WIF-B cultures were treated in the presence or absence of ethanol (25 mmol/L) for 48 h after pretreatment with (C pyr and E pyr) or without (C and E) pyrazole. Glutathione was depleted by the inclusion of BSO in the culture media when indicated (+ BSO). A: The amount of reduced glutathione (GSH) was detected as described in “Material and Methods”; B: Measure of apoptosis in WIF-B cells following glutathione depletion. Cell lysates were assayed for caspase-3 activity and the release of fluorescent product was detected and expressed for five independent experiments; C: Phase contrast images of the treated WIF-B cultures. aP < 0.05 vs control; cP < 0.05 vs ethanol-treated.
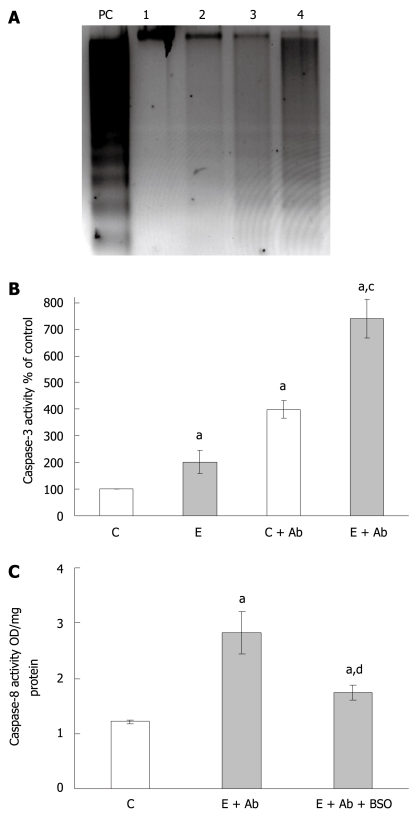
Fas-mediated apoptosis in ethanol treated WIF-B cells: regulation by GSH
Since the depletion of glutathione by BSO resulted in an abrogation of caspase 3 activity, we next analyzed how such events would affect Fas-mediated apoptotic mechanisms which we have previously reported as a potential contributing pathway involved in ethanol-mediated cell death[5]. WIF-B cultures were treated with ethanol for 24 h prior to overnight challenge with anti-Fas antibody/ActD. Following treatment with anti-Fas antibody, both control and ethanol-treated cells greatly increased the Fas-dependent DNA ladder formation (Figure 4A). However, maximal DNA degradation was observed in the cells exposed to ethanol prior to Fas antibody challenge (Figure 4A). Similarly, the increase in caspase-3 activity observed after ethanol treatment alone was found to be further exacerbated when cells exposed to alcohol were treated with Fas antibody (Figure 4B). And finally, when the activation of caspase-8 was measured, the inclusion of BSO in the media was found to significantly reduce the activation of this upstream initiator protease which is linked to Fas-mediated pro-apoptotic events (Figure 4C).
Figure 4.
Fas-mediated apoptosis following ethanol administration and the effects of BSO treatment. A: Representative gel depicting apoptosis identified by DNA fragmentation. WIF-B cultures were left untreated or treated with 25 mmol/L ethanol for 24 h prior to challenge with anti-Fas antibody/Act D (Ab) for 16 h. The samples represented are untreated control cells (lane 1), ethanol alone-treated cells (lane 2), control cells incubated with Ab (lane 3), and ethanol-treated cells incubated with Ab (lane 4). UV-treated WIF-B cells were used as a positive control (lane PC); B: The analysis of caspase-3 activation following Fas antibody treatments. WIF-B cells were cultured for 24 h in the absence and presence of ethanol followed by the additional overnight incubation with Ab as described above. The activity of caspase-3 was assayed in four independent experiments as previously described; C: The effect of glutathione depletion by BSO on caspase-8 activation. WIF-B cultures were treated with ethanol and Fas antibody with or without the inclusion of BSO. The activity of caspase-8 was determined in three independent experiments as described in “Material and Methods”. aP < 0.05 vs untreated control cells; cP < 0.05 vs corresponding controls; dP < 0.05 vs cells treated with ethanol plus Ab.
DISCUSSION
There is ample evidence indicating that ethanol administration results in hepatocellular apoptosis, yet a comprehensive understanding of contributing mechanisms remains incomplete. Previously, we have shown that ethanol induces apoptosis in WIF-B cells partly as a consequence of ADH-mediated ethanol metabolism, and was associated with death receptor-mediated events, particularly the membrane localization of Fas and the subsequent activation of caspases[5]. In the present study, we have corroborated the role of death-receptor triggers as ethanol-treated WIF-B cells were found to be sensitive to Fas-mediated apoptosis. Additionally, we further defined that ethanol-mediated apoptosis of the WIF-B cells requires an adequate cellular level of glutathione, and that increases in ROS generation are not necessarily associated with the promotion of proapoptotic pathways.
The generation of oxidative stress and mitochondrial related alterations have been implicated as a key mechanism in various pathological systems, including ethanol-mediated hepatocellular damage. Particularly relevant to ALD is the fact that the liver expresses an ethanol-inducible form of one of the cytochrome P450 isoforms (CYP2E1), that is involved in the generation of ROS[7], which may contribute to hepatocellular injury. In the current work, we demonstrated that ethanol treatment of WIF-B cells increases ROS and lipid peroxidation products and that this could be prevented by the presence of DAS or antioxidants, confirming the role CYP2E1 plays in ethanol-mediated oxidative stress in hepatocytes. However, protection from apoptotic cell death was not observed when CYP2E1 was inhibited or when antioxidants were present. To further clarify these observations, the expression of CYP2E1 was manipulated in the WIF-B cells by pyrazole pre-treatment prior to ethanol administration. The results of those experiments demonstrated that the content and activity of CYP2E1 could be increased and that the enhanced induction was correlative to an observed increase of ROS detected in the cells. However, the CYP2E1-related enhancement of oxidative stress in the WIF-B cells did not correlate with an increase in apoptotic cell injury, but rather to necrosis. These results support the hypothesis that when oxidative stress is enhanced, a concomitant decrease in viability and caspase activation occurs that changes the mode of hepatocellular death from apoptosis to necrosis.
Also associated with oxidative stress mechanisms and the adverse pathology present in ALD are alterations that occur to the antioxidant defense mechanisms within the liver. In particular, a vital defense mechanism against oxidative stress is the tripeptide glutathione, the cellular level of which has been implicated as a critical factor in whether a cell survives or succumbs to death mechanisms induced by toxins. However, the effect of ethanol on GSH levels as well as the role of glutathione in apoptosis remains controversial as ethanol administration has been reported to change hepatic GSH levels in both positive and negative ways[9–11]. Differences between studies included the mode and amount of ethanol administered, as well as the animal or cell culture system utilized. The present study represents a hepatic cell culture model (WIF-B cells) that adequately mimics in vivo hepatocyte functions, a trait that is sought for model systems for accurate comparisons to human pathology. Specifically, WIF-B cells efficiently metabolize ethanol and express signs of ethanol-mediated damage while using physiologically relevant levels of alcohol (25 and 50 mmol/L). This is often in contrast to other in vitro models that utilize upwards of 100 mmol/L ethanol treatments. In using the WIF-B cell model, we have demonstrated in this study that GSH levels were found to be unaltered by ethanol treatment alone. Additionally, a decline in GSH levels in ethanol-treated cells was observed only after intracellular oxidation was increased due to enhanced activation of CYP2E1 following pyrazole pretreatment. Furthermore, inclusion of the glutathione synthesis inhibitor BSO resulted in the suppression of caspase activation, thereby providing protection against ethanol-induced apoptosis. A potential contributing factor that may be involved in this observed GSH-mediated protection from apoptotic cell death is the fact that caspase-8 activity was found to be diminished when GSH was depleted. This observation is supported by other works demonstrating that sustained glutathione levels may be required for caspase-dependent apoptosis because of the redox-sensitive nature of the cysteine proteases[12,15]. Also, the mode and extent of glutathione removal used in this study may be an important factor as it has been noted that acute GSH depletion can result in the inhibition of Fas-mediated apoptosis, whereas prolonged GSH depletion can override protective anti-apoptotic actions and thus mediate an enhancement of apoptotic cell death[16].
In summary, the liver has been shown to be highly susceptible to Fas-mediated injury and this study has demonstrated that ethanol administration to WIF-B cells can trigger signals associated with this pathway as well as induce susceptibility to this form of apoptotic cell damage. Also, this work has determined that apoptosis induced as a consequence of ethanol metabolism is not completely dependent upon ROS status but is dependent on sustained GSH levels. Thus, factors that regulate apoptotic/necrotic cell death mechanisms and signal the selection of one over the other during ethanol treatment are in part related to the concentration of ROS generated as well as to the ability of antioxidant defenses to cope with the elevated oxidative stress. Overall, the data presented here support the hypothesis that hepatocellular damage which occurs during the early stages of alcohol-mediated injury (i.e. steatosis) may involve the preferential signaling of apoptotic mechanisms whereas stages of more advanced disease (e.g. steatohepatitis and fibrosis) may involve more necrotic rather than apoptotic cell death of hepatocytes as the liver’s sensitivity to oxidative stress mechanisms is enhanced.
COMMENTS
Background
It has been documented that the clinical progression of alcoholic liver disease is associated with an increase in hepatocellular damage which may involve the promotion and execution of apoptotic death mechanisms. It has also been shown that as a consequence of ethanol metabolism, oxidative stress is induced in hepatocytes through the generation of reactive oxygen species (ROS), such as hydrogen peroxide and superoxide. These oxidants can promote hepatotoxicity by inducing protein oxidation, enzyme inactivation, lipid peroxidation, and the production of reactive aldehydes. However, the relationship between hepatocellular oxidative stress and the promotion of apoptotic cell injury is not completely understood. This study is part of current efforts aimed at clarifying pathways and mechanisms associated with ethanol-mediated hepatotoxic events.
Research frontiers
As part of the effort to dissect parameters that are involved in ethanol-related signaling and its adverse effects, researchers have utilized a variety of models. This study utilizes hepatic hybrid WIF-B cells as an in vitro model for the study of alcohol-associated hepatocellular alterations. This is an emerging model for studying the effects of ethanol on cellular processes that is showing immense promise as these cells endogenously express ethanol metabolizing enzymes (alcohol dehydrogenase and CYP2E1), and as a consequence, have been shown to exhibit classic alcohol-mediated adverse effects such as triglyceride accumulation. In this work, the use of the WIF-B cells has brought forth new information concerning the relationship of oxidative stress and cell death following ethanol treatment in a model that provides a more accurate comparison to human pathology than other culture systems.
Innovations and breakthroughs
This study demonstrates that ethanol administration not only results in the trigger of signals associated with the Fas death receptor pathway, but that ethanol also primes hepatocytes, making them more susceptible to apoptotic damage. Also, we showed that apoptosis induced as a consequence of ethanol metabolism in the hepatoma cultures was not completely dependent upon oxidative stress mechanisms and was related to sustained cellular glutathione levels. Thus, this work implies that the status of thiol levels in hepatocytes may predict what hepatotoxic signaling events (i.e. apoptotic or necrotic) are triggered by the corresponding level of ethanol exposure and oxidative stress.
Applications
The prevalence and progression of alcohol-induced liver disease is a major health concern worldwide. Many prior studies have shown that the enhancement of adverse outcomes and pathological damage is associated with several parameters, including the induction of oxidative stress and the promotion of hepatocellular death mechanisms. This study provides evidence indicating that as oxidative stress in hepatocytes is enhanced (a condition related to increased ethanol consumption and/or duration of use); a change in the mode of cell injury occurs from mechanisms that support proapoptotic events to those involved in passive necrosis of the cell. This information may aid in the development of therapeutic interventions for use at appropriate stages of the disease process.
Terminology
WIF-B cells: Highly differentiated cells that are a clone of a human fibroblast (WI 38) and a Fao rat hepatoma cell. These cells develop a hepatocellular-polarized phenotype in culture and efficiently metabolize ethanol.
Peer review
This study is a continuation of a series of studies reported by this group. In this extended study they have proved that ethanol induced enhanced susceptibility of Fas mediated apoptosis of polarized hepatic cells (WIF-B) is dependent on sustained glutathione levels and only partially dependent on ROS status.
Acknowledgments
The authors thank Jacy Kubik for her excellent technical assistance.
Supported by The National Institute on Alcohol Abuse and Alcoholism and by the Department of Veterans Affairs
Peer reviewers: Dr. Natalie J Torok, UC Davis Medical Center, Patient Support Services Building, 4150 V Street, Suite 3500, Sacramento, CA 95817, United States; Parimal Chowdhury, Professor, Department of Physiology and Biophysics, College of Medicine University of Arkansas for Medical Sciences, 4301 W Markham Street Little Rock, Arkansas 72205, United States
S- Editor Li LF L- Editor Logan S E- Editor Ma WH
References
- 1.Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34:248–253. doi: 10.1016/s0168-8278(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 2.Ihrke G, Neufeld EB, Meads T, Shanks MR, Cassio D, Laurent M, Schroer TA, Pagano RE, Hubbard AL. WIF-B cells: an in vitro model for studies of hepatocyte polarity. J Cell Biol. 1993;123:1761–1775. doi: 10.1083/jcb.123.6.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender V, Bravo P, Decaens C, Cassio D. The structural and functional polarity of the hepatic human/rat hybrid WIF-B is a stable and dominant trait. Hepatology. 1999;30:1002–1010. doi: 10.1002/hep.510300436. [DOI] [PubMed] [Google Scholar]
- 4.Schaffert CS, Todero SL, McVicker BL, Tuma PL, Sorrell MF, Tuma DJ. WIF-B cells as a model for alcohol-induced hepatocyte injury. Biochem Pharmacol. 2004;67:2167–2174. doi: 10.1016/j.bcp.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 5.McVicker BL, Tuma DJ, Kubik JL, Tuma PL, Casey CA. Ethanol-induced apoptosis in polarized hepatic cells possibly through regulation of the Fas pathway. Alcohol Clin Exp Res. 2006;30:1906–1915. doi: 10.1111/j.1530-0277.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 6.Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000;174:160–171. doi: 10.1034/j.1600-0528.2002.017411.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu D, Cederbaum AI. Oxidative stress mediated toxicity exerted by ethanol-inducible CYP2E1. Toxicol Appl Pharmacol. 2005;207:70–76. doi: 10.1016/j.taap.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival-death switch. J Gastroenterol Hepatol. 2006;21 Suppl 3:S3–S6. doi: 10.1111/j.1440-1746.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- 9.Oh SI, Kim CI, Chun HJ, Park SC. Chronic ethanol consumption affects glutathione status in rat liver. J Nutr. 1998;128:758–763. doi: 10.1093/jn/128.4.758. [DOI] [PubMed] [Google Scholar]
- 10.Rouach H, Fataccioli V, Gentil M, French SW, Morimoto M, Nordmann R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–355. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- 11.Shaw S, Jayatilleke E, Ross WA, Gordon ER, Leiber CS. Ethanol-induced lipid peroxidation: potentiation by long-term alcohol feeding and attenuation by methionine. J Lab Clin Med. 1981;98:417–424. [PubMed] [Google Scholar]
- 12.Hentze H, Latta M, Kunstle G, Lucas R, Wendel A. Redox control of hepatic cell death. Toxicol Lett. 2003;139:111–118. doi: 10.1016/s0378-4274(02)00425-3. [DOI] [PubMed] [Google Scholar]
- 13.Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos. 1985;13:548–552. [PubMed] [Google Scholar]
- 14.McVicker BL, Tuma DJ, Kubik JA, Hindemith AM, Baldwin CR, Casey CA. The effect of ethanol on asialoglycoprotein receptor-mediated phagocytosis of apoptotic cells by rat hepatocytes. Hepatology. 2002;36:1478–1487. doi: 10.1053/jhep.2002.37137. [DOI] [PubMed] [Google Scholar]
- 15.Hentze H, Kunstle G, Volbracht C, Ertel W, Wendel A. CD95-Mediated murine hepatic apoptosis requires an intact glutathione status. Hepatology. 1999;30:177–185. doi: 10.1002/hep.510300111. [DOI] [PubMed] [Google Scholar]
- 16.Haouzi D, Lekehal M, Tinel M, Vadrot N, Caussanel L, Letteron P, Moreau A, Feldmann G, Fau D, Pessayre D. Prolonged, but not acute, glutathione depletion promotes Fas-mediated mitochondrial permeability transition and apoptosis in mice. Hepatology. 2001;33:1181–1188. doi: 10.1053/jhep.2001.24235. [DOI] [PubMed] [Google Scholar]