Abstract
AIM: To investigate the prognostic factors in patients with hepatocellular carcinoma (HCC) accompanied by microscopic portal vein invasion (PVI).
METHODS: Of the 267 patients with HCC undergoing hepatic resection at Aso Iizuka Hospital, 71 had PVI. After excluding 16 patients with HCC that invaded the main trunk and the first and second branches of the portal vein, 55 patients with microscopic PVI were enrolled.
RESULTS: The patients with HCC accompanied by microscopic invasion were divided into two groups: solitary PVI (PVI-S: n = 44), and multiple PVIs (PVI-M: n = 11). The number of portal vein branches invaded by tumor thrombi was 5.4 ± 3.8 (2-16) in patients with PVI-M. In cumulative survival, PVI-M was found to be a significantly poor prognostic factor (P = 0.0019); while PVI-M and non-anatomical resection were significantly poor prognostic factors in disease-free survival (P = 0.0213, and 0.0115, respectively). In patients with PVI-M, multiple intrahepatic recurrence was more common than in the patients with PVI-S (P = 0.0049). In patients with PVI-S, non-anatomical resection was a significantly poor prognostic factor in disease-free survival (P = 0.0370). Operative procedure was not a significant prognostic factor in patients with PVI-M.
CONCLUSION: The presence of PVI-M was a poor prognostic factor in patients with HCC, accompanied by microscopic PVI. Anatomical resection is recommended in these patients with HCC. Patients with HCC and PVI-M may also be good candidates for adjuvant chemotherapy.
Keywords: Hepatocellular carcinoma, Microscopic portal vein invasion, Hepatectomy, Prognosis, Recurrence
INTRODUCTION
Hepatocellular carcinoma (HCC) is a malignant tumor with periportal venous metastasis. Vascular invasion, especially portal vein invasion (PVI), is a major determinant of outcome after hepatic resection in patients with HCC[1–7].
Magnetic resonance imaging (MRI) and ultrasonography can detect tumor invasion of the major branches of the portal or hepatic veins, 81%-95% of the time[8–10]. However, the presence of microscopic PVI, limited to the subsegmental portal vein branches, cannot be diagnosed before hepatic resection. Recently reported predictors of microscopic PVI include size, number, and histological grade of tumors, as well as serum level of des-γ-carboxy prothrombin (DCP)[11–13]. Miyata
et al[14] have demonstrated that tumorous arterioportal (A-P) shunt formation on computed tomography (CT) during hepatic arteriography is an important predictive value for PVI. A recent study by Nishie et al[15] has shown that an area exhibiting low attenuation on CT during arterio-portography, and high attenuation on CT during hepatic arteriography around the tumor, is a good predictor of PVI. Thus, recent studies have suggested that the presence of microscopic PVI can be predicted.
Nevertheless, the prognostic factors of HCC with microscopic PVI have remained elusive and the operative procedures for this type of HCC have not been determined. In the present study, we evaluated the prognostic factors in cumulative and disease-free survival in patients with HCC, accompanied by microscopic PVI.
MATERIALS AND METHODS
Patients
From April 1992 to December 2005, 267 patients underwent their first liver resection for HCC at the Department of Hepatogastro-enterological Surgery at Aso Iizuka Hospital in Japan. From a retrospective database, 55 patients were enrolled in this study, according to the following criteria: (1) an absence of HCC invading the main trunk and the first and second branches of the portal vein, upon preoperative radiological evidence and intraoperative findings; (2) no remnant cancer after surgery, as confirmed by ultrasonography, CT and/or MRI; and (3) the presence of microscopic PVI upon histological examination.
There were 41 male and 14 female patients with an average age of 64 years (median: 66 years). Among these patients, 35 (64 %) were infected with hepatitis C virus, which leads to chronic liver disease. The indocyanine green retention test at 15 min was 15.5% ± 10.2%. On pathological examination, the tumor size was 5.0 ± 1.1 cm and the main grade of cancer cell was moderately differentiated in 38 patients (69%) and poorly differentiated in 17 (31%). Microscopic intrahepatic metastasis was found in 24 patients (44%).
Methods
The prognostic factors were examined in cumulative and disease-free survival, using the following variables: age (older or younger than 67 years); gender (male versus female); platelet numbers (greater than versus less than or equal to 150 000/mm3 ); serum albumin levels (greater than versus less than or equal to 3.8 g/dL); tumor size (greater than versus less than or equal to 4.2 cm); serum levels of alpha-fetoprotein (AFP) (greater than versus less than or equal to 28 ng/mL); DCP (greater than versus less than or equal to 300 mAU/mL); operative procedures (anatomical versus non-anatomical resection); histological grading of cancer cell differentiation (moderate versus poor); presence of intrahepatic metastases (negative versus positive); and microscopic PVI (solitary or multiple). The measurement of serum DCP has been described previously[13]. The measurement of serum DCP was started at our hospital in 1999 and was therefore only available in the latest 36 patients.
Anatomical resection included hemi-hepatectomy, segmentectomy, and subsegmentectomy, based on Couinaud’s classification. Non-anatomical resection was partial hepatectomy, including the tumor.
Histological study
All of the resected specimens were cut into serial 5-10-mm thick slices and fixed in 10% formalin. After macroscopic examination, the slice with the greatest dimension was trimmed for paraffin blocks and cut into 4-μm microscopic sections. The slices were then stained with hematoxylin and eosin (HE). When clusters of cancer cells were present in the extra tumoral portal vein, accompanied with bile duct and hepatic artery, it was defined as positive for extra tumoral PVI. When more than two clusters of cancer cells were present in different portal vein branches, it was defined as multiple PVI (PVI-M) (Figure 1). When only one cluster was present in a single portal vein branch, it was defined as solitary PVI (PVI-S).
Figure 1.
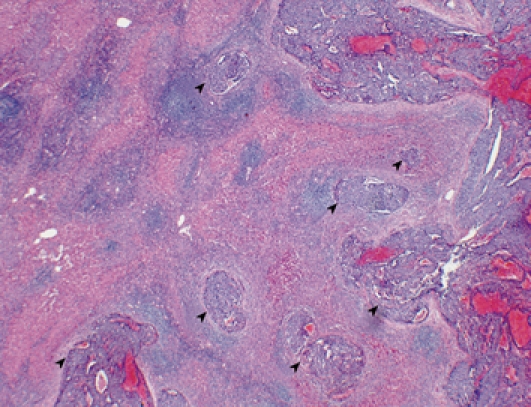
Multiple microscopic PVI surrounding a tumor (arrow heads). HE staining (Original magnification, × 20).
Follow-up strategy and recurrence pattern
After discharge, all patients were examined for recurrence by ultrasonography and tumor markers, such as AFP and DCP every month, and by CT every 6 mo. When recurrence was suspected, additional examinations such as hepatic angiography were performed. The recurrence pattern was determined by ultrasonography, CT, MRI and hepatic angiography and was defined as previously reported[7]. Briefly, none was the absence of HCC recurrence, nodular recurrence was fewer than four recurrent nodules, and multiple recurrence was four or more recurrent nodules.
Impact of operative procedures in patients with PVI-S and PVI-M
In patients with PVI-S and PVI-M, the impact of operative procedures (non-anatomical versus anatomical resection) was compared on cumulative and disease-free survival.
Statistical analysis
All data were expressed as mean ± SD. The χ2 test of independence was used with categorical variables. The continuous variables were divided by their median values. The survival and disease-free survival curves were generalized using the Kaplan-Meier method and then compared using the log-rank test. The Stat view software (Version 4.11; Abacus Concepts Inc., Berkeley, CA, USA) was used for the analysis on a Macintosh computer. P < 0.05 was considered to be statistically significant.
RESULTS
Histological examination of PVI-S and PVI-M
Of the 267 patients, 55 (21%) had microscopic PVI. The overall incidence of PVI-S was 16% (44 patients) and that of PVI-M was 4% (11 patients). Of the 55 patients with PVI, the 11 patients with PVI-M represented 20%. The number of portal vein branches invaded by tumor thrombi was 5.4 ± 3.8 (2-16) on the slices, stained with HE in patients with PVI-M.
Significant prognostic factors in cumulative survival
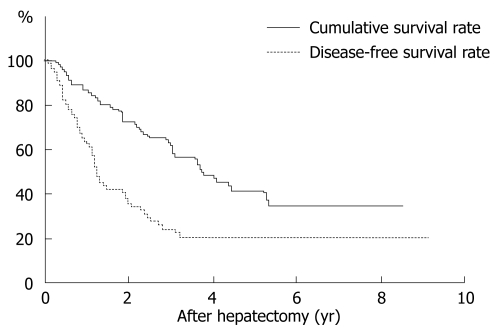
The overall survival after hepatectomy in 55 patients with microscopic PVI was 89.0% at 1 year, 66.6% at 3 years, 46.0% at 5 years, and 36.1% at 10 years (Figure 2). The median survival was 4.6 ± 0.6 years. Among 11 clinicopathological factors, only the extent of PVI was significant (Table 1). Thus, the cumulative survival curve for patients with PVI-M was significantly worse than that of those with PVI-S (P = 0.0019). The median survival of patients with PVI-S and PVI-M was 5.4 ± 1.8 and 1.3 ± 0.7 years, respectively. In operative procedures, the survival rate for anatomical resection tended to be better than that for non-anatomical resection, although the difference was not significant (P = 0.0620).
Figure 2.
Cumulative and disease-free survival rate in patients with HCC, accompanied by microscopic PVI. The overall cumulative and disease-free survival after hepatectomy in 55 patients was 89.0% and 68.7% at 1 year, 66.6% and 27.3% at 3 years, 46.0% and 22.7% at 5 years.
Table 1.
Univariate analysis of clinicopathological prognostic factors for cumulative survival rate
| Factors |
Survival rate (%) |
P value | ||
| 1 yr | 3 yr | 5 yr | ||
| Age (yr) | ||||
| < 67 (n = 27) | 80.6 | 60.4 | 42.1 | 0.9546 |
| ≥ 67 (n = 28) | 96.6 | 75.7 | 50.0 | |
| Gender | ||||
| Male (n = 41) | 92.6 | 72.3 | 45.2 | 0.7614 |
| Female (n = 14) | 78.6 | 50.0 | 42.9 | |
| Platelets (104/mm3) | ||||
| < 1.5 (n = 27) | 92.6 | 61.7 | 46.6 | 0.7341 |
| ≥ 1.5 (n = 28) | 85.7 | 67.5 | 46.0 | |
| Albumin (g/dL) | ||||
| < 3.9 (n = 27) | 100 | 76.9 | 47.2 | 0.6123 |
| ≥ 3.9 (n = 28) | 79.0 | 56.8 | 47.5 | |
| Tumor size (cm) | ||||
| < 4.3 (n = 27) | 92.4 | 72.8 | 35.9 | 0.8752 |
| ≥ 4.3 (n = 28) | 85.7 | 60.7 | 52.0 | |
| AFP (ng/mL) | ||||
| 0-27 (n = 28) | 96.4 | 71.3 | 55.4 | 0.1001 |
| > 27 (n = 27) | 81.2 | 61.6 | 35.8 | |
| DCP (mAU/mL) | ||||
| 0-300 (n = 18) | 94.1 | 75.1 | 75.1 | 0.1834 |
| ≥ 300 (n = 18) | 83.3 | 55.6 | 55.6 | |
| Operative procedure | ||||
| Anatomical resection (n = 32) | 90.5 | 74.2 | 51.4 | 0.0620 |
| Non-anatomic resection (n = 23) | 87.0 | 55.9 | 39.5 | |
| Tumor grade of differentiation | ||||
| Moderate (n = 38) | 97.3 | 70.0 | 54.9 | 0.1076 |
| Poor (n = 17) | 70.6 | 58.8 | 30.3 | |
| IM (-) (n = 31) | 96.8 | 66.7 | 56.5 | 0.2625 |
| IM (+) (n = 24) | 79.2 | 66.7 | 32.8 | |
| PVI-S (n = 44) | 97.7 | 76.5 | 51.2 | 0.0019 |
| PVI-M (n = 11) | 54.5 | 27.3 | 27.3 | |
IM: Intrahepatic metastasis.
Significant prognostic factors in disease-free survival
The disease-free survival in 55 patients was 68.7% at 1 year, 27.3 % at 3 years, 22.7 % at 5 years, and 22.7 % at 10 years (Figure 2). Among 11 clinicopathological factors, the extent of PVI and operative procedures were significant (Table 2). The disease-free survival curve for patients with PVI-M was significantly worse than that for patients with PVI-S (P = 0.0072). The median disease-free survival in PVI-S and PVI-M was 1.5 ± 0.8 and 0.4 ± 0.2 years, respectively. In operative procedures, the disease-free survival rate for anatomical resection was significantly better than that for non-anatomical resection (P = 0.0074). The median disease-free survival for anatomical and non-anatomical resection was 2.0 ± 0.2 and 0.8 ± 0.1 years, respectively.
Table 2.
Univariate analysis of clinicopathological prognostic factors for disease-free survival rate
| Factors |
Survival rates (%) |
P value | ||
| 1 yr | 3 yr | 5 yr | ||
| Age (yr) | ||||
| < 67 (n = 27) | 54.7 | 31.9 | 31.9 | 0.7438 |
| ≥ 67 (n = 28) | 72.5 | 21.3 | ||
| Gender | ||||
| Male (n = 41) | 68.7 | 27.3 | 22.7 | 0.2716 |
| Female (n = 14) | 48.6 | 19.4 | 19.4 | |
| Platelets (104/mm3) | ||||
| < 1.5 (n = 27) | 52.8 | 7.9 | 7.9 | 0.1703 |
| ≥ 1.5 (n = 28) | 74.2 | 33.7 | 33.7 | |
| Albumin (g/dL) | ||||
| < 3.9 (n = 27) | 61.5 | 22.6 | 16.9 | 0.8555 |
| ≥ 3.9 (n = 28) | 65.8 | 29.4 | 29.4 | |
| Tumor size (cm) | ||||
| < 4.3 (n = 27) | 62.9 | 35.0 | 35.0 | 0.4474 |
| ≥ 4.3 (n = 28) | 64.8 | 17.0 | 11.3 | |
| AFP (ng/mL) | ||||
| 0-27 (n = 28) | 59.1 | 39.4 | 39.4 | 0.1692 |
| > 27 (n = 27) | 68.0 | 14.6 | 9.7 | |
| DCP (mAU/mL) | ||||
| < 300 (n = 18) | 72.5 | 43.5 | 43.5 | 0.2995 |
| ≥ 300 (n = 18) | 63.0 | 21.0 | 14.0 | |
| Operative procedure | ||||
| Anatomical resection (n = 32) | 82.3 | 33.0 | 33.0 | 0.0074 |
| Non-anatomical resection (n = 23) | 40.9 | 17.0 | 11.4 | |
| Tumor grade of differentiation | ||||
| Moderate (n = 38) | 69.3 | 36.7 | 31.4 | 0.0661 |
| Poor (n = 17) | 52.9 | 7.4 | 7.4 | |
| IM (-) (n = 31) | 67.1 | 28.2 | 23.5 | 0.4761 |
| IM (+) (n = 24) | 59.8 | 22.1 | 22.1 | |
| PVI-S (n = 44) | 74.6 | 27.4 | 23.5 | 0.0072 |
| PVI-M (n = 11) | 20.0 | 20.0 | ||
Comparison of PVI-S and PVI-M recurrence patterns
A comparison of the recurrence pattern is shown in Table 3. The incidence of multiple recurrence in patients with PVI-M was 82% and 30% in patients with PVI-S (P = 0.0049).
Table 3.
Comparison of recurrence patterns for 55 HCC patients with single or multiple PVI n (%)
| Recurrence pattern | None | Nodular | Multiple |
| PVI-S (n = 44) | 16 (36) | 15 (34) | 13 (30) |
| PVI-M (n = 11) | 2 (18) | 0 | 9 (82) |
The incidence of multiple recurrence in PVI-M was significantly higher than that in PVI-S (P = 0.0049).
Impact of operative procedures in patients with PVI-S and PVI-M
In patients with PVI-S, non-anatomical resection tended to be a poor prognostic factor for cumulative survival (P = 0.0782), while non-anatomical resection was significantly poor prognostic factor in disease-free survival (P = 0.0370) (Table 4). In patients with PVI-M, operative procedures were not significant in disease-free survival (Table 5).
Table 4.
Impact of operative procedures (non-anatomical versus anatomical resection) was examined in patients with PVI-S, depending on cumulative survival rate and disease-free survival rates
| Factors |
Survival rates (%) |
P value | ||
| 1 yr | 3 yr | 5 yr | ||
| Cumulative survival | ||||
| Anatomical resection (n = 26) | 96.2 | 83.9 | 55.0 | 0.0782 |
| Non-anatomical resection (n = 18) | 100 | 65.8 | 46.1 | |
| Disease-free survival | ||||
| Anatomical resection (n = 26) | 90.9 | 32.2 | 32.2 | 0.0370 |
| Non-anatomical resection (n = 18) | 52.9 | 22.1 | 14.7 | |
Table 5.
Impact of operative procedures (non-anatomical versus anatomical resection) was examined in patients with PVI-M, depending on cumulative survival and disease-free survival rates
| Factors |
Survival rates (%) |
P value | ||
| 1 yr | 3 yr | 5 yr | ||
| Cumulative survival | ||||
| Anatomical resection (n = 6) | 66.7 | 33.3 | 33.3 | 0.4497 |
| Non-anatomical resection (n = 5) | 40.0 | 20.0 | 20.0 | |
| Disease-free survival | ||||
| Anatomical resection (n = 6) | 40.0 | 20.0 | 20.0 | 0.2651 |
| Non-anatomical resection (n = 5) | 0 | |||
DISCUSSION
The incidence of microscopic PVI has been reported to be more than 20% in resected HCC[1,2]. Even in small HCCs, up to 2 cm in diameter, the incidence of PVI is 15%[3]. In this study, the incidence of PVI was found in 55 of 267 (20%) patients.
Vascular invasion, especially PVI, is a major determinant of the outcome after hepatic resection in patients with HCC[1–7]. In the present study, the survival rate was poorer for HCC patients with PVI than those without (data not shown). Nevertheless, the prognosis of patients with PVI varied. With regard to recurrence patterns, 18 (33%) of the 55 patients with HCC accompanied by PVI had no recurrence, and 22 patients (40%) had multiple recurrence. Clearly, the outcome in patients with no recurrence was better than that of patients with multiple recurrence.
Determination of the prognostic factors in patients with PVI is important for postoperative therapeutic strategy. There has been no study of prognostic factors in patients with HCC accompanied by PVI. In the present study, detailed histological examination of resected specimens revealed that PVI-M was a significantly poor prognostic factor after hepatectomy for cumulative and disease-free survival. With regard to operative procedures, anatomical resection tended to improve survival rates and significantly improve disease-free rates.
Histologically, the number of portal vein branches invaded by tumor thrombi was 5.4 ± 3.7 (2-16) in patients with PVI-M. Although PVI was limited to the subsegment of the liver, multiple portal vein branches that surrounded the tumor were invaded. In these patients, the biological behavior of HCC with PVI-M may be similar to that of HCC, with invasion to the first branches or main trunks of the portal vein. The survival rate in patients with PVI-M was only 54.5% at 1 year after hepatectomy. The mean survival and disease-free survival after hepatectomy in patients with PVI-M was 1.3 and 0.4 years, respectively. Multiple recurrence was more common in patients with PVI-M than those with PVI-S. This clinical outcome was similar to that previously shown for patients with HCC, accompanied by portal vein thrombi of the first branches or main trunks[16,17].
Anatomical resection of the liver significantly improved the disease-free rates for HCC with PVI in the present study. Hasegawa et al[18] have reported that anatomical resection, such as segmentectomy and subsegmentectomy for HCC, is a reasonable treatment option and yields more favorable results than non-anatomical resection. A recent comparison of the outcomes of anatomical subsegmentectomy and non-anatomical minor hepatectomy for single HCC, based on a Japanese nationwide survey, recommends anatomical resection, especially when the size of HCC ranges 2-5 cm[19]. In our study, non-anatomical resection was a significantly poor prognostic factor in disease-free survival and tended to be a poor prognostic factor in cumulative survival in patients with PVI-S. Therefore, anatomical resection is preferable in patients with HCC accompanied by PVI. Recent studies have demonstrated that HCC with PVI can be predicted by several factors[11–15]. Therefore, patients with HCC that have high risk factors for PVI preoperatively should be recommended for anatomical resection. To clarify this hypothesis, further examination is necessary.
In PVI-M patients, there was no significant difference in the outcome between anatomical and non-anatomical resection. Recently, in patients with portal vein thrombi in major portal branches, adjuvant chemotherapy has been reported to be effective following hepatectomy and thrombectomy[16,17,20,21]. Patients with HCC and PVI-M may also be good candidates for adjuvant chemotherapy.
In conclusion, in patients with HCC, accompanied by microscopic PVI, the presence of PVI-M is a poor prognostic factor. In PVI-S patients, anatomical resection is preferable to non-anatomical resection. Patients with HCC and PVI-M may be good candidates for adjuvant chemotherapy. Further studies aimed at improving the outcome of patients with PVI after hepatectomy are necessary.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is a malignant tumor with periportal venous metastasis. Vascular invasion, especially portal vein invasion (PVI), is a major determinant of outcome after hepatic resection in patients with HCC. Nevertheless, the prognostic factors of HCC with microscopic PVI have remained elusive and the operative procedures for this type of HCC have not been determined.
Research frontiers
The prognostic factors in cumulative and disease-free survival in patients with HCC, accompanied by microscopic PVI, were evaluated in the present study.
Innovations and breakthroughs
The presence of multiple PVI (PVI-M) was a poor prognostic factor in patients with HCC, accompanied by microscopic PVI. Anatomical resection is recommended in these patients with HCC. Patients with HCC with PVI-M may be good candidates for adjuvant chemotherapy.
Applications
In patients with HCC, accompanied by microscopic PVI, the presence of PVI–M is a poor prognostic factor. In solitary PVI patients, anatomical resection is preferable to non-anatomical resection. Patients with HCC and PVI-M may be good candidates for adjuvant chemotherapy.
Terminology
When clusters of cancer cells were present in the extra tumoral portal vein, accompanied with bile duct and hepatic artery, it was defined as positive for extra tumoral PVI. When more than two clusters of cancer cells (PVI) were present in different portal vein branches, it was defined as PVI-M. When only one cluster was present in a single portal vein branch, it was defined as solitary PVI.
Peer review
This paper was correctly planned to investigate the prognostic factors in patients with HCC accompanied by microscopic PVI. This research is of clinical importance in post-resection management of patients with HCC. The results provide robust clinical evidence to suggest firm scientific conclusions.
Peer reviewer: Roberto Testa, Professor, Department of Internal Medicine, University of Genoa, Viale Benedetto XV 6, Genoa 16132, Italy
S- Editor Li LF L- Editor Kerr C E- Editor Zheng XM
References
- 1.Shirabe K, Kanematsu T, Matsumata T, Adachi E, Akazawa K, Sugimachi K. Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology. 1991;14:802–805. doi: 10.1002/hep.1840140510. [DOI] [PubMed] [Google Scholar]
- 2.Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996;131:71–76. doi: 10.1001/archsurg.1996.01430130073014. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda S, Itamoto T, Nakahara H, Kohashi T, Ohdan H, Hino H, Ochi M, Tashiro H, Asahara T. Clinicopathologic features and prognostic factors of resected solitary small-sized hepatocellular carcinoma. Hepatogastroenterology. 2005;52:1163–1167. [PubMed] [Google Scholar]
- 4.Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 1998;227:424–432. doi: 10.1097/00000658-199803000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao WH, Ma ZM, Zhou XR, Feng YZ, Fang BS. Prediction of recurrence and prognosis in patients with hepatocellular carcinoma after resection by use of CLIP score. World J Gastroenterol. 2002;8:237–242. doi: 10.3748/wjg.v8.i2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802. doi: 10.1002/cncr.20426. [DOI] [PubMed] [Google Scholar]
- 7.Shirabe K, Wakiyama S, Gion T, Motomura K, Koyanagi T, Sakamoto S, Nagaie T. Clinicopathological risk factors linked to recurrence pattern after curative hepatic resection for hepatocellular carcinoma--results of 152 resected cases. Hepatogastroenterology. 2007;54:2084–2087. [PubMed] [Google Scholar]
- 8.Nelson RC, Chezmar JL, Sugarbaker PH, Murray DR, Bernardino ME. Preoperative localization of focal liver lesions to specific liver segments: utility of CT during arterial portography. Radiology. 1990;176:89–94. doi: 10.1148/radiology.176.1.2353115. [DOI] [PubMed] [Google Scholar]
- 9.Bach AM, Hann LE, Brown KT, Getrajdman GI, Herman SK, Fong Y, Blumgart LH. Portal vein evaluation with US: comparison to angiography combined with CT arterial portography. Radiology. 1996;201:149–154. doi: 10.1148/radiology.201.1.8816536. [DOI] [PubMed] [Google Scholar]
- 10.Hann LE, Schwartz LH, Panicek DM, Bach AM, Fong Y, Blumgart LH. Tumor involvement in hepatic veins: comparison of MR imaging and US for preoperative assessment. Radiology. 1998;206:651–656. doi: 10.1148/radiology.206.3.9494482. [DOI] [PubMed] [Google Scholar]
- 11.Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, Ikai I, Yamaoka Y, Regimbeau JM, Belghiti J, Curley SA, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–232; discussion 232. doi: 10.1016/s1091-255x(01)00015-4. [DOI] [PubMed] [Google Scholar]
- 12.Adachi E, Maeda T, Kajiyama K, Kinukawa N, Matsumata T, Sugimachi K, Tsuneyoshi M. Factors correlated with portal venous invasion by hepatocellular carcinoma: univariate and multivariate analyses of 232 resected cases without preoperative treatments. Cancer. 1996;77:2022–2031. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2022::AID-CNCR9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Shirabe K, Itoh S, Yoshizumi T, Soejima Y, Taketomi A, Aishima S, Maehara Y. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol. 2007;95:235–240. doi: 10.1002/jso.20655. [DOI] [PubMed] [Google Scholar]
- 14.Miyata R, Tanimoto A, Wakabayashi G, Shimazu M, Nakatsuka S, Mukai M, Kitajima M. Accuracy of preoperative prediction of microinvasion of portal vein in hepatocellular carcinoma using superparamagnetic iron oxide-enhanced magnetic resonance imaging and computed tomography during hepatic angiography. J Gastroenterol. 2006;41:987–995. doi: 10.1007/s00535-006-1890-2. [DOI] [PubMed] [Google Scholar]
- 15.Nishie A, Yoshimitsu K, Asayama Y, Irie H, Tajima T, Hirakawa M, Ishigami K, Nakayama T, Kakihara D, Nishihara Y, et al. Radiologic detectability of minute portal venous invasion in hepatocellular carcinoma. AJR Am J Roentgenol. 2008;190:81–87. doi: 10.2214/AJR.07.2810. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka S, Shimada M, Shirabe K, Maehara S, Harimoto N, Tsujita E, Sugimachi K, Maehara Y. A novel intrahepatic arterial chemotherapy after radical resection for advanced hepatocellular carcinoma. Hepatogastroenterology. 2005;52:862–865. [PubMed] [Google Scholar]
- 17.Liang LJ, Hu WJ, Yin XY, Zhou Q, Peng BG, Li DM, Lu MD. Adjuvant intraportal venous chemotherapy for patients with hepatocellular carcinoma and portal vein tumor thrombi following hepatectomy plus portal thrombectomy. World J Surg. 2008;32:627–631. doi: 10.1007/s00268-007-9364-0. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252–259. doi: 10.1097/01.sla.0000171307.37401.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, Ikai I, Kudo M, Kojiro M, Makuuchi M, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2008;143:469–475. doi: 10.1016/j.surg.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Nagano H, Sakon M, Eguchi H, Kondo M, Yamamoto T, Ota H, Nakamura M, Wada H, Damdinsuren B, Marubashi S, et al. Hepatic resection followed by IFN-alpha and 5-FU for advanced hepatocellular carcinoma with tumor thrombus in the major portal branch. Hepatogastroenterology. 2007;54:172–179. [PubMed] [Google Scholar]
- 21.Imura S, Ikemoto T, Morine Y, Fujii M, Miyake H, Tashiro S, Shimada M. Effect of a new adjuvant systemic interferon alpha, 5-fluorouracil and cisplatin on advanced hepatocellular carcinoma with macroscopic portal invasion. Hepatogastroenterology. 2008;55:615–620. [PubMed] [Google Scholar]