Abstract
Purpose
Sigma receptors are implicated in memory and cognitive functions, drug addiction, depression and schizophrenia. In addition, sigma receptors are strongly overexpressed in many tumours. Although the natural ligands are still unknown, steroid hormones are potential candidates. Here, we examined changes in binding of the sigma-1 agonist 11C-SA4503 in C6 glioma cells and in living rats after modification of endogenous steroid levels.
Methods
11C-SA4503 binding was assessed in C6 monolayers by gamma counting and in anaesthetized rats by microPET scanning. C6 cells were either repeatedly washed and incubated in steroid-free medium or exposed to five kinds of exogenous steroids (1 h or 5 min before tracer addition, respectively). Tumour-bearing male rats were repeatedly treated with pentobarbital (a condition known to result in reduction of endogenous steroid levels) or injected with progesterone.
Results
Binding of 11C-SA4503 to C6 cells was increased (~50%) upon removal and decreased (~60%) upon addition of steroid hormones (rank order of potency: progesterone > allopregnanolone = testosterone = androstanolone > dehydroepiandrosterone-3-sulphate, IC50 progesterone 33 nM). Intraperitoneally administered progesterone reduced tumour uptake and tumour-to-muscle contrast (36%). Repeated treatment of animals with pentobarbital increased the PET standardized uptake value of 11C-SA4503 in tumour (16%) and brain (27%), whereas the kinetics of blood pool radioactivity was unaffected.
Conclusions
The binding of 11C-SA4503 is sensitive to steroid competition. Since not only increases but also decreases of steroid levels affect ligand binding, a considerable fraction of the sigma-1 receptor population in cultured tumour cells or tumour-bearing animals is normally occupied by endogenous steroids.
Keywords: Steroid hormones, Positron emission tomography (PET), Glioma, Sigma receptor ligand, 11C-SA4503
Introduction
The use of positron emission tomography (PET) for imaging of cancer has a successful history spanning more than three decades. Suitable radiopharmaceuticals have been designed to track molecular events in the body, to monitor the time course of disease and to assess treatment outcome. Although 18F-FDG and other metabolic PET tracers (18F-FLT, 11C-methionine, 11C-choline, etc.) are widely available for the diagnosis of cancer and monitoring of treatment response, these tracers are not tumour specific and/or have only moderate cellular uptake [1, 2]. For this reason, there is much interest in the development and validation of novel radiopharmaceuticals with greater tumour selectivity. Attractive candidates are sigma ligands since sigma receptors are strongly overexpressed in rapidly proliferating cells [3–6].
Sigma receptors are proteins with a highly conserved sequence (87–92% identity and 90–93% homology for sigma-1 receptor) [7]. They are found in kidney, liver, immune, endocrine and reproductive organs [8]. Furthermore, sigma receptors are widely distributed in the brain and are implied in memory function, cognition, drug addiction, depression and schizophrenia [9]. Importantly, sigma receptors were demonstrated to play a role in tumour cell proliferation and cancer cell death. These binding sites are therefore important targets in the development of novel anti-cancer drugs [10]. Although the endogenous ligands for sigma receptors have not yet been identified, steroid hormones (in particular progesterone) are potential candidates [11, 12].
Recent work has demonstrated regulation of sigma-1 receptor availability by endogenous steroids. Such steroids modulate the efficacy of a sigma-1 receptor agonist in stress and depression [12]. Binding of neurosteroids to sigma receptors affects the release of substance P from nociceptor endings in mice [13] and the release of glutamate and norepinephrine in rat prelimbic cortex and hippocampus [14, 15]. Furthermore, such binding reduces subjective craving in cocaine addiction [16]. Based on their effects on substance P and norepinephrine release, progesterone was proposed to be a sigma antagonist, whereas dehydroepiandrosterone-3-sulphate (DHEA-S) and pregnenolone sulphate were considered as sigma agonists [13, 15]. Multiple sequence analyses revealed the presence of two steroid-like binding domains (SBDLI and SBDLII) in the guinea pig sigma-1 receptor (amino acids 91–109 and 176–194, respectively) [17, 18]. Recently, it was shown that administration of steroids reduces in vivo binding of the sigma-1 receptor ligand 18F-FPS in rodent brain [19].
Although an interaction between cerebral sigma receptors and steroids has been established, our knowledge about competition between sigma ligands and endogenous steroids in cancer cells is still rudimentary. Moreover, it remains necessary to examine how steroid hormones affect the binding of radiopharmaceuticals other than 18F-FPS, since sigma ligands may bind to different receptor subtypes [20] and to different binding sites within the sigma-1 receptor molecule (e.g. the agonist binding site and the phenytoin-binding site [21]).
Here, we examine the impact of steroid competition on binding of the sigma-1 agonist 11C-SA4503. 11C-SA4503 has been used extensively for PET studies of animal tumours [2, 22–24] and the human brain [25–28]. For in vitro tests of steroid competition, we used C6 rat glioma cells, a tumour line which expresses both sigma-1 and sigma-2 receptors [3]. As an in vivo model we used C6 glioma-bearing Wistar rats. Tumour cells were either repeatedly washed and incubated in steroid-free medium or exposed to five different exogenous steroids. Rats were either repeatedly injected with pentobarbital, a treatment known to reduce the levels of endogenous steroids [29], or injected with progesterone. If the binding of 11C-SA4503 is sensitive to competition by endogenous steroids, we expected to find increased tracer binding after prolonged anaesthesia and decreased binding after steroid addition. Such competition, if present, could result in intrasubject variability of the binding potential of 11C-SA4503 in women during the menstrual cycle and also in intersubject variability in aged subjects which may show widely different endogenous steroid concentrations.
Materials and methods
Culture media and drugs
Dulbecco’s minimum essential medium (DMEM), fetal calf serum (FCS) and trypsin were products of Invitrogen. Allopregnanolone acetate (5α-pregnan-3β-ol-20-one 3β-acetate), dehydroepiandrosterone 3-sulphate sodium salt (DHEA-S), haloperidol, progesterone (cell culture tested, P8783) and trypan blue (0.4% solution in phosphate-buffered saline) were purchased from Sigma. Androstanolone (5α-androstan-17β-ol-3-one) and testosterone were obtained from Fluka. MatrigelR came from Becton Dickinson. Arachid oil was a product of Levo BV, Franeker, Holland. Stock solutions of steroids and of haloperidol were prepared in ethanol unless otherwise indicated. The radioligand 11C-SA4503 was prepared by reaction of 11C-methyl iodide with the appropriate 4-O-methyl precursor [30]. The decay-corrected radiochemical yield was 9–11%, the specific radioactivity >11 TBq/mmol at the moment of injection and radiochemical purity >95%.
Cell culture
C6 rat glioma cells obtained from the American Type Culture Collection were grown as monolayers in DMEM (high glucose) supplemented with 7.5% FCS in a humidified atmosphere of 5% CO2/95% air at 37°C. Before each experiment, the cells were seeded in 12-well plates (Costar). An equal number of cells were dispensed in each well in 1.1 ml of serum-containing medium: DMEM (high glucose) supplemented with 7.5% FCS.
Binding studies
Binding studies were performed 48 h after seeding cells in 12-well plates when confluency had reached 80–90%. In some experiments, cells were steroid-depleted by removal of the normal medium, repeated (3 ×) washing with phosphate-buffered saline (PBS, 1 ml) and addition of DMEM (high glucose) without serum and phenol red, 1 h before addition of the radiotracer. Various concentrations of an unlabelled competitor (haloperidol or a steroid) were dispensed to the culture medium in the wells. Steroids investigated were allopregnanolone, androstanolone, DHEA-S, progesterone and testosterone. After 2 min, 4 MBq of 11C-SA4503 in <30 µl of saline (containing 30% ethanol) were added to each well. After 45–60 min of incubation, the medium was quickly removed and the monolayer was washed 3 times with PBS. Cells were then treated with 0.2 ml of trypsin. When the monolayer had detached from the bottom of the well, 1 ml of DMEM (high glucose) supplemented with 7.5% FCS was added to stop the proteolytic action. Cell aggregates were resolved by repeated (at least tenfold) pipetting of the trypsin/DMEM mixture. Radioactivity in the cell suspension (1.2 ml) was assessed using a gamma counter (Compugamma 1282 CS, LKB-Wallac, Turku, Finland). A sample of the suspension was mixed with trypan blue solution (1:1 v/v) and was used for cell counting. Cell numbers were determined manually, using a phase contrast microscope (Olympus, Tokyo, Japan), a Bürker bright-line chamber (depth 0.1 mm; 0.0025 mm2 squares) and a hand tally counter. All experiments were performed as a quadruplicate study with at least two repeats.
Animal model
The animal experiments were performed by licensed investigators in compliance with the Law on Animal Experiments of The Netherlands. The protocol was approved by the Committee on Animal Ethics of the University of Groningen.
C6 glioma cells [2.5 × 106, in a 1:1 mixture of Matrigel and DMEM (high glucose with 7.5% FCS)] were subcutaneously injected into the right shoulder of male Wistar rats. The animals were maintained at a 12 h light/12 h dark regime and were fed standard laboratory chow ad libitum. They were scanned after 9–10 days, when tumours had grown to above 0.5 g.
MicroPET scanning
Two rats were scanned simultaneously in each scan session, using a Siemens/Concorde microPET camera (Focus 220). A list mode protocol was used (60 min, brain, tumour and upper half of both lungs in the field of view). These organs were clearly visualized, as reported previously [24]. The scanning was started during injection into the first rat; the second rat was injected 30 s later. Body temperature of anaesthetized animals in the scanner was kept at 37.5 ± 0.5°C, using rectal probes, individual temperature controllers and electronic heating (M2M Imaging).
Two microPET scans of five rats were made in order to explore the effect of anaesthesia duration on the in vivo binding of 11C-SA4503 (23–27 MBq administered as a 0.3 ml bolus, tail vein catheter, mass <2 nmol). For the first scan, the tracer was injected shortly (<20 min) after the induction of pentobarbital anaesthesia (first scan = control condition). When this scan had been finished, the animal was kept under anaesthesia and remained at a fixed position in the microPET scanner. 11C-SA4503 was once more injected after prolonged anaesthesia [four intraperitoneal injections of a sodium pentobarbital solution in water (60 mg/ml): first injection 1 ml/kg body weight, and then three subsequent 0.3 ml/kg body weight at 30–40 min intervals, total anaesthesia duration >3.5 h] in order to acquire the second scan.
For examination of the effect of an exogenous steroid on 11C-SA4503 binding, microPET scans were made of four pairs of rats. One rat of each pair was treated with progesterone (three intraperitoneal injections of 10 mg each at 1.5-h intervals, in arachid oil, progesterone-treated group), whereas the other animal was treated with carrier (arachid oil) only (control group). In the arachid oil- and progesterone-treated rats, the tracer 11C-SA4503 was injected shortly (< 20 min) after the induction of pentobarbital anaesthesia.
List mode data were reframed into a dynamic sequence of 4 × 60 s, 3 × 120 s, 4 × 300 s and 3 × 600 s frames. The data were reconstructed per time frame employing an interactive reconstruction algorithm (OSEM2D). The final data sets consisted of 95 slices with a slice thickness of 0.8 mm and an in-plane image matrix of 128 × 128 pixels of size 1 × 1 mm2. Data sets were fully corrected for random coincidences, scatter and attenuation. A separate transmission scan was acquired for attenuation correction. This scan was performed right after the last emission scan.
Three-dimensional regions of interest (3-D ROIs) were manually drawn around the entire tumour, brain and peripheral area of the right lung, avoiding hilar structures, as described previously [24]. Time-activity curves (TACs) and volumes (cm3) for the ROIs were calculated, using standard software (AsiPro VM 6.2.5.0, Siemens-Concorde, Knoxville, TN, USA). TACs were normalized for body weight and injected dose as indicated in the figure legends.
Biodistribution studies
After the scanning period, the anaesthetized animals were terminated. Blood was collected, and plasma and a cell fraction were obtained from the blood sample by short centrifugation (5 min at 1,000 g). Several tissues (see Table 1) were excised. The complete tumour was removed and separated from muscle and skin. All tissue samples were weighed. Radioactivity in tissue samples was measured using a gamma counter, applying a decay correction. The results were expressed as dimensionless standardized uptake values (SUVs). The parameter SUV is defined as: [tissue activity concentration (MBq/g) * body weight (g) / injected dose (MBq)].
Table 1.
Effect of prolonged anaesthesia and progesterone administration on biodistribution of 11C-SA4503
| Tissue | Controla (n = 4) | Prolonged anaesthesiab (n = 5) | Progesterone-treated* (n = 4) | Effect of long anaesthesia (p) | Effect of progesterone (p) |
|---|---|---|---|---|---|
| Cerebellum | 1.66 ± 0.18 | 2.41 ± 0.33 | 1.69 ± 0.30 | < 0.01 | NS |
| Cerebral cortex | 1.50 ± 0.40 | 2.38 ± 0.40 | 1.85 ± 0.33 | 0.02 | NS |
| Rest of brain | 1.51 ± 0.38 | 2.12 ± 0.41 | 1.69 ± 0.37 | NS | NS |
| Adipose tissue | 0.25 ± 0.12 | 0.38 ± 0.15 | 0.25 ± 0.08 | NS | NS |
| Bladder | 0.67 ± 0.16 | 0.80 ± 0.31 | 0.55 ± 0.17 | NS | NS |
| Bone | 0.27 ± 0.08 | 0.42 ± 0.09 | 0.34 ± 0.11 | < 0.05 | NS |
| Bone marrow | 1.56 ± 0.36 | 2.15 ± 0.23 | 1.17 ± 0.20 | < 0.05 | NS |
| Heart | 0.35 ± 0.09 | 0.48 ± 0.07 | 0.31 ± 0.04 | NS | NS |
| Large intestine | 1.82 ± 0.36 | 2.62 ± 0.39 | 1.87 ± 0.39 | < 0.05 | NS |
| Small intestine | 2.35 ± 0.28 | 3.48 ± 0.86 | 2.73 ± 0.53 | NS | NS |
| Kidney | 4.07 ± 0.45 | 4.60 ± 0.19 | 4.07 ± 0.63 | NS | NS |
| Liver | 7.53 ± 0.77 | 5.20 ± 0.28 | 7.16 ± 1.39 | 0.001 | NS |
| Lung | 2.21 ± 0.59 | 2.51 ± 0.62 | 1.92 ± 0.50 | NS | NS |
| Muscle | 0.18 ± 0.04 | 0.21 ± 0.01 | 0.20 ± 0.05 | NS | NS |
| Pancreas | 3.78 ± 1.55 | 5.06 ± 1.22 | 5.16 ± 1.60 | NS | NS |
| Plasma | 0.09 ± 0.03 | 0.10 ± 0.02 | 0.09 ± 0.01 | NS | NS |
| Blood cells | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.01 | < 0.05 | NS |
| Spleen | 2.26 ± 0.39 | 3.76 ± 0.95 | 2.07 ± 0.41 | < 0.05 | NS |
| Submandibular gland | 2.91 ± 0.91 | 2.08 ± 0.17 | 2.59 ± 0.85 | NS | NS |
| C6 tumour | 0.83 ± 0.11 | 1.52 ± 0.36 | 0.57 ± 0.14 | < 0.05 | < 0.05 |
| Urine | 0.48 ± 0.20 | 0.75 ± 0.76 | 0.92 ± 0.29 | NS | < 0.05 |
Results
Cell experiments
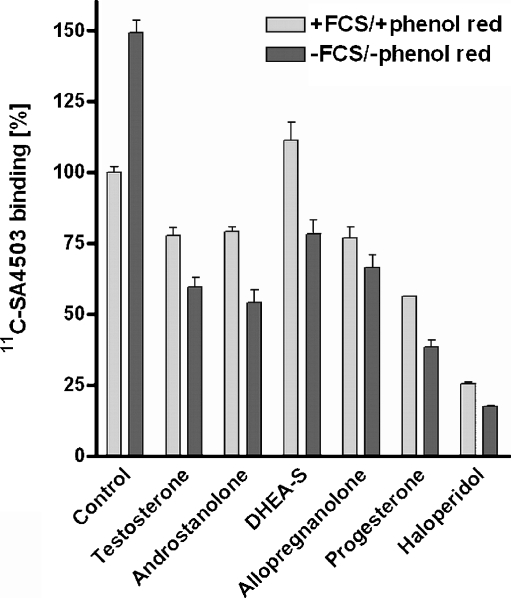
The growth medium of C6 cells (DMEM-high) contains steroid hormones since it is supplemented with FCS (7.5% v/v). This medium also contains the indicator phenol red which may interact with hormone binding sites, particularly oestrogen receptors [31]. Yet, the addition of non-radioactive steroids to normal growth medium (50 µM of progesterone, allopregnanolone, testosterone or androstanolone) resulted in measurable competition with the radioligand 11C-SA4503 for binding to cellular sigma receptors (Fig. 1). In contrast, the administration of 50 µM DHEA-S had no statistically significant effect.
Fig. 1.
Competition of exogenous steroids (concentration 50 µM) and haloperidol (50 µM) with 11C-SA4503 binding to C6 cells in normal culture medium and in steroid-free medium (expressed as a percentage of the binding to untreated cells in normal culture medium). All treatments resulted in a statistically significant effect on tracer binding, with exception of the DHEA-S effect in normal culture medium
C6 cells were depleted from endogenous steroids by removal of growth medium, repeated washing of the monolayer with PBS and addition of medium without FCS and phenol red. Such steroid depletion resulted in a significant (49 ± 4%) increase of the specific binding of 11C-SA4503 to intact cells (Fig. 1).
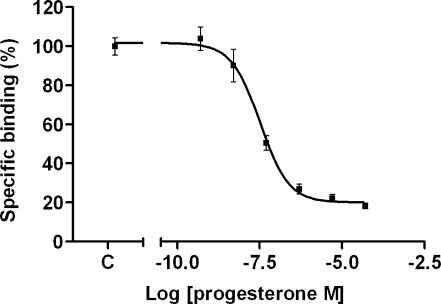
When the steroid supplementation experiment was repeated in steroid-free medium, a stronger effect of exogenous steroids was observed than in the normal growth medium. Even 50 µM DHEA-S caused now a significant decrease of cellular 11C-SA4503 binding (Fig. 1). Both in normal and in steroid-depleted medium, the observed rank order of potency was: progesterone > androstanolone = testosterone = allopregnanolone > DHEA-S. A dose-response plot was made for the most potent steroid competitor, progesterone. The IC50 value of this compound for inhibition of cellular 11C-SA4503 binding was 33 nM (Fig. 2).
Fig. 2.
Dose-response plot of progesterone competition with 11C-SA4503 binding to C6 cells in steroid-free medium. Specific binding was defined as total binding minus the residual binding observed in the presence of an excess of haloperidol (50 µM)
Addition of the anaesthetic pentobarbital to C6 cells (up to concentrations of 1 mM in normal medium) did not result in any inhibition of 11C-SA4503 binding (values not shown).
Animal data
No behavioural changes were observed in the animals up to 10 days after inoculation of C6 cells. Tumours appeared after 5 days and grew exponentially, as reported previously [24]. Body weight of the animals at the time of PET scanning was 326 ± 22 g. Tumour size was then 1.3 ± 0.7 g (mean ± SD, range 0:.48–2.39 g). No significant tumour necrosis was observed, as reported previously [2].
MicroPET scans
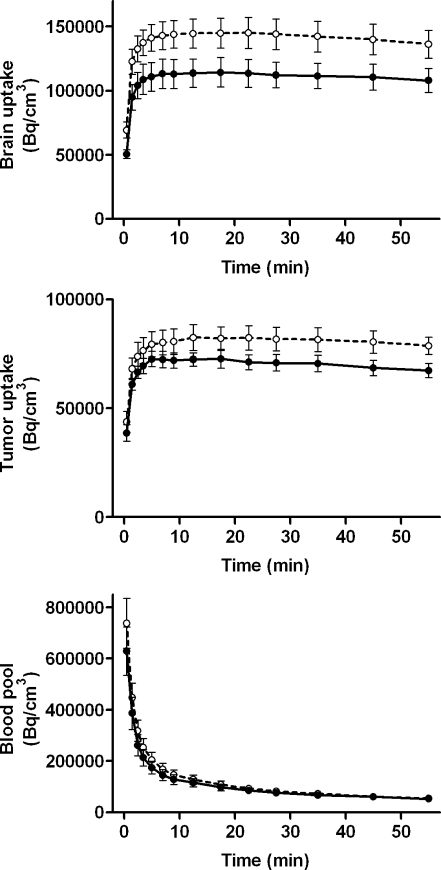
Pentobarbital anaesthesia is known to affect steroid metabolism. A strong (> 70%) decline of endogenous steroid levels in the brain (progesterone, pregnenolone) occurs upon treatment of male rats with anaesthetic amounts of sodium pentobarbital (0.2 mmol/kg i.p., [29]). Plasma progesterone in female rats is also decreased after pentobarbital administration [32]. Thus, competition of endogenous steroids with 11C-SA4503 binding in target organs was expected to be reduced after repeated treatment of Wistar rats with pentobarbital. A significant increase of tracer SUV in target organs (brain 27%, C6 tumour 16%) was indeed observed after prolonged anaesthesia (Fig. 3). However, anaesthesia duration did not significantly affect the kinetics of radioactivity in a blood pool from the right lung (Fig. 3).
Fig. 3.
Time-activity curves of 11C-SA4503-derived radioactivity in tumour, brain and blood pool. Solid line: animals scanned after short pentobarbital anaesthesia (< 20 min, control condition, n = 5); dotted line: same animals scanned after protracted pentobarbital anaesthesia (>3.5 h, n = 5). The PET data were normalized to a body weight of 330 g and an injected radioactivity dose of 20 MBq, as described previously [24]. The effect of protracted anaesthesia on brain uptake (after >10 min) and tumour uptake (after >20 min) was statistically significant, in contrast to the effect on radioactivity in the blood pool
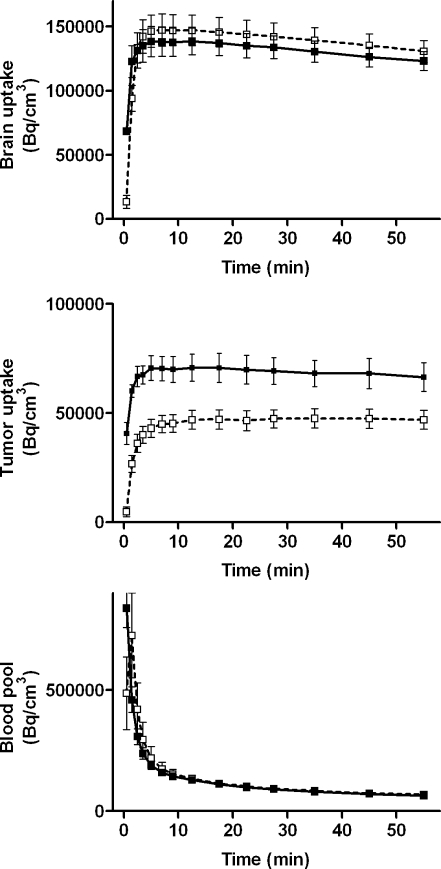
Pretreatment of animals with a high dose of progesterone resulted in a significant decrease of ligand SUV in the C6 tumour (36 %), but did not significantly affect tracer kinetics in the brain or blood pool (Fig. 4).
Fig. 4.
Time-activity curves of 11C-SA4503-derived radioactivity in tumour, brain and blood pool. Solid line: animals scanned at baseline (treated with arachid oil only, control group, n = 4); dotted line: other animals scanned after repeated injections of progesterone in arachid oil (progesterone-treated group, n = 4). The PET data were normalized to a body weight of 330 g and an injected radioactivity dose of 20 MBq, as described previously [24]. The effect of progesterone addition on tumour uptake (but not brain or blood pool uptake) was statistically significant
MicroPET images of a single rat acquired after short and prolonged anaesthesia are shown in Fig. 5. Images of solvent (arachid oil)- and progesterone-pretreated animals are presented in Fig. 6.
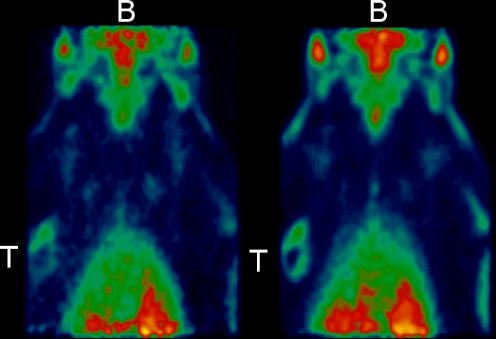
Fig. 5.
MicroPET images of a single animal scanned with 11C-SA4503 after short (<20 min, upper row) and protracted (>3.5 h, lower row) pentobarbital anaesthesia. Images are summed data from 1 to 60 min after injection. The positions of tumour and brain are indicated by capitals T and B
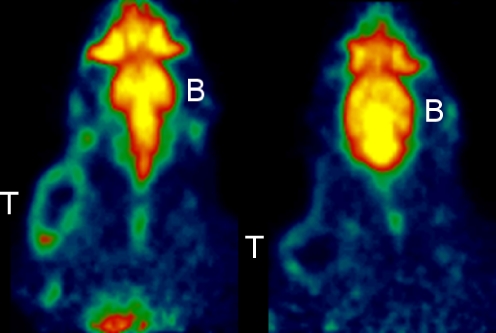
Fig. 6.
MicroPET images of two different rats scanned with 11C-SA4503 after pretreatment with solvent (arachid oil, upper row) or progesterone (lower row). Images are summed data from 1 to 60 min after injection. The positions of tumour and brain are indicated by capitals T and B
Biodistribution studies
Biodistribution experiments confirmed the outcome of the microPET studies (Table 1). Prolonged anaesthesia with pentobarbital resulted in a significant increase of sigma ligand binding in C6 tumour, brain (particularly cerebellum and cerebral cortex), bone and bone marrow, large intestine, blood cells and spleen. Plasma levels of radioactivity were not changed under these conditions and the amount of radioactivity in the liver was significantly decreased.
Pretreatment of animals with progesterone caused a 31% decrease of sigma ligand binding in the C6 tumour, but did not significantly affect tracer uptake in any other organ or in plasma (Table 1).
Discussion
In vitro steroid competition
Although an interaction of steroid hormones with sigma receptors has been described in many publications, there are few reported observations of competition between endogenous steroids and sigma receptor ligands in intact cancer cells. Here, we demonstrate that altered steroid levels result in changes of 11C-SA4503 binding to sigma receptors in the C6 glioma cell line. Steroid depletion (removal of FCS from the growth medium and washing of cells with steroid-free medium) resulted in a 49% increase of 11C-SA4503 binding. Apparently, high steroid levels in the normal growth medium result in reduced tracer binding and can obscure the effects of steroids which bind with rather low affinity to sigma receptor proteins. Enhanced competition of DHEA-S and other steroids with 11C-SA4503 binding was observed when cells were washed and incubated in steroid-free medium, 1 h before tracer addition.
Testosterone (50 µM), an androgen secreted by the testis, caused a 40% reduction of radioligand binding to C6 cells in steroid-free medium. Androstanolone (dihydrotestosterone), a metabolite of testosterone which mediates many of the biological actions of testosterone, had a similar effect as the parent compound. Dehydroepiandrosterone sulphate, a steroid sigma agonist with antidepressant actions and an allosteric modulator of γ-aminobutyric acid (GABA)A receptors, was the weakest competitor for 11C-SA4503 binding. The most potent steroid inhibitor proved to be progesterone (secreted by the corpus luteum in females) which reduced cellular 11C-SA4503 binding by 60%. Allopregnanolone, an important metabolite of progesterone, was less potent than its parent compound, but still caused a 33% reduction of radioligand binding. Non-specific binding (defined as binding of the radioligand to C6 cells in the presence of an excess of haloperidol) was about 25% in normal growth medium and 18% in steroid-free medium (Fig. 1).
The results of our experiments in cancer are in accordance with previous studies which have employed different radioligands and have focused on healthy tissue. Progesterone and testosterone were shown to compete with the binding of 3H-SKF-10,047 and 3H-haloperidol to sigma receptors in homogenates from guinea pig brain and spleen [33]. These steroids were also potent inhibitors of the binding of 3H-haloperidol to sigma-1 receptors in human placenta [11], and they reduced binding of the sigma-1 ligand 18F-FPS to isolated membrane fragments and to the brain of intact rats [19]. Progesterone acted like a sigma-1 antagonist in the rat hippocampus, mimicking the effects of haloperidol on 3H-norepinephrine release [15]. Progesterone but not dehydroepiandrosterone sulphate reduced the in vivo binding of 3H-SKF-10,047 to sigma-1 receptors in the mouse hippocampus and cortex [34].
In vivo steroid competition
Progesterone supplementation reduced tumour SUV (from 0.83 ± 0.11 to 0.57 ± 0.14) and the tumour-to-muscle concentration ratio (from 4.6 to 2.8), as indicated by our biodistribution data (Table 1). TACs in microPET indicated a progesterone-mediated decrease of 11C-SA4503 binding in the C6 tumour (by 36%), but not in the brain (Fig. 4). The reason for the absence of an effect of progesterone supplementation on radioligand binding in the brain of intact rats is unknown. P-glycoprotein (Pgp) function in the blood-brain barrier hampers the entry of exogenous progesterone into the brain [35], whereas C6 tumours do not express Pgp (personal observations). Therefore, exogenous progesterone can freely enter the implanted C6 tumour, but can enter the brain only to a limited extent.
Our data suggest that not only steroid supplementation—as reported previously for other sigma ligands—but also removal of steroid is accompanied by changes of the in vivo binding of 11C-SA4503. Repeated treatment of animals with pentobarbital increased tumour SUV (from 0.83 to 1.52) and the tumour to muscle ratio (from 4.6 to 7.2, Table 1). Tracer SUV in cerebellum and cerebral cortex was also increased (from 1.66 to 2.41 and from 1.50 to 2.38, respectively, Table 1). TACs in the microPET experiments indicated increased 11C-SA4503 binding both in the C6 tumour (by 16%) and in the brain (by 27%, Fig. 3). Prolonged pentobarbital anaesthesia and progesterone supplementation were not accompanied by any significant change of the blood activity curves of 11C-SA4503 (Figs. 2 and 3). Altered tracer binding to sigma-1 receptors in tumour and brain appears therefore to be related to changes within target tissue rather than to altered tracer delivery to the target organs. Since pentobarbital has multiple effects on cerebral biochemistry, our data do not prove that the effect of prolonged anaesthesia is due to an interference of pentobarbital with steroid metabolism. However, the information from our microPET and biodistribution studies is consistent with the hypothesis that prolonged pentobarbital anaesthesia results in reduced levels of endogenous steroid hormones.
Conclusion
Steroid competition affects binding of the sigma ligand 11C-SA4503 in tumour cells and tumour-bearing rats. Progesterone, the most potent steroid competitor of 11C-SA4503 binding, showed an IC50 value in tumour cells of 33 nM, or 10 ng/ml (Fig. 2). Similar levels of progesterone (3–28 ng/ml) are reached in human plasma during the luteal phase of the menstrual cycle [36] and even higher levels occur during hormone treatment. Moreover, steroid levels may change during chemotherapy, both in male and female patients [37–40]. Therefore, (patho)physiological variations of these levels can result in variability of the binding potential of 11C-SA4503 and should be taken into account in PET studies of patients and healthy volunteers.
Acknowledgments
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Anna A. Rybczynska, Email: a.rybczynska@ngmb.umcg.nl
Aren van Waarde, Email: a.van.waarde@ngmb.umcg.nl.
References
- 1.Strauss LG. Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med 1996;23:1409–15. doi:10.1007/BF01367602. [DOI] [PubMed]
- 2.van Waarde A, Jager PL, Ishiwata K, Dierckx RA, Elsinga PH. Comparison of sigma-ligands and metabolic PET tracers for differentiating tumor from inflammation. J Nucl Med 2006;47:150–4. [PubMed]
- 3.Vilner BJ, John CS, Bowen WD. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res 1995;55:408–13. [PubMed]
- 4.Mach RH, Smith CR, al-Nabulsi I, Whirrett BR, Childers SR, Wheeler KT. Sigma 2 receptors as potential biomarkers of proliferation in breast cancer. Cancer Res 1997;57:156–61. [PubMed]
- 5.al-Nabulsi I, Mach RH, Wang LM, Wallen CA, Keng PC, Sten K, et al. Effect of ploidy, recruitment, environmental factors, and tamoxifen treatment on the expression of sigma-2 receptors in proliferating and quiescent tumour cells. Br J Cancer 1999;81:925–33. doi:10.1038/sj.bjc.6690789. [DOI] [PMC free article] [PubMed]
- 6.Wheeler KT, Wang LM, Wallen CA, Childers SR, Cline JM, Keng PC, et al. Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br J Cancer 2000;82:1223–32. doi:10.1054/bjoc.1999.1067. [DOI] [PMC free article] [PubMed]
- 7.Maurice T, Gregoire C, Espallergues J. Neuro(active)steroids actions at the neuromodulatory sigma1 (sigma1) receptor: biochemical and physiological evidences, consequences in neuroprotection. Pharmacol Biochem Behav 2006;84:581–97. doi:10.1016/j.pbb.2006.07.009. [DOI] [PubMed]
- 8.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol 1994;268:9–18. doi:10.1016/0922-4106(94)90115-5. [DOI] [PubMed]
- 9.Hashimoto K, Ishiwata K. Sigma receptor ligands: possible application as therapeutic drugs and as radiopharmaceuticals. Curr Pharm Des 2006;12:3857–76. doi:10.2174/138161206778559614. [DOI] [PubMed]
- 10.Vilner BJ, de Costa BR, Bowen WD. Cytotoxic effects of sigma ligands: sigma receptor-mediated alterations in cellular morphology and viability. J Neurosci 1995;15:117–34. [DOI] [PMC free article] [PubMed]
- 11.Ramamoorthy JD, Ramamoorthy S, Mahesh VB, Leibach FH, Ganapathy V. Cocaine-sensitive sigma-receptor and its interaction with steroid hormones in the human placental syncytiotrophoblast and in choriocarcinoma cells. Endocrinology 1995;136:924–32. doi:10.1210/en.136.3.924. [DOI] [PubMed]
- 12.Maurice T, Phan VL, Urani A, Kamei H, Noda Y, Nabeshima T. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol 1999;81:125–55. doi:10.1254/jjp.81.125. [DOI] [PubMed]
- 13.Ueda H, Inoue M, Yoshida A, Mizuno K, Yamamoto H, Maruo J, et al. Metabotropic neurosteroid/sigma-receptor involved in stimulation of nociceptor endings of mice. J Pharmacol Exp Ther 2001;298:703–10. [PubMed]
- 14.Dong Y, Fu YM, Sun JL, Zhu YH, Sun FY, Zheng P. Neurosteroid enhances glutamate release in rat prelimbic cortex via activation of alpha1-adrenergic and sigma1 receptors. Cell Mol Life Sci 2005;62:1003–14. doi:10.1007/s00018-005-5004-8. [DOI] [PubMed]
- 15.Monnet FP, Mahe V, Robel P, Baulieu EE. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci USA 1995;92:3774–8. doi:10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed]
- 16.Romieu P, Lucas M, Maurice T. Sigma1 receptor ligands and related neuroactive steroids interfere with the cocaine-induced state of memory. Neuropsychopharmacology 2006;31:1431–43. doi:10.1038/sj.npp.1300885. [DOI] [PubMed]
- 17.Chen Y, Hajipour AR, Sievert MK, Arbabian M, Ruoho AE. Characterization of the cocaine binding site on the sigma-1 receptor. Biochemistry 2007;46:3532–42. doi:10.1021/bi061727o. [DOI] [PubMed]
- 18.Pal A, Hajipour AR, Fontanilla D, Ramachandran S, Chu UB, Mavlyutov T, et al. Identification of regions of the sigma-1 receptor ligand binding site using a novel photoprobe. Mol Pharmacol 2007;72:921–33. doi:10.1124/mol.107.038307. [DOI] [PubMed]
- 19.Waterhouse RN, Chang RC, Atuehene N, Collier TL. In vitro and in vivo binding of neuroactive steroids to the sigma-1 receptor as measured with the positron emission tomography radioligand [18F]FPS. Synapse 2007;61:540–6. doi:10.1002/syn.20369. [DOI] [PubMed]
- 20.Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, et al. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci 1992;13:85–6. doi:10.1016/0165-6147(92)90030-A. [DOI] [PubMed]
- 21.Cobos EJ, Lucena G, Baeyens JM, Del Pozo E. Differences in the allosteric modulation by phenytoin of the binding properties of the sigma1 ligands [3H](+)-pentazocine and [3H]NE-100. Synapse 2006;59:152–61. doi:10.1002/syn.20230. [DOI] [PubMed]
- 22.van Waarde A, Buursma AR, Hospers GA, Kawamura K, Kobayashi T, Ishii K, et al. Tumor imaging with 2 sigma-receptor ligands, 18F-FE-SA5845 and 11C-SA4503: a feasibility study. J Nucl Med 2004;45:1939–45. [PubMed]
- 23.Kawamura K, Kubota K, Kobayashi T, Elsinga PH, Ono M, Maeda M, et al. Evaluation of [11C]SA5845 and [11C]SA4503 for imaging of sigma receptors in tumors by animal PET. Ann Nucl Med 2005;19:701–9. doi:10.1007/BF02985120. [DOI] [PubMed]
- 24.van Waarde A, Shiba K, de Jong JR, Ishiwata K, Dierckx RA, Elsinga PH. Rapid reduction of sigma1-receptor binding and 18F-FDG uptake in rat gliomas after in vivo treatment with doxorubicin. J Nucl Med 2007;48:1320–6. doi:10.2967/jnumed.107.042085. [DOI] [PubMed]
- 25.Ishikawa M, Ishiwata K, Ishii K, Kimura Y, Sakata M, Naganawa M, et al. High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: A positron emission tomography study using [11C]SA4503. Biol Psychiatry 2007;62:878–83. doi:10.1016/j.biopsych.2007.04.001. [DOI] [PubMed]
- 26.Sakata M, Kimura Y, Naganawa M, Oda K, Ishii K, Chihara K, et al. Mapping of human cerebral sigma1 receptors using positron emission tomography and [11C]SA4503. Neuroimage 2007;35:1–8. doi:10.1016/j.neuroimage.2006.11.055. [DOI] [PubMed]
- 27.Mishina M, Ishiwata K, Ishii K, Kitamura S, Kimura Y, Kawamura K, et al. Function of sigma1 receptors in Parkinson’s disease. Acta Neurol Scand 2005;112:103–7. doi:10.1111/j.1600-0404.2005.00432.x. [DOI] [PubMed]
- 28.Mishina M, Ohyama M, Ishii K, Kitamura S, Kimura Y, Oda K, et al. Low density of sigma1 receptors in early Alzheimer’s disease. Ann Nucl Med 2008;22:151–6. doi:10.1007/s12149-007-0094-z. [DOI] [PubMed]
- 29.Korneyev AY, Costa E, Guidotti A. During anesthetic-induced activation of hypothalamic pituitary adrenal axis, blood-borne steroids fail to contribute to the anesthetic effect. Neuroendocrinology 1993;57:559–65. doi:10.1159/000126405. [DOI] [PubMed]
- 30.Kawamura K, Elsinga PH, Kobayashi T, Ishii S, Wang WF, Matsuno K, et al. Synthesis and evaluation of 11C- and 18F-labeled 1-[2-(4-alkoxy-3-methoxyphenyl)ethyl]-4-(3-phenylpropyl)piperazines as sigma receptor ligands for positron emission tomography studies. Nucl Med Biol 2003;30:273–84. doi:10.1016/S0969-8051(02)00439-0. [DOI] [PubMed]
- 31.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA 1986;83:2496–500. doi:10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed]
- 32.Lee CJ, Do BR, Kim JK, Yoon YD. Pentobarbital and ketamine suppress serum concentrations of sex hormones in the female rat. J Anesth 2000;14:187–90. doi:10.1007/s005400070003. [DOI] [PubMed]
- 33.Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science 1988;240:219–21. doi:10.1126/science.2832949. [DOI] [PubMed]
- 34.Maurice T, Roman FJ, Privat A. Modulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to sigma 1 receptors in the mouse forebrain. J Neurosci Res 1996;46:734–43. doi:10.1002/(SICI)1097-4547(19961215)46:6<734::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed]
- 35.Cisternino S, Rousselle C, Debray M, Scherrmann JM. In situ transport of vinblastine and selected P-glycoprotein substrates: implications for drug-drug interactions at the mouse blood-brain barrier. Pharm Res 2004;21:1382–9. doi:10.1023/B:PHAM.0000036911.49191.da. [DOI] [PubMed]
- 36.Chiron Diagnostics ACS. Centaur Progesterone Assay Manual. 1998.
- 37.LeBlanc GA, Kantoff PW, Ng SF, Frei E, Waxman DJ. Hormonal perturbations in patients with testicular cancer treated with cisplatin. Cancer 1992;69:2306–10. doi:10.1002/1097-0142(19920501)69:9<2306::AID-CNCR2820690917>3.0.CO;2-F. [DOI] [PubMed]
- 38.Nader S, Schultz P, Deinzer D, Samaan NA. Acute hormonal changes following chemotherapy for Hodgkin’s disease in man. J Androl 1983;4:293–7. [DOI] [PubMed]
- 39.Pribylova O, Springer D, Svobodnik A, Kyr M, Zima T, Petruzelka L. Influence of chemotherapy on hormonal levels in postmenopausal breast cancer patients. Neoplasma 2008;55:294–8. [PubMed]
- 40.Padmanabhan N, Wang DY, Moore JW, Rubens RD. Ovarian function and adjuvant chemotherapy for early breast cancer. Eur J Cancer Clin Oncol 1987;23:745–8. doi:10.1016/0277-5379(87)90272-0. [DOI] [PubMed]