Abstract
The present experiment was designed to investigate the effect of housing conditions on task performance and corticosterone response. Two groups of male F344BNF1 rats were housed on a ventilated rack with ad libitum access to water and a restricted feeding regime. Group 1 was housed in solid-bottom caging with corn cob bedding, whereas group 2 was housed in wire-bottom caging. After learning an operant task, each rat was exposed to acute restraint followed 48 h later by exposure to continuous light. Corticosterone concentrations were determined before and after exposure to each intervention. Contrary to assumptions, housing did not affect task performance. Baseline corticosterone concentrations were similar for the 2 experimental groups, but corticosterone concentrations were significantly higher for the wire-bottom group than the solid-bottom group immediately after the restraint and remained elevated 2 d later. Corticosterone levels decreased in both groups after exposure to continuous light. Overall, the data indicate that subtle but significant differences occur in responses of rats housed on wire-bottom versus solid-bottom caging when the animals are exposed to acute restraint.
Abbreviation: CL, continuous light; R, restraint; SC, solid-bottom caging; WC, wire-bottom caging
A 1999 survey estimated that 80% of the rodents in the group of pharmaceutical and toxicology laboratories that responded were housing rodents in wire bottom caging.25 Ease of cleaning and research study requirements were among the factors that contributed to the widespread use of this caging type. Specifically, wire-bottom caging was believed to limit coprophagia in food-deprived animals, maintaining their motivation to work for food pellets. In addition, ingestion of feces can interfere with the measurement of drug levels and thus complicate toxicology or pharmacology studies; depending on the mechanism of drug elimination, feces may contain active drug or metabolites.25 Wire-bottom caging presumably limits this variable. Concomitant with research needs, the animal well-being should also be considered when choosing housing. Preference testing showed that rats preferred a solid floor to a wire grid for rest, which comprises approximately 70% to 75% of a rat's day in a colony setting.14 Another study reported that both rats and mice preferred various types of bedding material, such as wood chips, shredded paper, and sawdust, on a solid-bottom cage to a wire-mesh floor.14Foot lesions and sores have been reported in rodents housed long-term on wire-bottom caging.21 A similar study evaluating housing on wire grates reports that tactile hyperesthesia, intraepidermal changes, and plantar nerve injury completely resolved when wire grates were removed.17
The combination of the preference studies and the assumption that preference indicates the best type of cage for an animal have been used to support limiting the use of wire caging for rodents. However, the hypothesis that solid-bottom cages improve the general psychologic health of the rodents as compared with wire bottom cages has not been tested.25 Enrichment attempts often are based more on anthropomorphic feelings than on biological relevance and functional utility to the animals. Enrichment research would greatly be improved by developing well-focused and well-founded hypotheses and predictions and by testing these hypotheses in a systematic manner with appropriate controls.19
The introduction of environmental enrichment (modifications to the environments of animals to improve their biologic functioning) into laboratory animal research facilities is increasing.27 As reflected in the federal Animal Welfare Act amendments of 1985, the role of environmental enrichment in enhancing animal welfare has been well established in larger species such as primates, dogs, and rabbits.29 Environmental enrichment was defined specifically as exercise for dogs and, for non-human primates, environmental enhancement with the goal of promoting psychologic well-being.12 The Guide encourages housing animals with a goal of maximizing species-specific behaviors and minimizing stress-induced behaviors. Although not specifically termed an enriched environment, solid-bottom caging with bedding for rodents is recommended in the 1996 revision of The Guide, because evidence suggests that this type of caging is preferred by mice and rats18 Furthermore, The Report of the Rodent Refinement Working Party recommends solid floors over wire-grid floors, because solid floors allow the provision of a substrate that allows animals to carry out many of their normal activities.9 Bedding and nesting material provide an easy, economical, and highly beneficial form of environmental enrichment that considerably increases cage utilization.9
Environmental enrichment may ameliorate some of the problems associated with containment by altering the environment to allow animals to engage in a wider range of behaviors within the species-specific repertoire. In turn, provision of environmental enrichment may improve well-being and decrease stereotypic behaviors.27 For example, rats provided with a behavioral response to avoid electric shock had significantly less weight loss and developed fewer gastric ulcerations than did those unable to avoid the electric shock.28 Well-designed housing systems that allow for effective coping behavior may enhance well being, whereas caging that limits opportunities for active behavioral responses may cause stress.11 When unable to perform species-specific behaviors, animals may show abnormal behavior or other pathology.10
To evaluate the effects of wire-bottom caging, we analyzed both glucocorticoid concentration and behavior, as assessed by performance on an operant sustained attention task, requiring the detection and discrimination of signal and nonsignal events. Sustained attention is a psychologic construct that has been defined as the “state of readiness to detect and respond to certain specified changes occurring rarely and unpredictably” or the ability to maintain mental responsiveness or performance for prolonged periods of time.16 Use of an operant conditioning task as a behavioral assessment enabled assessment of the need for wire-bottom caging as part of the food restriction required for this task. Task performance was defined by the speed of task acquisition, by using clearly defined criteria specific to the sustained attention task. This study was designed to determine the effect of housing conditions on the speed of operant task acquisition and the hypothalamic–pituitary–adrenal axis response to stressors as measured by corticosterone levels. Specifically, this experiment tested 2 main hypotheses: that housing conditions (that is, wire versus bedding) would not affect the latency to achieve task acquisition but would differentially affect corticosterone profiles in response to acute restraint (R) and chronic alteration in circadian rhythm.
Materials and Methods
Subjects.
Thirty-two male Fischer 344 × Brown Norway (F344BNF1) rats, 4 mo of age and weighing 325 to 350 g, were obtained from Harlan Sprague Dawley (Indianapolis, IN) Rats were maintained in a specific pathogen-free facility that was monitored quarterly and tested free of the following agents: rat parvovirus, Toolan H1 virus, Kilham rat virus, rat minute virus, parvovirus NS1, sialodacryoadenitis virus, Sendai virus, pneumonia virus of mice, reovirus types 1 and 2, Mycoplasma pulmonis, Theiler murine encephalomyelitis virus, lymphocytic choriomeningitis virus, endoparasites, and ectoparasites. All experiments were approved by the institutional animal care and use committee and were performed at The Ohio State University (Columbus, OH) in an AAALAC-accredited facility.
Housing.
All rats were housed individually in ventilated caging (Tecniplast, Italy). Rats in 1 group were housed in solid-bottom cages with corncob bedding (the SC group; n = 17), whereas those in the other group were housed on wire inserts over bedding (the WC group; n = 15). The insert consisted of 1-mm-diameter round wires spaced 1 cm apart and was elevated 2 cm from the bedding. The caging rack provided automatic water available ad libitum, and rats were fed a standard rodent diet (diet 8640, Harlan) for the duration of the study. Rats were weighed 3 times weekly, and the number of daily pellets provided was adjusted to maintain them at 90% free-feeding weight based on a mean body weight curve for male F344BNF1 rats.24. An altered light cycle was used (lights on, 0200; lights off, 1400) so that the task training could occur during normal working hours and still maintain a normal circadian rhythm for the rats. During the last 7 d of the study, each animal was moved to a continuous-light (CL) room to chronically disrupt the circadian rhythm.
Operant chamber training boxes.
Sound-attenuated chambers (Med Associates, East Fairfield, VT) equipped with a central panel light (2.8 W), 2 retractable levers, a pellet dispenser, and a house light (2.8 W) were used for behavioral training. The pellet dispenser cup was located opposite the levers, so that rats were required to turn around to retrieve pellets as reward. Operation of levers, pellet dispensers, houselights, and panel lights and recording of behavioral responses was controlled by Med-PC software (Med Associates, East Fairfield, VT).
The operant chambers were equipped with wire-bottom flooring for ease of sanitation. The round wire rods were 4.8 mm in diameter and spaced 1.25 cm apart. A metal tray located at the bottom of the chamber contained corncob bedding and absorbed feces and urine produced during the training session. The floor of the chamber was markedly different from the wire insert used in cages and was considered novel to both groups of rats. In addition, only 3% of the animal's weekly time was spent inside the chamber. Any effect of this novelty on task acquisition would be similar for all rats and therefore was considered negligible.
Behavioral training.
Rats were acclimated for 2 wk to the animal room, after which time each rat began training to acquire a sustained-attention task. The task consisted of 4 sequential shaping steps. Animals trained 6 d each week during the second half of the light phase (between 800 and 1200). Data were analyzed biweekly, and each rat was advanced to the next step of shaping once performance criterion was met. Rats remained in the training box for a maximum of 50 min each session. Typically, rats were given 160 to 180 trials each session, which lasted between 35 to 45 min, depending on individual response latencies. During the first shaping step, animals were trained to bar press for a food reward (1 Noyes pellet, 45 mg, PJ Noyes, Lancaster, NH) by using a modified FR1 schedule, which provided a single food pellet (food reward) to the animal immediately after a lever press. Corrections were built into the program to prevent side bias by the animal.16 Animals were advanced to the second step of shaping after achieving 120 rewarded bar presses per session over 3 consecutive sessions.
The second shaping step required detection and discrimination of signal events (1-s flash of light) and nonsignal events (no flash). Two seconds after presentation of a signal or nonsignal event, levers were extended into the operant chamber and remained active for 4 s. Animals were trained to press the left lever in the event of a signal trial and the right lever in response to nonsignal trials. Correct responses to signal trials were scored as hits, whereas correct responses to nonsignal trials were scored as correct rejections. Incorrect responses to signal and nonsignal trials were not rewarded and scored as false alarms and misses, respectively. In the event that an animal responded incorrectly to a trial, the trial was repeated as many as 3 times (correction trials). If an animal responded incorrectly to 3 consecutive correction trials, a forced-choice trial was initiated. During a forced-choice trial, only the correct lever was extended and remained active for 90 s or until a response was made. In the event that the forced-choice trial was a signal trial, the signal light remained illuminated as long as the lever remained active. Failure to respond to a trial was scored as an omission. Presentation of signal and nonsignal events were randomized over the session. The house light remained off for the duration of the session to facilitate the initial training of signal discrimination. Animals advanced to the subsequent step of shaping once they achieved 59% hits and correct rejections for 3 consecutive days.
During the third phase of shaping, the house light remained unlit, and correction and forced-choice trials were no longer presented. The duration of signal trials was shortened to 500, 50, or 25 ms. Sessions consisted of 162 trials (half nonsignal, half signal; 27 trials per signal duration for each signal type) and the sequence of signal and nonsignal events was presented in random order. Rats advanced to the final stage of shaping on achieving performance criteria of at least 70% hits for the 500-ms signals, 70% correct rejections for the nonsignal events, and fewer than 25 omissions for 3 consecutive days.
The final stage of shaping was identical to stage 3, except that the house light was illuminated throughout the entire session. This critical step required animals to constrain their behavior and attend to the specific location of the signal light. Sessions consisted of 162 trials, 81 of which were signal trials (500, 50, or 25 ms in duration) presented randomly with 81 nonsignal events. Complete acquisition of the task was defined by training on the fourth step for 10 wk.
Restraint\.
After completion of that day's task session, each rat was placed in an acrylic tube (Braintree Scientific, Braintree, MA) for a 60-min period of restraint. Blood was collected within 1 min of placement in the tube (pre-restraint [preR] samples) by using a 25-gauge needle and a 1-ml syringe. Another sample (restraint [R] sample) was collected after 60 min of restraint, just prior to returning the animal to its cage. 48 h after collection of the R sample, a post-restraint (postR) blood sample was collected after the daily task session. The rat was then moved to a room for continuous light exposure. After 7 d of continuous light exposure, a final blood sample (continuous light [CL] sample) was collected prior to euthanasia by CO2 overdose. Euthanasia was confirmed by opening the chest cavity. All blood samples were collected by means of tail venipuncture, with the rat maintained in the tube restraint. Samples were obtained after the daily work session at least 2 h before the end of the light phase of the circadian cycle (between 0900 and 1200) order to minimize the effect of natural changes in corticosterone concentrations throughout the day. Blood samples were placed on ice immediately after collection in 500-µl microfuge tubes. Samples were centrifuged for 30 min at 140 x g. Serum was collected and stored at –80 °C.
Corticosterone radioimmunoassay.
Serum samples were analyzed for corticosterone concentration at the conclusion of the study by using a commercially available radioimmunoassay (ICN, Costa Mesa, CA). The standard curve was run in triplicate, and samples were run in duplicate. This assay is highly specific, crossreacting at less than 1% with other hormones.
Statistical analysis.
The threshold for statistical significance was chosen as a P value of less than or equal to 0.05. Mean rank of days to task acquisition was compared for statistical significance by using Kruskal–Wallis nonparametric ANOVA. Corticosterone concentrations were analyzed by repeated-measures ANOVA and Huynh–Feldt correction with time points as a within-subject factor and housing as a between-subject factor. Kruskal–Wallis nonparametric ANOVA of the mean ranks was used to further analyze differences between groups. Dunn multiple comparisons tests were used to evaluate within-subject time points.
Results
Days to task acquisition.
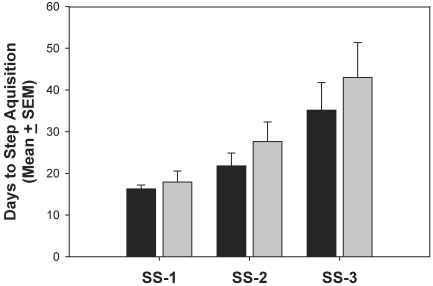
Data were compiled by using the group average of days to completion at each of the 3 shaping steps. No significant differences were detected between the SC and WC groups of rats (Figure 1).
Figure 1.
Group mean of days to task criterion at 3 shaping steps (SS). Intergroup differences were not statistically significant. Black bars, SC; gray bars, WC.
Corticosterone levels.
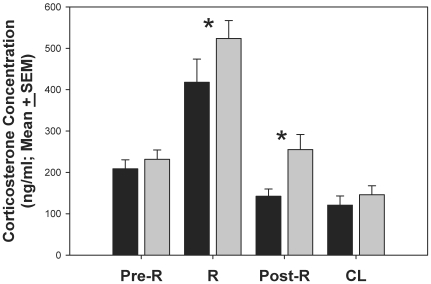
Corticosterone concentrations were included in statistical analysis only when the results of duplicate assays were congruent. Samples per time point (N) were 13, 15, 13, and 14 for the SC group and 11, 12, 11, and 13 for the WC group at the preR, R, postR, and CL time point respectively equating to 77% to 89% of the data. Compared with the SC group, the WC group had higher corticosterone concentrations at the R (P = 0.045) and postR (P = 0.001) time points. Housing condition did not alter baseline (preR) and CL corticosterone concentrations (Figure 2).
Figure 2.
Group means of corticosterone concentration at 4 time points: preR, R, postR, and CL. Corticosterone concentrations in the WC group (gray bars) were significantly increased at R (Kruskal–Wallis test, P = 0.045) and postR (Kruskal–Wallis test, P = 0.001) compared with those in SC animals (black bars).
Within-group analysis of the SC group showed that corticosterone values were significantly lower (P < 0.05) at both the postR and CL time points compared with the R time point. Within the WC group, values were significantly lower (P < 0.05) at the CL time point only compared with the R time point. (Table 1).
Table 1.
Results of Dunn multiple comparisons test evaluating differences in corticosterone concentration within each housing group at various time points
| Group | Time point comparison | Mean rank difference | P |
| SC | preR versus R | −29.006 | >0.05 |
| SC | preR versus CL | 22.701 | >0.05 |
| SC | R versus postR | 47.331 | <0.01 |
| SC | R versus CL | 51.707 | <0.001 |
| SC | postR versus CL | 4.376 | >0.05 |
| WC | preR versus R | −37.894 | >0.05 |
| WC | preR versus CL | 22.773 | >0.05 |
| WC | R versus postR | 35.212 | >0.05 |
| WC | R versus CL | 60.667 | <0.001 |
| WC | postR versus CL | 25.455 | >0.05 |
Corticosterone concentrations dropped significantly in the SC group from R to both postR and CL. Corticosterone concentrations dropped significantly in the WC group from R to CL.
Discussion
The conclusions obtained as the result of environmental enrichment research should always be evaluated in the context of study design. Many studies have used complex environments, special caging, or social interactions as the enriched environment.2, 23 Our study compared wire-bottom caging to our standard solid-bottom cage with corncob bedding because the results could be more easily applied to our daily animal care routine. Bedding provides a substrate for rats to manipulate within solid-bottom cages, which are considered ‘enriched’ compared with barren wire-bottom cages.
The findings of this study indicate that performance of a sustained-attention task was not influenced by the housing conditions of SC or WC. Collectively these data indicate that housing conditions (that is, wire- and solid-bottom caging) did not differentially affect the rate of acquisition of an operant sustained-attention task. In particular, we found no evidence to support the hypothesis that housing on bedding affects task acquisition.
The response of an organism to stressful situations includes both physical and behavioral adaptations. Almost any type of stress, whether physical or psychologic, causes an immediate and marked increase in hormones of the hypothalamic–pituitary–adrenal axis.7 This endocrine cascade is triggered initially by the production of corticotrophin-releasing hormone from the hypothalamus, which in turn elicits an increase in adrenocorticotropin hormone secretion from the anterior pituitary gland, followed within minutes by increased adrenocortical secretion of glucocorticoids. 7. Glucocorticoids rapidly mobilize amino acids and fats from cellular stores to be available immediately for energy and for synthesis of other compounds, including glucose, needed by the different tissues of the body.7 This compensatory response can have protective and adaptive as well as damaging effects.15 Eliminating the stressor typically decreases the need for glucocorticoids and leads to a return to the original homeostasis. However, intense long-lasting stress may result in a new biological equilibrium due to downregulation of glucocorticoid receptors.5 If the exposure to glucocorticoids is prolonged, a variety of pathologic outcomes become more likely, including insulin-resistant diabetes, hypertension, immunosuppression, and reproductive impairments.22 Adrenal hormones, catecholamines, and glucocorticoids also may play roles in the noxious effects of stress in the central nervous and cardiovascular systems. Specifically, rats injected repeatedly with glucocorticoids develop hippocampal neuronal loss, perhaps due to enhanced neuronal vulnerability to glutamate toxicity.1 Physical measurements, such as food consumption or altered behavior, and physiologic measurements, such as hormone and glucocorticoid concentrations, can be used as an indication of stress and ultimately of animal welfare.20
The second part of this study evaluated the corticosterone concentrations at 4 time points to determine whether housing conditions affected responses to 2 different stressful conditions. WC and SC baseline (preR) corticosterone concentrations did not differ, and were within the typical range for a 12-mo-old male rat (150 to 230 ng/ml).6 Restraint for 1-h increased corticosterone levels in all animals in the study. Compared with the SC group, the WC group showed higher concentrations of corticosterone immediately after restraint. By the postR timepoint (48 h), corticosterone concentrations dropped in all animals but levels in the WC group remained significantly higher than those in the SC group suggesting that the rats housed on WC displayed an enhanced and prolonged physiologic response to acute restraint. Typically, corticosterone stimulates a negative feedback loop involving 2 pathways. It acts directly on the hypothalamus to decrease the formation of corticotrophin-releasing factor and on the anterior pituitary gland to decrease the formation of adrenocorticotropin hormone. During chronic stress, stress stimuli can overcome the direct inhibitory feedback of corticosterone leading to prolonged secretion.7
Animals exposed to chronic stress, whether continuous or intermittent, exhibit normal or enhanced hypothalamic–pituitary–adrenal axis responses to novel, acute stressors.3 Prior stress may leave a central facilatory trace that, on exposure to a novel stressor, balances or overcomes the negative feedback effects of circulating glucocorticoids.3 The greater glucocorticoid response to restraint seen in the WC group is consistent with ongoing chronic stress.
The second part of the corticosterone data is more challenging to interpret. Continuous exposure to bright light has been shown to strongly suppress diurnal rhythms of the sleep-wake cycle, drinking, locomotion, and body temperature in rats.8 In fact, light is the most important environmental signal regulating the temporal patterns of animal behavior.8 One study showed that disruption of biological rhythms increased plasma corticosterone levels.13 We predicted that altered light exposure would differentially affect the corticosterone response under the 2 housing conditions. By the final time point (CL), corticosterone concentrations for both housing groups were significantly lower than the R concentration, and corticosterone levels of the WC and SC rats were not statistically different. Similarly, corticosterone concentrations were not significantly different from the original baseline concentration (preR).
One study suggested that successful behavioral adaptation to stress may be associated with altered circadian rhythmicity.26 All samples were collected immediately after the daily work session. For the standard 12:12 room, the time would equate to at least 2 h before the end of the light phase of the diurnal cycle. Disruption of the light cycle may have altered diurnal synchrony in individual rats. The nadir or lowest point of corticosterone concentration normally occurs at the end of the daily active cycle; 4 for a male rat at that time point, the value is 10 to 60 mg/ml.6 Additional samples taken at multiple time points during the continuous light portion of the study could reveal whether any shifting of the active cycle occurred.
Taken together, the findings of this study refute the belief that rats used in operant conditioning studies must be housed in wire-bottom caging in order to maintain their motivation to work. In addition, our findings show that rats in the 2 housing conditions differed in their corticosterone profiles in response to acute restraint. The prolonged elevation in corticosterone in WC rats after restraint suggests that this housing condition exacerbates this physiologic effect in rats. These findings provide important objective information that can be used to support the choice of housing conditions for rats used in biomedical research.
Acknowledgments
I would like to sincerely thank Monique Morgan, Dr. Judy Hickman-Davis, Dr. Stefan Niewiesk, and Wanda Kirk for their contributions to this study. This work was financially supported by an ACLAM foundation grant, in addition to NIH SOAR funding.
References
- 1.Armanini MP, Hutchins C, Stein BA, Sapolsky RM. 1990. Glucocorticoid endangerment of hippocampal neurons is NMDA receptor-dependent. Brain Res 532:7–12 [DOI] [PubMed] [Google Scholar]
- 2.Auvergne R, Lere C, El Bahh B, Arthaud S, Lespinet V, Rougier A, Le Gal La Salle G. 2002. Delayed kindling epileptogenesis and increased neurogenesis in adult rats housed in an enriched environment. Brain Res 954:277–285 [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar S, Dallman M. 1998. Neuroanatomical basis for facilitation of hypothalamic–pituitary–adrenal responses to a novel stressor after chronic stress. Neuroscience 84:1025–1039 [DOI] [PubMed] [Google Scholar]
- 4.Cavigelli SA, Monfort SL, Whitney TK, Mechref YS, Novotny M, McClintock MK. 2005. Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. J Endocrinol 184:153–163 [DOI] [PubMed] [Google Scholar]
- 5.Fleshner M, Deak T, Spencer RL, Laudenslager ML, Watkins LR, Maier SF. 1995. A long-term increase in basal levels of corticosterone and a decrease in corticosteroid-binding globulin after acute stressor exposure. Endocrinology 136:5336–5342 [DOI] [PubMed] [Google Scholar]
- 6.Fox JG. 2002. Laboratory animal medicine, 2nd ed. San Diego: Academic Press [Google Scholar]
- 7.Guyton AC, Hall JE. 2000. Textbook of medical physiology. p 1064 [Google Scholar]
- 8.Ikeda M, Sagara M, Inoue S. 2000. Continuous exposure to dim illumination uncouples temporal patterns of sleep, body temperature, locomotion, and drinking behavior in the rat. Neurosci Lett 279:185–189 [DOI] [PubMed] [Google Scholar]
- 9.Jennings M, Batchelor GR, Brain PF, Dick A, Elliott H, Francis RJ, Hubrecht RC, Hurst JL, Morton DB, Peters AG, Raymond R, Sales GD, Sherwin CM, West C. 1998. Refining rodent husbandry: the mouse. report of the rodent refinement working party. Lab Anim 32:233–259 [DOI] [PubMed] [Google Scholar]
- 10.Jensen P, Toates FM. 1993. Who needs ‘behavioural needs’? Motivational aspects of the needs of animals. Appl Anim Behav Sci 37:161–181 [Google Scholar]
- 11.Krinke G. 2000. The laboratory rat. San Diego: Academic Press [Google Scholar]
- 12.Kulpa-Eddy JA, Taylor S, Adams KM. 2005. USDA perspective on environmental enrichment for animals. ILAR J 46:83–94 [DOI] [PubMed] [Google Scholar]
- 13.Ma WP, Cao J, Tian M, Cui MH, Han HL, Yang YX, Xu L. 2007. Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats. Neurosci Res 59:224–230 [DOI] [PubMed] [Google Scholar]
- 14.Manser CE, Morris TH, Broom DM. 1995. An investigation into the effects of solid or grid cage flooring on the welfare of laboratory rats. Lab Anim 29:353–363 [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS, Seeman T. 1999. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci 896:30–47 [DOI] [PubMed] [Google Scholar]
- 16.McGaughy J, Sarter M. 1995. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 117:340–357 [DOI] [PubMed] [Google Scholar]
- 17.Mizisin AP, Kalichman MW, Garrett RS, Dines KC. 1998. Tactile hyperesthesia, altered epidermal innervations, and plantar nerve injury in the hindfeet of rats housed on wire grates. Brain Res 788:13–19 [DOI] [PubMed] [Google Scholar]
- 18.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 19.Newberry RC. 1995. Environmental enrichment: increasing the biological relevance of captive environments. Appl Anim Behav Sci 44:229–243 [Google Scholar]
- 20.Olsson IAS, Dahlborn K. 2002. Improving housing conditions for laboratory mice: a review of ‘environmental enrichment’. Lab Anim 36:243–270 [DOI] [PubMed] [Google Scholar]
- 21.Peace TA, Singer AW, Niemuth NA, Shaw ME. 2001. Effects of caging type and animal source on the development of foot lesions in Sprague Dawley rats (Rattus norvegicus). Contemp Top Lab Anim Sci 40:17–21 [PubMed] [Google Scholar]
- 22.Sapolsky RM. 2003. Stress and plasticity in the limbic system. Neurochem Res 28:1735–1742 [DOI] [PubMed] [Google Scholar]
- 23.Soffie M, Hahn K, Terao E, Eclancher F. 1999. Behavioural and glial changes in old rats following environmental enrichment. Behav Brain Res 101:37–49 [DOI] [PubMed] [Google Scholar]
- 24.Sprott RL. 1997. Diet and calorie restriction. In: Experimental gerontology. New York: Elsevier Science; p 205–214 [DOI] [PubMed] [Google Scholar]
- 25.Stark DM. 2001. Wire-bottom versus solid-bottom rodent caging issues important to scientists and laboratory animal science specialists. Contemp Top Lab Anim Sci 40:11–14 [PubMed] [Google Scholar]
- 26.Stewart KT, Rosenwasser AM, Hauser H, Volpicelli JR, Adler NT. 1990. Circadian rhythmicity and behavioral depression: I. effects of stress. Physiol Behav 48:149–155 [DOI] [PubMed] [Google Scholar]
- 27.Van de Weerd HA, Aarsen EL, Mulder A, Kruitwagen CL, Hendriksen CF, Baumans V. 2002. Effects of environmental enrichment for mice: variation in experimental results. J Appl Anim Welf Sci 5:87–109 [DOI] [PubMed] [Google Scholar]
- 28.Weiss JM. 1968. Effects of coping responses on stress. J Comp Physiol Psychol 65:251–260 [DOI] [PubMed] [Google Scholar]
- 29.Zinn JA. 1986. Library of Congress, Congressional Research Service. The Food Security Act of 1985:17 [Google Scholar]