Abstract
Acute respiratory distress syndrome (ARDS) is an important and potentially life-threatening complication in humans that arises subsequent to a variety of primary insults including noxious fume inhalation, infection, and trauma. Here we describe the first two cases of ARDS reported in association with postoperative complications in rhesus macaques. In agreement with the multifactorial nature of the human syndrome, ARDS in one monkey was attributed to sepsis, whereas in the other it was ascribed to neurogenic trauma. Despite the different etiologies, both monkeys demonstrated clinical features of ARDS, including progressive dyspnea and pulmonary edema, and syndrome-defining histopathologic criteria including edema with intraalveolar neutrophils, fibrinohemorrhagic effusions with crescentic membranes, and interstitial vascular degeneration. Recognition and aggressive treatment of ARDS at an early stage may improve survival rates in dyspneic nonhuman primates with underlying extrapulmonary diseases.
Abbreviation: ARDS, acute respiratory distress syndrome
Acute respiratory distress syndrome (ARDS) is a leading cause of morbidity and mortality in humans, with approximately 200,000 diagnoses annually.13 This syndrome can be initiated by either indirect or direct pulmonary injury. Indirect causes include sepsis, severe trauma such as long bone fractures, blood transfusion, acute pancreatitis, drug overdose, and shock. Direct injury includes viral, bacterial, and fungal pneumonias, near drowning, toxin or gas inhalation, gastric aspiration, fat and amniotic-fluid inhalation, pulmonary contusion, alveolar hemorrhage, reperfusion injury, and unilateral lung reimplantation.26
Acute respiratory distress syndrome is characterized by 2 phases. The initial phase, known as the ‘exudative phase,’ occurs within 24 to 48 h of the initial insult. Grossly, this phase is characterized by dusky reddish-blue lungs with frothy, often blood-tinged, fluid in the airways.10 On histopathology there is diffuse protein-rich pulmonary edema admixed with variable numbers of neutrophils, alveolar hemorrhage, and atelectasis.26 Alveolar edema exhibits organization with fibrin and hyaline membrane formation. The disease progresses to the ‘fibrinoproliferative phase’ approximately 48 h after the insult.10 At autopsy, lungs may be firm and fail to collapse fully after incisure of the diaphragm. Vessels may be occluded by fibrin thrombi and exhibit intramural fibrinoid necrosis. Pulmonary hypertension may result in thickening of the tunica muscularis, luminal constriction and compensatory capillary proliferation, and neovascularization. Parenchymal changes include interstitial fibrosis and type II pneumocyte hypertrophy.10 In the fibrinoproliferative phase, hypoxemia may result from arteriovenous shunting or impaired ventilation and perfusion.7 Therefore, patients that survive the initial insult remain at risk for catastrophic pulmonary insufficiency due to alveolar fibrosis.10
The pathogenesis of ARDS is complex and incompletely understood. Early in the disease course, inflammatory mediators are released from activated neutrophils and macrophages.7,26 Cytokines and elastases increase capillary permeability and damage vessel walls, with extravasation of leukocytes, fluid accumulation in interstitial and alveolar spaces, and emphysematous change.7,26 Reactive endothelial cells, neutrophils, and macrophages produce large quantities of tissue factor, an important initiator of the extrinsic clotting cascade.7 Decreased concentrations of anticoagulant proteins C and S and increased concentrations of antifibrinolytic proteins and plasminogen activator inhibitor 1 further promote coagulation.9,11 This scenario results in increased formation and deposition of fibrin into the microvasculature causing an obstruction of blood flow.7 In addition, inflammatory mediators reduce and neutralize surfactant production, contributing to atelectasis.26 Physiologically, ARDS is characterized by severe hypoxemia (partial arterial pressure of oxygen [PaO2]/fractional concentration of oxygen in inspired air [FIO2] < 200).2 Treatment is supportive and can be unrewarding. Prognosis depends on the age of the patient, severity of the disease at onset, and the presence of comorbidities such as shock and renal or hepatic failure.26
Nonhuman primates are used frequently for neurologic research because of their trainability and close similarity to human anatomy and neurophysiology. Unfortunately, the invasive instrumentation and procedures required for some experiments have the potential to incite localized or multisystem disease. Here we describe the development of ARDS in 2 rhesus macaques that developed complications after experimental craniotomy. The submitted protocols were compliant with the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and approved by the Massachusetts Institute of Technology Committee on Animal Care. As true for humans, ARDS in these monkeys was attributed to extrapulmonary diseases, including meningitis-associated septicemia and neurogenic injury. Our findings suggest that ARDS is an underdiagnosed condition in laboratory nonhuman primates.
Case Report 1
An adult male, intact, 10.6-kg rhesus macaque underwent craniotomy superior to the right hippocampus. Presurgical medication included atropine (0.04 mg/kg IM) and ketamine (10 mg/kg IM). The monkey was maintained on 1% to 2% isoflurane anesthesia with balanced oxygen. Surgery and recovery were uneventful. The postoperative treatment regimen included buprenorphine (0.01 mg/kg SC or IM q 8-12 h) for pain and trimethoprim–sulfadiazine (30 mg/kg SQ or IM) once a day to prevent infection. Two weeks after surgery, the monkey had an episode of self-inflicted head trauma in the cage, resulting in bleeding around the surgical implant. One week later, the monkey was placed on approved water restriction in advance of a behavioral exercise.
The next day, the monkey was found hunched in its cage and poorly reactive; it was sedated lightly for physical examination. Mucous membranes were pale gray and pulses were weak. No abnormality was reported on lung auscultation, and the temperature was 38.1 °C. Blood was collected, and the monkey was rapidly given 250 ml 5% dextrose in lactated Ringer solution, followed by 50 ml lactated Ringer solution for circulatory support and maintenance of blood glucose. In addition, the animal received methylprednisolone sodium succinate (30 mg/kg IM) for cerebral swelling and shock, and ceftriaxone (50 mg/kg IM) as a broad-spectrum antibiotic.
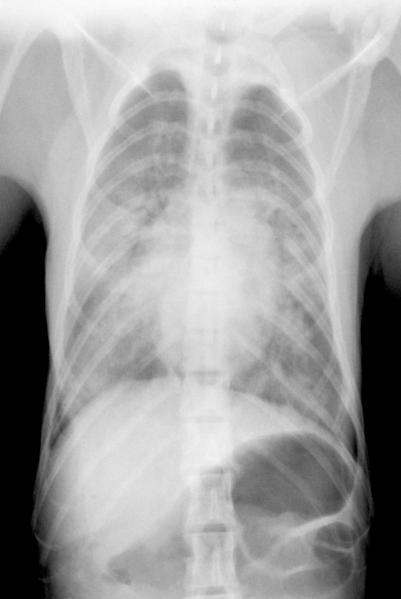
After transitory improvement, the monkey became more lethargic and developed dyspnea. Bloodwork revealed marked leukocytosis (25.5 cells/mm3) with a left shift (3060 absolute count), mild erythropenia (5.41 × 106/mm3), and normoglycemia (105 mg/dl). Wet lung sounds were reported on auscultation, and pulmonary edema was confirmed by radiographs (Figure 1). To treat pulmonary edema, furosemide (2 mg/kg IV) was administered. The monkey was intubated and given ventilatory support with balanced O2 intermittently for approximately 11 h. On multiple occasions, frothy serosanguinous fluid was aspirated from the tracheal tube. Atropine for circulatory support and drying of mucous membranes, intravenous lactated Ringer solution for circulatory support, and oxygen for respiratory support were administered. Blood glucose was 211 mg/dl and electrocardiogram revealed sinus tachycardia. Furosemide, ceftriaxone, and atropine were continued overnight, and cimetidine (5 mg/kg IM) was administered to prevent acid reflux. In addition, flunixin meglumine (1.1 mg/kg) for endotoxic shock, diazepam (1 ml, 5mg/ml IV) for pulmonary fluid mobilization, dobutamine (50 μg/min IV) for circulatory support and pulmonary fluid mobilization, and doxapram (5 mg/kg IV) for respiratory stimulation were administered. Episodes of cardiac arrest occurred throughout the evening and eventually pupillary and conscious reactions disappeared. Resuscitation was unsuccessful, and the monkey was euthanized with intravenous pentobarbital sodium.
Figure 1.
Dorsoventral thoracic radiograph, case 1. Note coalescing pulmonary edema obscuring small airway detail.
At necropsy, granulation tissue was noted at the craniotomy site, accompanied by purulent material, dural thickening, and hemorrhage. The right lungs were diffusely wet and reddened. The left caudodorsal lung was mottled. Histopathology of the brain revealed severe, focally extensive fibrinosuppurative meningitis with multifocal extension into the gray matter (Figure 2). Multifocal chronic active meningoencephalitis was characterized by fibrosis, granulation tissue, hemorrhage, and neutrophils. Bacterial culture of the meninges was positive for Staphylococcus aureus, group A Streptococcus pyogenes, and Corynebacterium ulcerans. The group A Streptococcus pyogenes was resistant to gentamycin and trimethoprim–sulfadiazine.
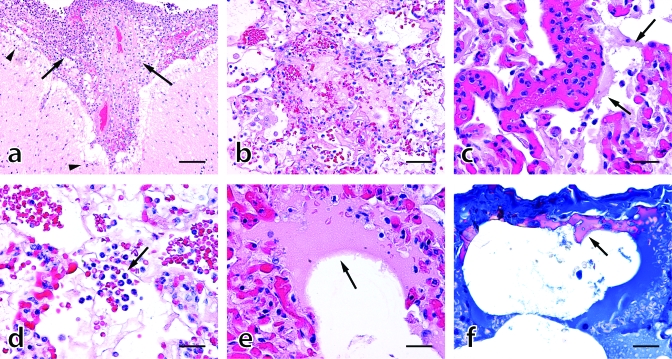
Figure 2.
Brain and lung histopathology, case 1. (A) Severe fibrinosuppurative meningitis (arrows) with localized infiltration of polymorphonuclear cells (PMN) into subjacent gray matter (arrowheads). (B) Focally extensive fibrinoproteinaceous transudate and hemorrhage occluding terminal airways; note reactive changes to peripheral alveoli including transalveolar fibrillar bridging, epithelioid macrophages, and type II pneumocyte hypertrophy. (C) Severe interstitial congestion with numerous intravascular PMN and tattered fibrinoid material lining alveolar walls (arrows). (D) Alveolar cellular exudate composed of PMN and erythrocytes (arrow). (E) Crescentic condensation of fibrinoproteinaceous material (arrow) lining airway epithelium, an acute precursor to hyaline membranes. (F) Carstair stain demonstrating organized fibrin (pink; arrow) lining subpleural alveoli. Hematoxylin and eosin stain (A through E), Carstair stain (F); bar: 160 μm (A), 80 μm (B), 40 μm (C through F).
Grossly, the lungs exhibited regionally severe pulmonary edema, airway hemorrhage, and early consolidation. Histologically, there was coalescing alveolar serofibrinous edema that sometimes formed early crescents, hemorrhage, neutrophil exudation, reactive macrophages, and type II pneumocyte hypertrophy (Figure 2). Pleuritis, atelectasis, emphysema, and serosal to transmural edema of the large arteries were evident. Carstair-positive fibrin-rich hyaline membranes were concentrated in subpleural terminal airways. The morphologic diagnosis of acute serofibrinous pulmonary edema with neutrophilic exudates and early crescent formation was consistent with criteria for human ARDS. Aerobic lung culture was negative after 48 h.
Case Report 2
An adult male, intact, 9.1-kg rhesus macaque underwent surgical craniotomy for an imaging study. Premedication included atropine (0.04 mg/kg IM), injectable cephalosporin (20 mg/kg IV repeated q2 h), and tiletamine–zolazepam (5 mg/kg IM). Surgery lasted 8.5 h, with assisted ventilation. Tidal volume ranged from 130 to 165 ml with 6 to 10 breaths/min. The following parameters were maintained during surgery: isoflurane, 0.5% to 2%; O2, 2 l/min; SpO2, 80% to 100%; and CO2, 25 to 35 mm Hg. The monkey was given furosemide (2 mg/kg IV) for diuresis, mannitol (1.5 g/kg IV) and dexamethasone sodium phosphate (2 to 4 mg/kg IM) to reduce intracranial pressure during the procedure and buprenorphine (0.01 mg/kg SQ orIM q8-12 h) for pain and enrofloxacin (5 mg/kg IM) to prevent infection postoperatively. The monkey recovered uneventfully.
The next morning, swelling of the skin and subcuntaneous tissue at the left side of the craniotomy site was noted. In addition to the standard postoperative regimen just described, methylprednisolone sodium succinate (15 mg/kg IM) to prevent brain swelling and cimetidine (5 mg/kg IM) for nausea and to counteract the gastric side effects from methylprednisolone sodium succinate were implemented. The monkey remained inappetant and quiet, alert, and responsive throughout the day. On the morning of the second day after surgery, the monkey was quiet and nonresponsive, and vomitus without blood was present in the cage. The animal was lightly sedated with ketamine (10 mg/kg IM) for a complete physical examination, with no abnormalities noted. At this time, methylprednisolone sodium succinate was discontinued and cimetidine was increased to 7 mg/kg. The animal did not improve, and subsequent physical examination revealed: body temperature, 36.7 °C; heart rate, 120 beats/min; respiratory rate, 45 breaths/min; capillary refill time, less than 2 s; mucous membranes, pink; hematocrit, 32%, and blood glucose, 80 mg/dl. In addition, buprenorphine (0.02 mg/kg) and cimetidine (7 mg/kg) were administered, and clinical improvement of the animal was noted later in the evening.
The next morning (day 3 after surgery), the monkey was found dead in the cage. A necropsy was performed. No lesions were associated with a previously established head post over the parietal cortex. However, the recent craniotomy site over the occipital lobe had a 1-cm area of erythema over the cerebral cortex. In the thorax, the left cranioventral lung was mottled and thickened. Histopathologically, focally extensive and severe astrocytic and oligodendrocytic intracellular edema, neuronal degeneration, and mild fibrinosuppurative leptomeningitis with extension into subjacent neuropil was present at the at the occipital surgery site. The brainstem showed unifocal glial cell ballooning degeneration and neuronal vacuolation. Lung lesions were similar but less severe to those just described. Bacterial cultures of the meningesere were negative.
Discussion
Here we describe the development of ARDS associated with complications of surgical craniotomy in 2 rhesus macaques. Acute respiratory distress syndrome in case 1 was attributed to polymicrobial suppurative meningoencephalitis accompanied by marked neutrophilia with a left shift. Organisms recovered from the meninges included Staphylococcus aureus and group A Streptococcus pyogenes. S. aureus has been associated with meningitis and bacteremia with septic shock in humans.16 In the lungs, the S. aureus alphatoxin stimulates the pulmonary vascular arachidonic acid–lipoxygenase pathway directly and indirectly through the classical humoral cascade and leukocyte stimulation, leading to increased vascular leakage.20 In addition, S. aureus has an IL1β receptor for the proinflammatory cytokine IL1β, which is secreted from innate immune cells during inflammation. The presence of this receptor enhances the extracellular growth and potential pathogenesis of this organism.14 Group A Streptococcus spp. are also responsible for development of meningitis in humans24 and can lead to shock, bacteremia, and ARDS.22 Corynebacterium ulcerans has been reported as a cause of pyogranulomatous meningoencephalitis in a goat.16 Although the organism has been recovered from the oropharynx and cephalic implants of macaques in the facility in which the animals of this report were housed, it has not been implicated in meningitis.1 Whereas some species of the bacterium are pneumotropic,4 none have been described in association with ARDS. All of the recovered bacteria are common skin flora. Antibiotic sensitivity results demonstrated that the group A Streptococcus pyogenes was resistant to trimethoprim–sulfadiazine.
In contrast to case 1, the second animal lacked evidence of clinically significant bacterial infection. Rather, the monkey in case 2 exhibited focally severe neurodegenerative changes in the occipital lobe at the site of craniotomy and focally severe fibrinohemorrhagic interstitial pneumonia with intraalveolar neutrophils and perivascular edema in the lung. In this case, ARDS was attributed to direct neurogenic injury with reactive pulmonary disease. Acute cerebral trauma results in an acute rise in intracranial pressure that may increase pulmonary vascular hydrostatic pressure through multiple mechanisms, including pulmonary vasoconstriction from sympathetic innervation, increased left-atrial pressure from systemic arterial hypertension, and increased pulmonary capillary permeability.19 Whereas ARDS was considered the proximate cause of death in case 1, the less widely disseminated and more acute pulmonary disease in case 2 suggested that central neuropathy was the most important lesion. Nevertheless, the incidental finding of pulmonary lesions consistent with ARDS in case 2 suggests that this syndrome may occur more often than has been recognized in nonhuman primates that undergo invasive procedures.
Numerous treatment modalities exist for ARDS in humans, and many of these therapies can be applied in the nonhuman primate laboratory setting. Because ARDS is usually a sequel to an extrapulmonary insult, the underlying disease must be sought and treated aggressively. Antibiotic therapy based on culture and sensitivity results is indicated in cases of septicemia. Supportive care for ARDS includes mechanical ventilation with or without oxygen supplementation, intravenous colloidal or crystalloid fluids (or both), sedatives, diuretics, bronchodilators, respiratory stimulants, intrabronchial surfactant administration, corticosteroids, and prone positioning.
Respiratory support is the mainstay of ARDS treatment. Oxygen therapy by means of nasal cannula, face mask, oxygen cage, or intubation in obtunded animals is essential. If necessary, mechanical ventilation for delivery of positive end-expiratory pressure (to prevent alveolar collapse is ideal.7 However, tidal volumes must be calculated cautiously, because high tidal volumes with compounding atelectasis can cause neighboring patent alveoli to become overly distended, leading to disruption of the alveolar epithelium and capillary endothelium as well as induction of inflammatory mediators and subsequent cytokine release. Lungs subsequently become congested and edematous after this direct physical damage.12 Historically, the recommended tidal volume for humans has been 12 ml/kg predicted body weight and for domestic animals has been 10 to 20 ml/kg.12,25 Safe tidal volumes in monkeys have not been published but generally are extrapolated from those for humans. Concern for tidal volume induced pulmonary damage has stimulated recent research, and recommendations of smaller tidal volumes in domestic animals (4 to 5 ml/kg; positive end-expiratory pressure, 2 cm) and humans (6 ml/kg predicted body weight) to preserve cardiac output and prevent atelectasis have been proposed.12,25 Hypercapnia and hypoxia must be monitored closely in cases of head trauma. In our setting, tidal volumes ranging of 6 to 10 ml/kg with close monitoring are used for mechanical ventilation of nonhuman primates during elective surgery and for respiratory support in cases of dyspnea.
Pulmonary edema is a defining feature of ARDS and is due to increased permeability of the alveolar epithelium and capillary endothelium. The pulmonary endothelium is more permeable to proteins than are other vessels. Because colloids such as albumin and hetastarch rapidly equilibrate with the interstitial space, colloidal fluids may offer no better protection against vascular leakage than do standard crystalloids such as lactated Ringer solution. Protein permeability becomes even more pronounced when there are insults to both the alveolar and capillary endothelia.
Colloid therapy should be used with caution in patients with damaged alveolar epithelium and capillary endothelium because interstitial edema can progress rapidly to alveolar flooding.6 Therefore, in cases of shock compounded with pulmonary edema, fluids should be administered judiciously to maintain cardiac output and arterial blood pressure. Dopamine may be indicated to maintain systemic blood pressure.7 Other drugs such as dobutamine drip (2 μg/kg hourly) and morphine (1 to 2 mg/kg SC, IM, or IV every 4 h) also have been used with the goal of displacing fluid from the lungs into the systemic vasculature.18,15 Intrabronchial fluid and foam, as observed in case 1, can be treated by intubation and aspiration with or without nebulization with a 20% ethanol solution into the airways.5 Animals should be monitored continually by assessing arterial blood pressure, capillary refill time, mucous membrane color, peripheral pulse quality, packed cell volume, total protein concentration, electrolyte levels, and BUN and creatinine concentration.7
Sedatives should be considered in conscious animals with hypoxia-associated anxiety, but these drugs should be used with caution due to dose-dependent respiratory depression. If indicated, one drug of choice is morphine due to its combined sedative effects and reported mobilization of pulmonary fluid. Extra care is required in New World primates, because these species may exhibit extreme sensitivity to respiratory depression associated with opioids. Other side effects of this class of drugs in primates include intense facial pruritis due to histamine release.3,7
Diuretics such as furosemide (1 to 2 mg/kg IV every 4 to 12 h) are indicated for the treatment of pulmonary edema. However, this drug may prove of minimal benefit in the case of ARDS, where there is increased pulmonary vascular permeability.3,7 Caution should be taken in animals with existing progressive renal disease, electrolyte and water imbalances, impaired hepatic function, and hypovolemia.18 In addition, blood urea nitrogen, creatinine, and urine output should be monitored during therapy. Bronchodilators such as aminophylline (25 to 100 mg/animal PO or 10 mg/kg IV) and theophylline (5 mg/kg initially then 2 to 4 mg/kg) can be administered to increase airway volume.3 These drugs have diuretic activity, counteract bronchospasm, enhance mucocilliary function, and decrease diaphragmatic fatigue and pulmonary edema.7,18 Side effects include CNS stimulation and gastrointestinal irritation.18 Doxapram (2 mg/kg IV) can be given as a respiratory stimulant in cases where respiratory function is diminished.3,18
In ARDS, there is a deficit of surfactant due its inactivation by plasma proteins, inflammatory mediators, and cellular debris. This deficit in surfactant exacerbates atelectasis and hypoxemia.12,23,26 A proven therapy for neonatal respiratory distress syndrome includes the administration of surfactant directly into the lung by means of an endotracheal tube followed by mechanical ventilation stabilization.23 In pediatric populations with ARDS, treatment with surfactant decreased the mortality rate, but the number of days receiving supplemental oxygen, length of hospital stay, and hospital charges were unchanged.23 In adults with ARDS, surfactant therapy has shown no confirmed benefit.23 Another controversial treatment involves prone positioning, with patients placed face down for approximately 6 h each day for 10 d. Proposed benefits of prone positioning include an increase in end-expiratory lung volume, improved ventilation–perfusion matching, regional changes in ventilation, redistribution of blood flow and ventilation to less-affected areas of lung, improved secretion clearance, and recruitment of atelectatic regions of lung.8,26 Concerns with this treatment include increased risk for regurgitation, extubation of endotracheal tubes, and facial edema.3,13 Patients with cardiogenic pulmonary edema, cerebral edema, or intracranial hypertension should not be placed in this position.26 Although studies have shown that prone positioning improves arterial oxygenation,8 it has not been shown to improve survival time or decrease ventilator or intensive care unit time.26 There are no reports of the application of this strategy to nonhuman primates with respiratory distress.
Corticosteroids are an integral component of the standard ARDS treatment regimen in the acute phase. However, a controversy in the field surrounds the use of this class of drugs over a long period of time. Well-known side effects of chronic glucocorticoid administration include immunosuppression, hyperglycemia, poor wound healing, psychosis, pancreatitis, and prolonged muscle weakness.18 One study found that over the course of the disease, nonsurvivors of ARDS had significantly higher levels of proinflammatory cytokines TNFα, IL1β, and IL6 than did survivors. High concentrations of these cytokines lead to the impairment of bacterial killing by monocytes allowing progression of the disease induced by bacterial pathogenic factors. Methylprednisolone reduces the mRNA expression of these cytokines and restores monocyte killing. Accordingly, one study found that prolonged (7 d or more) administration of low doses of methylprednisolone minimized excessive inflammation.22 However, another large clinical trial involving moderate-dose methylprednisolone did not support the long-term administration of methylprednisolone to patients with ARDS and found that treatment actually could be harmful if initiated within 2 wk after disease onset20. Therefore, short-acting corticosteroid treatment is indicated for acute ARDS in nonhuman primates, but risk-versus-benefit must be weighed in the case of chronic glucocorticoid administration, and prophylactic broad-spectrum antibiotics should be considered to reduce the chance of opportunistic infection.
To our knowledge, this article is the first description of ARDS in rhesus macaques. Both animals exhibited respiratory distress as a component of multisystemic disease and exhibited gross and histologic lesions consistent with ARDS, including pulmonary edema with neutrophils, fibrinohemorrhagic effusion, and interstitial vascular degeneration. In agreement with the multifactorial nature of the disease in humans, we attributed one case of ARDS to septicemia and the other to direct neurogenic trauma. These findings suggest that ARDS in rhesus macaques is underreported and may be common in other nonhuman primates as well. Aggressive supportive treatment in humans has reduced death rates from ARDS dramatically in recent years. Although not all of these interventions are practical in a veterinary setting, increased awareness of ARDS as a potential sequel to a variety of injuries may lead to earlier and more aggressive treatment in nonhuman primates with respiratory distress, thereby improving survival rates in these valuable animals.
References
- 1.Bergin IL, Chien CC, Marini RP, Fox JG. 2000. Isolation and characterization of Corynebacterium ulcerans from cephalic implants in macaques. Comp Med 50:530–535 [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, Carlet J, Flake K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. 1994. Report of the American–European Consensus Conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. J Crit Care 9:72–81 [DOI] [PubMed] [Google Scholar]
- 3.Carpenter JW. 2001. Exotic animal formulary, 3rd ed St Louis (MO): Elsevier Saunders [Google Scholar]
- 4.Colt HG, Morris JF, Marston BJ, Sewell DL. 1991. Necrotizing tracheitis caused by Corynebacterium pseudodiphtheriticum: unique case and review. Rev Infect Dis 13:73–76 [DOI] [PubMed] [Google Scholar]
- 5.Davis LE. 1985. Handbook of small animal therapeutics. New York: Churchill Livingstone [Google Scholar]
- 6.DiBartola SP. 2000. Fluid therapy in small animal practice, 2nd ed Philadelphia: WB Saunders Company [Google Scholar]
- 7.Ettinger SJ, Feldman EC. 2005. Textbook of veterinary internal medicine, 6th ed St Louis (MO): Elsevier Saunders [Google Scholar]
- 8.Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, Malacrida R, Di Giulio P, Fumagalli R, Pelosi P, et al. 2001. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 345:568–573 [DOI] [PubMed] [Google Scholar]
- 9.Gunther A, Mosavi P, Heinemann S. 2000. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia: comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med 161:454–462 [DOI] [PubMed] [Google Scholar]
- 10.Hasleton PS, Roberts TE. 1999. Adult respiratory distress syndrome—an update. Histopathology 34:285–294 [DOI] [PubMed] [Google Scholar]
- 11.Idell S. 2003. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med 31:S213–S220 [DOI] [PubMed] [Google Scholar]
- 12.Malhotra A. 2007. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med 357:1113–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthay MA, Zimmerman GA. 2005. Acute lung injury and the acute respiratory distress syndrome. Am J Respir Cell Mol Biol 33:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meduri GU. 2002. Clinical review: a paradigm shift—the bidirectional effect of inflammation on bacterial growth. Clinical implications for patients with acute respiratory distress syndrome. Crit Care 6:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molloy WD, Lee KY, Girling L, Prewitt RM. 1985. Treatment of canine permeability pulmonary edema: short-term effects of dobutamine, furosemide, and hydralazine. Circulation 72:1365–1371 [DOI] [PubMed] [Google Scholar]
- 16.Morris WE, Uzal FA, Cipolla AL. 2005. Pyogranulomatous meningoencephalitis in a goat due to Corynebacterium ulcerans. Vet Rec 156:317–318 [DOI] [PubMed] [Google Scholar]
- 17.Pintado V, Meseguer MA, Fortun J, Cobo J, Navas E, Quereda C, Corral I, Moreno S. 2002. Clinical study of 44 cases of Staphylococcus aureus meningitis. Eur J Clin Microbiol Infect Dis 21:864–868 [DOI] [PubMed] [Google Scholar]
- 18.Plumb DC. 2005. Plumb's veterinary drug handbook, 5th ed Stockholm (WI): PharmaVet [Google Scholar]
- 19.Schwarz S, Schwab S, Keller E, Bertram M, Hacke W. 1997. Neurogenic disorders of heart and lung function in acute cerebral lesions. Nervenarzt 68:956–962 [DOI] [PubMed] [Google Scholar]
- 20.Seeger W, Lasch HG. 1987. Septic lung. Rev Infect Dis 9(Suppl 5):S570–S579 [DOI] [PubMed] [Google Scholar]
- 21.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network 2006. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354: 1671–1684 [DOI] [PubMed] [Google Scholar]
- 22.Stevens DL. 1992. Invasive group A streptococcus infections. Clin Infect Dis 14:2–11 [DOI] [PubMed] [Google Scholar]
- 23.Stevens TP, Sinkin RA. 2007. Surfactant replacement therapy. Chest 131:1577–1582 [DOI] [PubMed] [Google Scholar]
- 24.van de Beek D, de Gans J, Spanjaard L, Sela S, Vermeulen M, Dankert J. 2002. Group A streptococcal meningitis in adults: report of 41 cases and a review of the literature. Clin Infect Dis 34:e32–e36 [DOI] [PubMed] [Google Scholar]
- 25.Veterinary Information Network (VIN) 2003 Artificial ventilation: Australian College of Veterinary Scientists Science Week [Internet]. Available from: http://www.vin.com/Members/Proceedings/Proceedings.plx?CID=acvsc2003&PID=pr04913&O=VIN.
- 26.Wheeler AP, Bernard GR. 2007. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369:1553–1564 [DOI] [PubMed] [Google Scholar]