Abstract
Bronchoalveolar lavage (BAL) by means of bronchoscopy is a diagnostic tool frequently used for clinical and research purposes in nonhuman primates. Although many institutions use this procedure, the technique is not standardized. One technical aspect that can vary is the method by which fluid is recovered. The purpose of this study was to evaluate differences between 2 different BAL aspiration techniques. Bronchoscopy and BAL fluid collection were performed on 20 rhesus macaques (Macaca mulatta). Data collected for comparison included heart rate, oxygen saturation levels, rectal temperature, volume of fluid collected, total cell count, cell viability, differential cell count, and flow cytometry. Results showed no significant differences in the heart rate, oxygen saturation, or body temperature between the 2 groups. Likewise, differential cell counts and cell viability studies of the retrieved fluid did not differ between methods. Compared with the conventional technique, the modified aspiration technique led to an 8.3% increase in overall fluid yield and a higher concentration of cells recovered. These differences are statistically significant and likely will be clinically relevant in the context of diagnosis.
Abbreviation: BAL, bronchoalveolar lavage; BALPF, bronchoalveolar lavage pooled fluid; cBAL, conventional bronchoalveolar lavage; mBAL, modified bronchoalveolar lavage
Bronchoalveolar lavage (BAL) is a useful and commonly implemented tool in research and clinical medicine.6,8,14 This valuable diagnostic procedure is used to evaluate the pathomechanisms of airway diseases.10 The technique involved in performing BAL consists of inserting a flexible videoscope into the trachea under direct visualization and manipulating the instrument to the chosen site.2,3,4 The bronchoscope then is wedged into a subsegmental bronchus to create a seal, which then allows fluid to be instilled and aspirated from the area of interest.3,4,7,10
Although BAL is used frequently, the procedure is limited by lack of standardization. Aspects of the technique such as amount of instillate used, size of syringes used, amount of ‘dwell’ time of instillate before aspiration, and the suction application technique vary widely.3,4,12,13 Sources in the literature recommend fluid instillation volume ranging from 10 to 300 ml in human patients and 50 to 600 ml in dogs.1,3,12,15 Likewise, there are no standard recommendations regarding syringe size, which affects the amount of vacuum applied during aspiration. One randomized trial focusing on syringe size included 30 patients with chronic obstructive pulmonary disease and found that performing BAL with 50-ml syringes acquires more fluid with less oxygen desaturation than can be attained by using 20-ml syringes.5 No other studies addressing syringe sizes were identified during literature searches. The time that instillate remains in the airway before aspiration is yet another variable. Most literature sources recommend immediate aspiration of instillate, but some authors advocate leaving lavage fluid in the airway for the period of a normal breath prior to aspiration.1,3,7,12 Various methods of fluid aspiration including manual suction, drainage by gravity, and mechanical suction at a judicious negative pressure (50 to 100 mm Hg) also have been described. 2,4,7,11-13 Due to a lack of comparative studies, no method is specifically recommended.
Although attempts have been made to achieve some standardization, primarily by way of professional consensus, few hypothesis-driven studies have been completed to optimize BAL technique.13 Many aspects of the BAL procedure require further testing to achieve consistency. The aim of this study was to compare a specific technical modification of the aspiration process with a method used more conventionally during manual recovery of BAL fluid. These techniques were evaluated for differences in fluid composition, animal morbidity, and ergonomics.
Materials and Methods
Animals.
All subjects were healthy adult (16 male, 4 female) rhesus macaques (Macaca mulatta) of Indian origin, ranging in age from 4.2 to 9.4 y (average, 6.6 y) and in body weight from 5.1 to 14.6 kg (average, 9.9 kg). Animals were housed in stainless steel cages, according to the regulations of the Animal Welfare Act and recommendations of The Guide for the Care and Use of Laboratory Animals.9 Animal rooms were maintained on a 12:12-h light:dark cycle (lights on at 0600). All cages were equipped with resting perches and other enrichment devices. The monkeys were fed commercial nonhuman primate biscuits (Purina Diet 5037, PMI Feeds, St Louis, MO) twice daily, provided water ad libitum, and given supplemental fruit and forage throughout the week. Food was removed 12 h prior to anesthesia and BAL procedures. The Institutional Animal Care and Use Committee approved all aspects of this study, and the facility is accredited by AAALAC. Before the start of the experiment, each monkey was determined to be healthy on the basis of a normal physical exam, blood biochemistry analysis, and complete blood count analysis.
Bronchoscopy.
A pediatric fiberoptic bronchoscope with biopsy channel (BF 3C20/BF3C30; outer diameter, 3.6 mm; biopsy channel diameter, 1.2 mm; or Olympus BF type 3C160; outer diameter, 3.8 mm; biopsy channel diameter, 1.2 mm; Olympus Optical, Lake Success, NY) equipped with a light source and video processor (Evis CLV-U20 or Evis Exera II CV-180, Olympus Optical) was used to perform BAL fluid collection. A color monitor (Trinitron OEV203, Olympus Optical) was used to display video of the airways during the procedure. Images of the lavage sites in the lung were captured by using a digital image recorder (Evis Exera II CV-180, Olympus Optical).
Experimental methods.
Two aspiration techniques were evaluated. The conventional method (cBAL) involved gentle manual instillation and aspiration of fluid by using a syringe directly inserted into the biopsy channel of the flexible bronchoscope (Figure 1). In the modified aspiration technique (mBAL), a 70-cm section of sterile standard intravenous extension tubing with male and female ends (International Win, Limited) is inserted between the syringe tip and biopsy channel of the bronchoscope (Figure 2 A, B). In both techniques, the bronchoscope is manipulated into a subsegmental bronchus by using the same method. While the bronchoscopist maintained the seal between the bronchus and the distal end of the scope, a second operator attached the syringe to the bronchoscope by using one of the 2 techniques.
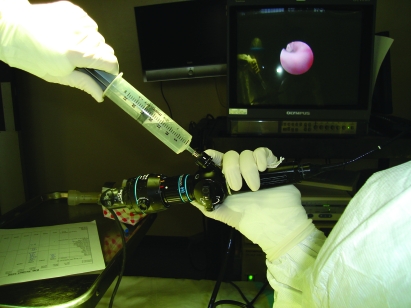
Figure 1.
The conventional BAL (cBAL) procedure. The aspiration syringe is attached directly to the biopsy and suction channel of the bronchoscope. Note the awkward wrist position of the bronchoscopist holding the bronchoscope during aspiration.
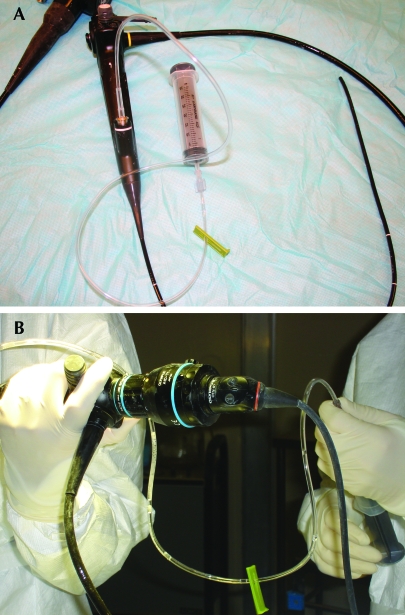
Figure 2.
(A) Setup for the modified BAL (mBAL) method. The distal portion of a 70-cm section of sterile tubing is attached to the biopsy and suction channel of the bronchoscope, and a syringe is attached at the proximal portion of the tubing. (B) The mBAL procedure. Note the more comfortable positioning of bronchcoscopist's wrist and the technician's ability to use both hands to control the amount of suction applied to the syringe.
The 20 animals were divided into 2 equal groups. All animals from each group had 2 BAL procedures performed at 2-week intervals. Group 1 underwent the cBAL technique followed by the mBAL technique 2 wk later. Group 2 underwent mBAL followed by cBAL technique 2 wk later.
All nonhuman primates were anesthetized with tiletamine–zolazepam (8 mg/kg IM; Telazol, Fort Dodge Animal Health, Fort Dodge, IA), after which respiratory rates, heart rates, and rectal temperatures were recorded. At the time of each BAL, peripheral blood collected by femoral vein venipuncture was used to evaluate complete blood count and biochemical values. A physical exam was performed prior to each BAL procedure. Animals then were seated in a custom-designed aluminum chair. Thermal support was provided by using a heat-therapy pump and water blanket (TP400, Gaymar Industries, Orchard, NY). Oxygen (100%, 2 to 4 l) was provided to animals through a nasal cannula throughout the BAL procedure.14 Oxygen saturation and heart rate were monitored continuously through pulse oximetry (Nellcor Puritan Bennet, Pleasanton, CA; Surgivet, Smiths Medical, Waukesha, WI).
To maintain consistency, procedures were performed by the same veterinarian and technician at each time point. A laryngoscope was used to assist in visualization of the epiglottis while lidocaine (2%; Phoenix Scientific, St Joseph, MO) was administered topically by drop instillation from a 3-ml syringe. The bronchoscope was manipulated through the larynx, beyond the vocalis muscle, and into the trachea under direct visualization and advanced to the carina, where it was maneuvered to facilitate orientation to determine right versus left side of lungs. The tip of the bronchoscope was advanced into the right diaphragmatic lung lobe past the second- and third-generation bifurcations and wedged into a subsegmental bronchus.15
Once the tip of the bronchoscope was seated, 2 aliquots of 25-ml physiologic saline at room temperature were introduced into the lung by 1 of the 2 instillation methods. Fluid was aspirated manually by using the syringe, either attached directly to the bronchoscope or to the tubing. All fluid was combined into a single pooled sample in a 50-ml conical centrifuge tube and placed on ice.
Volume determination.
The total volume of BAL fluid recovered was measured. For both methods of aspiration, volume yield was calculated by dividing the total volume of lavage fluid recovered by the initial volume of instillate in the syringes. All cellular calculations were based on a pooled sample, which included all fluid in syringes regardless of their volume capacity.
Cytologic preparations.
For each sample, 3 ml of bronchoalveolar lavage pooled fluid (BALPF) was aliquoted into 4.9-ml gel K2EDTA plastic tubes (Becton Dickinson, Franklin Lakes, NJ) and maintained on ice. All samples were batch-processed within 1 h of collection. Two direct smears of each sample were made from the BALPF and air-dried. For each sample, 1 cytospin smear was made by the addition of 100 µL BALPF into a sample chamber (Shandon Single Cytofunnel, Thermo Electron Corporation, Waltham, MA). The cytospin preparation was centrifuged at 200 × g for 6 min in a cytocentrifuge (Shandon Cytospin 3, Thermo Electron Corporation). The cytospin smear was air-dried. Both the direct and cytospin smears were stained with modified Wright stain by using an automated stainer (Hema-Tek, Miles Scientific, Naperville, IL). A single cytologist evaluated all stained smears for morphologic and staining properties. A 150- to 200-cell nucleated differential count from 5 or more fields of the stained cytospin preparation was made by using the 100× oil objective.
Cell preparation.
The remaining BALPF fluid of each sample was aliquoted into multiple 15-ml polypropylene centrifuge tubes (Corning, Corning, NY) and maintained on ice until processing. All samples were batch-processed within 1 h of collection. Samples were centrifuged at 400 × g for 7 min (Allegra 6R Centrifuge, Beckman Coulter, Fullerton, CA). The supernatant was discarded, and the cell pellet was resuspended in 0.5 ml of a mixed-media solution made by combining 500 ml RPMI 1640 without glutamine (Mediatech, Herndon, VA), 25 ml FBS (HyClone, Logan, UT), 5 ml penicillin–streptomycin solution (10,000 U penicillin/ml and 10,000 μg streptomycin/ml; Cambrex, Walkersville, MD), 5 ml L-glutamine (200 mM in 0.85% NaCl; Cambrex), and 5 ml 1M HEPES (HyClone). Resuspended BALPF samples were maintained on ice until total nucleated cells were counted or samples were prepared for flow cytometry.
Total nucleated cell count.
Viability testing and a total nucleated cell count were performed by diluting resuspended BALPF in trypan blue dye (1:9). Dye exclusion and cell counts were performed by using KOVA Glasstic Slides 10 with Grids (Hycor Biomedical, Garden Grove, CA). The reported total nucleated cell count was calculated by using the formula supplied by the slide manufacturer:
No. cells/µl = Total no. cells counted (live + dead)/9 (no. of small grids counted) × 90 (slide factor) × 9 (dilution factor) × total volume of BALPF (ml) collected/volume of aliquot (ml).
Flow cytometry immunophenotyping.
Resuspended BALPF was filtered through nylon mesh to remove mucus and debris. Antibodies used for 4-color lymphocyte immunophenotyping were mouse antihuman antibodies for CD3–fluorescein isothiocyanate (clone SP34), CD4–allophycocyanin (clone SK3), CD8–peridinin-chlorophyll-protein complex (clone SK1), and CD20–peridinin-chlorophyll-protein complex (clone L27) from BD Biosciences (San Jose, CA). Filtered BALPF and antibodies were added to 5-ml polystyrene round-bottom tubes (BD Falcon, San Jose, CA) and incubated for 30 min at 4 °C in the dark. Red blood cells were lysed with a 1:10 dilution of 1× BD FACS Lysing Solution (BD Biosciences) as directed by the manufacturer. After a PBS wash, the final cell pellet was fixed with a 2% paraformaldehyde solution (reagent-grade paraformaldehyde powder, Sigma-Aldrich, St Louis, MO). Flow cytometry (FACSCalibur [BD Biosciences] with Macintosh Operating System 10.4.1 [Apple, Cupertino, CA]) was performed within 24 h of cell preparation. Data were acquired by using Cell Quest Pro 5.2.1 (BD Biosciences); a minimum of 20,000 events was recorded for each sample. Data were analyzed by using FlowJo for Windows (Tree Star, Ashland, OR). Each sample was evaluated by forward scatter versus side scatter for identification of the lymphocyte population; CD3+ T lymphocytes and CD20+ B lymphocytes were gated within the lymphocyte population. The CD3+ gate was used to identify CD4+ and CD8+ T lymphocytes.
Statistical analysis.
Data are expressed as mean ± SD. All data were analyzed by using Statistica software (StatSoft, Tulsa, OK). Paired t tests were used to compare cBAL and mBAL results for all except morbidity measures. Morbidity measures were analyzed by repeated-measures ANOVA, with post hoc pairwise comparisons after Bonferroni correction if significant main effects were obtained. Significance level was set at a P value of less than 0.05 for all analyses.
Results
BAL samples collected from 20 healthy Indian origin rhesus macaques by 2 different techniques were examined for significant differences in regards to sample quality, animal morbidity, and ease of use by operators. Both BAL techniques were performed on each monkey, with a 2-wk interval between methods.
The mBAL technique yielded a greater volume of BAL fluid than did cBAL. The mean amount of fluid recovered in the mBAL group was 41.38 ml (range, 36 to 46 ml; 72% to 92% of the instilled volume) compared with a mean of 37.78 ml (28 to 44 ml; 56% to 88%) obtained by using the cBAL method (Figure 3); this 3.6-ml difference was statistically significant (t19 = 3.55, P = 0.002). The mean volume collected by using the mBAL method was 83.92% (range, 72% to 96%) of fluid was recovered compared with only 75.63% (range, 57% to 88%) with the cBAL method; this difference was statistically significant (t19 = 3.71, P = 0.001).
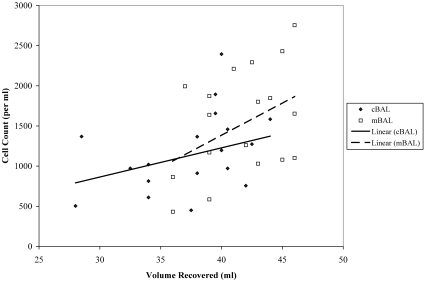
Figure 3.
Scatterplot with linear trend lines of volume recovered and total cell count for each BAL method. Both methods reveal a positive association between the total volume recovered and the total cell count.
A higher concentration of cells was obtained by using the mBAL technique (t19 = 2.24, P = 0.037; Figure 3). No significant differences were found between groups for the percentage of live cells, dead cells, macrophages, lymphocytes, eosinophils, mast cells, neutrophils, or goblet cells. All bacterial cultures of BALPF samples yielded normal oral contaminants or negative cultures. No significant differences were found in flow cytometry measures (Table 1).
Table 1.
Comparison of cBAL and mBAL data
| cBAL | mBAL | P | |
| Volume recovered (ml) | 37.78 ± 4.51 | 41.38 ± 3.52 | 0.002 |
| Volume yield (%) | 0.76 ± 0.09 | 0.84 ± 0.07 | 0.001 |
| Cell data | |||
| Total cell count (cells/μl) | 1146.38 ± 109.35 | 1495.31 ± 646.27 | 0.037 |
| Live nucleated cells (% of total nucleated cells) | 92.00 ± 14.52 | 93.35 ± 9.98 | 0.760 |
| Dead nucleated cells (% of total nucleated cells) | 6.55 ± 13.17 | 6.65 ± 9.98 | 0.980 |
| Eosinophils (% of total nucleated cells) | 5.60 ± 9.52 | 3.05 ± 3.69 | 0.268 |
| Goblet cells (% of total nucleated cells) | 0.10 ± 0.31 | 0.25 ± 0.44 | 0.186 |
| Leukocytes (% of total nucleated cells) | 5.05 ± 2.04 | 5.95 ± 2.48 | 0.160 |
| Lymphocytes (% of total nucleated cells) | 9.70 ± 7.00 | 8.85 ± 3.98 | 0.582 |
| Macrophages (% of total nucleated cells) | 80.75 ± 13.39 | 84.55 ± 9.67 | 0.114 |
| Mast cell (% of total nucleated cells) | 2.50 ± 4.21 | 2.45 ± 4.61 | 0.934 |
| Neutrophils (% of total nucleated cells) | 1.25 ± 1.07 | 0.90 ± 0.72 | 0.201 |
| Flow cytometry | |||
| Granulocytes (% of total nucleated cells) | 12.97 ± 11.60 | 10.60 ± 5.04 | 0.423 |
| Monocytes (% of total nucleated cells) | 40.17 ± 11.56 | 36.40 ± 12.19 | 0.337 |
| Lymphocytes (% of total nucleated cells) | 38.55 ± 11.33 | 39.81 ± 12.17 | 0.706 |
| CD3+ T cells (% of lymphocytes) | 90.55 ± 2.99 | 90.43 ± 3.18 | 0.899 |
| CD4+ T cells (% of CD3+ lymphocytes) | 40.88 ± 8.72 | 42.60 ± 9.06 | 0.550 |
| CD8+ T cells (% of CD3+ lymphocytes) | 62.20 ± 8.71 | 64.69 ± 5.38 | 0.275 |
| CD20+ B cells (% of lymphocytes) | 4.72 ± 2.23 | 5.48 ± 2.47 | 0.273 |
| Respiration rate (breaths/min) | 0.942 | ||
| Before procedure | 31.90 ± 9.61 | 30.90 ± 7.15 | |
| Immediately after procedure | 32.05 ± 8.67 | 30.40 ± 9.39 | |
| 15 min after procedure | 31.20 ± 7.85 | 30.80 ± 8.24 | |
| Heart rate (beats/min) | 0.862* | ||
| Before procedure | 144.85 ± 26.33 | 149.35 ± 21.76 | |
| Immediately after procedure | 164.40 ± 23.43 | 166.30 ± 24.67 | |
| 15 min after procedure | 130.40 ± 21.50 | 131.45 ± 25.50 | |
| Oxygen saturation (%) | 0.680 | ||
| Before procedure | 95.05 ± 2.19 | 95.85 ± 2.25 | |
| Immediately after procedure | 95.75 ± 3.08 | 95.25 ± 3.18 | |
| 15 min after procedure | 97.55 ± 7.74 | 97.05 ± 2.42 | |
| Temperature (ºC) | 0.909* | ||
| Before procedure | 37.55 ± 17.16 | 37.67 ± 17.27 | |
| Immediately after procedure | 37.05 ± 17.17 | 37.00 ± 17.23 | |
| 15 min after procedure | 36.70 ± 17.18 | 36.59 ± 17.25 |
Data were compared by using paired t tests, except for morbidity measures (repeated- measures ANOVA)
*, interaction effect of group x time was not significant, however there was a significant main effect of time regardless of group
Morbidity, respiratory rate, heart rate, oxygen saturation, and rectal temperature data were collected prior to, immediately after, and 15 min after BAL procedures. Clinical morbidity such as bronchial collapse, bronchospasm, and contusion formation did not occur. There were no significant interaction effects of BAL technique by time (before, immediately after, and 15 min after) for respiratory rate, heart rate, oxygen saturation, and rectal temperature (Table 1).
A survey comprising multiple-choice questions was presented orally to the technician and veterinarian performing procedures at the end of the study revealed that both participants felt mBAL enabled more precise control of syringe-generated suction and easier syringe changing. The veterinarian reported less stress of hand and wrist and more comfortable positioning with mBAL, with subsequently reduced hand, wrist, and arm fatigue.
Discussion
The goal of this study was to compare the cBAL and mBAL methods of applying mechanical suction while performing BAL in nonhuman primates. Lavage fluid volumes and cellular components, morbidity, and operator ergonomics were evaluated. The BAL fluid data demonstrate the advantages of the mBAL technique. The mBAL method resulted in less residual fluid in the lungs after aspiration. This feature may have implications for lowering morbidity associated with BAL. The data also suggest that mBAL increased the comfort and efficiency of the staff performing the procedure.
The lavage fluid obtained by using mBAL was significantly greater in volume and cell concentration than that from cBAL. This increase in fluid recovery could lead to more accurate diagnoses by providing more cells for culture and analysis. This increased yield also generates a larger sample for research studies from the same amount of instillate as the cBAL, potentially providing researchers with superior sample quality while posing no increased risk to research animals. In addition to improved recovery of cells and fluid volume, results of cell viability analysis, flow cytometry, bacterial culture, and differential counts did not differ between mBAL and cBAL. Overall, mBAL provides increased fluid recovery with increased cell concentration without altering typical cellular ratios.
Based on the data collected in this study, we speculate that the mBAL method improves on the cBAL collection method and may reduce the number of animals required for studies involving BAL fluid sampling. Fewer animals and smaller volumes of instillate may be required to achieve scientific and diagnostic goals. In addition, associated morbidity may decline with the recovery of more lavage fluid after instilling less volume. In human patients, common complications of BAL include temporary decrease in lung function parameters, alveolar infiltrates, fever, bronchial hyperactivity, and bronchospasm. In human studies, complication rates were higher with the cBAL method versus the mBAL method. Due to the limitations of the present study to accurately quantify the afore-mentioned complications in nonhuman primates, clinical morbidity such as bronchial collapse, bronchospasm, and contusion formation were monitored at the time of the procedures and were not observed. Although we noted no clinical differences between groups in our study, we hypothesize that with more technically advanced monitoring systems, mBAL actually may prove to cause fewer complications than does the cBAL method, as shown in human studies.12 Heart rate, respiratory rate, oxygen saturation, and temperatures were recorded for each animal prior to BAL, immediately after the procedure, and 15 min later. These data revealed no significant differences between groups, indicating no disadvantages of mBAL with respect to animal health.
When presented with a series of multiple choice questions, the technician and veterinarian performing the procedures indicated that mBAL afforded a more comfortable arm position, resulting in less fatigue, and enabled participants more precise control of syringe-applied suction and easier syringe changes. These advantages are especially important in research medicine, given the large number of animals in which BAL is used to collect samples, often in a single day. Both the technician and veterinarian performing the procedures in this study preferred the mBAL technique.
As in a previous comparison of BAL techniques,12 the aim of the current study was not to investigate the biophysical principles that may explain the ability of tubing to increase BAL fluid recovery. Perhaps the presence of the tubing between the syringe and bronchoscope mitigates the high negative peak pressures originating from hand suction. This factor would influence fluid dynamics by partially converting turbulent flow into laminar flow. Further perhaps the more comfortable position of the syringe allows the user to control the suction force applied more accurately. In addition, future studies are required to compare mBAL and cBAL with other methods such as gravity drainage and mechanical suction.
The mBAL method has several advantages over cBAL. First, more fluid is recovered using the same amount of instillate. In addition, mBAL enhances cell counts without changes to differential cell count percentages established by using cBAL as a control. Increasing the cell concentration of the recovered fluid creates a more valuable diagnostic or research sample. Using less lavage fluid, decreasing turbulent flow, and controlling suction more precisely most likely will reduce BAL-associated tissue damage and morbidity. The mBAL method provides more control and improved operator comfort and is better ergonomically. Given the results of this study, we recommend the use of mBAL to scientists using the handheld syringe method for BAL fluid recovery in rhesus monkeys.
Acknowledgments
We would like to thank the technicians and animal care staff at the Tulane National Primate Research Center, especially Samantha Groce and Victoria Williams. This work supported by the Tulane National Primate Research Center Base Grant 5P51 RR00164-41 and grants G20 RR016930-01, G20 RR018397-01, G20 RR019628-01, G20 RR013466-01, G20 RR012112-01, G20 RR05169-01, AI49080, and AA13563 from the National Institutes of Health.
References
- 1.Andreasen CB. 2003. Bronchoalveolar lavage. Vet Clin North Am Small Anim Pract 33:69–88 [DOI] [PubMed] [Google Scholar]
- 2.Balfour-Lynn IM. 2002. Bronchoscopy—how and when? Paediatr Respir Rev 3:255–264 [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP. 1992. Bronchoalveolar lavage. In: Manning S, Petrash, Thorp D. The handling and analysis of bronchoalveolar lavage specimens. Bronchoalveolar lavage in infectious disease. St Louis: Mosby Year Book; p 3–64 [Google Scholar]
- 4.Crystal RG, Reynolds HY, Kalica AR. 1986. Bronchoalveolar lavage. The report of an international conference. Chest 90:122–131 [DOI] [PubMed] [Google Scholar]
- 5.De Blasio F, Rotondetto S, Sarno M, Pezza A. 1995. Arterial oxygen desaturation as a consequence of different bronchoalveolar techniques. J Bronchology 2:107–112 [Google Scholar]
- 6.Haley PJ, Muggenburg BA, Rebar AH, Shopp GM, Bice DE. 1989. Bronchoalveolar lavage cytology in cynomolgus monkeys and identification of cytologic alterations following sequential saline lavage. Vet Pathol 26:265–273 [DOI] [PubMed] [Google Scholar]
- 7.Hawkins EC, DeNicola DB, Kuehn NF. 1990. Bronchoalveolar lavage in the evaluation of pulmonary disease in the dog and cat. State of the art. J Vet Intern Med 4:267–274 [DOI] [PubMed] [Google Scholar]
- 8.Laviolette M, Carreau M, Coulombe R. 1988. Bronchoalveolar lavage cell differential on microscope glass cover. A simple and accurate technique. Am Rev Respir Dis 138:451–457 [DOI] [PubMed] [Google Scholar]
- 9.National Research Council 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 10.Novak Z, Petak F, Banfi A, Toth-Szuki V, Barati L, Kosa L, Bari F, Szekely E. 2006. An improved technique for repeated bronchoalveolar lavage and lung mechanics measurements in individual rats. Respir Physiol Neurobiol 154:467–477 [DOI] [PubMed] [Google Scholar]
- 11.Reynolds HY. 1987. Bronchoalveolar lavage. Am Rev Respir Dis 135:250–263 [DOI] [PubMed] [Google Scholar]
- 12.Rosell A, Xaubet A, Agusti C, Castella J, Puzo C, Curull V, de Gracia J. 2006. A new BAL fluid instillation and aspiration technique: a multicenter randomized study. Respir Med 100:529–535 [DOI] [PubMed] [Google Scholar]
- 13.Smith PA, Kohli LM, Wood KL, Hage CA, Twigg HL, 3rd, Knox KS. 2006. Cytometric analysis of BAL T cells labeled with a standardized antibody cocktail correlates with immunohistochemical staining. Cytometry B Clin Cytom 70:170–178 [DOI] [PubMed] [Google Scholar]
- 14.Tate MK, Rico PJ, Roy CJ. 2004. Comparative study of lung cytologic features in normal rhesus (Macaca mulatta), cynomolgus (Macaca fasicularis), and African green (Chlorocebus aethiops) nonhuman primates by use of bronchoscopy. Comp Med 54:393–396 [PubMed] [Google Scholar]
- 15.Thomas MJ, Flanary LR, Brown BA, Katze MG, Baskin CR. 2006. Use of human nasal cannulas during bronchoscopy procedures as a simple method for maintaining adequate oxygen saturation in pigtailed macaques (Macaca nemestrina). J Am Assoc Lab Anim Sci 45:44–48 [PubMed] [Google Scholar]