Abstract
The objective of this study was to determine the level of anesthesia attained in Xenopus laevis frogs using a propofol bath administration. Thirty-three nonbreeding female Xenopus laevis frogs were used. At 175 mg/l, all frogs died after bath administration. An appropriate anesthetic dose was determined to be 88 mg/l for 15 min. After administration of this dose, the acetic acid test, withdrawal reflex, righting reflex, heart rate, and respiratory frequency were used to evaluate central nervous system depression. Pharmacokinetics of propofol were calculated after blood concentration determination by tandem liquid chromatography–mass spectrometry analyses. Short-duration anesthesia (less than 30 min) was obtained, and in many frogs, muscular fasciculation was seen during the acetic acid test. The area under the time–concentration curve (AUC0–t) was 24.07 μg·min/ml, and AUCinf was 24.71 μg·min/ml. The elimination half-life was 1.18 h. When administered as a single-bath immersion for 15 min, propofol does not appear to be a safe and effective anesthetic for Xenopus laevis frogs, due to a narrow dose–effect window, short duration, and shallow level of anesthesia obtained.
Abbreviation: AUC, area under the time–concentration curve; bpm, beat per minute; Clast, last measurable plasma concentration; Cmax, maximal concentration; GABA, γ-aminobutyric acid; kel, terminal rate constant of elimination; MS222, tricaine methanesulfonate; rpm, respirations per minute; T1/2, half life
Amphibian medicine and surgery are of increasing interest in veterinary practice. Many drugs have been used as anesthetics in amphibians, including systemic injections of ketamine4 and other cyclohexanones, such as tiletamine combined with zolazepam;13 benzocaine;5,21,26 volatile anesthetics such as methoxyflurane, halothane, and isoflurane administered topically or via a water bath;18,19,23,25 intracoelomic injection of medetomidine or barbiturates;4,11,22 and bath administration of clove oil or eugenol.5,6,11 Historically, tricaine methanesulfonate (MS222) has been the most commonly used agent for inducing anesthesia in frogs.3,12,14 Propofol has rarely been used in amphibians. In frogs, sublingual plexus injection (10 mg/kg) of propofol rapidly achieved sedation but not surgical anesthesia.11 Intraceolomic administration of propofol (30 mg/kg) may provide adequate anesthesia,22 but this result was achieved in only 1 Pelodryas caerula frog. Therefore, although many anesthetic substances have been used in amphibians, they have produced variable planes and durations of anesthesia.
Anesthesia of amphibians is controversial with regard to defining the state of anesthesia. Pharmacokinetic studies of analgesics and anesthetics used in frogs are still needed, as are standardized methods to evaluate the depth of anesthesia in frogs. Onset and depth of anesthesia can be determined through the observation of several distinct phases: prephase of paradoxical excitement, loss of righting reflex, cessation of abdominal respiration movements, cessation of spontaneous movements, loss of corneal reflex, loss of guttural movements, and cessation of withdrawal movements after nociceptive stimulation (in order of progressive anesthesia depth).2,12,19 Based on a recent publication, amphibians can be considered anesthetized if loss of the righting and withdrawal reflexes is achieved.25
Because bath administration has shown some success for anesthetizing frogs and because few studies have been done to evaluate propofol as an anesthetic, the primary goal of this study was to determine the anesthetic properties of propofol in Xenopus laevis frogs using a bath administration.
Materials and Methods
Animals.
A total of 33 nonbreeding female Xenopus laevis frogs (Xenopus I, Dexter, MI) with body weights ranging from 115 to 165 g were used. Frogs were housed in water-filled (>4 l/frog) polycarbonate cages (40 cm × 20 cm × 15 cm; Ancare, Bellmore, NY). The purified water was obtained from filtering through activated charcoal and UV treatment (pH, 6.8 to 7.3; chlorine and chloramines, 0 mg/ml; ammonia, 0.2 mg/ml (normal range, 0.4 to 0.6 mg/ml); nitrites, 0 mg/ml (normal, <1 mg/ml); hardness, 0 (normal range, 70 to 150 mg/ml); copper, 0 mg/ml [all tubing was polyvinyl chloride]). Water and room temperatures were kept at 21 ± 2 °C at all times. Every 2 d, the water was changed, and the containers were cleaned. Animals were fed every other day with blood worms (Bloodworms Tropical Fish Food, Hayward, CA). The experimental protocol was approved by the institutional Animal Care and Use Committee of the Faculty of Veterinary Medicine of the University of Montreal prior to animal use and is in accordance with the guidelines of the Canadian Council on Animal Care.
Chemicals.
Propofol (purity, 97%) in its liquid form (0.962 g/ml) was purchased from Sigma (St Louis, MO).
Pharmacodynamic study.
In a pilot study, 9 frogs were used (n = 3 per dose). Immersion for 15 min in a bath containing propofol at 350, 175, and 88 mg/l were used sequentially to evaluate anesthesia. With the higher doses (350 and 175 mg/L), all frogs died. An apparently acceptable level of anesthesia was seen with 88 mg/l (data not shown), and this dose was chosen for the main study.
Six frogs were used in the efficacy study. Frogs were placed individually into a polycarbonate cage with the depth of purified water adjusted (approximately 1 to 2 cm) to keep the nostrils out of the water after propofol administration. During experiments, animals were regularly (2 to 3 min) sprayed with purified water to ensure optimal gas exchange.11,12 For the immersion bath, the propofol solution (88 mg/l; pH, 7.3) was placed in a metal container (15 cm diameter × 13 cm depth). The animal was immersed for 15 min and the container was covered so that the frog was kept in total darkness for the duration of induction. Frogs then were thoroughly rinsed with purified water and placed into polycarbonate cages with 2 to 3 cm of purified water.
Various behavioral tests were used to evaluate the effects of propofol. The acetic acid test,20 righting reflex, withdrawal reflex, heart rate, and respiratory frequency were used to assess the effects of propofol before immersion and at 0 (end of immersion bath), 15, 30 min, and 1 and 2 h after exposure to propofol. All tests have been described previously.6 Frogs first were assessed with the acetic acid test, which gives an indication of the analgesic properties in response to chemical induced pain stimuli.16 Application of single drops (20 µl) of incrementally increasing concentrations (0, 5, 10, 15, 20, 25, 30, 40, 80, and 100%) of glacial acetic acid to the dorsum of the frog's thigh or leg. A contact time of 5 s was allowed before thoroughly rinsing the tested skin area with purified water. The test was considered positive if the frog exhibited the wiping response, which consists of a motor reflex whereby the frog dislodged the drop by using its other leg. If no reaction was observed, the next higher concentration was applied, alternating between right and left legs and thighs. The level of desensitization was rated as the lowest concentration of glacial acetic acid at which a wiping response occurred.
Heart and respiratory rates, as well as the righting reflex and withdrawal reflex, were evaluated after completion of the acetic acid test. Heart rate was evaluated by using a flow detector (Doppler Ultrasonic Flow Detector, 8.1 mHz, model 811B, Parks Medical Electronics, Aloha, OR). Frogs were placed with the probe over the sternum. Abdominal or nare movements were counted for respiratory frequency.10 The righting reflex was evaluated by assessing the animal's ability to turn onto its ventrum when placed on its back. The withdrawal reflex was tested by pinching a phalangeal articulation of the pelvic limb with surgical forceps for a maximum of 2 s. Heart and respiratory rates were evaluated prior to all reflexes.
Pharmacokinetic study.
A total of 18 frogs (n = 3 per time point) were used for this study. They were placed individually in an immersion bath containing 88 mg/l propofol. Terminal blood samples (0.3 ml) were collected from the heart at 0, 0.5, 1, 2, 4, and 6 h after administration. When insufficiently anesthetized for intracardiac blood sampling, the frogs were anesthetized with a 0.2% solution of MS222.19 Whole blood content of propofol was measured by tandem liquid chromatography–mass spectrometry (PESciex API 3+, Applied Isosystem–MDS Sciex, Concord, ON, Canada) according to previously published methods.1
Pharmacokinetic parameters of propofol in blood were calculated for each animal by using noncompartmental methods.17 The area under the time–concentration curve from time 0 to the last measurable concentration values (AUC0–t) was calculated by using the linear trapezoidal rule. A terminal rate constant of elimination (kel) was calculated by using a minimum of 3 measurable plasma concentrations, and the terminal elimination half-life (T1/2) was calculated by using 0.693/kel. AUC extrapolated to infinity (AUCinf) was calculated by using AUC0–t + Clast/kel, where Clast was the last measurable plasma concentration.
Statistics and pharmacokinetics.
Statistical analysis was performed with SAS software (version 8.2, SAS Institute, Cary, NC). Statistical significance was set at a P level of 0.05. The acetic acid test, heart rate, and respiratory frequency results were evaluated by using a repeated-measures linear model with time as a within-subject factor. Posthoc Dunnett statistical tests were used to evaluate whether postanesthesia results at selected time points were significantly different from control (–15 min) values.
Results
All 6 animals used in the efficacy study showed darkened skin color, decreased muscle tone, but no signs of ill health at 24 h after immersion in propofol at 88 mg/l.
Pharmacodynamic study.
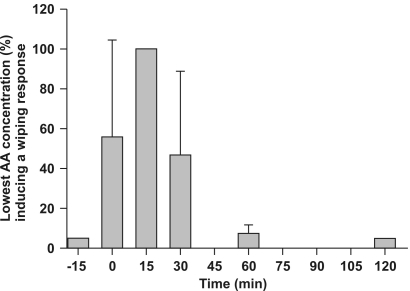
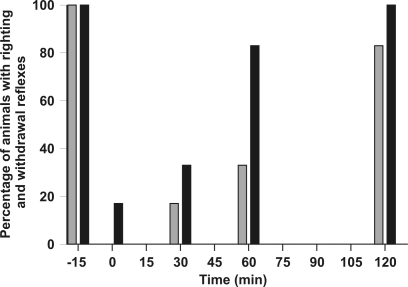
Figure 1 presents the wiping response results after bath immersion of frogs in propofol (88 mg/l). Loss of the nociceptive response occurred on removal of the frogs from the bath until 30 min thereafter. The wiping response was absent (P < 0.0001) only at 15 min after immersion. Even though a high acetic acid concentration (mean, 40% to 60%) was necessary to induce a wiping response at 0 and 30 min (P < 0.001), muscle fasciculations were present in 4 of 6 animals after application of the acetic acid drop; similar muscle contractions occurred in the remaining 2 frogs at 15 min after induction in the absence of a wiping response. The wiping response at 60 and 120 min did not differ from baseline. Figure 2 shows that most animals showed a depressed righting reflex at 0, 30, and 60 min (100%, 83%, and 66%, respectively, of animals tested) and a depressed withdrawal reflex at 0 and 30 min (83% and 66% respectively). The righting and withdrawal reflexes were nearly fully recovered at 60 and 120 min, respectively.
Figure 1.
Lowest acid acetic concentration (AA, %) inducing the wiping response in Xenopus laevis frogs (n = 6) after immersion in a propofol solution (88 mg/l). Baseline values 15 min before propofol bath immersion are shown as well as responses for 0 to 120 min after 15-min immersion in the propofol solution.
Figure 2.
Percentage of Xenopus laevis frogs (n = 6) with a righting reflex (gray bars) and withdrawal reflex (black bars) after immersion in a propofol solution (88 mg/l). Baseline values 15 min before propofol bath immersion are shown as well as responses for 0 to 120 min after 15-min immersion in the propofol solution.
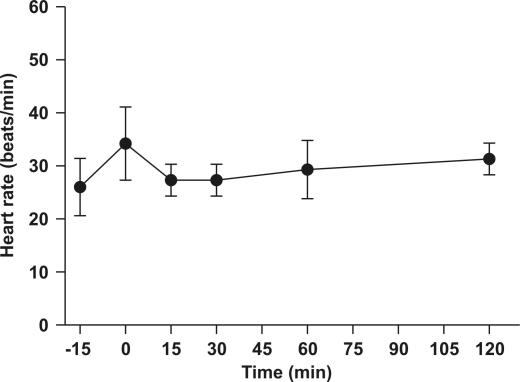
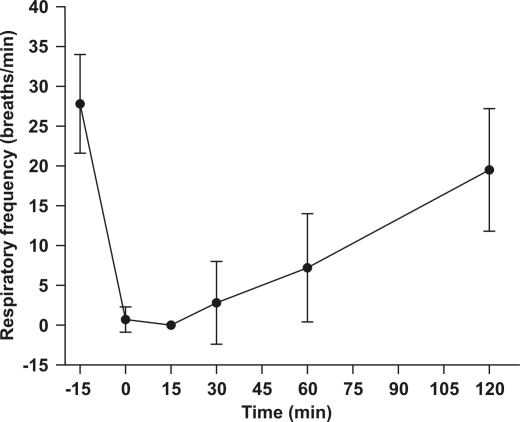
Propofol did not cause significant effects on heart rate (mean baseline, 29.9 ± 2.9 beats per minute [bpm]; minimum, 24 bpm; maximum, 45 bpm; Figure 3. However, propofol did induce respiratory depression (P < 0.01; Figure 4). The mean respiratory rate significantly decreased from baseline (27.8 ± 6.2 respirations per minute [rpm]) to an average of 0.7 ± 1.6 and 0 rpm at 0 and 15 min, respectively, and progressively returned to 19.5 ± 7.7 rpm at 2 h after induction.
Figure 3.
Mean (± SD) heart rate 15 min before and after immersion of Xenopus laevis frogs (n = 6) in a propofol solution (88 mg/l).
Figure 4.
Mean (± SD) respiratory frequency 15 min before and after immersion of Xenopus laevis frogs (n = 6) in a propofol solution (88 mg/l).
Pharmacokinetic study.
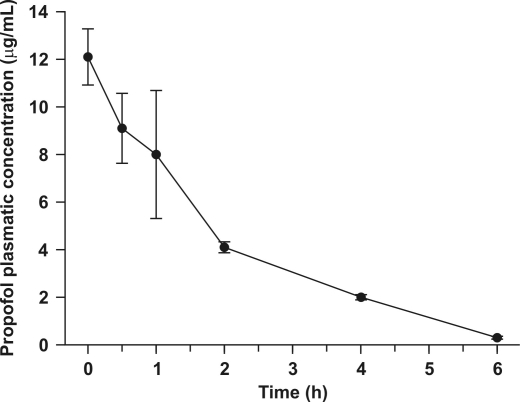
Pharmacokinetics data (Figure 5) were calculated from whole-blood concentrations only. The maximal blood concentration was 12.1 µg/ml at 0 min after induction, AUC0-t was 24.07 μg·min/ml, and AUCinf was 24.71 μg·min/ml. Further T1/2 was 1.18 h, suggesting rapid elimination and little likelihood of drug accumulation with daily administration.
Figure 5.
Semilogarithmic representation of the mean (± SD) propofol blood concentration at selected time points. Xenopus laevis frogs (n = 3/time point) were immersed in a propofol solution (88 mg/l).
Discussion
Propofol produces brief, shallow anesthesia in African clawed frogs when administered by immersion at a concentration of 88 mg/l. High concentrations (175 and 350 mg/l) caused death in all animals. Behavioral tests revealed that the duration of analgesia was at least 30 min, but the depth of anesthesia was considered to be light. During the acetic acid test, frogs (4 of 6) showed muscle fasciculations at 15 min after induction, and some frogs (1 of 6 and 2 of 6) reacted at 0 and 30 min after induction, respectively, during the withdrawal test. In a previous study,9 propofol (10 mg/kg) injected extravascularly into the sublingual plexus area of Rana pipiens induced sedation, but anesthesia was present in only 58% of the tested population without achieving surgical anesthesia. In accordance with our study, propofol does not appear to be a suitable anesthetic agent for anesthesia in frogs when administered as a bath immersion. Other routes of administration should be evaluated because propofol appears to provide adequate anesthesia after intraceolomic administration.22 Because bath immersion with propofol led to light anesthesia at 88 mg/l and death at 175 mg/l (22 and 44 mg per 250 ml, respectively), the safety margin for efficacy is narrow, and this drug–route combination is not a good candidate for surgical anesthesia.
Propofol is a general anesthetic that enhances the function of γ-aminobutyric acid (GABA) receptors.7,16 Binding to this receptor causes inhibitory activity of the central nervous system leading to immobility and unconsciouness.15,24 Although GABA is a leading inhibitory neurotransmitter in vertebrates, differences may be seen across species. For example, markedly more GABA immunoreactive cells are present in the brains of bullfrogs than in those of Xenopus.8 This difference suggests different levels of anesthesia in amphibians for a given administered concentration.
Clove oil has been used to anesthetize Rana pipiens frogs;5,11 bath immersion at 315 mg/l achieves anesthesia in most animals. Eugenol, the active molecule of clove oil, can be used to anesthetize frogs by using a concentration of 350 mg/ml; frogs are placed in approximately 250 ml of immersion solution for 15 min.6 Surgical anesthesia is obtained for approximately 30 min, and recovery is seen within 1 to 2 h. The 4-h half-life of 4 eugenol suggests little accumulation with repeated administration and prolonged duration of anesthetic effects than seen with propofol (T1/2 = 1.18 h). The short duration and light anesthesic level in Xenopus frogs may be explained by rapid elimination or metabolism of propofol when compared with a similar phenol-like chemical (eugenol) which is also high lipophilic and because Xenopus appear to have fewer GABAergic neurons than other frogs.
Anesthetics known to work well in frogs are MS222, eugenol, and benzocaine. MS222 is a water-soluble acid salt present as an unionized form that has successfully been used to immobilize and anesthetize amphibians.2,3,12,26 When dissolved in water at concentrations required for anesthesia (10 to 20 min), the solution has a pH of approximately 3. For this reason a buffered solution is prepared: 1 g MS222 in 1 l water plus 25 ml 0.5 M Na2HPO4 to yield a 0.1% solution. Even with pH adjustments, erythema of light skin areas, such as the ventrum, may still occur during MS222 anesthetic induction. Although MS222 is often the first choice for anesthetizing frogs and fish, it is toxic to handlers and should be used with care.6
A solution of 0.02% to 0.03% of benzocaine (1% alcohol) can be used to achieve surgical anesthesia in amphibians.21,26 Recovery typically occurs within 60 min. Another method is to apply 20% benzocaine cream (0.1 ml/10 g; e.g., Orajel, Del Laboratories, Uniondale, NY) topically,5 which safely immobilized frogs and anesthetized 50% of the animals for approximately 15 min.
Other anesthetics have been used in frogs with variable success. Isoflurane topically (bubbling in water or skin application) or administered by injection has been used,18 but injections did not induce sedation and caused death of some animals. Topical application of isoflurane induced brief (10 to 30 min) in a small percentage of animals. Barbiturates should be used with caution in amphibians due to the low safety margins of these drugs.26 Intramuscular injections of ketamine or tiletamine with zolazepam failed to induce anesthesia at low doses and produced unacceptable mortality rates at high doses.13
Propofol bath immersion of Xenopus leavis frogs does not appear to be a safe and effective anesthetic approach in light of its narrow dose–effect window as well as the brief and shallow anesthesia obtained.
Acknowledgments
The authors would like to thank Guy Beauchamp (Faculty of Veterinary Medicine, University of Montreal) for statistical analyses and Marie-Therese Parent for the preparation of the figures.
References
- 1.Beaudry F, Guénette SA, Winterborn A, Marier JF, Vachon P. 2005. Development of a rapid and sensitive LC-ESI/MS/MS assay for the quantification of propofol using a simple off-line dansyl chloride derivatization reaction to enhance signal intensity. J Pharm Biomed Anal 39:411–417 [DOI] [PubMed] [Google Scholar]
- 2.Crawshaw GJ. 1993. Amphibian medicine. In: Fowler M. Zoo and wild animal medicine: current therapy 3. Philadelphia: WB Saunders; p 131–139 [Google Scholar]
- 3.Downes U. 1995. Tricaine methanesulfonate in amphibian: a review. Bull Assoc Rept Amph Vet 5:11–16 [Google Scholar]
- 4.Green CJ. 1979. Classes amphibia, reptilia and aves. In: Green CJ. Animal anesthesia, Laboratory animal handbook 8. London: Laboratory Animals Ltd; p 111–113 [Google Scholar]
- 5.Guénette SA, Lair S. 2006. Anesthesia of the leopard frog, Rana pipiens: a comparative study between four different agents. J Herpetol Med Surg 16:38–44 [Google Scholar]
- 6.Guénette SA, Hélie P, Beaudry F, Vachon P. 2007. Eugenol for anesthesia of African clawed frogs (Xenopus laevis). Vet Anaesth Analg 34:164–170 [DOI] [PubMed] [Google Scholar]
- 7.Hales TG, Lambert JJ. 1991. The action of propofol on inhibitory aminoacid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol 104:619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollis DM, Boyd SK. 2005. Distribution of GABA-like immnoreactive cell bodies in the brain of two amphibians, Rana catesbeiana and Xenopus leavis. Brain Behav Evol 65:127–142 [DOI] [PubMed] [Google Scholar]
- 9.Hutchison V, Whitford W, Kohl M. 1968. Relation of body size and surface area to gas exchange in anurans. Physiol Zool 41:65–85 [Google Scholar]
- 10.Jørgensen CB. 2000. Amphibian respiration and olfaction and their relationships: from Robert Townson (1794) to the present. Biol Rev Camb Philos Soc 75:297–345 [DOI] [PubMed] [Google Scholar]
- 11.Lafortune M, Mitchell MA, Smith JA. 2001. Evaluation of medetomidine, clove oil, and propofol for anesthesia of leopard frogs, Rana pipiens. J Herpetol Med Surg 11:13–18 [Google Scholar]
- 12.Letcher J. 1992. Intracelomic use of tricaine methanesulfonate for anesthesia of bullfrogs (Rana catesbeiana) and leopard frogs (Rana pipiens). Zoo Biol 11:243–251 [Google Scholar]
- 13.Letcher J, Durante R. 1995. Evaluation of use of tiletamine–zolazepam for anesthesia of bullfrogs and leopard frogs. J Am Vet Med Assoc 207:80–82 [PubMed] [Google Scholar]
- 14.Mader DR. 1996. Amphibian husbandry and medicine. In: Wright KM.Reptile medicine and surgery. Philadelphia: WB Saunders; p 436–459 [Google Scholar]
- 15.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. 2002. The sedative component of anesthesia is mediated by GABAA receptors in an endogenous sleep pathway. Nat Neurosci 5:979–984 [DOI] [PubMed] [Google Scholar]
- 16.Orser BA, Wang LY, Pennefather PS, MacDonald JF. 1994. Propofol modulates activation and desensitization of GABAA receptors in cultured murine hippocampal neuron. J Neurosci 5:979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowland M, Tozer TN. 1995. Clinical pharmacokinetics: concepts and applications. Philadelphia: Lippincott Williams and Wilkins; p 367–389 [Google Scholar]
- 18.Smith JM, Stump KC. 2000. Isoflurane anesthesia in the African clawed frog (Xenopus laevis). Contemp Top Lab Anim Sci 39:39–42 [PubMed] [Google Scholar]
- 19.Stetter MD, Raphael B, Indiviglio F, Ross R. Isoflurane anesthesia in amphibians: comparison of five application methods. Proceedings of the American Association of Zoo Veterinarians; 1996. ; Puerto Vallarta, Mexico; Yulee (FL): AAZV p 255–257 [Google Scholar]
- 20.Stevens CW. 1992. Alternative to the use of mammals for pain research. Life Sci 50:901–912 [DOI] [PubMed] [Google Scholar]
- 21.Vanable JJ. 1985. Benzocaine: an excellent amphibian anesthetic. Axolotol Newslett 14:19–21 [Google Scholar]
- 22.Von Esse FV, Wright KM. 1974. Effect of intracoelomic propofol in White's tree frogs, Pelodryas caerulea. Bull Assoc Rept Amph Vet 9:7–8 [Google Scholar]
- 23.Wass JA, Kaplan HM. 1974. Methoxyflurane anesthesia for Rana pipiens. Lab Anim Sci 24:669–671 [PubMed] [Google Scholar]
- 24.Williams DB, Akabas MH. 2002. Structural evidence that propofol stabilizes different GABAA receptor states at potentiating and activating concentrations. J Neurosci 22:7417–7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright KM. 1996. Amphibian husbandry and medicine. In: Wright KM, Whitaker BR.Amphibian medicine and captive husbandry. Malabar (FL): Krieger Publishing Company; p 436–458 [Google Scholar]
- 26.Wright KM. 2001. Restraint techniques and euthanasia. InWright KM, Whitaker BR. Amphibian medicine and captive husbandry. Malabar (FL): Krieger Publishing Company; p 111–122 [Google Scholar]