Abstract
Objectives
Final pathologic specimen free of detectable disease (P0) is not uncommon in patients undergoing cystectomy for bladder cancer, especially in the era of neoadjuvant chemotherapy. To improve our understanding of its significance in a contemporary series, we performed an outcomes analysis of this cohort of patients.
Methods
Over the last 15 yr, 1104 patients with bladder cancer underwent radical cystectomy at our institution. Of these, 120 (11%) were pT0N0M0 (P0) in the surgical specimen and form the basis of this report. Survival data were estimated by method of Kaplan and Meier, with Cox proportional hazards regression model used to evaluate associations between survival and variables studied.
Results
Clinical stages were cT1, 21 patients; cT2, 65; cT3b, 20; cT4a, 11; and cT4b, 3. The 5-yr estimates of overall (OS), disease-specific (DSS), and recurrence-free survival (RFS) rates were 84%, 88%, and 84%, respectively. With mean follow-up of 43 mo, 11 patients developed recurrences, 9 of whom died of disease. Median time to recurrence was 7.7 mo (range: 2.2–45 mo). On multivariate analysis, presence of lymphovascular invasion and concomitant carcinoma in situ on the transurethral resection of the bladder tumor specimen were the only significant prognostic factors associated with shorter OS (p = 0.04) and RFS (p = 0.049), respectively. Notably, patients who received preoperative chemotherapy (n = 77) had 5-yr survival rates similar to those of patients who did not.
Conclusion
Although patients who are P0 at cystectomy have a good prognosis, not all can be cured. The favorable prognosis conferred by a P0 state appears to be independent of whether this is achieved by neoadjuvant chemotherapy or by thorough transurethral resection before cystectomy.
Keywords: Bladder cancer, Cystectomy, Prognosis, pT0N0, Stage
1. Introduction
Radical cystectomy with pelvic lymph node dissection (PLND) remains the gold standard for treatment of muscle-invasive bladder cancer. In approximately 10% of patients (range: 6–41%, depending on risk factors before cystectomy), no tumor is found in the specimen (pT0N0M0, “P0”) at the time of radical cystectomy and PLND [1–4]. This P0 status can be due to a complete transurethral resection of the bladder tumor (TURBT) prior to cystectomy or a result of “down-staging” in patients who received preoperative chemotherapy. Most data on patients with P0 disease have been in pure cystectomy series and not reflective of the current climate of multimodal therapy. We evaluated the prognostic significance of P0 stage in a contemporary series of patients treated with radical cystectomy including those who received preoperative chemotherapy.
2. Patients and methods
An institutional review board-approved search of our bladder cancer database identified 1104 patients who underwent radical cystectomy and PLND for bladder cancer at our institution between 1990 and 2005. Each patient had a preoperative work-up that included blood tests, a chest x-ray, computed tomography (CT) of the abdomen and pelvis, and a bone scan (if indicated). It is also our practice to perform a cystoscopy, exam under anesthesia (EUA), and complete resection of all visible tumor prior to making a decision regarding upfront cystectomy or preoperative chemotherapy. At our institution most patients are considered for preoperative chemotherapy if they have “high-risk” disease, which we define as the presence of three-dimensional palpable mass (cT3b) on bimanual EUA, invasion of adjacent organs (cT4a), cT2 lesion with lymphovascular invasion (LVI) on transurethral biopsy specimen, or clinically evident pelvic node metastasis on abdominal imaging [2]. Of all patients, 290 (26%) received preoperative chemotherapy. After surgery, patients were followed with routine blood tests, chest x-rays, CT of the abdomen and pelvis, and bone scans (if indicated) to assess for recurrence of disease at 3- to 6-mo intervals. Collected data points included age, sex, clinical stage, histology, LVI, presence of concomitant carcinoma in situ (CIS), the use of preoperative chemotherapy, type of urinary diversion, pathologic stage, number of nodes excised, recurrence, and survival. Patients who were found to have no tumor in the surgical specimen (pT0N0M0 or P0) were included in this study.
Survival estimates were calculated using the Kaplan-Meier method. The Cox proportional hazards regression model was used to assess the prognostic significance of each variable studied in a univariate fashion. All potential prognostic factors with a p < 0.25 from the univariate analyses were then included in a saturated model, and backward elimination was used to remove factors from the model based on the likelihood ratio test in the multiple regression analysis using the Cox proportional hazards regression model. A p < 0.05 was considered statistically significant.
3. Results
Of 1104 patients identified in our time period, 120 (11%) were P0 at cystectomy and PLND. The clinical characteristics of these patients are summarized in Table 1. Clinical stages were cT1: 21 patients; cT2: 65; cT3b: 20; cT4a: 11; and cT4b: 3. Seventy-seven patients received preoperative chemotherapy; 90% were platinum-based regimens. None of the patients received postoperative adjuvant therapy. Forty patients (33%) had transitional cell carcinoma with a component of variant histology (6 sarcomatoid, 9 micropapillary, 8 glandular, 12 squamous, 3 small cell, and 2 microcystic). Patients who received preoperative chemotherapy had higher incidence of LVI on TURBT (48% vs. 2%, p < 0.001), higher incidence of concomitant variant histology (40% vs. 21%, p = 0.043), and more advanced clinical T stage (≥ cT3b) (43% vs. 2%, p < 0.001), as well as cN stage (9% vs. 0%, p < 0.049) compared to those who did not receive preoperative chemotherapy, which reflects our practice of selecting patients for preoperative chemotherapy based on the presence of high-risk clinical features.
Table 1.
Characteristics of 120 patients
| No. of patients (%) | |
|---|---|
| Median age, yr | 64 (range: 34–82) |
| Sex | |
| Male | 97 (81) |
| Female | 23 (19) |
| Tumor grade | |
| High | 114 (95) |
| Low | 6 (5) |
| Clinical T stage | |
| T1 | 21 (17) |
| T2* | 65 (54) |
| T3b† | 20 (17) |
| T4a‡ | 11 (9) |
| T4b | 3 (3) |
| Clinical N stage | |
| N0 | 113 (94) |
| N+ | 7 (6) |
| Histology | |
| TCC | 80 (67) |
| TCC + other | 40 (33) |
| Concomitant CIS | 21 (17) |
| LVI | 38 (32) |
| Hydronephrosis | 12 (10) |
| Neoadjuvant chemotherapy | 77 (64) |
| Type of diversion | |
| Ileal conduit | 66 (55) |
| Ileal neobladder | 46 (38) |
| Continent cutaneous | 8 (7) |
| Median no. of nodes examined | 11 |
TCC = transitional cell carcinoma; CIS = carcinoma in situ; LVI = lymphovascular invasion.
Four patients had cN+.
Two patient had cN+
One patient had cN+
With a range of follow-up of 1–177 mo (mean, 43 mo; median, 32 mo), the 5-yr overall (OS), disease-specific survival (DSS), and recurrence-free survival (RFS) rates were 84%, 88%, and 84%, respectively (Fig. 1). Eleven patients (9.2%) developed recurrences (cT1: 3, cT2: 3, cT3b: 3, and cT4: 2), 9 of whom died of disease (Table 2). The majority of patients who developed recurrences (7 of 11, 64%) had either LVI or CIS on the TURBT specimen and the majority of patients (9 of 11) had received preoperative chemotherapy. Of those who developed recurrences, two had pelvic recurrence, eight had distant disease, and one had both; none developed upper tract recurrence. Median time to recurrence of these patients was 7.7 mo (range: 2.2–45 mo). Patients who received preoperative chemotherapy had similar 5-yr OS (80% vs. 90%, p = 0.31), DSS (86% vs. 92%, p = 0.65), and RFS (80% vs. 90%, p = 0.18) compared with those who did not (Fig. 2). The presence of LVI on transurethral biopsy was significantly associated with shorter OS on multivariate analysis (hazard ratio [HR], 2.66; confidence interval [CI], 1.04–6.88; p = 0.04). The 5-yr OS for patients who had LVI on transurethral biopsy was 69.6% compared to 89.2% for those who did not (Fig. 3). Further, the presence of concomitant CIS was significantly associated with shorter RFS (HR, 2.47; CI, 1.01–6.08, p = 0.049) on multivariate analysis but not with OS (p = 0.08) or DSS (p = 0.22; Fig. 4). Notably, 16 of 21 patients (76%) who had concomitant CIS had received preoperative chemotherapy. Clinical stage was not associated with OS, DSS, or RFS (Fig. 5). Other variables including age, sex, hydronephrosis, presence of variant histology, history of prior superficial disease, and number of nodes excised were not significantly associated with OS, DSS, or RFS on multivariate analysis.
Fig. 1.
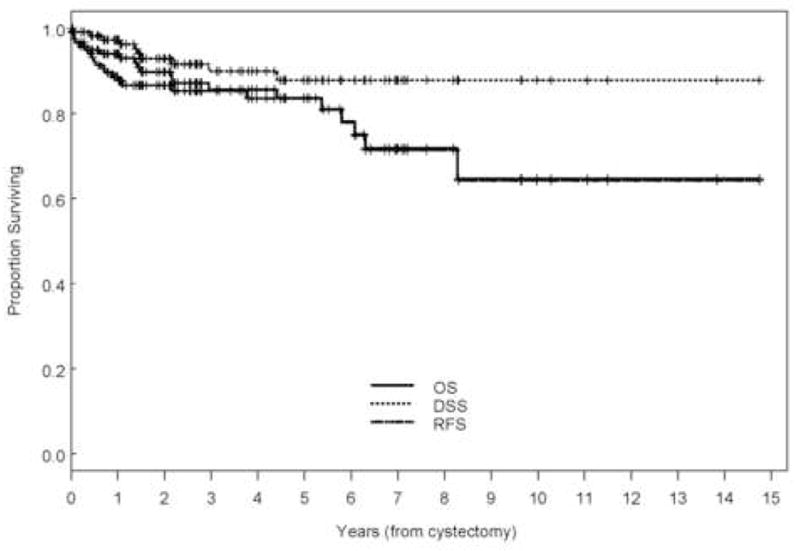
Kaplan-Meier estimate of overall survival (OS), disease-specific survival (DSS), and recurrence-free survival (RFS) for all patients who were P0 at cystectomy.
Table 2.
Characteristics of 11 patients who developed disease recurrence
| No. of patients (%) | |
|---|---|
| Sex | |
| Male | 10 (91) |
| Female | 1 (9) |
| Clinical T stage | |
| T1 | 3 (27) |
| T2 | 3 (27) |
| T3b | 3 (27) |
| T4a | 1 (9) |
| T4b | 1 (9) |
| Clinical N stage | |
| N0 | 10 (91) |
| N+ | 1 (9) |
| Histology | |
| TCC | 7 (64) |
| TCC + other | 4 (36) |
| Concomitant CIS | 5 (45) |
| LVI | 6 (55) |
| Hydronephrosis | 3 (27) |
| Neoadjuvant chemotherapy | 9 (82) |
| Median no. of nodes examined | 14 |
TCC = transitional cell carcinoma; CIS = carcinoma in situ; LVI = lymphovascular invasion.
Fig. 2.
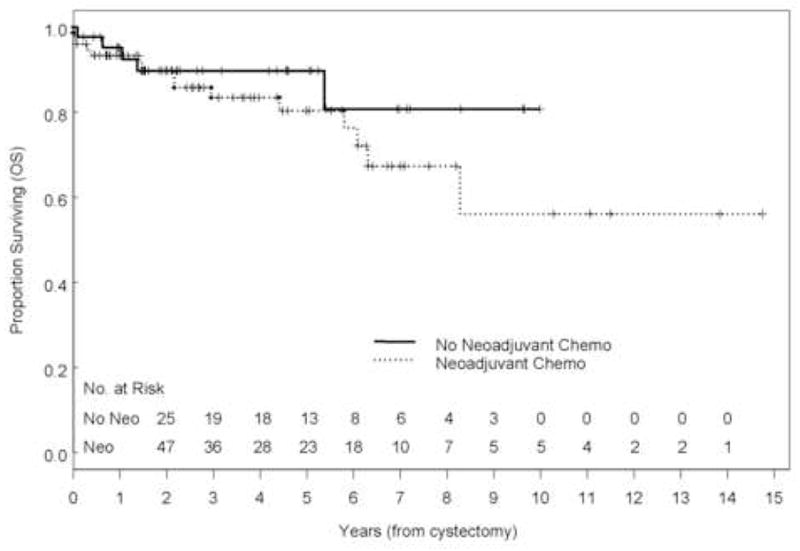
Kaplan-Meier estimate of overall survival (OS) stratified by the use of preoperative chemotherapy (p = 0.32).
Fig. 3.
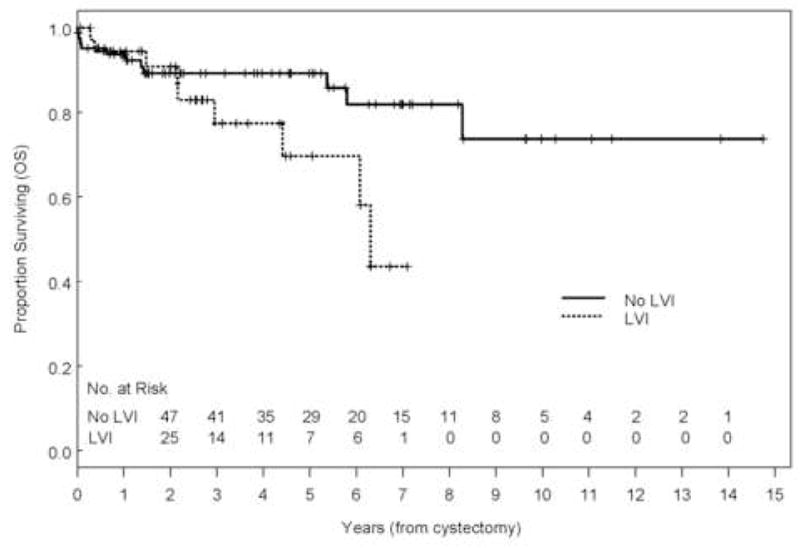
Kaplan-Meier estimate of overall survival (OS) stratified by the presence of lymphovascular invasion (p = 0.08, multivariate p = 0.04).
Fig. 4.
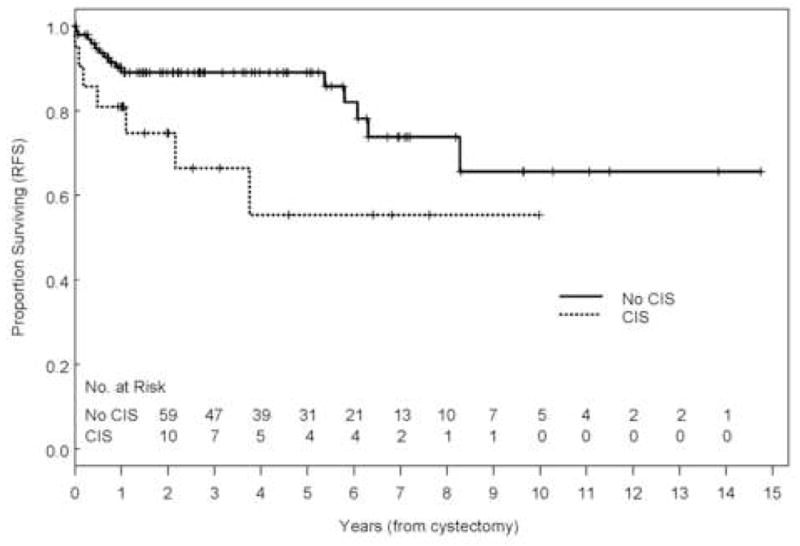
Kaplan-Meier estimate of recurrence-free survival (RFS) stratified by the presence of concomitant carcinoma in situ (p= 0.049).
Fig. 5.
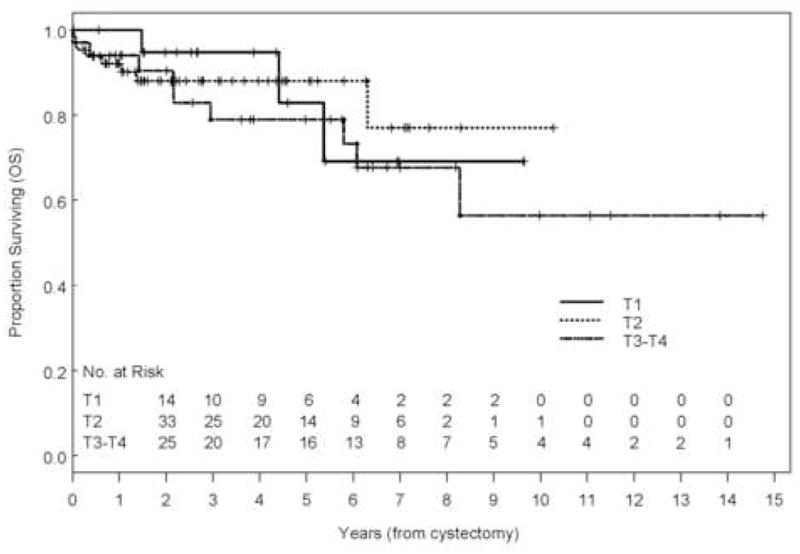
Kaplan-Meier estimate of overall survival (OS) stratified by clinical stage (p = 0.693).
4. Discussion
P0 stage at cystectomy is an indicator of good prognosis and appears to be an independent predictor of favorable outcome in patients with bladder cancer. However, not all can be considered cured of disease.
Pathologic stage is a major prognostic factor for survival in patients undergoing radical cystectomy for urothelial carcinoma [1]. P0 rates across several series have ranged from 6% to 41% [1,4–7] varying, in part, due to different types of neoadjuvant chemotherapy used, aggressiveness of the surgeon to perform a “complete re-TUR,” patient characteristics, or meticulous assessment by the pathologist to detect focal microscopic focus of disease. Our P0 rate of 11%, though on the lower end of reported rates, is probably reflective of our referral pattern in that we are a tertiary cancer center. Despite the high-risk clinical population, we found that patients with P0 stage have a good prognosis with 5-yr OS and DSS of 84% and 88%, respectively. However, 11 patients (9.2%) did develop either pelvic or distant recurrences, and recurrences occurred even beyond 3 yr following definitive surgery. This emphasizes the importance of lifelong surveillance for disease recurrence in patients with P0 stage.
Previous reports have attempted to evaluate the significance of not finding viable tumor on pathologic examination of the cystectomy specimen. Thrasher et al reported on 66 patients who were pT0 at radical cystectomy and found that survival was affected by clinical stage. Because this was an older series (from 1969 to 1990), none received neoadjuvant chemotherapy and 22 received neoadjuvant radiotherapy; furthermore, the study did not analyze the impact of other variables such as lymph node disease, extent of dissection, or LVI [6]. Similarly, the recent multicenter study reported by Palapattu et al had only 57 patients who were pT0N0 between 1984 and 2003 and analyzed only the impact of clinical stage in these patients [4]. Volkmer et al [3] reported that P0 confers a survival advantage only in patients with clinical T2a disease; however, none of their patients received preoperative chemotherapy. The patient population from the above-mentioned three studies were different from our series because a considerable number of patients had clinical non-muscle-invasive (≤ cT1) disease (65%, 45%, and 42% vs. only 17% of the patients in our series), which might be a reflection of our referral pattern. Furthermore, none of the reports analyzed for associations between other parameters and outcome to identify prognostic factors that may be useful in the management of such patients after their surgery.
Our report is the first to identify prognostic factors that might help sub-stratify patients who are P0 at radical cystectomy. In the multivariate analysis, we found that LVI on the transurethral biopsy was significantly associated with shorter survival. Although our report was not meant to evaluate the significance of LVI per se, our findings are consistent with reports that identify LVI as a prognostic factor in bladder cancer [8]. Recent reports have shown that the presence of LVI is an independent predictor of advanced tumor stage, grade, and shorter OS and RFS in patients with pT×N0 disease. However, this is the first report to show that LVI is also prognostic in patients who are pT0N0 at cystectomy and further support the hypothesis that LVI may be reflective of a disease state where the malignant cells have attained a metastatic phase. As such, we routinely offer neoadjuvant chemotherapy for patients with LVI on TUR biopsy. Although it can be argued that the number of nodes excised in our series was relatively low, it is still comparable with other contemporary series where the median number of nodes excised was 13 [9]. Furthermore, we do not routinely send the nodes in packets, a method that has been previously shown to almost triple the number of nodes reported by the pathologist [10]. Interestingly, our study also showed that the presence of concomitant CIS was associated with shorter time to recurrence, suggesting that CIS may be a marker for more aggressive tumor biology [11,12].
Though there were more patients rendered P0 by systemic chemotherapy than by TURBT, it is important to note that at our institution not all patients with muscle invasive (cT2) bladder cancer receive neoadjuvant chemotherapy. In light of the morbidity from current systemic chemotherapy, only those considered as having a high risk of occult metastatic disease receive neoadjuvant chemotherapy. Our definition of those at high risk of occult metastatic disease include the presence of a three-dimension mass on EUA (cT3b), invasion of an adjacent organ (cT4a), the presence of LVI, and hydronephrosis. What is apparent from our findings, however, is that even in these patients at high risk of occult metastatic disease, a P0 (pT0N0M0) “status” confers a clear benefit to survival, regardless of whether neoadjuvant chemotherapy was used or not. Interestingly, this was also seen in the large prospective, randomized trial reported by Grossman et al, in which patients were randomized to radical cystectomy or three cycles of neoadjuvant chemotherapy. In this report, 26% of patients overall were pT0 at cystectomy, and there was no survival difference in patients rendered P0 by neoadjuvant chemotherapy (48 of 126 patients, 38%) versus those rendered P0 by TUR (18 of 121 patients, 15%) [13]. Thus, it appears that the biologic and prognostic significance of P0 state is independent of whether this was achieved by means of a surgeon’s knife (TURBT) or a medical oncologist’s chemotherapy.
We acknowledge certain inherent limitations of our report. First, it is a single-center experience. However, this allows us to report on patients who were treated in a “uniform” manner and had their specimens evaluated by a core group of pathologists. At the same time the presence of multiple surgeons makes our results applicable to other urologic oncology practices. Also, we specifically concentrated on a contemporary period (1990–2005) to avoid variations in treatment parameters. Second, comparing clinical stages between patients who underwent neoadjuvant therapy and those who underwent upfront cystectomy is accompanied by a potential for “staging error” because the tumor stage before neoadjuvant therapy is based on the results of the TUR specimen, EUA, and imaging data. However, the purpose of our analysis was not to draw comparisons between the two treatment modalities, but to report on the outcome of patients who have a tumor-free cystectomy specimen and identify prognostic factors in these patients. In this context, our results suggest that patients who achieve a P0 status may have a surveillance schedule independent of whether or not they received chemotherapy before cystectomy. Patients who have LVI or concomitant CIS on transurethral biopsy and achieve a P0 stage at surgery are at higher risk of recurrence and hence may require closer surveillance
5. Conclusion
Patients who are P0 on cystectomy specimen have a good prognosis. However, not all can be considered cured of their disease. The favorable prognosis conferred by a P0 state appears to be independent of whether this is achieved by administration of neoadjuvant chemotherapy or by thorough transurethral resection before cystectomy. The presence of LVI and concomitant CIS are associated with adverse outcomes.
Table 3.
Multivariate analysis of variables that predict overall, disease-specific, and recurrence-free survival
| OS | DSS | RFS | |
|---|---|---|---|
| Age | NS | NS | NS |
| Prior tumors | NS | NS | NS |
| Clinical stage | NS | NS | NS |
| Concomitant variant histology | NS | NS | NS |
| LVI | 0.04 | NS | NS |
| Concomitant CIS | NS | NS | 0.049 |
| Hydronephrosis | NS | NS | NS |
| Total no. of nodes | NS | NS | NS |
| Neoadjuvant chemotherapy | NS | NS | NS |
| Distant metastasis | < 0.01 | < 0.01 | — |
OS = overall survival; DSS = disease-specific survival; PFS = progression-free survival; NS = not significant (p ≥ 0.05); LVI = lymphovascular invasion; CIS = carcinoma in situ.
Acknowledgments
Supported by the M.D. Anderson Bladder SPORE (5P50CA091846-03) and Department of Urology T32 Training Grant (CA079449-06) and Core grant.
Footnotes
Take Home Message: Patients with P0 at cystectomy have a good prognosis whether P0 is achieved with neoadjuvant chemotherapy or transurethral resection before cystectomy; however, not all are cured. Presence of lymphvascular invasion and concomitant carcinoma in situ are associated with adverse outcomes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Millikan R, Dinney C, Swanson D, et al. Integrated therapy for locally advanced bladder cancer: final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J Clin Oncol. 2001;19:4005–13. doi: 10.1200/JCO.2001.19.20.4005. [DOI] [PubMed] [Google Scholar]
- 3.Volkmer BG, Kuefer R, Bartsch G, Jr, et al. Effect of a pT0 cystectomy specimen without neoadjuvant therapy on survival. Cancer. 2005;104:2384–91. doi: 10.1002/cncr.21475. [DOI] [PubMed] [Google Scholar]
- 4.Palapattu GS, Shariat SF, Karakiewicz PI, et al. Cancer specific outcomes in patients with pT0 disease following radical cystectomy. J Urol. 2006;175:1645–9. doi: 10.1016/S0022-5347(05)00995-X. discussion 1649. [DOI] [PubMed] [Google Scholar]
- 5.Yiou R, Patard JJ, Benhard H, Abbou CC, Chopin DK. Outcome of radical cystectomy for bladder cancer according to the disease type at presentation. BJU Int. 2002;89:374–8. doi: 10.1046/j.1464-4096.2001.001020.x. [DOI] [PubMed] [Google Scholar]
- 6.Thrasher JB, Frazier HA, Robertson JE, Paulson DF. Does of stage pT0 cystectomy specimen confer a survival advantage in patients with minimally invasive bladder cancer? J Urol. 1994;152(2 pt 1):393–6. doi: 10.1016/s0022-5347(17)32746-5. [DOI] [PubMed] [Google Scholar]
- 7.Wijkstrom H, Norming U, Lagerkvist M, Nilsson B, Naslund I, Wiklund P. Evaluation of clinical staging before cystectomy in transitional cell bladder carcinoma: a long-term follow-up of 276 consecutive patients. Br J Urol. 1998;81:686–91. doi: 10.1046/j.1464-410x.1998.00637.x. [DOI] [PubMed] [Google Scholar]
- 8.Lotan Y, Gupta A, Shariat SF, et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol. 2005;23:6533–9. doi: 10.1200/JCO.2005.05.516. [DOI] [PubMed] [Google Scholar]
- 9.Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol. 2003;169:943–5. doi: 10.1097/01.ju.0000032474.22093.06. [DOI] [PubMed] [Google Scholar]
- 10.Bochner BH, Herr HW, Reuter VE. Impact of separate versus en bloc pelvic lymph node dissection on the number of lymph nodes retrieved in cystectomy specimens. J Urol. 2001;166:2295–6. [PubMed] [Google Scholar]
- 11.Shariat SF, Palapattu GS, Amiel GE, et al. Impact of concomitant carcinoma in situ on clinical outcomes after radical cystectomy. J Urol. 2006;175:403. [Google Scholar]
- 12.Di Stasi SM, Giannantoni A, Giurioli A, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: a randomised controlled trial. Lancet Oncol. 2006;7:43–51. doi: 10.1016/S1470-2045(05)70472-1. [DOI] [PubMed] [Google Scholar]
- 13.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]


