Abstract
Neurons in monkey medial superior temporal cortex selectively respond to the patterned visual motion in optic flow that simulates observer self-movement. We trained monkeys in a task that required behavioral responses indicating the location of a precue or the simulated heading direction in a subsequent optic flow stimulus. Medial superior temporal neuronal responses contained transient peaks at latencies proportionate to the distance from the precue to the heading direction represented by the subsequent optic flow. We conclude that these response transients reveal neural mechanisms underlying covert shifts of spatial attention and that the varying latency of these transients reflect the time required for reorientation between attentional targets.
Keywords: attention, cerebral cortex, motion, neurons, vision
Introduction
We covertly shift the focus of our attention to locations of interest without externally observable evidence of our surreptitious reorientation [1], but for our greater sensitivity to stimuli presented at a precued site [2]. Covert shifts are not instantaneous; human behavioral [3] and neurophysiological [4] findings suggest that attentional transitions require 200–400ms, relying on more rapid posterior parietal neuronal responses to cues marking the behaviorally relevant location [5,6]. Here, we show that covert reorientation is accompanied by a transient neuronal response that is delayed proportional to the distance traversed by the shift in spatial attention.
The cortical analysis of visual motion relies on a dorsal extrastriate processing pathway that extends from primary sensory receptive areas to posterior parietal centers for spatial cognition. In the macaque monkey, medial superior temporal cortex forms the junction of occipital and parietal cortices. Dorsal medial superior temporal neurons respond preferentially to the large patterns of visual motion in optic flow [7] with selectivity for the radial centers of motion in optic flow [8] that simulate particular directions of observer self-movement [9].
The medial superior temporal area is adjacent to the middle temporal area within the macaque superior temporal sulcus. Medial temporal neurons show substantial changes in neuronal responsiveness depending on the location of the spatial focus of visual attention relative to the individual neuron’s visual receptive field location. Similarly, the responses of medial superior temporal neurons to visual motion in their receptive fields are enhanced by directing the animal’s attention to the stimulus [10]. We studied the effects of spatial attention on the optic flow responses of dorsal medial superior temporal neurons, finding selective effects of cueing tasks, and cue location [11]. We now report observations on optic-flow-evoked neuronal response dynamics related to the spatial reorientation of attention.
Materials and methods
Stimuli and behavioral tasks
The monkeys fixated the center of a 90° square tangent screen viewing radial optic flow stimuli while fixation was monitored using magnetic search coils. The optic flow had one of eight centers of motion at 30° eccentricity and at 45° intervals around the fixation point. The optic flow contained approximately 1000 dots moving at an average speed of 40°/s. Dot speed increased from the center of motion as cos(a)× sin(a) [7], where a is the angle between the line of sight and each dot. Each dot was 75° wide with a brightness of 1.8 cd/m2 on a background of 0.2 cd/m2.
The exogenous cueing task contained two types of interleaved trials. Trials presenting behaviorally relevant optic flow began with centered fixation maintained for 2 s followed by optic flow for 1 s. These stimuli were replaced by a multiple choice array with eight targets requiring a saccade within 500ms to the target corresponding to the center of motion in the preceding optic flow. Trials presenting behaviorally irrelevant optic flow began with centered fixation for 1 s, followed by a 1 s flash of a 5° square precue at one of eight 30° eccentric positions, a 150–350 ms delay, and an optic flow for 1 s. These stimuli were replaced by the same multiple-choice array, but in this case requiring a saccade to the remembered location of the flashed precue.
The location of the radial centers of motion in the optic flow and the location of flashed precue stimuli were pseudorandomly selected. As a result, some of the precues would happen to be located near the location of the subsequent optic flow’s radial center, whereas others would happen to be located far from the subsequent radial center. The monkeys were required to maintain central fixation within a 2°× 2° centered fixation window throughout all stimulus presentation epochs, and to successfully complete the saccade task, to earn a liquid reward.
Neuronal recording
All procedures were approved by the University of Rochester Committee on Animal Research and were consistent with policies of Society for Neuroscience. Bilateral recording cylinders were placed over trephine holes in the parietal calvarium (anterioposterior − 2 mm, mediolateral ±15 mm, angle 0) over medial superior temporal cortex. Tungsten microelectrodes (FHC, Inc., Bowdoinham, Maine, USA) were passed through transdural guide tubes into cortex to record single neuron spikes using a dual window discriminator and the REX experimental control system (LSR/NEI/NIH, Bethesda, Maryland, USA). Receptive fields were hand mapped with bar and textured stimuli and judged to be from medial superior temporal cortex based on accepted criteria. After pentobarbital euthanasia and transcardiac formalin perfusion, histological analysis confirmed that all of these neurons were recorded on the anterior bank of the superior temporal sulcus in the more densely myelinated zone associated with dorsal medial superior temporal cortex.
Statistical analysis
The amplitudes of neuronal responses to optic flow were entered into repeated-measures two-way analyses of variance with factors main effects of task (relevant, irrelevant) and center of motion (eight sites, each with eight presentations) in the relevance comparison and main effects of near/far square locations and radial centers of motion (eight sites, each with three presentations each for the near and far relative positions) in the precue comparison. Greenhouse–Geyser correction for the nonsphericity of variances was used to identify significant effects. Analyses used the 50–300ms interval after the onset of optic flow in agreement with our earlier findings of a 50ms minimal response latency for optic flow in dorsal medial superior temporal cortex [12] and our earlier conclusion that a 250ms averaging interval provides stable representations of optic flow heading selectivity in these neurons [13].
Our analyses included all neurons with significant main effects of optic flow relevance and task by radial center interaction effects (P<0.05). We used a cutoff of P value of less than 0.1 for near/far trials as this effect was based on half as many trials as the comparison for main effect of flow relevance. In this regard, our perspective followed the approach suggested by an effect–size criterion, the difference of the two conditions divided by the root mean square of their averaged standard deviations [14].
Spike density functions were computed using a Gaussian filter with a standard deviation of 20 ms [15]. Sample spike density functions were computed as the point-wise average of individual neuron spike density functions normalized by average firing rate. Spike density functions were visualized as mean spike density function ± the standard error. Statistical significance of peak firing was determined by analyzing a window including the time of peak firing ± 50ms, observed to be the interval over which response transients were sustained. A one-tailed t-test was used to compare firing rates in corresponding intervals.
Gaussian fits
We fit Gaussian curves to the average responses across center of motion locations using amplitude, baseline, width, and preferred radial center parameters. Goodness of fit was measured as an F-ratio of the residuals in the best Gaussian versus best linear fit; only neurons showing fits with P value of less than 0.05 were accepted [16]. In creating the sample response Gaussians we normalized the responses of each neuron to its preferred response amplitude, assigning the preferred average firing rate over the 50–300ms interval and a normalized firing rate of 100 spikes/s.
Results
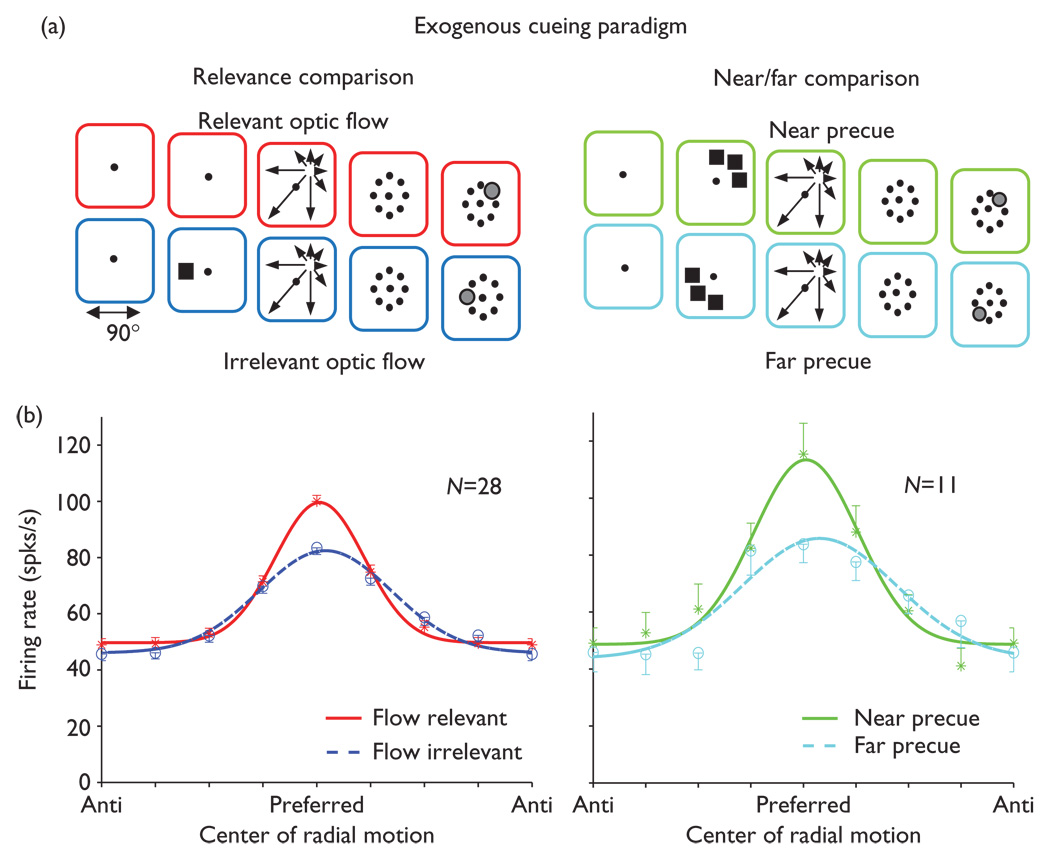
We recorded the optic flow responses of 410 MSTd neurons in two Rhesus monkeys as part of a larger study of attentional effects [11]. In the exogenous cueing task (n=135 neurons), relevant optic flow trials presented one of eight radial centers of optic flow and then required a saccade to the site of the preceding radial center (see Methods and Fig. 1a, left, top). Irrelevant optic flow trials first presented a flashed square precue at one of the eight radial center locations (Fig. 1a, left, bottom), then an independently selected radial optic flow stimulus near to or far from the precue site (Fig. 1a, right), and then required a saccade to the site of the preceding flashed precue.
Fig. 1.
Behavioral effects on neuronal responses in an exogenous cueing paradigm. (a) Left: relevant (red) and irrelevant (blue) optic flow trial types. In relevant optic flow trials, centered fixation is followed by optic flow with one of eight radial centers of motion and then an array requiring a saccade to the remembered radial center location. In irrelevant optic flow trials, a flashed square preceded the irrelevant optic flow and a saccade was required to the remembered square location. Right: precue effects were seen in the flow-irrelevant condition by comparing trials in which the precue was nearby (green) versus far from (light blue) the radial center location. (b) Left: neuronal responses (mean ± s.e.) to optic flow with Gaussian fits to flow-relevant (solid, red) and flow-irrelevant (dashed, blue) trials showing greatest flow-relevant enhancement at the preferred radial center. Right: neuronal responses to optic flow in near (solid, green) and far (dashed, light blue) precue trials showing greatest near precue enhancement at the preferred radial center. Gaussian fits are to the averaged, normalized responses aligned on the radial center of motion that evoked the largest response in each neuron.
Considering all 135 neurons together does not reveal substantial preferences for one condition over others. However, a different view is accorded by considering the quarter of all neurons (24%, 32 of 135) that show significant effects of behavioral condition on the optic flow responses. These effects were of two varieties: in 21% (28 of 135) of the neurons, there were significant differences between responses to relevant and irrelevant optic flow (Fig. 1b, left); 16 having a main effect of task; six having a task by center of motion interaction effect; and six showing both effects. In 8% (11 of 135) of the neurons, there was a significant effect of near versus far precue location: four having a main effect of task, six having a task by center of motion interaction effect, and one showing both effects (Fig. 1b, right). We cannot conclude that relevance effects are larger or more prevalent than near/far effects because half as many trials contribute to the near/far comparison, the irrelevant optic flow trials. This view is reinforced by the comparable relevance and near/far effect sizes seen using the 22 neurons with significant (P<0.1) near/far effects.
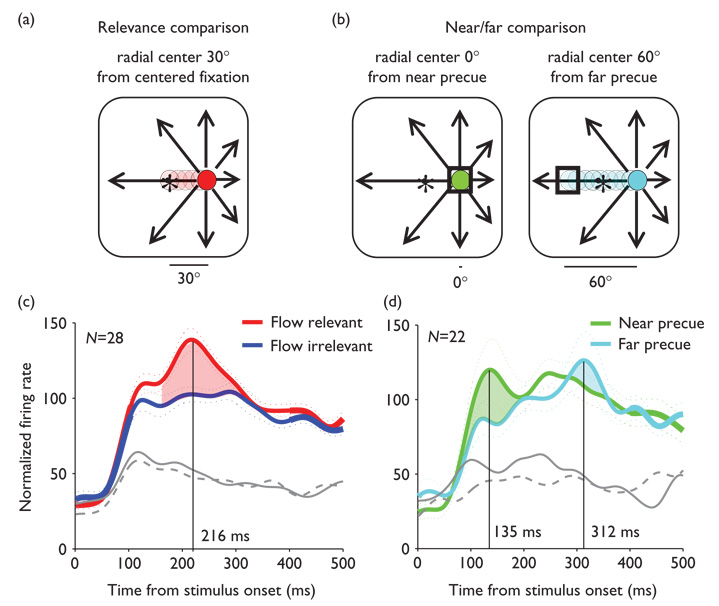
Spike density functions from neurons with significant behavioral effects reveal transient bursts of activity superimposed on each neuron’s sustained responses to their preferred optic flow stimuli. These response transients peak at times that differ across the three behavioral conditions, varying with the distance between task-related targets in trials with behaviorally relevant optic flow (Fig. 2a) versus near and far precued irrelevant optic flow (Fig. 2b). In relevant optic flow trials, the response transients peak 216ms after the onset of the optic flow stimulus (Fig. 2c). In the near precue trials the response transients peak 135 ms after the onset of the optic flow stimulus, and in far precue trials they peak at 312 ms (Fig. 2d).
Fig. 2.
Effects of exogenous cueing on transient response latency. (a) 30° traversal of covert spatial attention (red) from centered fixation to the location of the optic flow’s radial center (arrows). (b) Sustained covert spatial attention (green) when the flashed precue (square) overlies the location of the radial center (arrows). 60° traversal of covert spatial attention (blue) when the flashed precue (square) is opposite to the location of the radial center (arrows). (c and d) Spike density functions of responses to the preferred (colored) and antipreferred radial centers for neurons having significant effects (gray, solid is relevant optic flow and near precued; dashed is irrelevant optic flow and far pre-cued). (c) Average normalized responses (±s.e.) as spike density functions during flow-relevant (red) and flow-irrelevant (blue) trials diverge from 120–300 ms, reaching maximal flow-relevant enhancement at 216 ms. (d) Spike density functions of responses in the near precue trials (green) peak at 135 ms after stimulus onset, whereas responses in the far precue trials (light blue) peak at 312 ms.
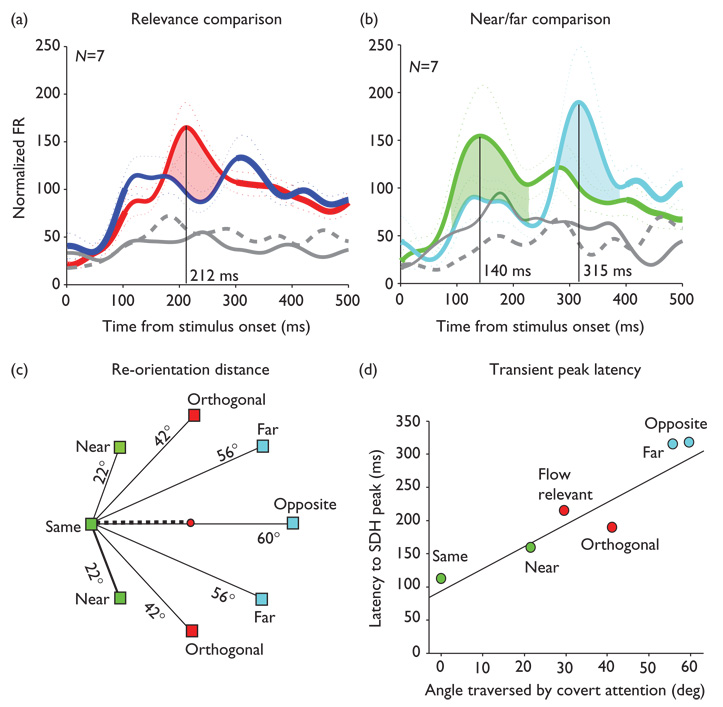
Transient response latency differences across behavioral conditions can be compared directly in the seven neurons that were included both in the 28 neurons with significant optic flow relevance effects and the 11 neurons with significant near/far relative location effects (Fig. 3a and b). Peak latency is shortest (140 ms, P=0.01) in the near precue trials, in which attention was drawn in the direction of the optic flow’s radial center before the optic flow was presented. Peak latency is longest (315 ms, P=0.03) in the far precue trials, in which attention was drawn away from the location of the optic flow’s radial center before the optic flow was presented. Peak latency is intermediate (212 ms, P<0.001) in the flow relevant trials, in which attention remained focused at the centered fixation point until the optic flow was presented (Fig. 3c and d). (The distance of shifts in covert spatial attention from all of the behavioral conditions are summarized in Fig. 3c and their observed relationship to transient peak latencies in Fig. 3d).
Fig. 3.
Linear relationship between transient peak latency and distance traversed by covert spatial attention in the exogenous cueing paradigm (a) and (b). Format as in Fig. 2, for the seven neurons having significant optic flow relevance and significant near versus far flashed precue effects showing clear shifts in the latency of response transients. (c) Illustration of visual angle between the eight precues and a radial center of optic flow on the horizontal meridian in the left visual field. The dashed line indicates the 30° of visual angle traversed by covert spatial attention in flow relevant trials. (d) Relationship between the latency to the peak of the transient response and the distance (visual angle) between the flashed precue and the location of the radial center in the subsequent optic flow stimulus. Spike density functions were derived for each of the six attentional shift distances created by these stimuli. The best-fit line through these data has a slope of 3.64 and an intercept of 94.6, with r2 = 0.89 and P value of less than 0.005. FR, firing rate. SDH, spike density histogram.
We considered that a consistent time lag between the onset of a neuronal response and the latency to the transient response peak would suggest that the peak is part of the basic visual processing of the optic flow stimulus. Alternatively, a variable time lag between onset and peak would suggest that the peak reflects a second process, an outcome one more consistent with the peak’s reflecting covert reorientation. We measured population response onset as the time at which the standard error envelope around the response exceeded that around the control firing rate. This measure provided good visual agreement with the plotted spatial distribution functions. In the seven neurons with significant relevance and near–far effects we found onset latencies of: near precued = 68ms, flow relevant = 61 ms, and far precued = 112ms.
Thus, the time lag between onset and peak varied across conditions as: near = 72 ms, flow relevant = 151 ms, and far = 203 ms. The onset difference between near and far responses is 44 ms, about 25% of the 175 ms difference in peak latencies. Thus, we conclude that peak latency is not closely linked to the onset of the response and that the transient peaks recorded in these neurons are likely to reflect a separate process.
The exogenous cueing task presented precue, optic flow, and behavioral response array stimuli in discrete, fixed intervals so that stimulus transitions and overt behavior would not obscure effects attributable to covert reorientation. As saccadic responses were paced by the variable onset of the saccade target array, we could not derive direct links between attentional effects and saccadic behavioral response times. Recognizing these limitations, we examined the saccade latency behavioral response data to identify any parallels with the dynamics of neuronal response transients. Relevant optic flow evoked saccade latencies of 223±29 and 267±45ms in the two monkeys. The near and far precue irrelevant optic flow trials evoked saccade latencies of 219±30 and 220±29ms in the first monkey, and 345±58 and 330±53ms in the second monkey. Thus, we saw no consistent relationship between saccade latencies and reorientation target distance as seen in the neuronal response peak latencies.
The most parsimonious view of the saccade latency data may be that both monkeys performed the relevant optic flow trials similarly, but the second monkey took a different approach to the precued trials. The second monkey’s approximately 70 ms delay in the precued trials may reflect its making a second covert shift back to the fixation point after the precue was extinguished so that the response transient effects might be less robust in this monkey. This inference is consistent with the observation that six of the neurons with the most robust relevance and near/far effects were from the first monkey. The current experiment only allows us to conclude that behavioral task effects might be influenced by the monkey’s strategy in the task, an effect we have seen in related studies [17], and that further insight into these issues will require alternative experimental designs.
Discussion
The proportionality of the distance between attentional targets and the latency of transient responses in the exogenous cueing paradigm supports the suggestion that attention moves across visual space at a fixed speed [18], rather than with a constant latency independent of distance [19]. Our findings suggest a speed of attentional movement as the reciprocal of the slope of the regression line, 0.27°/ms.
It is noteworthy that the linear trend in the peak latency data is apparent even when the flow relevant condition is removed and only the average latency to the peak in neural activity of responses in the exogenously cued, irrelevant optic flow trials are included in the linear regression. We should emphasize that the monkey is warned that the optic flow is irrelevant in the precue trials and there is no benefit in reorienting to the center of motion in the optic flow that follows the precue. However, the rapid succession of the interleaved relevant and irrelevant optic flow trials might encourage the monkey to use the single strategy of attending to all stimuli and then make the saccade choice when there is ample time at the end of the trial. When we view these stimuli, we note that it is hard to resist a seemingly reflexic reorientation to stimuli that are the relevant cue in half of the trials.
The speed of 0.27°/ms derived from our studies is about twice that derived from human behavioral studies, that is, 0.13°/ms [18]. The faster speed suggested by our findings may reflect species differences, task differences, or medial superior temporal cortex’s role in optic flow analysis rather than the complex process of directing the behavioral responses studied in humans. The comparability of attentional mechanisms in monkeys and humans, and of the processes studied in this work and earlier attentional studies, is evidenced by identical estimates of the shortest latencies to attentional engagement of 140ms both in our exogenous cueing data and in human studies of attentional dynamics [20].
The proportionality of the distance between attentional targets and the latency of transient responses in medial superior temporal cortex suggests a gradual shifting of covert spatial attention across visual space. Attentional response transients in medial superior temporal cortex might originate in an adjacent posterior parietal task-dependent saliency map [21]. Changes in the spatial distribution of activity in the parietal saliency map, delayed in proportion to the distance between activated sites, may drive the transient enhancement of activity in medial superior temporal cortex neurons that represent self-movement headings directed toward corresponding locations in visual space.
Posterior parietal N2pc electrophysiological transients are associated with covert attentional shifts in humans [22]. Human posterior parietal activation during attentional shifts is also evident in functional imaging studies that suggest the involvement of a fronto-parietal network overlapping on a cortical distributed system for gaze control [23]. Together, these findings support the premotor theory of attention that emphasizes similarities between covert attentional shifts and overt saccadic eye movements [24]. A link between attentional reorientation and the redirection of gaze is consistent with adjacent or shared frontal cortical control mechanisms for attention and saccades having top-down projections to extrastriate visual areas [25].
Conclusion
We view medial superior temporal cortex neuronal response transients in the exogenous cueing paradigm as reflecting the arrival of a spatial attentional signal that enhances activity encoding a behaviorally relevant self-movement heading direction in medial superior temporal cortex. The proportionality between the distance of covert shifts and the latency of the transient responses suggests gradual transformations across topographically organized representations of visual space within neural centers generating top-down signals.
Acknowledgements
The authors are grateful for the colleagueship ofDrs.Roberto Fernandez, Voyko Kavcic, Mark Mapstone, and William K. Page, as well as William Vaughn. MJD is currently T32 Research Fellow in Child Psychiatry at New York State Psychiatric Institute, Columbia University, New York, NY. This work was supported by grants from the National Eye Institute (EY10287) and the National Institutes on Aging (AG17596). MJD was a Medical Scientist Trainee (T32-GM07356) and was also supported by an NIH training grant to The University of Rochester Center for Visual Science (T2-EY07125C).
References
- 1.Posner MI, Snyder CRR. Facilitation and inhibition in the processing of signals. In: Rabbitt PM, Dornic S, editors. Attention and performance V. London: Academic Press; 1975. pp. 669–682. [Google Scholar]
- 2.Egly R, Homa D. Sensitization of the visual field. J Exp Psychol Hum Percept Perform. 1984;10:778–793. doi: 10.1037//0096-1523.10.6.778. [DOI] [PubMed] [Google Scholar]
- 3.Weichselgartner E, Sperling G. Dynamics of automatic and controlled visual attention. Science. 1987;238:778–780. doi: 10.1126/science.3672124. [DOI] [PubMed] [Google Scholar]
- 4.Muller MM, Picton TW, Valdes-Sosa P, Riera J, Teder-Salejarvi WA, Hillyard SA. Effects of spatial selective attention on the steady-state visual evoked potential in the 20–28 Hz range. Brain Res Cogn Brain Res. 1998;6:249–261. doi: 10.1016/s0926-6410(97)00036-0. [DOI] [PubMed] [Google Scholar]
- 5.Bushnell MC, Goldberg ME, Robinson DL. Behavioral enhancement of visual responses in monkey cerebral cortex. I. modulation in posterior parietal cortex related to selective visual attention. J Neurophysiol. 1981;46:755–772. doi: 10.1152/jn.1981.46.4.755. [DOI] [PubMed] [Google Scholar]
- 6.Mountcastle VB, Andersen RA, Motter BC. The influence of attentive fixation upon the excitability of the light sensitive neurons of the posterior parietal cortex. J Neurosci. 1981;1:1218–1235. doi: 10.1523/JNEUROSCI.01-11-01218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. I.A continuum of response selectivity to large-field stimuli. J Neurophysiol. 1991;65:1329–1345. doi: 10.1152/jn.1991.65.6.1329. [DOI] [PubMed] [Google Scholar]
- 8.Duffy CJ, Wurtz RH. Response of monkey MST neurons to optic flow stimuli with shifted centers of motion. J Neurosci. 1995;15:5192–5208. doi: 10.1523/JNEUROSCI.15-07-05192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy CJ. MST neurons respond to optic flow and translational movement. J Neurophysiol. 1998;80:1816–1827. doi: 10.1152/jn.1998.80.4.1816. [DOI] [PubMed] [Google Scholar]
- 10.Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 11.Dubin MJ, Duffy CJ. Behavioral influences on cortical neuronal responses to optic flow. Cereb Cortex. 2007;17:1722–1732. doi: 10.1093/cercor/bhl083. [DOI] [PubMed] [Google Scholar]
- 12.Duffy CJ, Wurtz RH. Multiple temporal components of optic flow responses in MST neurons. Exp Brain Res. 1997;114:472–482. doi: 10.1007/pl00005656. [DOI] [PubMed] [Google Scholar]
- 13.Logan DJ, Duffy CJ. Cortical area MSTd combines visual cues to represent 3-D self-movement. Cerebral Cortex. 2006;16:1494–1507. doi: 10.1093/cercor/bhj082. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 15.Richmond BJ, Optican LM. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. II. Quantification of response waveform. J Neurophysiol. 1987;57:147–161. doi: 10.1152/jn.1987.57.1.147. [DOI] [PubMed] [Google Scholar]
- 16.McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page WK, Duffy CJ. Cortical neuronal responses to optic flow are shaped by visual strategies for steering. Cereb Cortex. 2008;18:727–729. doi: 10.1093/cercor/bhm109. [DOI] [PubMed] [Google Scholar]
- 18.Tsal Y. Movements of attention across the visual field. J Exp Psychol: Hum Percept Performance. 1983;9:523–530. doi: 10.1037//0096-1523.9.4.523. [DOI] [PubMed] [Google Scholar]
- 19.Remington R, Pierce L. Moving attention: evidence for time-invariant shifts of visual selective attention. Percept Psychophys. 1984;35:393–399. doi: 10.3758/bf03206344. [DOI] [PubMed] [Google Scholar]
- 20.Braun D, Breitmeyer BG. Relationship between directed visual attention and saccadic reaction times. Exp Brain Res. 1988;73:546–552. doi: 10.1007/BF00406613. [DOI] [PubMed] [Google Scholar]
- 21.Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- 22.Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400:867–869. doi: 10.1038/23698. [DOI] [PubMed] [Google Scholar]
- 23.Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, et al. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- 24.Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong KM, Fitzgerald JK, Moore T, Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]