Abstract
The high affinity (Kd = 0.2 nM) of the soybean agglutinin (SBA), a tetrameric GalNAc specific lectin, for a modified form of porcine submaxillary mucin, a linear glycoprotein, that possessing a molecular mass of ~106 daltons and ~2300 GalNAcα1-O-Ser/-Thr residues (Tn-PSM) has been ascribed to an internal diffusion mechanism that involves binding and jumping of the lectin from GalNAc to GalNAc residue of the mucin (Dam, T. K., et al. (2007) J. Biol. Chem. 282, 28256-28263). Hill plot analysis of the raw ITC data shows increasing negative cooperativity, which correlates with increasing lectin-mucin cross-linking interactions and decreasing favorable binding entropies. However, the affinity of bound SBA for other Tn-PSM molecules during cross-linking is much higher than that of free SBA for GalNAcα1-O-Ser, a monovalent analog. The high affinity of bound SBA for GalNAc residues on other Tn-PSM molecules appears to be due to the favorable entropy of binding associated with the internal diffusion mechanism. Furthermore, the increasing negative cooperativity of SBA binding to Tn-PSM correlates with decreasing internal diffusion of the lectin on the mucin as cross-linking occurs. These findings indicate the importance of the internal diffusion mechanism in generating large, favorable entropies of binding that drive lectin-mucin cross-linking interactions. The results are important for understanding the energetics of lectin-mucin cross-linking interactions that are associated with biological signaling on the surface of cells, and the role of the internal diffusion mechanism in ligand-biopolymers in general.
Lectins are multivalent carbohydrate-binding proteins that are present in animals, plants and microorganisms (1). The biological activities of lectins include receptor-mediated endocytosis of glycoproteins, cellular recognition and adhesion (2), inflammation (3), and cell growth and metastasis (4, 5). As a consequence of their multivalent structures (6, 7), lectin binding leads to cross-linking and aggregation of specific multivalent glycoprotein receptors on cells. This, in turn, results in signal transduction effects that include apoptosis of T cells (8, 9), regulation of the T-cell receptor (10, 11), regulation of the location and activity of the Glut-2 transporter in pancreatic beta cells (12), and regulation cell cycling kinetics and activities of cytokine receptors (13). Hence, it is important to understand the mechanisms of binding and cross-linking of lectins with multivalent glycoprotein receptors in order to gain insight into their structure-activity properties in biological systems.
While the structures of many lectin cross-linked complexes with multivalent carbohydrates and glycoproteins have been investigated (14–16), much less is known about the mechanisms of binding of lectins to multivalent glycoproteins that lead to cross-linking of the receptors. Most glycoprotein receptors on the surface of cells possess multiple copies of a single carbohydrate epitope, which can be expressed in individual linear or branched chain structures, and/or multiple glycosylation sites in the protein (17). For example, CD43, a galectin-1 counter receptor on T cells (18), is a linear glycoprotein (mucin) that possesses approximately 80 O-linked chains with terminal LacNAc epitopes (19). The globular glycoprotein receptor CD45 RO, which is also a galectin-1 counter receptor on T cells, possesses multiple branched N-linked carbohydrates with LacNAc residues (18). However, the mechanisms of binding of lectins such as galectin-1 to glycoprotein receptors like CD43 and CD45 RO are not well understood.
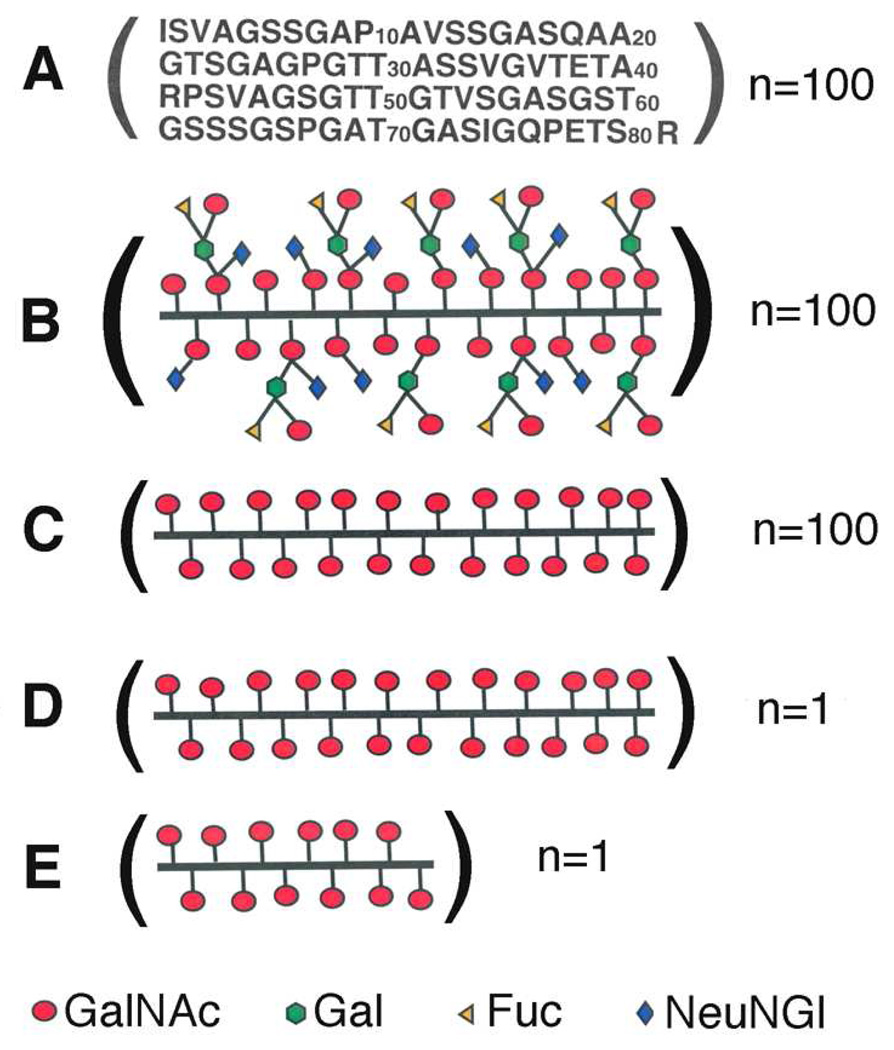
Recently, the thermodynamics of binding of the soybean agglutinin (SBA), a tetrameric Gal/GalNAc specific lectin, to enzymatically and chemically modified forms of porcine submaxillary mucin (PSM), a large linear glycoprotein, were investigated using isothermal titration microcalorimetry (ITC) (20). SBA was shown to bind with a Kd of 0.2 nM to a form of PSM possessing ~2300 α-GalNAc residues and a molecular mass of ~106 daltons (Tn-PSM) (Figure 1C), which is ~106 fold greater affinity as compared to GalNAcα1-O-Ser (Table 1), a monovalent analog (20). The naturally occurring mucin (Fd-PSM) (Figure 1B), which possesses longer chain carbohydrates, bound with ~100-fold lower affinity than Tn-PSM, while two shorter chain analogs of Tn-PSM (Figure 1D and 1E) bound with progressively lower affinities. The results shown in Table 1 from that study include the complete thermodynamics and stoichiometries of binding of SBA to the PSM analogs, which showed that the affinity of SBA increased with increasing polypeptide chain lengths of the mucins (20). Large favorable entropies of binding were associated with the enhanced affinities of the mucins (20).
Figure 1.
Structural representations of A) the amino acid sequence of the 100-repeat 81-residue polypeptide O-glycosylation domain of intact PSM; B) the fully carbohydrate decorated form (described in the text) of the 100-repeat 81-residue polypeptide O-glycosylation domain of PSM (Fd-PSM); C) the 100-repeat 81-residue polypeptide O-glycosylation domain of PSM containing only peptide linked α-GalNAc residues (Tn-PSM); D) the single 81-residue polypeptide O-glycosylation domain of PSM containing peptide linked α-GalNAc residues (81-mer Tn-PSM); E) the 38/40-residue polypeptide(s) derived from the 81-residue polypeptide O-glycosylation domain of PSM containing peptide linked α-GalNAc residues (38/40-mer Tn-PSM).
Table 1.
Thermodynamic binding parameters for SBA at pH 7.2, 27°C (36)
| Ligand | Kda (µM) | K(rel)b | −ΔGc (kcal/mol) | −ΔHd (kcal/mol) | −TΔSe (kcal/mol) | nf |
|---|---|---|---|---|---|---|
| GalNAcα1-O-Ser | 170 | 1 | 5.2 | 7.9 | 2.7 | 1.0 |
| 38/40-mer Tn-PSM | 1.4 | 120 | 8.0 | 32.2 | 24.2 | 0.2 |
| 81-mer Tn-PSM | 0.06 | 2,800 | 9.8 | 56.1 | 46.3 | 0.12 |
| Tn-PSM | 0.0002 | 850,000 | 13.1 | 4310 | 4297 | 0.0018 |
| Fd-PSM | 0.024 | 7,100 | 10.4 | 703 | 693 | 0.008 |
Errors in Kd range from 1–7%
Relative to GalNAcα1-O-Ser
errors in ΔG are less than 2%
errors in ΔH are 1% to 4%
errors in TΔS are 1% to 7%
errors in n are less than 4%.
Importantly, the thermodynamic data and dependence of affinity on the length of the mucin polypeptide chains suggested a dynamic binding mechanism in which lectin molecules bind and jump from carbohydrate epitope to epitope along the peptide backbone of the mucin polypeptide chain (20). This mechanism was previously suggested for lectins binding to multivalent carbohydrates (21) and to the multivalent globular glycoprotein asialofetuin (ASF) (22). This mechanism is also similar to the mechanism of binding of proteins to DNA (23).
The present study presents an analysis of the thermodynamics of SBA cross-linking with the mucins in Figure 1. The results suggest that entropy effects associated with the bind and jump mechanism play a major role in driving cross-linking interactions. These finding have important implications in understanding the structure-activity properties of mucins as glycoprotein receptors, and ligand-biopolymer interactions in general.
Structures of the Mucins
The structures of the PSM analogs in Figure 1 have been previously described in detail (20, 24). Briefly, PSM is composed of a very large central O-glycosylated domain flanked by small Cys-rich globular domains at both the N and C termini (25). The O-glycosylated domain consists of approximately 100 81-residue tandem repeats with the sequence shown in Figure 1A (26). Each tandem repeat contains 31 Ser/Thr O-glycosylation sites, and about 75% or more of the Ser and Thr residues are glycosylated to varying extents (24). Hence, there are ~2300 carbohydrate chains in the O-glycosylated domain of PSM. The carbohydrate side chains described for PSM range from the monosaccharide GalNAcα1-O-Ser/Thr, the pancarcinoma carbohydrate antigen Tn, to the core 1 blood group A tetrasaccharide α-GalNAc(1–3)[α-Fuc(1–2)]-β-Gal(1–3)-α-GalNAc-O-Ser/Thr (27). Each of the mono- through tetrasaccharides is potentially glycosylated by an NeuNGl residue attached α(2–6) to the peptide-linked GalNAc and therefore up to eight possible oligosaccharides are found in PSM.
Hill Plots of Raw ITC Data
The Hill plot, in which log {concentration of free ligand} is plotted versus log {fraction of bound protein}/{fraction of free protein} has been used to investigate cooperativity in a variety of ligand-protein systems (cf. 28, 29). Such plots may typically show evidence for positive or negative cooperative in the binding of monovalent ligands to multisubunit proteins. Ligand binding without cooperativity gives rise to a linear Hill plot with a slope of 1.0. Ligand binding with positive cooperativity gives rise to Hill plots with slopes greater than 1.0, while ligand binding with negative cooperativity gives Hill plots with slopes less than 1.0. Thus, the Hill plot has the advantage of assigning numerical values to the degree of cooperativity of the system, as compared to the Scatchard plot (30). The Hill plot also has the advantage of being a logarithmic representation which allows plotting of all theoretically obtainable data, unlike double reciprocal or half-reciprocal plots that often have open upper limits on the abscissa and ordinate (31). Hill plots can also reveal cooperativity associated with binding of multivalent ligands to non-cooperative multisubunit proteins such as concanavalin A (ConA) and Dioclea grandiflora lectin (DGL) (21). In using Hill plot analysis of the binding of multivalent sugars, the term for the fraction of bound ligand, Xb/Mt, is corrected for the valency of the sugar to give (Xb) x (functional valency of sugar)/Mt, which is a modification of the classical Hill plot.
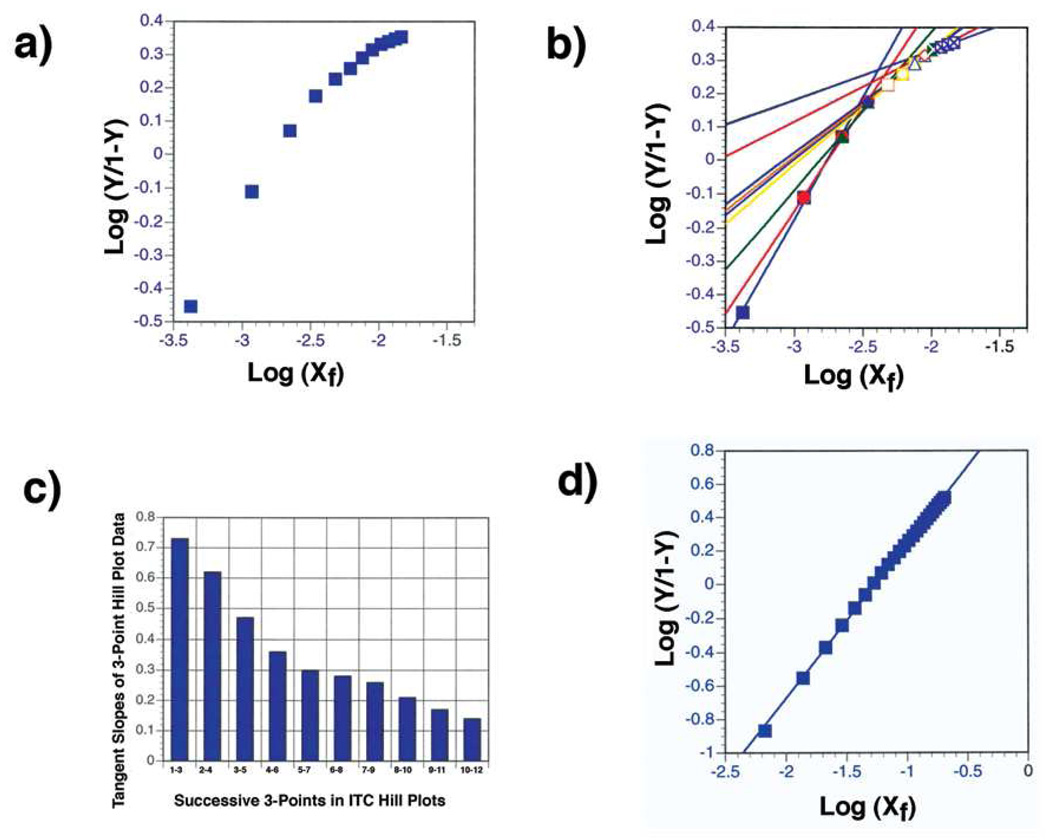
When the raw ITC binding data in Table 1 (20) are subject to Hill plot analysis, for example SBA binding to 38/40-mer Tn-PSM, the results are shown in Figure 2a, which shows increasing curvature with increased binding. For increased clarity, the data are shown as a progressive three point tangent slopes in Figure 2b, and a bar graph in Figure 2c. The data in Figures 2a–c show increasing negative cooperativity with increased binding of SBA to 38/40-mer Tn-PSM. Similar results are observed for the binding of SBA to Tn-PSM and 81-mer Tn-PSM. The ITC derived Hill plot for the binding of SBA to GalNAcα1-O-Ser in Figure 2d shows a straight line with a slope of 0.92, indicating essentially no binding cooperativity. Thus, the increasing negative cooperativity observed for the binding of SBA to the mucins is due to the ligands and not the lectin. Similar Hill plots have been observed for the binding of ConA) and DGL to synthetic bi-, tri- and tetraantennary N-linked carbohydrates (21), and the binding of galectins-1, -2, -3, -4, -5 and -7 to ASF (22).
Figure 2.
a) Hill plot of ITC data for SBA binding to 38/40-mer Tn-PSM; b) tangent slopes of progressive three-point intervals of the Hill plot for SBA binding to 38/40-mer Tn-PSM; c) bar graphs of the three-point tangent slopes of the ITC data Hill plots of SBA binding to 38/40-mer Tn-PSM; d) Hill plot of the ITC data of SBA binding to GalNAcα1-O-Ser (slope = 0.93).
Stoichiometry of Binding of SBA to Mucins
The ITC data in Table 1 provides the n value, the number of binding sites per SBA subunit, for each complex. We have previously shown that the reciprocal of n (1/n) represents the functional valence of the carbohydrate or glycoprotein binding to the lectin in the case where the number of binding sites per subunit of the lectin is known (32). In the case of SBA, the number of binding sites for GalNAc residues per subunit is one as determined by ITC (20) and x-ray crystallography (33). Using the 1/n value for Tn-PSM in Table 1 indicates that ~540 GalNAc residues out of the total of ~2300 GalNAc residues of the mucin bind to SBA under saturation conditions. SBA is a tetramer with four binding sites per molecule (33). Stoichiometric analysis indicates that all four sites of SBA are occupied at the end of the ITC experiment, and therefore, that SBA is completely cross-linked with Tn-PSM in solution. This is also true for the ITC experiments for SBA binding to the shorter mucin fragments.
Increasing Negative Cooperativity Correlates with Decreasing Favorable Entropy of Binding
The ΔH values for SBA binding to Tn-PSM and its fragments have been shown to be proportional to the number of GalNAc residues in the mucins that bind to the lectin (20). For example, ΔH is −4310 kcal/mol for SBA binding to Tn-PSM as compared to −7.9 kcal/mol for GalNAcα1-O-Ser (Table 1). If ΔH for Tn-PSM is divided by the 1/n value of 540 α-GalNAc residues in the mucin that bind to the lectin, the ΔH per α-GalNAc residue is −8.0 kcal/mol, which is similar to that of GalNAcα1-O-Ser. This indicates that each α-GalNAc residue of Tn-PSM that binds to SBA possesses essentially the same ΔH as GalNAcα1-O-Ser. The observed ΔH for SBA binding to Tn-PSM is thus the sum of the individual ΔH values of the α-GalNAc binding residues of the mucin.
The TΔS values for SBA binding to Tn-PSM and its fragments, on the other hand, have been shown to be non-proportional to the number of GalNAc residues in the mucins that bind to the lectin (20). For example, TΔS is −4297 kcal/mol for SBA binding to Tn-PSM as compared to −2.7 kcal/mol for GalNAcα1-O-Ser (Table 1). If TΔS for Tn-PSM is divided by the 1/n value of 540, the resulting TΔS per α-GalNAc residue is −7.96 kcal/mol, which is more unfavorable than −2.7 kcal/mol for GalNAcα1-O-Ser (Table 1). If TΔS were proportional to the number of binding epitopes in Tn-PSM, the observed TΔS would be −1458 kcal/mol, and ΔG would be −2852 kcal/mol, an impossibly large value. The observation that TΔS, unlike ΔH, does not scale in proportion to the number of binding epitopes has been previously observed in the binding of ConA and DGL to bi-, tri- and tetraantennary carbohydrates (21) and galectin-1, -2, -3, -4, -5 , and -7 to ASF, a multivalent glycoproteins (22). The non-proportional behavior of TΔS is characteristic of multiple lectin molecules binding to different epitopes of a multivalent carbohydrate, as opposed to a single lectin molecule binding to multiple epitopes of a single multivalent carbohydrate (34). In the latter case, both ΔH and TΔS increase in proportion to the number of binding epitopes in the multivalent ligand, with concomitantly larger increases in affinity (34).
Importantly, the increasing negative cooperativity observed for the binding of SBA to the mucins in the Hill plots must be due to the non-proportional TΔS for these interactions and not the proportional ΔH contributions, which remains a fixed value per GalNAc residue with increasing SBA binding.
Decreasing Favorable Entropy of Binding and the Bind and Jump Mechanism
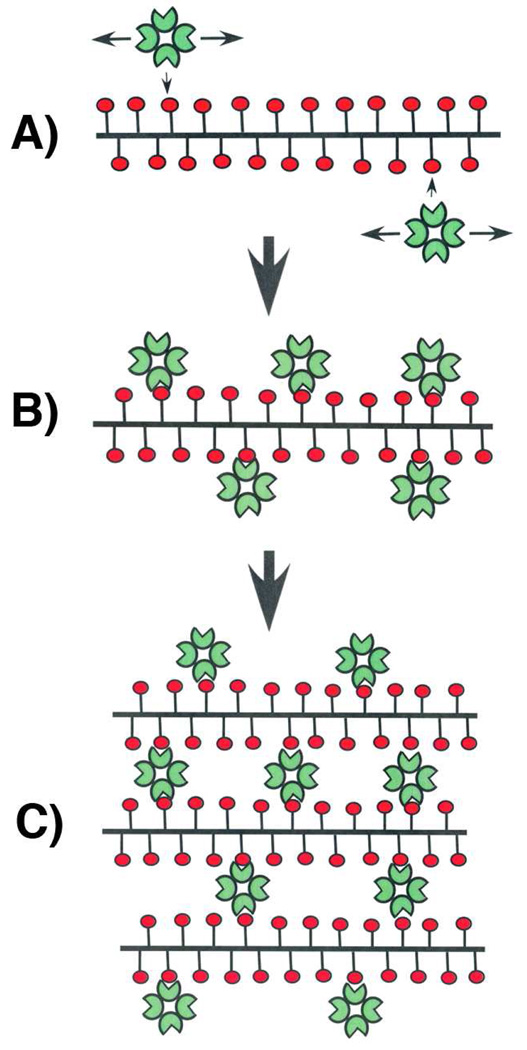
The initial binding of SBA to the mucins must involve very favorable TΔS contributions since the observed Kd values are composites of very high affinities and then lower affinities with increasing negative cooperativities. For example, if the first SBA molecule that binds to Tn-PSM (Figure 3A) possesses a Kd of 0.2 nM (or likely higher) (Table 1), ΔH for this interaction would be −8.0 kcal/mol (per GalNAc residue) and TΔS would be approximately +5.2 kcal/mol, as compared to −2.7 kcal/mol for SBA binding to GalNAcα1-O-Ser (Table 1). (The difference between TΔS values reflects the difference in −ΔG values for SBA binding to Tn-PSM and GalNAcα1-O-Ser). The highly favorable initial TΔS for SBA binding to Tn-PSM can be understood in terms of the dynamic bind and jump mechanism for SBA binding to the mucin (Figure 3A), as previously suggested (20). In essence, SBA binds and jumps from GalNac residue to GalNAc residue along the polypeptide chain of the mucin, resulting in increased residence time of the lectin on the mucin and a longer macroscopic off-rate. Since the dissociation constant, Kd, is the ratio of the reverse rate constant (off-rate) to the forward rate constant, the macroscopic Kd becomes much lower (greater affinity). This mechanism has been suggested to explain the higher affinity of SBA for Tn-PSM as compared to the shorter mucin fragments in Table 1 (20). This mechanism is also well known for the binding of protein ligands to DNA, which has been called the bind and slide or bind and hop mechanism (23).
Figure 3.
Schematic representations of A) SBA binding at low fractional occupany to Tn-PSM; B) SBA binding at higher fractional occupancy to Tn-PSM; C) SBA binding at high fractional occupancy with cross-linking of Tn-PSM.
The increasing negative cooperativity of SBA binding to the mucins with increasing concentration in the initial binding interactions can be explained on the basis of increasing the density of lectin molecules on the mucins, which would reduce their 1-dimensional diffusion paths, as previously suggested (20), and hence entropies of binding (Figure 3B).
SBA-Mucin Cross-linking Interactions Correlate with Increasing Unfavorable Entropy of Binding
Stoichiometric analysis indicates that increased binding of SBA to the mucins beyond the first subunit of the tetrameric lectin involves cross-linking interactions (Figure 3C), as discussed above. The Hill plots show increasing negative cooperativity under conditions of SBA-mucin cross-linking, and therefore decreasing favorable TΔS contributions. Hence, SBA/mucin cross-linking interactions are entropically less favorable as compared to the initial binding of the lectin to the mucin in Figures 3A and 3B.
However, the affinity of bound SBA for GalNAc residues on other mucin molecules during cross-linking is very high compared to that of free SBA to GalNAcα1-O-Ser. For example, the observed Kd of SBA for Tn-PSM is 0.2 nM (Table 1), at which 50% of the lectin is bound to the mucin. Under these conditions, at least two of the four subunits of SBA are cross-linked with Tn-PSM, and therefore the affinity of the lectin is nearly 106-fold greater than that of free SBA for GalNAcα1-O-Ser (Kd = 0.17 mM) (Table 1). The high affinity of SBA in these cross-linked complexes is not due to the ΔH of binding, which is fixed at −8.0 kcal/mol per binding GalNAc residue in Tn-PSM, but instead must be due to favorable TΔS contributions per GalNAc residue. Since favorable entropies of binding of SBA to Tn-PSM are ascribed to the bind and jump mechanism, it follows that SBA is dynamically bound to Tn-PSM in the process of cross-linking. As SBA undergoes further cross-linking, increasing negative cooperativity suggests a reduction in the internal diffusion of SBA on Tn-PSM as cross-linking restricts the movement of lectin molecules. Hence, high affinity cross-linking of Tn-PSM by SBA is driven by the highly favorable entropy of binding of the lectin to the mucin that is associated with the bind and jump mechanism.
Similar increasing negative cooperativity effects have been observed in the binding of ConA and DGL to bi-, tri- and tetraantennary carbohydrates (21) and galectin-1, -2, -3, -4, -5, and -7 to ASF (22), and much of these effects are likely to be due to cross-linking. Indeed, recent experiments with so-called monovalent galectins such as truncated galectin-3 and -5 show evidence of cross-linking activities.
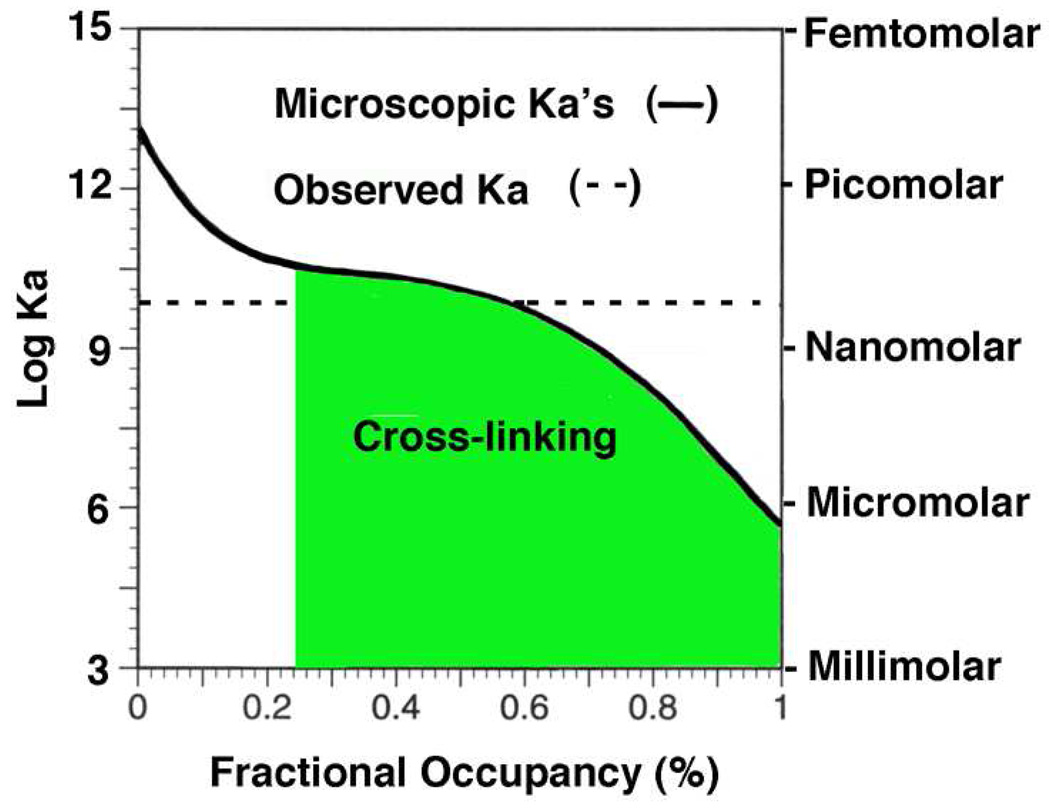
Estimated Range of Microscopic Kd Values for SBA Binding to Tn-PSM
Figure 4 shows a hypothetical plot of the microscopic Kd values versus fractional occupancy of SBA binding to Tn-PSM. The horizontal dashed line is the observed Kd value of 0.2 nM obtained from the ITC experiments (Table 1). However, due to increasing negative cooperativity the microscopic Kd values for SBA binding to Tn-PSM increase (decreasing affinity) with increasing fractional occupancy. The solid line is an estimate of the range of microscopic Kd values of SBA with increasing fractional occupany of Tn-PSM from 0 to 100 percent, which corresponds to 0 to 540 GalNAc residues. Estimates of the range of microscopic Kd values for the binding of ConA and DGL to a tetraantennary carbohydrate was ~2,500-fold from the first unbound carbohydrate epitope to the fourth epitope (21). Estimates of the range of microscopic Kd values for the binding of galectins-1, -2, -3, -4, -5 and -7 binding to ASF was ~3000- to 6000-fold from the first unbound epitope to the ninth unbound epitope (22). The range of microscopic Kd values for SBA binding to 540 GalNAc residues of Tn-PSM is estimated to be greater than the above examples, and as high as ~105 to ~106. This suggests that the first few molecules of SBA binding to Tn-PSM possess microscopic Kd values of picomolar or less, as shown in Figure 4. These SBA molecules have very favorable entropies of binding due their ability to bind and jump along the GalNAc residues of the Tn-PSM polypeptide chain. The TΔS value for these molecules would be ~+10 kcal/mol, as compared to −2.7 kcal/mol for the binding of free SBA to GalNAcα1-O-Ser. Thus, the large increase in microscopic Kd of SBA for Tn-PSM under these conditions is due to highly favorable entropy of binding effects that drive high affinity cross-linking interactions with increasing concentration of the lectin.
Figure 4.
Hypothetical plot (solid line) of the microscopic Kd values of SBA binding to Tn-PSM from 0 to 100 percent occupancy (0 to 540 GalNAc residues). The dashed line represents the observed Kd value of 0.2 nM for SBA binding to Tn-PSM in Table 1.
The diminished affinity of SBA for Tn-PSM over the full range of fractional occupancy in Figure 4 is thus due to decreasing favorable TΔS effects as the lectin undergoes increasing cross-linking with the mucin.
Implications
The high affinity binding and cross-linking interactions of SBA with the mucins in the present study involve increasing negative cooperativity, which extends the binding isotherms over a wide range of lectin concentrations. This suggests that lectin induced cross-linking of mucin receptors on the surface of cells, which is associated with many biological signal transduction processes such as galectin-1 induced apoptosis of T-cells (18), require a relatively large change in the concentration of lectin to initiate signaling. This provides a level of control for this important biological signaling mechanism. In addition, the concentration required for lectin induced cross-linking of mucin receptors would be a function of the mucin structure including the number and composition of carbohydrate binding epitopes, the number of tandem repeat polypeptide domains, the amino acid composition of the tandem repeat domains, and the density of the carbohydrate epitopes. These structural parameters would determine the microscopic affinity and hence entropy of binding of lectins to mucins that drive cross-linking interactions via the bind and jump mechanism. All of these variations are present in the family of animal mucins of which there are at least seventeen mucin gene products (35).
It has been recently been suggested that essentially all ligands may bind to biopolymers by the bind and jump mechanism (36). The results of the present study provide a general model for the enhance entropies of binding of ligands to biopolymers, and the effects on subsequent complex formation and activity of bound ligands.
Finally, a central question in carbohydrate biochemistry as to why lectins have relatively low affinities for individual carbohydrate epitopes such as GalNAc appears to be answered in part by the present study. Such low affinity interactions allow the lectin to gain large favorable entropies of binding to arrays of low affinity carbohydrate epitopes. This, in turn, permits high affinity dynamic binding, and high affinity of the bound protein for other molecules. It will be of interest to determine the effects of modulating the affinity and off-rates of proteins for individual epitopes in biopolymers on this highly favorable entropy of binding mechanism.
Abbreviations
- SBA
soybean agglutinin
- ConA
concanavalin A
- DGL
Dioclea grandiflora lectin
- PSM
porcine submaxillary mucin
- Fd-PSM
fully carbohydrate decorated porcine submaxillary mucin
- Tn-PSM
porcine submaxillary mucin containing GalNAcα1-O-Ser/Thr residues
- 81-mer Tn-PSM
81-residue amino acid repeat of domain of porcine submaxillary mucin containing GalNAcα1-O-Ser/Thr residues
- 38/40-mer Tn-PSM
38/40-residue amino acid cleavage product of 81-mer Tn-PSM containing GalNAcα1-O-Ser/Thr residues
- Tn-antigen
GalNAcα1-O-Ser/Thr
- ASF
asialofetuin
- GalNAc
N-acetyl-D-galactosamine
- Fuc
L-fucose
- Gal
D-galactose
- NeuNGl
N-glycoloylneuraminic acid or sialic acid
- LacNAc
N-acetyl-D-lactosamine
- ITC
isothermal titration microcalorimetry
- NMR
nuclear magnetic resonance
Footnotes
This work was supported by Grant CA-16054 from the National Cancer Institute, Department of Health, Education and Welfare, and Core Grant P30 CA-13330 from the same agency (C. F. B.) and Grant CA-78834 from the National Institutes of Health, National Cancer Institute (T. A. G).
REFERENCES
- 1.Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth J, editors. Essentials of Glycobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1999. [PubMed] [Google Scholar]
- 2.Drickamer K, Taylor ME. Biology of animal lectins. Annu. Rev. Cell Biol. 1993;9:237–264. doi: 10.1146/annurev.cb.09.110193.001321. [DOI] [PubMed] [Google Scholar]
- 3.Liu F-T. Galectins: A new family of regulators of inflammation. Clin. Immunol. 2000;97:79–88. doi: 10.1006/clim.2000.4912. [DOI] [PubMed] [Google Scholar]
- 4.Konstantinov KN, Robbins BA, Liu F-T. Galectin-3, a β-galactoside-binding animal lectin, is a marker of anaplastic large-cell lymphoma. Amer. J. Path. 1996;148:25–30. [PMC free article] [PubMed] [Google Scholar]
- 5.Akahani S, Nangia-Makker P, Inohara H, Kim H-RC, Raz A. Galectin-3: a novel antiapoptotic molecule with a functional BH1 (NHGR) domain of Bcl-2 family. Cancer Res. 1997;57:5272–5276. [PubMed] [Google Scholar]
- 6.Rini JM. Lectin Structure. Annu. Rev. Biophys. Biomolec. Struct. 1995;24:551–577. doi: 10.1146/annurev.bb.24.060195.003003. [DOI] [PubMed] [Google Scholar]
- 7.Loris R, Hamelryck T, Bouckaert J, Wyns L. Legume lectin structure. Biochim. Biophys. Acta. 1998;1383:9–36. doi: 10.1016/s0167-4838(97)00182-9. [DOI] [PubMed] [Google Scholar]
- 8.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 9.Perillo NL, Uittenbogaart CH, Nguyen JT, Baum LG. Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J. Exper. Med. 1997;185:1851–1858. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vespa GNR, Lewis LA, Kozak KR, Moran M, Nguyen JT, Baum LG, Miceli MC. Galectin-1 specifically modulates TCR signals to enhance TCR apoptosis but inhibit IL-2 production and proliferation. J. Immunol. 1999;162:799–806. [PubMed] [Google Scholar]
- 11.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsubo K, Takamatsu S, Minowa MT, Yoshida A, Takeuchi M, Marth JD. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;123:1307–1321. doi: 10.1016/j.cell.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 13.Partridge EA, Le Roy C, Di Gulielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. Regulation of cytokine receptors by golgi N-glycan processing and endocytosis. Science. 2005;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 14.Dam TK, Brewer CF. Carbohydrate-lectin cross-linking interactions: structural, thermodynamic, and biological studies. Methods Enzymol. 2003;362:455–486. doi: 10.1016/S0076-6879(03)01031-0. [DOI] [PubMed] [Google Scholar]
- 15.Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 2002;12:616–623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 16.Sacchettini JC, Baum LG, Brewer CF. Multivalent protein-carbohydrate interactions. A new paradigm for supermolecular assembly and signal transduction. Biochemistry. 2001;40:3009–3015. doi: 10.1021/bi002544j. [DOI] [PubMed] [Google Scholar]
- 17.Dam TK, Brewer CF. In: Fundamentals of lectin-carbohydrate interactions, In Comprehensive Glycoscience. Kamerling JP, Boons G-J, Lee YC, Suzuki A, Taniguichi N, Voragen AGJ, editors. Vol. 3. Oxford: Elsevier, Ltd.; 2007. pp. 397–452. [Google Scholar]
- 18.Pace KE, Lee C, Stewart PL, Baum LG. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J. Immunol. 1999;163:3801–3811. [PubMed] [Google Scholar]
- 19.Daniels MA, Hogquist KA, Jameson SC. Sweet ‘n’ sour: the impact of differential glycosylation on T cell responses. Nature Immunol. 2002;3:903–910. doi: 10.1038/ni1002-903. [DOI] [PubMed] [Google Scholar]
- 20.Dam TK, Gerken TA, Cavada BS, Nascimento KS, Moura TR, Brewer CF. Binding studies of α-GalNAc specific lectins to the α-GalNAc (Tn-antigen) form of porcine submaxillary mucin and its smaller fragments. J. Biol. Chem. 2007;282:28256–28263. doi: 10.1074/jbc.M704677200. [DOI] [PubMed] [Google Scholar]
- 21.Dam TK, Roy R, Pagé D, Brewer CF. Negative cooperativity associated with binding of multivalent carbohydrates to lectins. Thermodynamic analysis of the “multivalency effect”. Biochemistry. 2002;41:1351–1358. doi: 10.1021/bi015830j. [DOI] [PubMed] [Google Scholar]
- 22.Dam TK, Gabius H-J, Andre S, Kaltner H, Lensch M, Brewer CF. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry. 2005;44:12564–12571. doi: 10.1021/bi051144z. [DOI] [PubMed] [Google Scholar]
- 23.von Hippel PH. From “simple” DNA-protein interactions to the macromolecular machines of gene expression. Annu. Rev. Biophy. Biomolec. Struct. 2007;36:79–105. doi: 10.1146/annurev.biophys.34.040204.144521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerken TA, Gilmore M, Zhang J. Determination of the site-specific oligosaccharide distribution of the O-glycans attached to the porcine submaxillary mucin tandem repeat. J. Biol. Chem. 2002;277:7736–7751. doi: 10.1074/jbc.M111690200. [DOI] [PubMed] [Google Scholar]
- 25.Eckhardt AE, Timpte CS, DeLuca AW, Hill RL. The complete cDNA sequence and structural polymorphism of the polypeptide chain of porcine submaxillary mucin. J. Biol. Chem. 1997;272:33204–33210. doi: 10.1074/jbc.272.52.33204. [DOI] [PubMed] [Google Scholar]
- 26.Timpte CS, Eckhardt AE, Abernethy JL, Hill RL. Porcine submaxillary gland apomucin contains tandemly repeated, identical sequences of 81 residues. J. Biol. Chem. 1988;272:1081–1087. [PubMed] [Google Scholar]
- 27.Carlson D. Structures and immunological properties of oligosaccharides isolated from pig submaxillary mucins. J. Biol. Chem. 1968;243:616–626. [PubMed] [Google Scholar]
- 28.Stryer L. Biochemistry. 3rd ed. New York: W. H. Freedman and Company; 1988. [Google Scholar]
- 29.Di Cera E. Thermodynamic Theory of Site-Specific Binding Processes in Biological Macromolecules. New York: Cambridge University Press; 1995. [Google Scholar]
- 30.Scatchard G. The attractions of proteins for small molecules and ions. Annu. N. Y. Acad. Sci. 1949;51:660–672. [Google Scholar]
- 31.Weber G, Anderson S. Multiplicity of binding. Range of validity and practical test of Adair’s equation. Biochemistry. 1965;4:1942–1947. [Google Scholar]
- 32.Dam TK, Roy R, Das SK, Oscarson S, Brewer CF. Binding of multivalent carbohydrates to concanavalin A and Dioclea grandiflora lectin. Thermodynamic analysis of the “multivalency effect”. J. Biol. Chem. 2000;275:14223–14230. doi: 10.1074/jbc.275.19.14223. [DOI] [PubMed] [Google Scholar]
- 33.Dessen A, Gupta D, Sabesan S, Brewer CF, Sacchettini JC. X-ray crystal structure of the soybean agglutinin cross-linked with a biantennary analog of the blood group I carbohydrate antigen. Biochemistry. 1995;34:4933–4942. doi: 10.1021/bi00015a004. [DOI] [PubMed] [Google Scholar]
- 34.Dam TK, Brewer CF. Thermodynamic studies of lectin-carbohydrate interactions by isothermal titration calorimetry. Chem. Rev. 2002;102:387–429. doi: 10.1021/cr000401x. [DOI] [PubMed] [Google Scholar]
- 35.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 36.Dam TK, Brewer CF. Effects of clustered epitopes in multivalent ligand-receptor interactions. Biochemistry. 2008;47:8470–8476. doi: 10.1021/bi801208b. [DOI] [PubMed] [Google Scholar]