Abstract
Root development is extremely sensitive to variations in nutrient supply, but the mechanisms are poorly understood. We have investigated the processes by which nitrate (NO3−), depending on its availability and distribution, can have both positive and negative effects on the development and growth of lateral roots. When Arabidopsis roots were exposed to a locally concentrated supply of NO3− there was no increase in lateral root numbers within the NO3−-rich zone, but there was a localized 2-fold increase in the mean rate of lateral root elongation, which was attributable to a corresponding increase in the rate of cell production in the lateral root meristem. Localized applications of other N sources did not stimulate lateral root elongation, consistent with previous evidence that the NO3− ion is acting as a signal rather than a nutrient. The axr4 auxin-resistant mutant was insensitive to the stimulatory effect of NO3−, suggesting an overlap between the NO3− and auxin response pathways. High rates of NO3− supply to the roots had a systemic inhibitory effect on lateral root development that acted specifically at the stage when the laterals had just emerged from the primary root, apparently delaying final activation of the lateral root meristem. A nitrate reductase-deficient mutant showed increased sensitivity to this systemic inhibitory effect, suggesting that tissue NO3− levels may play a role in generating the inhibitory signal. We present a model in which root branching is modulated by opposing signals from the plant’s internal N status and the external supply of NO3−.
A plant’s ability to explore the soil and to compete effectively for soil resources is critically dependent on the architecture of its root system (1). Root architecture is determined by the pattern of root branching and by the rate and trajectory of growth of individual roots. These properties of a root system are not only under direct genetic control but are also highly plastic, being influenced by a wide range of physical, chemical, and biological factors (2, 3).
A striking example of plasticity in root development is seen in the way many plant species respond to an uneven distribution of nutrients (NO3−, NH4+ or inorganic phosphate) by proliferating their lateral roots preferentially within nutrient-rich zones (3, 4). This ability to “forage” for localized supplies of nutrients is believed to be important in determining a plant’s ability to compete for limiting resources (5). In cereals, the increased proliferation of laterals in the nutrient-rich zone is caused by an increase in both their numbers and their elongation rates (6–8). These localized responses have generally been explained in terms of either a direct or an indirect nutritional effect. Thus, it has been suggested that the roots directly exposed to a localized source of NO3− are stimulated because they benefit most from the increased N supply (6), or, alternatively, that increased metabolic activity in those same roots leads to a growth-stimulating influx of carbohydrates and auxin (7–10).
In contrast to the stimulatory effect of a localized NO3− supply, a high rate of N supply to the root system as a whole usually is associated with a reduced allocation of resources to root growth (i.e., decreased root/shoot ratios) (11). Similar apparently contradictory effects of NO3− on root growth were observed in Arabidopsis (12), which led to a model in which it was proposed that the NO3− supply modulates lateral root (LR) development in two distinct ways: through a systemic inhibitory effect that results from the accumulation of NO3− (and/or its metabolites) in the shoot and through a localized stimulatory effect that depends on the local concentration of NO3− at the LR tip. An analogous “feedback–feedforward” model for NO3− regulation of shoot–root allocation in tobacco has been postulated by Stitt and colleagues (13, 14).
The finding that LR proliferation in the nia1nia2 mutant of Arabidopsis [which is nitrate reductase (NR)-deficient and has a low capacity for NO3− assimilation] responds as strongly as the wild-type to a localized NO3− treatment (12), led to the suggestion that the stimulatory effect of NO3− on LR proliferation is triggered by a signal from the NO3− ion itself rather than by its nutritional properties. The Arabidopsis ANR1 gene, which encodes a NO3−-inducible and root-specific member of the MADS-box family of transcription factors, was identified as a key component of the signal transduction pathway by which NO3− stimulates LR proliferation (12).
Arabidopsis offers a number of advantages for the study of the nutritional control of root development, not least its small size (which allows root growth studies to be done in standard Petri dishes) and the availability of a wide range of nutritional and hormonal mutants to aid in the dissection of signal transduction pathways. In the present study we have examined in detail the effects of NO3− on root branching in Arabidopsis and present a model for how NO3− modulates LR growth and development via two distinct pathways.
MATERIALS AND METHODS
Plant Material.
Seed of Arabidopsis thaliana ecotype Columbia (Col) was from Lehle Seeds, (Round Rock, TX, catalogue no. WT-1A). The axr2–1 and axr4–2 mutants were kindly provided by O. Leyser (University of York, United Kingdom), the aux1–7 mutant by M. Bennett (University of Warwick, United Kingdom), END199 by P. Benfey (New York University), and the nia1nia2 mutant G′4-3 by N. Crawford (University of California, San Diego).
Growth of Seedlings.
The use of segmented agar plates to make localized applications of nutrients to Arabidopsis roots under aseptic conditions has been described (12). Unless otherwise stated, all plates contained 0.5% sucrose and 0.01 mM NH4NO3 and were incubated at 24–26°C under a 16:8 h light/dark regime. Seedlings were transferred to the segmented plates when their primary roots were long enough to extend into the middle segment (≈2 cm). For the uniform nutrient treatments, seedlings were germinated for 3 d and subsequently transferred to agar plates containing the appropriate nutrients. Lengths of individual primary and lateral roots were measured with a ruler directly or from images captured by using an Eagle Eye II Still Video System (Stratagene).
Cytology and Histochemistry.
Epidermal cells of LRs that had been fixed in 4% (vol/vol) formaldehyde were observed with differential interference contrast microscopy in either a Zeiss Photomicroscope III or a Leica DMRB microscope by using a ×40 objective. Mean mature cell lengths were determined from measurements on 7–10 cells per root in the zone where root hairs had recently emerged. β-glucuronidase (GUS) activity in roots of the GUS marker line END199 was detected histochemically by using 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gluc) as substrate (15).
RESULTS
Even Very Low NO3− Concentrations Applied Locally Can Stimulate LR Proliferation.
By using a technique in which Arabidopsis seedlings are grown aseptically on segmented vertical agar plates, we previously showed that a localized supply of 1 mM KNO3 to the primary roots of Arabidopsis seedlings stimulated LR proliferation specifically in the zone of treatment (12). To investigate the concentration dependence of this effect, a range of KNO3 concentrations from 0.05 to 50 mM was supplied to the middle segment of the segmented agar plates, the top and bottom segments receiving only the basal level of N (0.01 mM NH4NO3).
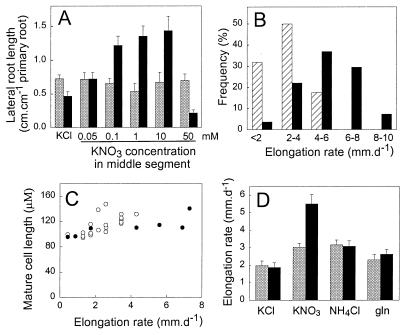
Surprisingly, even 0.05 mM NO3− was sufficient to produce a localized 50% increase in LR length in the zone of treatment (Fig. 1A). However, the strongest responses (2.5- to 3-fold stimulation) were seen at concentrations from 0.1 to 10 mM (Fig. 1A). At 50 mM NO3−, LR growth in the treated segment was inhibited by 50% compared with the control (which received 1 mM KCl). This effect, which is investigated in more detail below, is consistent with previous evidence that high concentrations of NO3− can inhibit LR development in Arabidopsis (12). In all cases, the effect of the localized NO3− supply was specific to the root segment receiving the treatment, LR lengths in the top segment not being significantly affected. (Over the short time span of this and the other experiments reported here, there was insufficient LR growth in the bottom segment of the plate for meaningful measurements to be made.)
Figure 1.
Effects of localized supplies of different N sources on LR growth. (A) Effect of different KNO3 concentrations in the “NO3−-rich” zone. Arabidopsis seedlings were grown on vertical agar plates that had been divided horizontally into three segments to allow different nutrient treatments to be applied to the basal, middle, and apical zones of the primary root (12). The top and bottom segments of the agar plate contained 0.01 mM NH4NO3 as sole N source, and the middle segment contained in addition the indicated concentrations of KNO3. The controls received 1 mM KCl in the middle segment (in preliminary experiments, the 1 mM KCl treatment was found to have no effect on LR growth compared with a water control). Nine days after transfer, LR lengths in the top (shaded bars) and middle (filled bars) segments were measured for each seedling (n = 23–33). (B) Effect of a localized supply of KNO3 on LR elongation rates in the NO3−-rich zone. Seedlings (12 per treatment) were grown at 25°C and under continuous light on segmented agar plates containing 0.01 mM NH4NO3 that were supplemented in the middle segment with either 1 mM KCl or 1 mM KNO3. The elongation rates of individual LRs in the middle segment were estimated by measuring their lengths on days 8 and 9 after transfer, and the frequency distribution of different elongation rates was plotted for the KCl (hatched bars) and KNO3 (filled bars) treatments. The mean rate of LR elongation in the KCl controls was 2.7 ± 0.26 and in the KNO3 treatment was 5.4 ± 0.38 mm⋅day−1. (C) Relationship between the elongation rate of a LR and the length of its mature cells. The elongation rates of individual LRs growing in a localized supply of 1 mM KCl or 1 mM KNO3 as described for B were determined for the 24-h period before they were excised and fixed for cytological examination. Mean mature cell lengths for the KCl (○) and KNO3 (●) treatments were estimated as described in Materials and Methods. (D) Effect of localized supplies of NH4+ and glutamine on LR elongation rates. Seedlings (11–13 per treatment) were grown on segmented agar plates containing 0.01 mM NH4NO3 and supplied in the middle segment with KCl, KNO3, NH4Cl, or glutamine (each at 0.1 mM). LR elongation rates in the top (shaded bars) and middle (filled bars) segments were measured between days 9 and 10 after transfer.
A Localized NO3− Supply Specifically Stimulates LR Elongation Rates Without Affecting LR Initiation.
In previous studies, mainly with monocots, localized NO3− treatments were found to stimulate both initiation and elongation of LRs (6–8). To investigate the nature of the response in Arabidopsis, roots were exposed to a localized supply of 1 mM KNO3 or 1 mM KCl, and the LR elongation rates were monitored. Although there was a wide variation in the growth rates of individual LRs within each treatment (Fig. 1B), the mean rate of LR elongation in the localized NO3− treatment was twice that in the controls. On the other hand, the numbers of emerged LRs in the treated segment did not differ significantly (3.1 ± 0.5 in the control and 2.8 ± 0.4 in the localized NO3− treatment). Thus, in Arabidopsis, a localized supply of NO3− specifically stimulates LR elongation.
The mean final cell length was determined for 29 individual lateral roots elongating at different rates either with or without the localized NO3− treatment (Fig. 1C). Only relatively small differences in the lengths of the mature cells were seen despite a wide variation in elongation rates, and there was no significant difference between the data for the NO3−-treated LRs and the controls. Thus, we can conclude that the stimulation of LR elongation by a localized NO3− treatment is primarily caused by a higher rate of cell production in the LR meristem rather than an increase in mature cell length.
Localized Supplies of Other N Sources Fail to Stimulate LR Elongation.
Ammonium and glutamine can each serve as alternative N sources for Arabidopsis, although at high concentrations (≥1 mM), they can inhibit growth (unpublished results). In preliminary experiments, we found that localized treatments with either 1 mM NH4Cl or 1 mM glutamine did not stimulate localized LR growth (data not shown). Because these results may have been influenced by the inhibitory effects of these relatively high concentrations, we repeated the experiment, using just 0.1 mM of each N source. The results (Fig. 1D) demonstrate that whereas this concentration of KNO3 stimulated LR elongation in the middle (treated) segment by almost 2-fold compared with the top segment, the same concentration of either NH4+ or glutamine produced no localized increase in LR elongation rates.
Evidence for an Overlap Between the Signal Transduction Pathways for NO3− and Auxin.
Elongation of both primary and lateral roots is highly sensitive to auxin, with either stimulatory or inhibitory effects being seen, depending on the auxin concentration (16). To look for evidence of interactions between the signal transduction pathways for NO3− and auxin, we tested the sensitivity of three auxin-resistant mutants, aux1 (17), axr2 (18), and axr4 (19), to a localized supply of 1 mM NO3−.
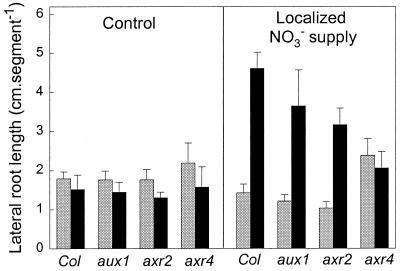
As shown in Fig. 2, LR growth in the aux1 and axr2 mutants responded to the NO3− treatment in a similar way as the wild type. On the other hand, LR growth in the axr4 mutant was not stimulated by the localized supply of NO3−. For axr4, as for the wild type, the localized NO3− treatment did not affect the numbers of emerged LRs (data not shown), indicating that the lack of response in the axr4 mutant was caused by a failure to stimulate LR elongation.
Figure 2.
The responses of three auxin-resistant mutants to a localized supply of KNO3. The auxin-resistant mutants (aux1–7, axr2–1, and aux4–2) and the wild type (Col) were grown under standard low-N conditions (see Fig. 1) on segmented agar plates, and the middle segment was supplied with either 1 mM KCl (control) or 1 mM KNO3 (localized NO3− supply). Nine days after transfer, LR lengths were measured in the top (shaded bars) and middle (filled bars) segments (13 seedlings of each line per treatment).
High Rates of NO3− Supply Inhibit LR Development at a Specific Stage.
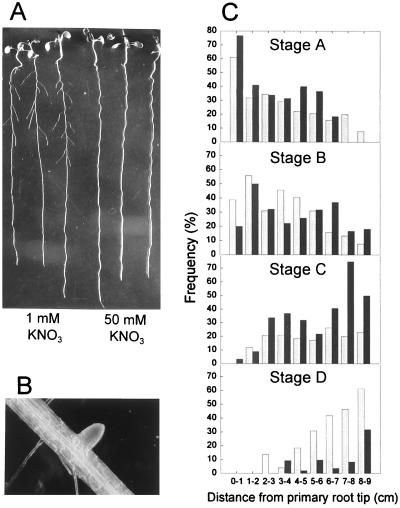
We previously reported that high NO3− concentrations (≥10 mM), when applied uniformly to the whole of the primary root, had a strong inhibitory effect on LR production without affecting the numbers of LRs initiated or the rate of primary root growth (12). Fig. 3A compares the root morphology of seedlings grown on 1 mM and 50 mM KNO3 illustrating the apparent absence of LRs at the higher NO3− concentration. However, closer examination of these seedlings showed the presence of many short but otherwise normal-looking LRs (Fig. 3B).
Figure 3.
Effect of a high rate of NO3− supply on LR development. (A) Photograph showing the suppression of LR development in seedlings grown for 7 days on 50 mM KNO3 compared with those grown on 1 mM KNO3. (B) Close-up of a typical stunted LR from a seedling grown on 50 mM KNO3. The primary root is approximately 0.2 mm in diameter. (C) LR development is specifically inhibited at a stage just after LR emergence. Seedlings of the END199 GUS marker line (30) were grown on 1 mM KNO3 (shaded bars) or 50 mM KNO3 (filled bars) for 7 days and then stained for GUS activity (9–15 seedlings per treatment). Each LR or LR primordium was classified according to its stage of development and its distance from the primary root tip. The relative frequency of each of the four developmental stages within each 1-cm segment of the primary root has been plotted. Stage A, up to 3 cell layers; Stage B, unemerged, >3 cell layers; Stage C, LR emerged, <0.5 mm long; Stage D, ≥0.5 mm long.
We used the GUS marker line END199 to identify the stage(s) of LR development that are blocked or delayed by high NO3− concentrations. Seedlings grown on 1 mM KNO3 or 50 mM KNO3 were stained for GUS activity and the emerged and unemerged LRs were classified into four developmental stages (see legend to Fig. 3). The mean number of LRs (emerged and unemerged) per seedling at 1 mM and 50 mM KNO3 was similar (33.2 and 30.6, respectively), but the mean length of LRs in the seedlings grown on 1 mM KNO3 was 4.5 cm per seedling, whereas on 50 mM it was only 0.3 cm, confirming that this line was as sensitive to the inhibitory effect of NO3− as the C24 line studied previously (12).
Fig. 3C shows the proportion of LRs at each stage that were found in successive 1 cm segments from the apex to the base of the primary root. The distribution along the primary root of unemerged LRs (stages A and B) was very similar in the two NO3− treatments, with the frequency of both stages generally declining with increasing distance from the root tip as more and more of the LR primordia emerged through the epidermis. There was, however, a marked difference between the two treatments in the distribution of stage C and stage D laterals: whereas most of the LRs growing on the lower NO3− concentration quickly progressed through stage C to stage D, LRs growing on 50 mM NO3− accumulated at stage C, with very few progressing to stage D by the day of measurement.
To investigate whether mature LRs are sensitive to 50 mM KNO3, seedlings were grown initially on 1 mM KNO3 and subsequently shifted to 50 mM KNO3. It was found that LRs that had already emerged at the time of the shift were insensitive to the high NO3− concentration and grew at the same average rate as LRs that were kept on 1 mM KNO3 throughout (data not shown). Thus, LRs appear to be sensitive to the inhibitory effect of a high rate of NO3− supply only during a very specific stage of their development, just after emergence and before maturation.
The phenotype of the alf3 mutant of Arabidopsis, which forms a primary root covered with stunted LRs (20), is superficially similar to that of wild-type seedlings growing on 50 mM KNO3 (Fig. 3). However, whereas the arrested alf3 LRs are dead and cannot be rescued once formed (20), many of the stunted LRs developing on 50 mM KNO3 will eventually grow out normally if left for long enough and will recover immediately if the seedlings are transferred to 1 mM NO3− (data not shown).
The Inhibitory Effect of NO3− Is Systemic and Is Not Alleviated in an NR-Deficient Mutant.
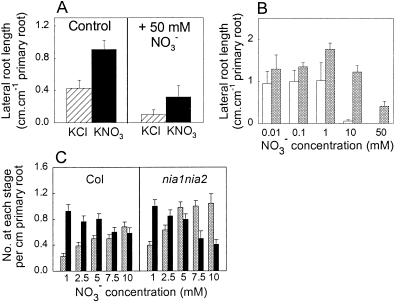
To investigate whether a high NO3− concentration has a localized or a systemic effect on LR development, we grew seedlings on segmented agar plates which contained 50 mM KNO3 in both the top and bottom segments and a much lower NO3− concentration in the middle segment (0.01 mM NH4NO3 plus either 1 mM KCl or 1 mM KNO3). Compared with the controls (which received only the basal level of N in the top and bottom segments), LR development in the middle segment was inhibited by about 70% in each case, despite the LRs not being directly exposed to the 50 mM KNO3 (Fig. 4A). Thus, in contrast to its stimulatory effect, the inhibitory effect of NO3− on LR development is systemic. When a similar experiment was performed by using 1 mM glutamine in the top and bottom segments, LR growth in the middle segment was inhibited by about 60% (data not shown), indicating that N sources other than NO3− are capable of producing a systemic inhibitory effect.
Figure 4.
Characterization of the inhibitory effect of NO3− in wild-type and an NR-deficient line. (A) The inhibitory effect of a high NO3− concentration is systemic. Seedlings were grown on segmented agar plates in which the top and bottom segments contained either 0.01 mM NH4NO3 (control) or 50 mM KNO3, whereas the middle segments received either 1 mM KCl (hatched bars) or 1 mM KNO3 (filled bars). LR lengths in the middle segment were measured 8 days after transfer (11–17 seedlings per treatment). (B) Increasing the sucrose concentration in the medium partially relieves the inhibitory effect of high NO3− concentrations. Seedlings were grown for 7 days on unsegmented agar plates containing a range of KNO3 concentrations and either 0.5% (open bars) or 2% (shaded bars) sucrose. Note that there was no significant LR growth in the 50 mM NO3− treatment at 0.5% sucrose. (C) An NR-deficient mutant is more sensitive than the wild type to the inhibitory effect of high NO3− concentrations. Seedlings of the wild type (Col) and of the nia1nia2 mutant (22) (12–18 per treatment) were grown for 7 days on agar plates containing a range of KNO3 concentrations, and the numbers of LRs at stages C (shaded bars) and D (filled bars) (see Fig. 3) were scored by bathing the roots in water and examining them at ×100 magnification with an inverted microscope.
In experiments with detached leaves, it was shown that feedback inhibitory effects of downstream metabolites (such as glutamine) on expression of the genes for NR and nitrite reductase can be counteracted by supplying a C source (21). To investigate whether a similar response occurred in the case of the inhibitory effect of NO3− on LR development, we measured the growth of LRs of seedlings grown on a range of NO3− concentrations and either the standard sucrose concentration (0.5%) or a higher concentration of sucrose (2%). Fig. 4B shows that the strong inhibitory effect of 10 mM and 50 mM KNO3 seen at 0.5% sucrose was markedly alleviated at 2% sucrose.
These experiments suggested that the inhibitory effect of 50 mM KNO3 was due to its influence on the N status of the plant. To determine the importance of NO3− assimilation in the process, we investigated the sensitivity of the NR-deficient nia1nia2 mutant (22) to high rates of NO3− supply. Seedlings of the wild type (Col) and the nia1nia2 mutant were grown on plates containing a range of NO3− concentrations from 1 to 10 mM, and the numbers of stage C and stage D laterals were determined. As is evident from the data in Fig. 3C, the relative numbers of stage C/stage D laterals provides a sensitive indicator of the specific inhibitory effect of NO3− on stage C laterals. As expected, the ratio of stage C/stage D laterals in both the wild-type and mutant lines increased as the NO3− concentration was increased. However, whereas at 1 mM KNO3, the proportion of stage C and stage D laterals in the mutant and wild-type seedlings was similar, higher concentrations of NO3− led to a greater accumulation of stage C laterals in nia1nia2 than in Col, with the differences between the lines becoming increasingly pronounced with increasing NO3− concentrations. Thus, the NR-deficient mutant has enhanced sensitivity to the inhibitory effect of high rates of NO3− supply on LR development.
DISCUSSION
The evidence reported here and elsewhere (12), demonstrates that the availability and distribution of the NO3− supply have very marked effects on LR growth and development in Arabidopsis. Most of these effects are consistent with a model in which NO3− has both positive and negative effects on LR proliferation (12). In the present study, we show that these opposing effects of NO3− not only operate by different pathways but also act at different stages of LR development.
Nitrate concentrations as low as 0.05 mM, if applied to just one zone of the primary root, were found to be able to stimulate LR proliferation within that zone, and this was because of an increased rate of cell production in the LR meristem (Fig. 1). In contrast to similar studies in other species (6–8), the localized supply of NO3− had no effect on LR initiation. Furthermore, whereas a localized supply of NH4+ is reported to stimulate LR initiation and elongation in barley (23), we found no effect of localized supplies of either NH4+ or glutamine on Arabidopsis.
We previously showed that an NR-deficient Arabidopsis mutant, with a much reduced capacity to assimilate NO3−, is able to respond normally to a localized NO3− supply (12). This result, together with the present finding that other N sources fail to stimulate LR elongation, does not support models in which localized LR proliferation is attributed either directly or indirectly to the nutritional role of NO3− (6–8, 10). Our data are more consistent with the previous suggestion that NO3− is acting as a signal rather than as a nutrient (12), i.e., that meristematic activity in the LR tip is responding directly to the external NO3− concentration. A similar signaling role for NO3− in stimulating root growth in tobacco was proposed on the basis of split-root experiments with an NR-deficient mutant (24).
The product of the NO3−-inducible ANR1 gene, a putative transcription factor of the MADS-box family, was recently identified as a likely component of the signal transduction pathway linking external NO3− to increased LR proliferation (12). Additional insight into this pathway comes from the finding that LR elongation in the axr4 mutant failed to respond to a localized NO3− supply, whereas two other auxin-resistant mutants resembled the wild type (Fig. 2). The axr4 mutant is unusual among auxin-resistant mutants, in that its sensitivity to other hormones such as ethylene and cytokinins is unaffected (19, 25), so its lack of responsiveness to the stimulatory effect of NO3− provides clear evidence for an overlap between the auxin and NO3− response pathways.
The inhibitory effect of NO3− on LR development differs in two important respects from its stimulatory effect. First, whereas the stimulatory effect acts on the mature LR, the inhibitory effect acts specifically on immature LRs, just after their emergence from the primary root (Fig. 3). Second, whereas the stimulatory effect is localized to the LRs directly exposed to the NO3−, the inhibitory effect is systemic (Fig. 4A).
The finding that the inhibitory effect of a treatment with 50 mM KNO3 is much diminished when it is applied to just one part of the root system (Figs. 1A and 4A) indicates that this effect depends not on its local concentration but on the total amount of NO3− taken up by the plant. The further finding that LR development in the NR-deficient nia1nia2 mutant is more sensitive rather than less sensitive to the inhibitory effects of high NO3− concentrations (Fig. 4C) suggests that the accumulation of NO3− itself within the plant is capable of generating the inhibitory effect. It is well established that NR-deficient mutants growing on NO3−-containing media accumulate high concentrations of NO3−, particularly in their leaves (26–28). By using tobacco lines with different degrees of NR deficiency, it has previously been found that there is a strong positive correlation between the leaf NO3− content and the shoot/root ratio (13), leading to the conclusion that NO3− levels in the shoot act as a signal to regulate the allocation of resources between shoots and roots. Root growth in a NR-deficient line growing on 12 mM NO3− was inhibited 2- to 3-fold compared with controls growing on 0.2 mM NO3−, and the resultant root system was shorter and less “bushy” than the controls (13). There therefore appear to be strong parallels between the inhibitory effects we observe in Arabidopsis and those seen in tobacco, but whether LR development is affected in the same stage-specific way in tobacco as in Arabidopsis has yet to be established.
Although the evidence supports a role for tissue NO3− in generating the systemic inhibitory signal, we cannot rule out a possible additional contribution by downstream metabolites of NO3−. When 1 mM glutamine was included in the medium, it too had a systemic inhibitory effect on LR development (data not shown), and in tobacco it was similarly found that shoot growth was stimulated and root growth was inhibited when the medium was supplemented with either NH4+ or glutamine (13). These results suggest that products of NO3− and NH4+ assimilation (such as glutamine or other amino acids) may serve as additional indicators of the plant’s internal N status, so that in plants that are not NR-deficient, the pools of these other N compounds may act in concert with the NO3− pool to determine the intensity of the inhibitory signal.
Previous work on LR development in Arabidopsis has identified the stage just after emergence of the LR primordium as a critical step (29). Evidence from cytological studies (30) and from the phenotype of the rml1 (root meristemless) mutant (31) indicates that although the structure of the LR meristem is already fully formed before emergence, it is only after emergence that the meristem is activated to allow continued growth of the mature LR. It appears that the stage between differentiation of the meristem and its activation is a time when the LR primordium becomes susceptible to the postulated systemic inhibitory signal. As a result, the duration of this developmental stage is extremely flexible, to the degree that under very high rates of NO3− supply, it can be extended by several days or more. Because not all LRs are delayed equally at any given NO3− concentration (see Fig. 3C) it may be that individual LRs differ in their sensitivity to the inhibitory signal. Studies on LR development in Vicia faba identified two periods of temporary mitotic quiescence or dormancy during early LR development, the second of which occurred just before emergence of the LR primordium (32).
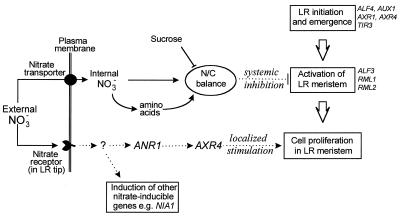
Our current model for the dual pathways by which NO3− regulates root branching in Arabidopsis is summarized in Fig. 5. The nature of the proposed systemic inhibitory signal is unknown, but it seems likely that it emanates from the shoot. Split-root experiments with tobacco indicate that shoot-derived signals are responsible for regulating shoot/root partitioning in tobacco in response to tissue NO3− levels (13). Similarly, the processes of NO3− uptake and symbiotic N2 fixation in plant roots are subject to feedback repression from regulatory signals that originate in the shoot (33–35).
Figure 5.
Dual-pathway model for regulation of LR growth and development by NO3−. Because the ANR1 gene is rapidly induced by NO3− (12), the putative NO3− receptor and the mechanism for transcriptional activation of ANR1 are likely to be shared with other NO3−-inducible genes such as the NIA1 genes encoding NR (39, 40). We have tentatively placed ANR1 upstream of AXR4 in the signal transduction pathway. This arrangement makes a number of predictions that can be tested experimentally by using axr4 mutants (19) and ANR1 antisense lines (12). Other genes implicated in controlling particular stages in LR initiation or development (29) are shown on the right. Broken arrows indicate signaling steps, solid arrows indicate transport or metabolic steps, and large open arrows indicate developmental steps.
The pool of amino acids that cycles between the shoot and the root is considered to be one possible means of transmitting information about the N requirements of the shoot (33, 35, 36). In tobacco, the high levels of leaf NO3− that were associated with an inhibition of root growth were correlated with a lower rate of sucrose export from the shoot and a lower sugar content in the root (24), leading to the suggestion that the reduced C allocation to the root may be responsible for the effects on root growth. Our finding that increasing the sucrose concentration in the medium from 0.5% to 2% partially relieved the inhibitory effect of 50 mM KNO3 (Fig. 4B) would appear to be consistent with this hypothesis. However, the highly specific way in which LR development in Arabidopsis is inhibited by a high NO3− supply argues against the notion that the roots are simply C starved. It seems more likely that the stimulatory effect of sucrose is due to its effect on the plant’s N status or its N/C ratio: there is evidence for regulatory mechanisms that monitor the balance between C and N metabolism in plants (21, 37), as there are in prokaryotes (38).
Although our observations have been made with Arabidopsis, an annual weed from disturbed habitats, the model we propose is consistent with the manner in which root architecture in species as diverse as cereals and trees is affected by the availability and distribution of NO3− (3). A key feature of the model is that it indicates how a plant could modify its pattern of root development in a way that integrates information about the spatial distribution of NO3− in the soil and the demand for N in the shoot. Whereas the localized stimulatory effect of NO3− allows for autonomous responses by individual lateral roots, the systemic inhibitory effect ensures that these responses are modulated according to the plant’s nutritional needs, thus optimizing resource allocation within the plant as a whole.
Acknowledgments
We thank M. Bennett, N. Crawford, O. Leyser, and P. Benfey for their generous gifts of seed and A. Williams for technical assistance. This work was supported in part by a grant from the European Union (contract no. BIO4-CT97–2231) in the Biotech programme of Framework IV. The Institute for Arable Crops Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council.
ABBREVIATIONS
- GUS
β-glucuronidase
- LR
lateral root
- NR
nitrate reductase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Lynch J. Plant Physiol. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiefelbein J W, Benfey P N. Plant Cell. 1991;3:1147–1154. doi: 10.1105/tpc.3.11.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson D. New Phytol. 1994;127:635–674. doi: 10.1111/j.1469-8137.1994.tb02969.x. [DOI] [PubMed] [Google Scholar]
- 4.Leyser O, Fitter A. Trends Plant Sci. 1998;3:203–204. [Google Scholar]
- 5.Hutchings M J, de Kroon H. Adv Ecol Res. 1994;25:159–238. [Google Scholar]
- 6.Hackett C. Aust J Biol Sci. 1972;25:1169–1180. [Google Scholar]
- 7.Drew M C. J Exp Bot. 1973;24:1189–1202. [Google Scholar]
- 8.Granato T C, Raper C D., Jr J Exp Bot. 1989;40:263–275. doi: 10.1093/jxb/40.2.263. [DOI] [PubMed] [Google Scholar]
- 9.Wiersum L K. Acta Bot Neerl. 1958;7:174–190. [Google Scholar]
- 10.Sattelmacher B, Gerendas J, Thoms K, Brück H, Bagdady N H. Environ Exp Bot. 1993;33:63–73. [Google Scholar]
- 11.Ericsson T. Plant Soil. 1995;168–169:205–214. [Google Scholar]
- 12.Zhang H, Forde B G. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- 13.Scheible W R, Lauerer M, Schulze E D, Caboche M, Stitt M. Plant J. 1997;11:671–691. [Google Scholar]
- 14.Stitt M, Scheible W-R. Plant Soil. 1998;201:259–263. [Google Scholar]
- 15.Jefferson R A, Kavanagh T A, Bevan M W. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans M L, Ishikawa H, Estelle M A. Planta. 1994;194:215–222. [Google Scholar]
- 17.Maher E P, Martindale S J B. Biochem Genet. 1980;18:1041–1053. doi: 10.1007/BF00484337. [DOI] [PubMed] [Google Scholar]
- 18.Wilson A K, Pickett F B, Turner J C, Estelle M. Mol Gen Genet. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]
- 19.Hobbie L, Estelle M. Plant J. 1995;7:211–220. doi: 10.1046/j.1365-313x.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- 20.Celenza J L, Grisafi P L, Fink G R. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- 21.Vincentz M, Moureaux T, Leydecker M T, Vaucheret H, Caboche M. Plant J. 1993;3:315–324. doi: 10.1111/j.1365-313x.1993.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson J Q, Crawford N M. Mol Gen Genet. 1993;239:289–297. doi: 10.1007/BF00281630. [DOI] [PubMed] [Google Scholar]
- 23.Drew M C. New Phytol. 1975;75:479–490. [Google Scholar]
- 24.Scheible W R, Gonzalez-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M. Plant Cell. 1997;9:783–798. doi: 10.1105/tpc.9.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leyser O. Physiol Plant. 1997;100:407–414. [Google Scholar]
- 26.Oostindier-Braaksma F, Feenstra W. Mutat Res. 1973;19:175–185. [Google Scholar]
- 27.Warner R L, Huffaker R C. Plant Physiol. 1989;91:947–953. doi: 10.1104/pp.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sueyoshi K, Kleinhofs A, Warner R L. Plant Physiol. 1995;107:1303–1311. doi: 10.1104/pp.107.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malamy J E, Benfey P N. Trends Plant Sci. 1997;2:390–396. [Google Scholar]
- 30.Malamy J E, Benfey P N. Development (Cambridge, UK) 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- 31.Cheng J C, Seeley K A, Sung Z R. Plant Physiol. 1995;107:365–376. doi: 10.1104/pp.107.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLeod R D, McLachlan S M. Protoplasma. 1975;85:291–304. [Google Scholar]
- 33.Parsons R, Stanforth A, Raven J A, Sprent J I. Plant Cell Environ. 1993;16:125–136. [Google Scholar]
- 34.Lainé P, Ourry A, Boucaud J. Planta. 1995;196:77–83. [Google Scholar]
- 35.Imsande J, Touraine B. Plant Physiol. 1994;105:3–7. doi: 10.1104/pp.105.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper H D, Clarkson D T. J Exp Bot. 1989;40:753–762. [Google Scholar]
- 37.Sivasankar S, Rothstein S, Oaks A. Plant Physiol. 1997;114:583–589. doi: 10.1104/pp.114.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Böhme H. Trends Plant Sci. 1998;3:346–351. [Google Scholar]
- 39.Crawford N M. Plant Cell. 1995;7:859–868. doi: 10.1105/tpc.7.7.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redinbaugh M G, Campbell W H. Physiol Plant. 1991;82:640–650. [Google Scholar]