Abstract
Testosterone moderates behavioral and physiological responses to the emotion anger. However, little is known about the effects of testosterone in the human brain in the context of the perception of anger. We used fMRI to measure BOLD responses to anger faces in the amygdala and ventromedial prefrontal cortex (vmPFC) as a function of endogenous testosterone levels in 24 participants (10 men). In one task, participants passively viewed anger faces and neutral faces and in another task, participants engaged in an oddball task while viewing anger and neutral faces. Men’s, but not women’s, amygdala BOLD response to anger faces was negatively correlated with their endogenous testosterone levels in both tasks. Men’s, but not women’s, vmPFC BOLD response to anger faces was positively correlated with their endogenous testosterone levels in the passive viewing task. In men, amygdala and vmPFC BOLD responses to anger faces were negatively associated. Our results extend past research by documenting associations between endogenous testosterone levels and BOLD responses to anger faces in the amygdala and vmPFC in men, and our results also support research that documents negative associations between amygdala and vmPFC activity.
Keywords: Testosterone, Amygdala, Prefrontal Cortex, fMRI, Anger, Emotion, Aggression, Dominance, Hormones, Neuroendocrine, Steroid
Introduction
High testosterone levels are associated with dominance behavior and pursuit of dominance status in men (Mazur & Booth, 1998). Men’s testosterone levels also rise as a function of winning dominance contests and feed back to the brain to prime future dominance behavior (Archer, 2006). Dominant male primates respond to dominance challenges with smaller cortisol changes (stress responses) and greater testosterone increases than non-dominant males, suggesting that dominant males find dominance signals less stressful and are more primed to engage in a dominance challenge (Sapolsky, 1987; 2005). In humans and non-human animals, testosterone also reduces central fear responses and stress axis reactivity (Hermans et al., 2006, 2007; Rubinow et al. 2005; van Honk et al., 2005; Viau, 2002). Testosterone may act on specific neural substrates in humans to reduce avoidance responses to threatening dominance signals.
In mammals, the amygdala is the “centerpiece of the subcortical networks involved in detecting and responding to threats” (LeDoux, 2000, p.301). In humans, amygdala responses to anger faces (interpersonal dominance threats) (Hess et al., 2000; Knutson, 1996; Oosterhof & Todorov, 2008) have been observed, but not consistently (Murphy et al., 2003). Clarifying previously inconsistent findings, some studies have shown that individual differences can be associated with amygdala responses to affective stimuli (Canli et al., 2002; Hariri et al., 2002). Testosterone’s positive association with men’s dominance behavior and physiology, as well as testosterone’s negative association with physiological responses to threat, make it plausible that testosterone levels and amygdala response to anger faces are associated in humans. Moreover, this is supported by evidence in male mammals showing that testosterone acts on the amygdala in conjunction with peptide hormones to mediate dominance behavior (Delville et al., 1996; de Vries & Miller, 1998; Ferris & Delville, 1994). The first purpose of the present research is to evaluate the relationship between amygdala BOLD response to anger faces and testosterone levels in humans.
The amygdala has multiple interconnections with the ventromedial prefrontal cortex (vmPFC), and the activation of these two regions in response to a variety of affective stimuli and affective tasks is commonly negatively correlated (Barbas, 2000; Blair, 2008). With relevance to the present study, Nomura and colleagues (2004) reported a negative correlation between amygdala activation to anger faces and vmPFC activation to anger faces. Importantly, recent animal studies have shown that testosterone acts on both the vmPFC and amygdala to reduce the threshold for engaging in behavioral aggression in males (Ambar & Chiavegatto, in press). The second purpose of the present research is to evaluate the relationship between vmPFC BOLD response to anger faces and testosterone levels in humans. Additionally, in the presence of significant effects of testosterone in both the amygdala and vmPFC, we aimed to assess the association between testosterone-related amygdala BOLD responses and testosterone-related vmPFC BOLD responses.
On the basis of fear- and stress-reducing properties of testosterone in response to threat, we hypothesized that high-testosterone men would show a lesser amygdala BOLD response to anger faces than low-testosterone men. On the basis of the common negative association between amygdala and vmPFC responses to affective stimuli, we hypothesized that high-testosterone men would show a greater vmPFC BOLD response to anger faces than low-testosterone men. We also hypothesized that we would not observe effects of testosterone on women’s BOLD responses in either the amygdala or the vmPFC, because research has not established a consistent link between endogenous testosterone and dominance in women (Mazur & Booth, 1998; Stanton & Schultheiss, 2007). Lastly, we chose to add Wernicke’s area as a control region of interest (ROI) in our analyses with the hypothesis that there should be no relationship between BOLD activation and testosterone levels in that ROI.
Methods
Participants
Participants (14 women and 10 men) had a mean age of 20.96 years (SD = 2.18) and were prescreened for mental and physical health problems that would have excluded them from fMRI testing. Participants were originally selected for high and low levels of a personality variable, implicit power motivation, in order to investigate other hypotheses (Schultheiss et al., 2008). However, participants’ implicit power motivation was not correlated with testosterone levels (r = −0.18, p = 0.62) and did not predict the presently reported effects.
Stimuli and task design
Stimuli consisted of pictures of 20 individuals (10 male, 10 female) × 3 expressions: surprise (open mouth), anger (bared teeth), and neutral (MacBrain Face Stimulus Set; Tottenham et al., in press). Faces were presented on a black background for 250 ms, followed by variable-duration interstimulus intervals (ISI; mean duration: 350 ms; range: 200 to 500 ms) consisting of fixation crosses centered at the height of the eyes in the presented faces. Blocks of each facial expression consisted of pictures from all 20 posers presented in randomized order and were presented in alternating order with blocks in which grey squares (same dimensions as faces) were presented in lieu of faces.
Face stimuli were presented in two tasks, a passive-viewing task and an oddball task. In the passive-viewing task, participants were instructed to keep their eyes directed at the fixation cross in the middle of the screen and to pay attention to all presented stimuli. In both tasks, an X randomly appeared instead of the fixation cross during the ISIs once per block in one half of the blocks, and twice in the other half. In the oddball task, participants were instructed to respond with a button press whenever an X appeared during the ISI. Other than the need to respond when an X was presented instead of a fixation cross during the ISI, the passive-viewing and oddball tasks were the same. Both the passive-viewing and oddball tasks consisted of two consecutive runs of 36 blocks each, and their order was counterbalanced across participants.
Scanning parameters
Participants lay supine in a 3.0 Tesla magnet (General Electric, standard quadrature head coil). We acquired functional images with a spiral in-out pulse sequence (repetition time [TR]: 2500ms; echo time [TE]; 30ms; flip angle: 90°). Twenty-nine contiguous horizontal slices of 4 mm thickness were acquired, encompassing the whole brain (field of view [FOV]: 220 × 220mm; voxel size: 3.44 mm × 3.44 mm × 4 mm). Structural images were acquired in the same slice locations using a T1-weighted gradient echo pulse sequence, with TR = 200 ms, TE = 3.6 ms, FOV = 220 × 220 mm, voxel size = 0.86 mm × 0.86 mm × 4 mm, and flip angle = 90°. Due to a programming error, volume acquisition stopped during the last block (surprise faces) on each run. Data from this block were therefore discarded from image analyses.
Image processing and data analysis
The first four volumes per run were discarded to allow the MRI signal to reach its steady state. Images were motion-corrected, realigned, normalized to the Montreal Neurological Institute (MNI) template (Evans et al. 1994), and then smoothed with an [8 8 8] mm kernel. We used SPM2 for data analyses (Wellcome Trust Centre for Neuroimaging, London, UK). Data were high-pass filtered at 100s (.01 Hz) and fitted to a canonical hemodynamic response function. Contrast images were created for each participant (Anger vs. Neutral) and submitted to 2nd level random effects analyses. The amygdala, vmPFC, and Wernicke’s area ROIs were defined using masks from the AAL library (Tzourio-Mazoyer et al., 2002). Results were thresholded using a combination of alpha (p < .05) and cluster size (k = 5) for the amygdala and alpha (p < .005) and cluster size (k = 10) for the vmPFC and Wernicke’s area.
Saliva sampling and testosterone assessment
Saliva samples were collected from participants directly before scanning sessions (range: 8:00am – 1:00pm) and stored using standard procedures (Schultheiss & Stanton, 2009). Salivary testosterone levels were assessed with solid-phase Coat-A-Count 125I radioimmunoassays for testosterone from Diagnostic Products Corporation following the protocol described in Schultheiss, Wirth, & Stanton (2004). Analytical sensitivity (B0 -3 SD) was 4.78 pg/mL. Analytical recovery was 118.55% for low (24.90 pg/mL) and 108.40% for high (151.8 pg/mL) testosterone levels. Mean intra-assay CV was 6.04%.
Results
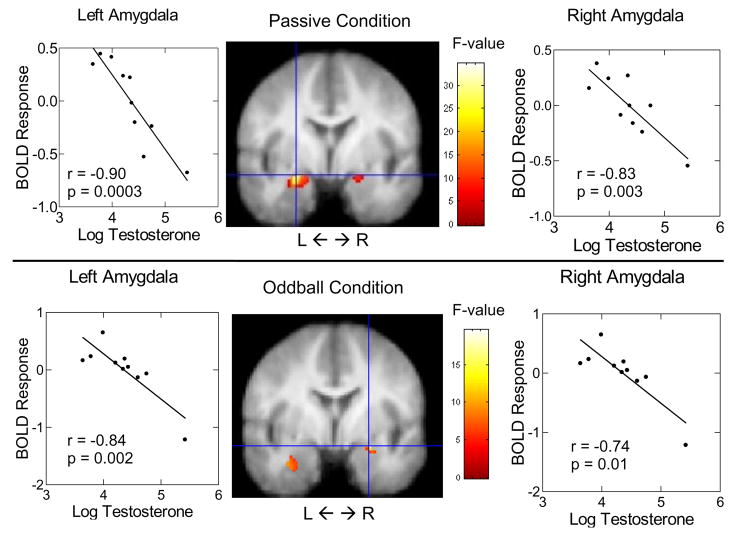
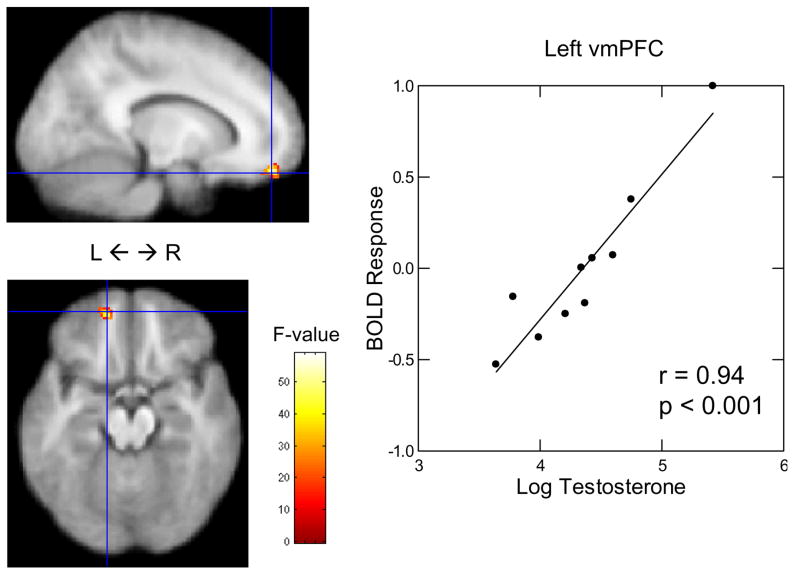
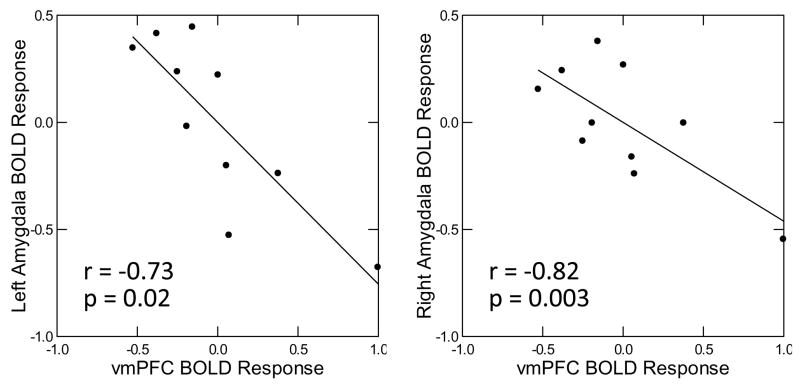
Participants’ testosterone concentrations were (mean ± SEM): Men: 88.51 ± 17.15 pg/mL; Women: 17.28 ± 1.52 pg/mL. Men’s testosterone values were log-transformed to correct for skewed raw values. Analyses were run separately for each sex due to the bimodal distribution of endogenous testosterone levels between sexes. A regression of men’s testosterone levels on amygdala BOLD response to anger-neutral face contrasts showed that men’s endogenous testosterone levels correlated negatively with left and right amygdala BOLD response to anger-neutral face contrasts in the passive viewing task1 (left: x = −26, y = 0, z = −12; r = −0.90, p = 0.0003), (right: x = 22, y = −6, z = −4; r = −0.84, p = 0.003) (see Fig. 1); there was no effect of testosterone on amygdala BOLD response to anger-neutral face contrasts in women (left: r = 0.42, p = 0.13), (right: r = −0.23, p = 0.44). A regression of men’s testosterone levels on vmPFC BOLD response to anger-neutral face contrasts showed that men’s endogenous testosterone levels correlated positively with left vmPFC BOLD response to anger-neutral face contrasts in the passive viewing task1 (x = −14, y = 52, z = −16; r = 0.94, p < 0.001) (see Fig. 2); there was no effect of testosterone on vmPFC BOLD response to anger-neutral face contrasts in women (r = −0.05, p = 0.87). For men, we then correlated the levels of BOLD response in the amygdala and VMPFC from the prior analyses and found that testosterone-related BOLD activation in the left vmPFC was negatively correlated with testosterone-related BOLD activation in the left amygdala (r = −0.82, p = .003) and right amygdala (r = −0.73, p = 0.02) (see Fig. 3).
Figure 1.
Correlations between men’s log-transformed testosterone levels and amygdala BOLD response to anger-neutral face contrasts in the passive viewing task (top panel) and the oddball task (bottom panel).
Figure 2.
Correlation between men’s log-transformed testosterone levels and left ventromedial prefrontal cortex BOLD response to anger-neutral face contrasts in the passive viewing task.
Figure 3.
Correlations between men’s testosterone-related ventromedial prefrontal cortex BOLD response and testosterone-related amygdala BOLD responses to anger-neutral contrasts in the passive viewing task.
In the oddball task, by regressing men’s testosterone levels on amygdala BOLD response to anger-neutral face contrasts, we also found a bilateral, negative correlation between testosterone and anger-neutral contrast BOLD activation in the amygdala in men1 (left: x = −36, y = 2, z = −2; r = −0.84, p = 0.002), (right: x = 24, y = 0, z = −12; r = −0.74, p = 0.01) (see Fig. 1), and again there was no effect of testosterone on amygdala BOLD response to anger-neutral face contrasts for women (left: r = 0.14, p = 0.63), (right: r = 0.23, p = 0.42). We found no effect of testosterone on vmPFC BOLD activation in the oddball task for men or women. We found no significant effects of testosterone on BOLD activation in Wernicke’s area for men or women in either task. We also re-ran all significant analyses partialing out variation in time of day for testing sessions. We found that the effects of testosterone were still present and of similar magnitude, with time of day not accounting for a significant portion of the variance in any result reported.
Discussion
In confirmation of our first hypothesis, amygdala BOLD responses to anger faces in both tasks were negatively correlated with endogenous testosterone levels in men. We also found that vmPFC BOLD responses to anger faces in the passive viewing task were positively correlated with endogenous testosterone levels in men, which confirmed our second hypothesis. In confirmation of our third hypothesis, testosterone levels were not associated with amygdala or vmPFC BOLD responses to anger faces in women. Lastly, our fourth hypothesis that testosterone levels would not be associated with Wernicke’s area BOLD responses to anger faces in men or women in either task was also confirmed.
Our results complement findings in males of mammalian species that have revealed functional links between testosterone and the amygdala and vmPFC in mediating dominance and aggressive behavior (Ambar & Chiavegatto, in press; Delville et al., 1996; Ferris & Delville, 1994). Several researchers have argued strongly that anger faces represent interpersonal dominance signals (Hess et al., 2000; Knutson, 1996; Oosterhof & Todorov, 2008). Our results provide evidence in humans of neural substrates where testosterone may drive differential activation patterns in the brain in response to dominance signals and challenges. We speculate that the negative correlation between testosterone and men’s amygdala response to anger suggests that high-testosterone men are less threatened by dominance challenges, and hence stimuli that are indicative of such challenges are perhaps less salient or aversive to them than to low-testosterone men. There is converging behavioral evidence showing that high-testosterone individuals show less behavioral aversion to angry faces in an instrumental learning task (Wirth & Schultheiss, 2007). This is also supported by the observation that testosterone reduces individuals’ ability to recognize anger in facial expressions of emotion (van Honk & Schutter, 2007). This lack of perceived threat may make high-testosterone men more likely to engage in testosterone-facilitated dominance behavior and approach dominance challenges.
We chose two tasks (passive-viewing and oddball) to test our hypotheses due to conflicting literature regarding whether the brain responds more to facial expressions of emotion when participants are focused on the emotion or when they are performing a “distraction” task (Critchley et al., 2000, Lieberman, 2003; Lieberman et al., 2007; Quirk, et al., 2003; Taylor, et al. 2003). The consistency of our findings showing a bilateral, negative correlation between amygdala BOLD response and testosterone levels in both tasks strengthens the conclusions that we drew from the data, because the effect is similar regardless of the presumed level of attention paid to the stimuli2.
The presently reported negative correlation between testosterone-related amygdala BOLD response and testosterone-related vmPFC BOLD response in the passive viewing task corroborates past research showing that amygdala responses and vmPFC responses to affective stimuli tend to be negatively correlated (cf. Blair, 2008), specifically in the context of perceiving anger faces (Nomura et al., 2004). Moreover, past research has shown that participants’ conscious attention to emotional components of stimuli leads to PFC activation that subsequently attenuates amygdala activation (Hariri et al., 2003; Lieberman et al., 2007). In the passive viewing task, subjects were not distracted by engaging in a simultaneous task and could pay greater attention to the emotional components of the anger faces. We speculate that the negative correlation between amygdala and vmPFC BOLD responses could possibly reflect high-testosterone participants paying greater attention to the anger stimulus, since they find anger faces to be less aversive (Wirth & Schultheiss, 2007), and this yielded a strong vmPFC BOLD response and corresponding attenuation of the amygdala BOLD response for high-testosterone men.
Our failure to find a relationship between amygdala or vmPFC BOLD response to anger faces and testosterone levels in women corroborates behavioral research that has failed to document a consistent relationship between testosterone and dominance in women (Mazur & Booth, 1998; Stanton & Schultheiss, 2007). Estradiol, which has been implicated in dominance motivation and responses to dominance contests in female primates and recently in women, also acts on the amygdala and could be examined in future research on women’s neural responses to dominance signals (Cottingham & Pfaff, 1986; Michael & Zumpe, 1993; Stanton & Schultheiss, 2007).
Although the present research has established a highly significant negative correlation between men’s endogenous testosterone levels and amygdala BOLD response to dominance signals and a highly significant positive correlation between men’s endogenous testosterone levels and vmPFC BOLD response to dominance signals, our study is not without its limitations. We cannot draw causal inferences regarding the effects of testosterone on amygdala and vmPFC BOLD response. Future research could also explore the relationships between testosterone and amygdala and vmPFC BOLD responses for several classes of emotional expressions as well as using trait measures of dominance as predictors of BOLD response to emotions. Additionally, our sample size is relatively small and replication studies are therefore particularly important. Despite these limitations, we think that our current findings are robust and provide a foundation for further research on endogenous testosterone levels’ associations with brain responses to emotional and dominance-related stimuli.
Acknowledgments
This research was supported by an NSF grant BCS-0444301 (to OCS), a research grant from the Office of the Vice President of Research at the University of Michigan (to OCS), and Rackham Predoctoral Fellowships (to SJS and MMW) from the University of Michigan. We thank Scott Liening for his help in scheduling and testing participants.
Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set.
Footnotes
We also re-ran the regression analyses for the amygdala and vmPFC using men’s raw (i.e. non log-transformed) testosterone levels, and we found that the pattern of effects is the same and of similar magnitude. In the passive-viewing task, the negative correlation between testosterone and amygdala BOLD response persisted when using raw testosterone values (left: −20, −2, −18; r = −0.88, p = 0.001) (right: 18, 0, −12; r = −0.91, p = < .001). The effect also persisted in the oddball task (left: −36, 0, −26; r = −0.88, p = 0.001) (right: 24, 0, −12; r = −0.93, p = < .001). In the passive-viewing task, the positive correlation between testosterone and vmPFC BOLD response persisted when using raw testosterone values (−14, 52, −16; r = 0.96, p < 0.001). Thus, the strength of the effects, independent of transformation, gives us confidence that the log-transformation of testosterone values did not “create” the reported effects.
Our reported patterns of amygdala BOLD response show reasonable variance in terms of spatial specificity. Thus, our patterns of BOLD response, particularly in the oddball task, could also be described as existing in peri-amygdaloid tissue, as opposed to the amygdala exclusively. This lack of spatial specificity is possibly due to the fact that our coordinates correspond to peak activations within a cluster of BOLD response that potentially has multiple local maxima of BOLD response, some of which may be slightly outside of the amygdala proper. These different peaks may have been more or less likely to emerge as the coordinate of peak activation in analyses of the different tasks or when using raw versus log-transformed testosterone as the predictor.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambar G, Chiavegatto S. Anabolic-androgenic steroid treatment induces behavioral disinhibition and downregulation of serotonin receptor mRNA in the prefrontal cortex and amygdala of male mice. Genes, Brain, and Behavior. doi: 10.1111/j.1601-183X.2008.00458.x. (in press) [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neuroscience Biobehavioral Review. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research bulletin. 2000;52:319–339. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of The Royal Society B. 2008;363:2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Sivers H, Whitfield SL, Gotlib IH, Gabrieli JDE. Amygdala response to happy faces as a function of extraversion. Science. 2002;296:2191. doi: 10.1126/science.1068749. [DOI] [PubMed] [Google Scholar]
- Cottingham SL, Pfaff D. Interconnectedness of steroid hormone-binding neurons: existence and implications. In: Ganten D, Pfaff D, editors. Morphology of Hypothalamus and Its Connections, Current Topics in Neuroendocrinology. Vol. 7. Berlin: Springer; 1986. pp. 223–249. [Google Scholar]
- Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: A functional magnetic resonance imaging study. Human Brain Mapping. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries GJ, Miller MA. Anatomy and function of extrahypothalamic vasopressin systems in the brain. Progress in Brain Research. 1998;199:3–20. doi: 10.1016/s0079-6123(08)61558-7. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour KM, Ferris CF. Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiology and Behavior. 1996;60:25–29. doi: 10.1016/0031-9384(95)02246-5. [DOI] [PubMed] [Google Scholar]
- Evans AC, Kamber M, Collins DL, Macdonald D. An MRI-based probabilistic atlas of neuroanatomy. In: Shorvon S, Fish D, Andermann F, Bydder GM, Stefan H, editors. Magnetic Resonance Scanning and Epilepsy. New York: Plenum; 1994. pp. 263–274. [Google Scholar]
- Ferris CF, Delville Y. Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology. 1994;19:593–601. doi: 10.1016/0306-4530(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hess U, Blairy S, Kleck RE. The influence of facial emotion displays, gender, and ethnicity on judgments of dominance and affiliation. Journal of Nonverbal Behavior. 2000;24:265–283. [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Koppeschaar HP, van Honk J. A single administration of testosterone reduces fear-potentiated startle in humans. Biological Psychiatry. 2006;59(9):872–874. doi: 10.1016/j.biopsych.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 2007;32:1052–1061. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Knutson B. Facial expressions of emotion influence interpersonal trait inferences. Journal of Nonverbal Behavior. 1996;20:165–182. [Google Scholar]
- LeDoux JE. The amygdala and emotion: a view through fear. In: Aggleton J, editor. The Amygdala. Oxford: Oxford University Press; 2000. pp. 289–310. [Google Scholar]
- Lieberman MD. Reflective and Reflexive Judgment Processes: A Social Cognitive Neuroscience Approach. In: Forgas JP, Williams KR, Hippel Wv, editors. Social judgments: Implicit and explicit processes. New York: Cambridge University Press; 2003. pp. 44–67. [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behavioral and Brain Sciences. 1998;21:353–397. [PubMed] [Google Scholar]
- Michael RP, Zumpe D. A review of hormonal factors influencing the sexual and aggressive behavior of macaques. American Journal of Primatology. 1993;30:213–241. doi: 10.1002/ajp.1350300306. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nomura M, Ohira H, Haneda K, Iidaka T, Sadato N, Okada T, Yonekura Y. Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expression primed by masked angry faces: an event-related fMRI study. NeuroImage. 2004;21:352–363. doi: 10.1016/j.neuroimage.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Oosterhof NN, Todorov A. The functional basis of face evaluation. PNAS. 2008;105:11087–11092. doi: 10.1073/pnas.0805664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience. 2003;23(25):8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, Roca CA, Schmidt PJ, Danaceau MA, Putnam K, Cizza G, Chrousos G, Nieman L. Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology. 2005;30:1906–1912. doi: 10.1038/sj.npp.1300742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, social status, and reproductive physiology in free-living baboons. In: Crews D, editor. Psychobiology and reproductive behavior: An evolutionary perspective. Englewood Cliffs, NJ: Prentice-Hall; 1987. pp. 291–322. [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC. A biobehavioral model of implicit power motivation: arousal, reward, and frustration. In: Harmon-Jones E, Winkielman P, editors. Social Neuroscience: Integrating biological and psychological explanations of social behavior. Guilford Press; New York: 2007. pp. 176–196. [Google Scholar]
- Schultheiss OC, Stanton SJ. Assessment of salivary hormones. In: Harmon-Jones E, Beer JS, editors. Methods in the neurobiology of social and personality psychology. New York, NY: Guilford; 2009. pp. 17–44. [Google Scholar]
- Schultheiss OC, Wirth MM, Stanton SJ. Effects of affiliation and power motivation arousal on salivary progesterone and testosterone. Hormones and Behavior. 2004;46(5):592–599. doi: 10.1016/j.yhbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Wirth MM, Waugh CE, Stanton SJ, Meier E, Reuter-Lorenz P. Exploring the motivational brain: Effects of implicit power motivation on brain activation in response to facial expressions of emotion. Social, Cognitive, and Affective Neuroscience. 2008;3:333–343. doi: 10.1093/scan/nsn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Schultheiss OC. Basal and dynamic relationships between implicit power motivation and estradiol in women. Hormones and Behavior. 2007;52(5):571–580. doi: 10.1016/j.yhbeh.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18(3):650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson CA. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. doi: 10.1016/j.psychres.2008.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ. Testosterone reduces conscious detection of signals serving social correction. Psychological Science. 2007;18:663–667. doi: 10.1111/j.1467-9280.2007.01955.x. [DOI] [PubMed] [Google Scholar]
- van Honk J, Peper JS, Schutter DJ. Testosterone reduces unconscious fear but not consciously experienced anxiety: implications for the disorders of fear and anxiety. Biological Psychiatry. 2005;58(3):218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. Journal of Neuroendocrinology. 2002;14(6):506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Schultheiss OC. Basal testosterone moderates responses to anger faces in humans. Physiology and Behavior. 2007;90(2–3):496–505. doi: 10.1016/j.physbeh.2006.10.016. [DOI] [PubMed] [Google Scholar]