Abstract
The biomechanical and wound healing properties of the cornea undermine the predictability and stability of refractive surgery and contribute to discrepancies between attempted and achieved visual outcomes after LASIK, surface ablation and other keratorefractive procedures. Furthermore, patients predisposed to biomechanical failure or abnormal wound healing can experience serious complications such as keratectasia or clinically significant corneal haze, and more effective means for the identification of such patients prior to surgery are needed. In this review, we describe the cornea as a complex structural composite material with pronounced anisotropy and heterogeneity, summarize current understanding of major biomechanical and reparative pathways that contribute to the corneal response to laser vision correction, and review the role of these processes in ectasia, intraocular pressure measurement artifact, diffuse lamellar keratitis (DLK) and corneal haze. The current understanding of differences in the corneal response after photorefractive keratectomy (PRK), LASIK and femtosecond-assisted LASIK are reviewed. Surgical and disease models that integrate corneal geometric data, substructural anatomy, elastic and viscoelastic material properties and wound healing behavior have the potential to improve clinical outcomes and minimize complications but depend on the identification of preoperative predictors of biomechanical and wound healing responses in individual patients.
Keywords: cornea, biomechanics, wound healing, refractive surgery, ectasia, haze, hyperopic shift, regression
1. Introduction
The structural and reparative properties of the cornea are essential to its function as a resilient, yet transparent, barrier to intraocular injury. Because the cornea is also the scaffold for the major refractive surface of the eye, any mechanical or biological response to injury will also influence optical performance. Consequently, the same mechanisms responsible for preserving ocular integrity can undermine the goals of achieving predictable and stable visual outcomes after keratorefractive surgery.
Even in an era of high-precision treatment algorithms, discrepancies between intended and realized visual outcomes are common. The shape-subtraction model of photokeratectomy that forms the basis of LASIK and PRK ablation routines (Munnerlyn et al., 1988) assumes a biologically and biomechanically inert cornea (Roberts, 2000) and does not account for non-idealities in the laser-tissue interaction. While empirical modifications to algorithms and major advances in laser delivery platforms have improved the statistical predictability of LASIK and PRK, the ability to anticipate confounding biological responses at the level of the individual patient remains limited. In some cases, a predisposition to mechanical instability or abnormal regulation of healing can lead to serious complications such as keratectasia or loss of corneal transparency (severe haze). The goal of research in this setting is to improve outcomes and reduce complications by discerning details of the biomechanical and wound healing pathways, identifying measurable predictors of individual responses and developing therapeutic models for controlling or compensating for these factors.
In this chapter, we highlight selected basic and practical considerations in corneal biomechanics and wound healing specific to the setting of photoablative corneal surgery, which accounts for the vast majority of all refractive surgery done today. These processes are approached temporally to distinguish between immediate biomechanical effects, later wound healing effects and ongoing biomechanical-wound healing interactions that help create a new steady state. Ultimately, biomechanical and wound healing responses are linked in time and space and are described separately only for the sake of clarity.
2. Corneal biomechanics
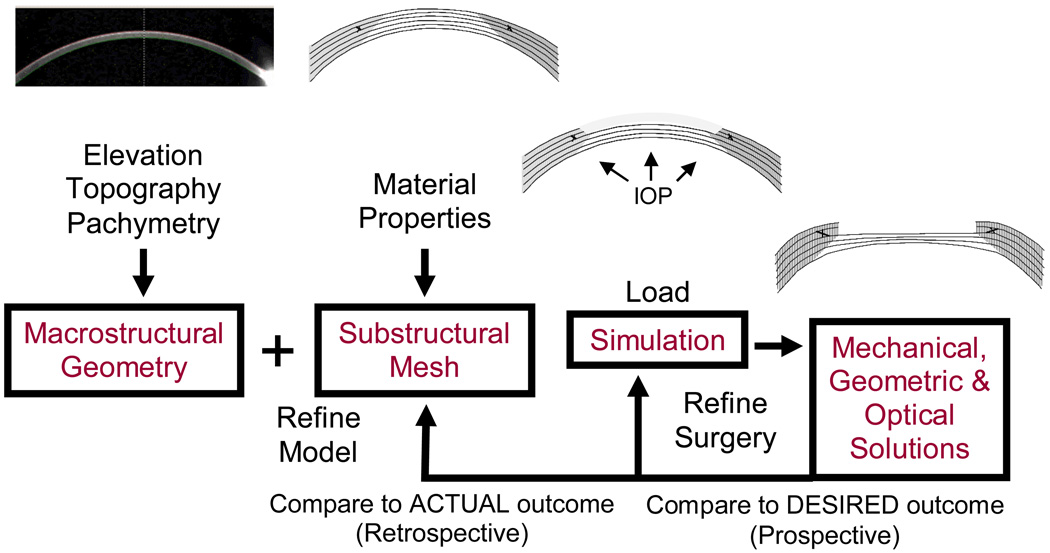
It is evident from incisional refractive surgery that the cornea is not mechanically inert. The role of biomechanics is therefore important to consider in routine LASIK or surface ablation procedures and in special cases where the biomechanical status of the cornea is abnormal (for example, after any previous refractive surgery or after penetrating keratoplasty). Biomechanical changes can manifest clinically as immediate corneal shape changes, shape instability over time and increased sensitivity to shape changes from stimuli such as altered hydration, hypoxia and subsequent injury or surgery. The relative impact of biomechanics and wound healing increases when wavefront-guided treatments of higher-order aberrations are attempted (Netto and Wilson, 2004; Roberts, 2002; Wilson et al., 2003b). Figure 1 describes an approach to biomechanical problems in the cornea that illustrates a relationship between the corneal structure, its material properties, the nature of the mechanical injury and the structural and optical responses. This approach is expanded in the following paragraphs.
Figure 1.
An approach to biomechanical modeling of surgery and disease in the cornea. Disease is simulated by alteration of the substructural components or their material properties. Surgery is simulated by imposing an ablation profile or incisions. The model is optimized retrospectively by comparing model simulations to analogous experiments in tissue or clinical models. A model optimized with clinical data can then be used prospectively to design and evaluate patient-specific treatment algorithms.
2.1. Foundations of the biomechanical response
2.1.1. Corneal architecture
Of the five anatomic layers of the cornea, only Bowman's layer and the stroma contain collagen fibrils. These layers thus provide the majority of the cornea’s tensile strength. The epithelium is attributed a minimal role in this tensile strength, and its removal causes little or no change in the anterior corneal curvature (Litwin et al., 1991). The extensibility and low stiffness of Descemet's membrane ensure its laxity over a broad range of intraocular pressures (IOP) (Jue and Maurice, 1986), which may serve to prevent transmission of stromal stresses to the endothelium. The role of Bowman's layer, an 8 to 12-um-thick acellular condensation of stroma with more randomly-oriented collagen fibrils (Komai and Ushiki, 1991), has been a subject of controversy (Seiler et al., 1992; Wilson and Hong, 2000). Although some have proposed a structural role distinct from that of the stroma, extensiometry studies suggest that removal of Bowman's layer does not measurably alter the mechanical properties of the cornea (Seiler et al., 1992).
The mechanical response of the cornea to injury is dominated by the stroma. On a weight basis, the stroma is approximately 78% water, 15% collagen and 7% non-collagenous proteins, proteoglycans and salts (Maurice, 1984). Three hundred to five hundred lamella run from limbus to limbus and are stacked with angular offsets; this orientation becomes increasingly random in the anterior stroma where significantly more oblique branching and interweaving are noted (Komai and Ushiki, 1991). Interlamellar branching is also more extensive in the corneal periphery than in its center (Polack, 1961; Smolek and McCarey, 1990). Interweaving of collagen bundles between neighboring lamellae provides an important structural foundation for shear (sliding) resistance (Ehlers, 1966) and transfer of tensile loads between lamellae (Figure 2) (Dupps and Roberts, 2001; Roberts, 2000). In addition, x-ray diffraction studies provide evidence of a predominantly circumferential fibril orientation in the corneal periphery (Meek and Newton, 1999) that may favor conservation of limbal circumferential dimensions even in ectatic disease (Edmund, 1989). Proteoglycans play a critical role in collagen fibril assembly and spacing (Chakravarti et al., 1998), and their mechanical importance may be greater than currently recognized.
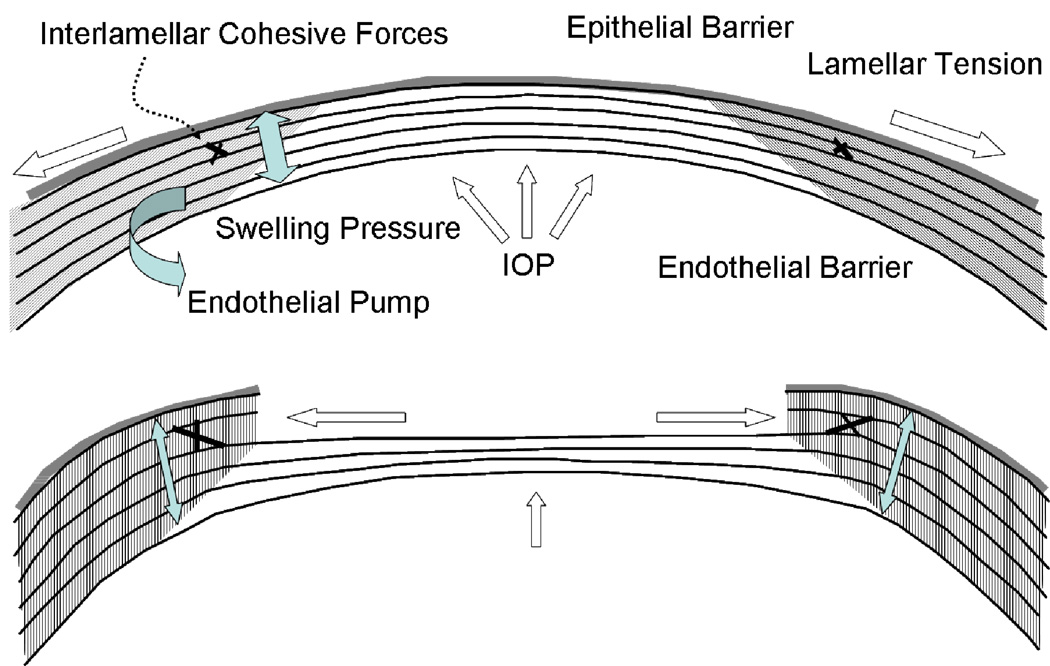
Figure 2.
Major biomechanical loading forces in the cornea and a model of biomechanical central flattening associated with disruption of central lamellar segments. A reduction in lamellar tension in the peripheral stroma reduces resistance to swelling and an acute expansion of peripheral stromal volume results (Dupps and Roberts, 2001; Roberts, 2000; Roberts, 2002). Interlamellar cohesive forces (Smolek, 1993) and collagen interweaving (Komai and Ushiki, 1991), whose distribution is greater in the anterior and peripheral stroma and is indicated by grey shading, provide a means of transmitting centripetal forces to underlying lamellae. Because the central portions of these lamellae constitute the immediate postoperative surface, flattening of the optical surface occurs, resulting in hyperopic shift. The degree of flattening is associated with the amount of peripheral thickening (Dupps and Roberts, 2001). This phenomenon is exemplified clinically by PTK-induced hyperopic shift but is important in any central keratectomy, including PRK and LASIK. Simultaneous elastic weakening of the residual stromal bed may occur (Guirao, 2005), and the threshold for inducing irreversible (plastic) or progressive (viscoelastic) steepening (or ectasia) is a matter of great clinical concern.
2.1.2. Corneal material properties
The mechanical properties of the cornea and its constituent materials are important for linking morphology to mechanical behavior (Figure 1). In the terminology of material science, the cornea is a complex anisotropic composite with nonlinear elastic and viscoelastic properties. It is a composite because its properties are determined by the interaction of disparate materials like collagen and a polyanionic ground substance, and anisotropic because its properties are not directionally uniform. The cornea is also highly heterogeneous in the central to peripheral, anterior to posterior and rotational dimensions. A generalized solution of the 3-dimensional equations describing such a complex system is untenable, and reduction of the problem to the linear, isotropic case is required to arrive at the more familiar definitions of Young’s modulus and other properties described below.
Friedenwald defined the ocular rigidity coefficient and performed some of the earliest characterizations of ocular biomechanical properties (Friedenwald, 1937). A pressure-volume curve is recorded during a volumetric distention experiment and provides a measure of whole-globe stiffness. This relationship is characterized by the slope of the pressure-volume curve (mmHg/µL). It is nonlinearly dependent upon IOP and has been shown to increase with age (Pallikaris et al., 2005). Its utility in refractive surgery remains to be demonstrated and may be limited to the extent that corneal contributions to rigidity are inseparable from scleral and uveal components.
The elastic modulus (Young’s modulus, E) provides an intrinsic indicator of material stiffness. An elastic material regains its original geometry when an imposed stress is removed and does so in a completely reversibly manner along the same stress-strain pathway. The elastic modulus is traditionally measured in excised tissue with an extensiometer that measures force generation during steady axial elongations of the sample. The slope of stress (force per unit area, Newton/m2) over strain (a dimensionless quantity defined by the current length divided by the starting length) is calculated for a representative portion of the curve. A high modulus indicates a stiff or low-compliance material. While most biological soft tissues approximate linear elastic behavior when a small range of stresses is considered, their overall elastic behavior is highly nonlinear. A linear approximation can be obtained from the instantaneous slope of the stress-strain curve (tangent modulus) or as a chord between two points on the curve (secant modulus) (Buzard, 1992).
In Figure 3, an example of nonlinear elastic behavior in a donor cornea specimen is presented. Nonlinearity arises from an initially slow uptake of load as the collagen takes up slack followed by strain-stiffening as maximal fibril recruitment is approached. Plastic responses such as yield and failure occur when a permanent strain is incurred and the material does not recover its original configuration upon unloading.
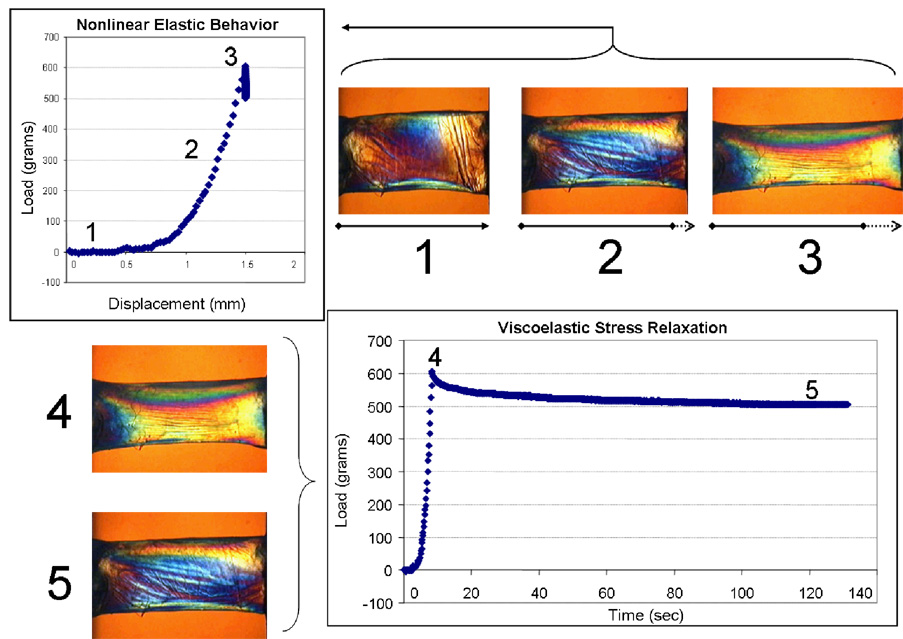
Figure 3.
Experiments illustrating elastic and viscoelastic properties in a 7mm, full-thickness horizontal corneal strip from a 63 year old donor. Elliptical polarization allows visualization of non-homogeneous internal stresses. Progressive stretching of the sample (1, 2 and 3) and measurement of the induced load (stress) allows calculation of the elastic (Young’s) modulus from the slope of the stress-strain relationship. The relationship is nonlinear. A second experiment in which a constant displacement is imposed in the same sample demonstrates time-dependent stress relaxation, a viscoelastic property of biological soft tissues (4 and 5). Courtesy of W.J. Dupps, Jr., MD, PhD and T. Doehring, PhD.
The range of reported values for the elastic modulus of the human cornea spans orders of magnitude (Bryant and McDonnell, 1996). Although some biological variability is expected, this scatter also reflects the challenges of obtaining representative data with a range of tissue hydrations, loading conditions and experimental techniques in ex vivo tissue. It should also be clear from the above discussion that a nonlinear function does not allow definition of a single modulus value, but instead requires its definition as a function of load or as a mean value over a specified loading interval. Membrane inflation experiments in normo-hydrated donor globes provide a more physiological model (Hjortdal and Jensen, 1995) but do not abrogate the ultimate need for in vivo measurement techniques.
Poisson’s ratio (v) is a conversion factor for relating strain in one direction to secondary strain in the transverse direction. A lamella under tensile stress will thin or narrow to some degree in its other dimensions. An out-of-plane/in-plane strain ratio of 0.49 is typically assumed because it approximates the cornea’s fluid-filled, near-incompressible status. In reality, Poisson’s ratio is a true physical property of the tissue and not a constant; as such, its role as a variable in elastic, plastic and viscoelastic thinning of the residual stromal bed after LASIK could be important.
Viscoelastic properties arise from the time-dependent nature of biomechanical responses in biological soft tissues. These properties are represented by the phenomena of hysteresis, stress relaxation and creep. Juxtaposed to the symmetric loading and unloading dynamics of purely elastic materials, viscoelastic materials return to their pre-stress configuration but via different stress-strain pathways that depend upon loading rates. This property is known as hysteresis. Viscoelastic creep is a time-dependent elongation that occurs under a sustained stress (such as IOP) and may be an important contributor to the pathomechanics of ectasia (Dupps, 2005). Finally, Figure 3 illustrates a viscoelastic stress relaxation experiment. Sample strain is increased then held constant while a slow but quantifiable time-dependent relaxation of the load is observed.
Shear strength describes stromal resistance to sublayer sliding and bending. The shear resistance provided by collagen interweaving and other matrix forces has been estimated from metrics such as the interlamellar cohesive strength (Smolek and McCarey, 1990; Smolek, 1993). Corneal shear strength is low relative to its tensile strength (Woo et al., 1972) but provides a mechanism for tensile load transfer between lamellae that may affect corneal shape after photoablation (Figure 2) (Dupps and Roberts, 2001). Abnormalities of bending strength and lamellar sliding have potential relevance in the pathogenesis of ectasia (Edmund, 1989; Meek et al., 2005; Smolek and Klyce, 2000).
2.1.2.1. Measurement of corneal material properties
Despite its limitations, extensiometry has revealed important deficits in elastic tensile strength in keratoconus (Andreassen et al., 1980) and remains the standard for ex vivo elastic modulus determination. Shear wave propagation velocity has been investigated as a potential in vivo marker of elastic modulus (Liu and Roberts, 2003; Wang et al., 1996). The commercially available Ocular Response Analyzer (Reichert, Inc.) utilizes a high-speed air-puff applanation technique to quantify the dynamics of corneal deformation and recovery as an indicator of global corneal hysteresis (Luce, 2005), and studies of this viscoelastic parameter in normal, keratoconus and post-LASIK patients are ongoing. Other technologies in early development include a measure of bending-resistance to stepwise indentations during Placido-ring topographic imaging (Grabner et al., 2005), interferometric determinations of apical displacement during IOP changes (Jaycock et al., 2005) and corneal optical coherence strain mapping (Ford et al., 2006).
2.1.3. Corneal loading forces and the acute response to keratectomy
Several forces contribute to the preoperative steady state and undergo complex disruptions during corneal refractive surgery (Figure 2). The hydrophilia of stromal glycosaminoglycans contributes to a negative intrastromal fluid pressure under which the entire stroma is heavily compressed (Klyce et al., 1971). The IOP manifests both as a centripetal force and as lamellar tension (Maurice, 1984) to counteract this stromal swelling pressure, which is also balanced by tear film evaporation, the epithelial and endothelial barriers and active endothelial transport (Mishima and Hedbys, 1968). Cohesive forces between lamellae provide further resistance to expansion of the interfibrillary space during swelling, and their greater relative strength in the peripheral and superior cornea may have implications for the infero-central predilection of keratoconus (Smolek, 1993) and in induced astigmatism after ablation and flap creation.
During LASIK, PRK or any other procedure involving central ablation, an immediate circumferential severing of corneal lamellae is produced. In simple elastic shell models, this results in a forward herniation that, if considered alone, would result in corneal steepening (Bryant MR, 1993). However, central ablation also relaxes lamellar tension in residual peripheral lamellar segments, which decreases local resistance to swelling and results in peripheral stromal thickening (Dupps and Roberts, 2001). Expansion of the peripheral stroma may generate centripetal stress in underlying lamellae through dense interlamellar connections at the margin of the ablation zone. Because the central portions of these lamellae comprise the new anterior surface, there is resulting central flattening (Figure 2). Empirically, the hyperopic response is dominant when ablation is limited to the anterior stroma, and progressively deeper circumferential insults result in a shift toward corneal steepening (Gilbert et al., 1990; Litwin et al., 1991). The elastic modulus of the residual stroma bed and shear modulus of the bordering peripheral stroma may be important factors in determining the pace of ablation depth-dependent flattening and the depth at which hyperopic effects lose ground to elastic steepening.
This model provides one rationale for hyperopic shifts that occur even before the onset of epithelial healing and in the absence of a concave ablation profile. This response is most clearly demonstrated by unintended hyperopic shift during PTK, in which an ablation depth-dependent flattening can be observed in donor and clinical studies despite attempts at plano ablation (Dupps and Roberts, 2001; Fagerholm et al., 1993). In a multivariate paired-control donor analysis of PTK-induced hyperopia, peripheral thickening was more strongly associated with the degree of flattening than the measured ablation pattern (Dupps and Roberts, 2001). While the relative influence of ablation profile is greater in ametropic treatments than in PTK, this analysis demonstrated in a controlled fashion that central curvature change is not solely a product of ablative shape subtraction. Changes in intrastromal fluid distribution and epithelial and stromal wound healing may contribute to partial regression of this hyperopic shift in the days and weeks following surgery.
In clinical practice, an intrinsic flattening response augments the effects of a myopic procedure and impedes efforts to correct hyperopia. This is supported by the fact that large overcorrections must be attempted to treat high levels of primary hyperopia (Lindstrom et al., 1999). If an identical algorithm is used to treat secondary hyperopia of the same magnitude (i.e., after previous myopic LASIK), significant overcorrection results (Lindstrom et al., 1999; Choi and Wilson, 2001). This difference in efficacy can be attributed to fundamental differences between the biomechanical status of a virgin cornea and that of a surgically-altered cornea that has adapted to an entirely different load-bearing milieu.
Creation of the LASIK flap itself may induce hyperopia, astigmatism and higher order aberrations that depend upon hinge position (Guell et al., 2005; Pallikaris et al., 2002). LASIK represents a more complex case than PRK because, in addition to the above effects, the flap itself is subject to shape changes induced by the circumferential keratotomy of flap creation. A decrease of lamellar tension within the flap may promote differential central flap thickening due to a peripheral predominance of interlamellar cohesive forces. This would favor a relative myopic shift within the flap that partially counteracts hyperopic changes in the underlying stromal bed. This is supported by the observation that post-LASIK myopic shifts during atmospheric hypoxia are associated with thickening of the central cornea (Nelson et al., 2001). It also follows that attempts at wavefront-driven correction of aberrations will likely be more inaccurate when using LASIK rather than PRK to correct a particular constellation of lower- and higher-order aberrations, especially for lower levels of myopia or hyperopia.
To account for the separate effects of flap creation and photoablation, some have investigated a staged procedure incorporating a re-measurement delay after flap creation (Porter et al., 2003; Waheed et al., 2005). This approach is more strongly advocated for correction of ametropia and astigmatism after penetrating keratoplasty, where the biomechanical and wound healing responses to flap creation are less predictable (Dada et al., 2001; Mularoni et al., 2006). Femtosecond lasers may allow more precise specification of flap morphology and could potentially reduce the astigmatic effects of flap creation and improve nomogram quality by reducing variability between patients. Transitions to larger treatment zones have reduced the tendency toward hyperopic overcorrection and postoperative regression in myopic LASIK (O'Brart et al., 1996).
2.2 Mathematical modeling of the cornea
Models of the cornea have taken many forms, from conceptual models to the schematic eye of Gullstrand to complex computational models that integrate structural, biomechanical and optical representations of the corneal response (Buzard, 1992). The predictive value of any model depends upon valid input, and recent progress in anterior segment imaging has improved our ability to accurately measure corneal geometry. By the scheme presented in Figure 1, geometric datasets can then be used as a scaffold for superimposing substructural features. Complex structures like the cornea can be divided into a mesh of representative geometries (“finite elements”) with their own material properties; the physical solutions during a surgical or disease simulation can then be obtained iteratively from element to element until the solution for the entire structure is obtained.
Creating an appropriate material properties overlay is a great challenge and is critical to surgical simulation and optimization. For example, if heterogeneity, swelling pressure and shear properties are neglected, then an elastic thin-shell model of the cornea fails to predict hyperopic shift in PTK as discussed above (Bryant MR, 1993). Models that incorporate these properties and their heterogeneous distribution (Katsube et al., 2002; Pinsky and Datye, 1991) are better capable of representing clinical results but are still exquisitely sensitive to errors in specified material properties, particularly the elastic modulus (Dupps, 2005; Guirao, 2005). Robust models require better techniques for measuring properties in individual patients.
2.3 Keretectasia
Keratoconus and post-LASIK ectasia have been discussed in previous sections but deserve a few additional comments. Clinical risk factors for post-LASIK ectasia include high myopia, forme fruste keratoconus and low residual stromal bed (RSB) thickness (Randleman et al., 2003). While a lower limit of 250 to 300 um has been recommended for the RSB (Seiler and Quurke, 1998), its ultimate thickness depends on microkeratome predictability and a stromal ablation rate that can vary with hydration and between patients. Even the most accurate estimates of RSB thickness will not fully account for elastic and viscoelastic risk factors, just as presence of a presumably normal central corneal thickness does not rule out keratoconus (Gherghel et al., 2004). Because focal weaknesses produce marked increases in local stress, spatial inhomogeneities in material strength may be more important than bulk properties in some cases. Conversely, evenly-distributed material strength may allow for long term stability of some corneas with a RSB thickness less than 250 um (Vinciguerra et al., 2005).
Previous sections have commented on possible biomechanical abnormalities in keratoconus and post-LASIK ectasia. Elastic steepening with progressive central thinning is inversely related to the elastic modulus of the residual central corneal bed (Guirao, 2005). However, an elastic forward protuberance of the cornea must be distinguished from ectasia. Although the former can be a precursor of ectasia (Guirao, 2005), ectasia requires a progressive deformation by definition and is therefore a viscoelastic phenomenon (Dupps, 2005). Furthermore, immediate postoperative increases in central posterior corneal elevation often noted on scanning slit topography do not necessarily represent a pre-ectatic anterior vaulting. Instead, posterior corneal steepening may reflect a relative posterior movement of the peripheral stroma in response to the differential swelling described previously (Dupps and Roberts, 2001; Grzybowski et al., 2005). Artifactual posterior steepening can also result from minification of the central posterior radius of curvature after myopic photokeratectomy (Nawa et al., 2005). LASIK may introduce a greater risk of viscoelastic failure than surface ablation procedures because of deeper forays into the posterior stroma. Lower keratocyte density, less collagen interweaving and more hydrophilic proteoglycans may all contribute to a region more prone to viscoelastic failure and abnormal repair. Intrastromal ring segments (Siganos et al., 2002) and UV-riboflavin collagen crosslinking (Wollensak et al., 2003) are two biomechanical approaches under investigation for restoration of structural stability in affected patients.
2.4. IOP measurement after refractive surgery
As early as 1937, Friedenwald acknowledged the importance of corneal resistance in applanation tonometry (Friedenwald, 1937). Many studies have demonstrated a decrease in applanation pressures after myopic PRK and LASIK that was initially attributed to decreases in central corneal thickness (Damji et al., 2003). Other studies demonstrating decreases in central applanation pressures after hyperopic LASIK—without a decrease in central corneal thickness (Munger et al., 2001)—suggest that a decrease in corneal resistance to applanation occurs and can affect IOP measurement independently of the central corneal thickness (CCT). A sensitivity analysis of the various factors influencing applanation pressure suggests that the elastic modulus may be considerably more influential than corneal thickness or curvature (Liu and Roberts, 2005). This issue and the important role of CCT in risk of progression from ocular hypertension to glaucoma (Brandt et al., 2001) has spawned efforts to distinguish corneal biomechanical properties from true IOP using devices such as the Dynamic Contour Tonometer (Kaufmann et al., 2003) and the Ocular Response Analyzer.
3. Wound healing
The immediate postoperative refractive results of LASIK or surface ablation are dominated by the programmed ablation zone geometry, the laser-tissue interaction and perioperative biomechanical responses. Healing ensues immediately, however, and the cornea’s optical properties are further modified. Biological diversity in this response is the norm, even in genetically similar individuals or contralateral eyes of the same patient. As such, it is a major factor in refractive overcorrection, undercorrection and regression, induction of irregular astigmatism and haze formation (Netto et al., 2005b).
3.1. Components of the wound healing response
Figure 4 summarizes corneal wound healing events of particular relevance to corneal refractive surgery. Though presented in the form of a linear cascade for simplicity, the interactions are complex, often occur simultaneously and are influenced by many other factors not depicted. This approach emphasizes the importance of stromal-epithelial and immune cell interactions (Netto et al., 2005b; Wilson et al., 2003b), which are mediated by cytokines, growth factors, chemokines and their receptors (Wilson et al., 1999b), and the central role of keratocyte apoptosis in activation of the wound healing cascade. The corneal nerves, lacrimal glands and tear film are also important participants (Tervo et al., 1997; Wilson et al., 1999a; Wilson et al., 2003b).
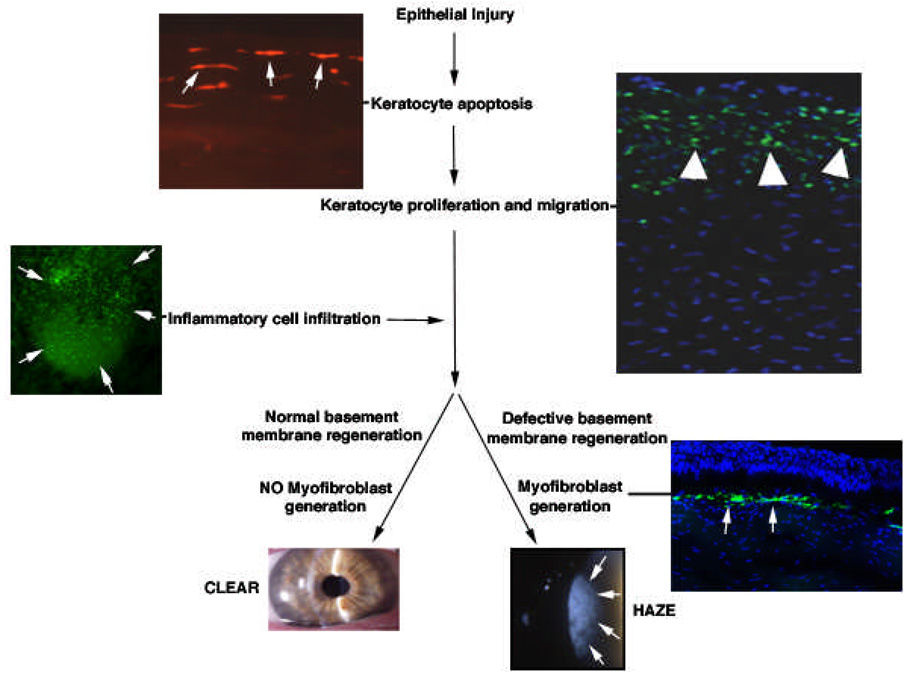
Figure 4.
Corneal wound healing cascade. This diagram provides a simplification of the corneal wound healing response. It can be described as a cascade, but many of the components noted here occur simultaneously or have temporal overlap and many other components that contribute are not depicted. Epithelial injury is the inciting event of wound healing in most cases. This injury can take the form of a scrape, incision, laser exposure (for example, the femtosecond laser), or other insult. The earliest observable stromal change following epithelial injury is the almost instantaneous programmed cell death (apoptosis) of underlying keratocyte cells. In this example, a section from a human cornea that underwent epithelial scrape prior to enucleation for choroidal melanoma is stained with the TUNEL assay to reveal keratocytes (arrows) undergoing apoptosis (500X mag.). Within a few hours, residual stromal keratocytes begin to undergo proliferation and migrate to restore stromal cellularity. The earliest mitosis in a cornea with a scrape injury is in the peripheral and posterior cornea. In this example, cells undergoing mitosis (arrowheads) are detected in a rabbit cornea at 24 hours after epithelial injury by performing immunocytochemistry for the Ki67 marker for mitosis (400X mag.). Within a few hours of injury, thousands of bone marrow-derived cells migrate into the cornea. These cells likely phagocytize remnants of dead cells and other debris, but may have other functions that have yet to be characterized. In this example, cells (arrows) expressing fluorescent green protein (FGP) can be seen migrating into the cornea at 24 hours after injury in a chimeric mouse in which only bone marrow-derived cells express the FGP marker. Depending on the type and extent of injury, myofibroblasts may be generated in the cornea. Current dogma is that these cells are derived from keratocytes that proliferate to form stromal fibroblasts, that then differentiate into myofibroblasts under the influence of transforming growth factor beta and other cytokines. However, studies have demonstrated that myofibroblasts in skin (Bhawan and Majno, 1989) and lung (Hashimoto et al., 2004) are derived from bone-marrow derived cells. Further work is needed to explore this possibility in the cornea. When myofibroblasts develop, they typically arise in stroma near the surface epithelium or epithelium that migrates into (or is placed ectopically within) stromal incisions – probably because the epithelium is a source of cytokines required for myofibroblast development. In the example provided, myofibroblasts (arrows) are detected using immunocytochemistry for the alpha smooth muscle actin marker in a rabbit cornea at one month after photorefractive keratectomy for 9 diopters of myopia. Corneas in which large numbers of myofibroblasts are generated develop haze, while those that do not remain clear–there tending to be a direct relationship between the level of haze and the density of myofibroblasts. Recent studies have demonstrated that surface irregularity (Netto et al., 2006), and likely associated abnormalities of the regenerated basement membrane (Netto et al., 2006; Stramer et al., 2003), are important factors in myofibroblast generation. There are also important species-related differences in the tendency to generate myofibroblasts. Rabbits have far greater tendency to develop myofibroblasts and corneal haze than humans and mice. The slit lamp photos of a clear cornea and a cornea with haze are both from humans (5X mag.).
The process begins with an epithelial insult, which may take the form of microkeratome or femtosecond laser-mediated disruption, alcohol exposure or a mechanical scrape. This is followed by release of cytokines from the injured epithelium and epithelial basement membrane, including interleukin (IL)-1 and tumor necrosis factor (TNF) alpha (Wilson et al., 1999b), bone morphogenic proteins (BMP) 2 and 4, epidermal growth factor (EGF) and platelet derived growth factor (PDGF) (Tuominen et al., 2001). These factors, and others derived from the tears, trigger a variety of responses in underlying stromal keratocytes, including an IL-1 mediated synthesis of Fas ligand. Keratocyte Fas ligand binds to Fas receptor on nearby keratocytes and induces apoptosis (Wilson et al., 1999b), a programmed form of cell death with minimal collateral damage due to a relative absence of cell lysis and lysozomal enzyme release, or might also induce autocrine suicide in keratocytes already expressing Fas. Apoptosis can be identified and localized in immunohistochemical preparations using TdT-mediated dUTP nick end labeling (TUNEL assay). A compromised epithelial barrier potentiates the effects of liberated epithelial and lacrimal cytokines by providing unhindered access to the stroma.
After the initial wave of keratocyte apoptosis, increasing numbers of cells undergo the more pro-inflammatory process of necrosis (Wilson et al., 2001b). Proliferation and migration of remaining keratocytes begins within 12 to 24 hours, giving rise to activated keratocytes, fibroblasts and possibly myofibroblasts responsible for repopulating the depleted stroma (Fini, 1999). Also, within the first 24 hours of injury, pro-inflammatory chemokines (Figure 4) from the epithelium or from keratocytes responding to IL-1 and TNF-alpha trigger stromal infiltration by macrophages/monocytes, T cells and polymorphonuclear cells. These cells, which arrive via the limbal blood supply as well as from the tear film (Helena et al., 1998), play a role in phagocytosis of apoptotic and necrotic debris and possibly other functions in the stroma.
One to 2 weeks following injury, cells that stain with antibody against alpha-smooth muscle actin (SMA) can be visualized in the anterior stroma directly below areas of epithelial basement membrane disruption in some corneas, depending on the level of correction, surface irregularity and other factors (Netto et al., 2006). These cells, known as myofibroblasts, are a critical component of the wound healing cascade. Presumed to be derivatives of keratocytes responding to transforming growth factor (TGF)-beta (Jester and Ho-Chang, 2003), myofibroblasts are notable for their contractile pseudopodia (the target of anti-SMA staining). They also exhibit reduced transparency due to altered corneal crystalline production (Jester et al., 1999) and play a comprehensive role in collagen and extracellular matrix remodeling through production of collagen, glycosaminoglycans, collagenases, gelatinases and MMPs. Myofibroblasts are clearly important effectors of corneal haze formation and regression due to stromal remodeling.
The delicate balance between stromal regeneration and fibrosis depends in large part upon the activity of these cells, and restoration of the epithelial basement membrane appears to favor the non-fibrotic phenotype (Stramer et al, 2003; Netto et al., 2005c). After an injury to the epithelial basement membrane, EGF facilitates the deposition of a fibronectin scaffold, atop which epithelial repair can proceed (Suzuki et al., 2003). Re-epithelialization after a broad defect like that associated with PRK typically occurs in 3–5 days. Myofibroblasts slowly disappear over the ensuing weeks, although the process may continue for months to years (Helena et al., 1998; Netto et al, 2006).
3.2. Wound healing in LASIK and surface ablation
Important differences exist in the pace, intensity and spatial distribution of wound healing activity as a function of the surgical approach to laser vision correction. Whereas PRK involves broad injury and removal of the epithelium, epithelial basement membrane, Bowman’s layer and a portion of the anterior stroma, LASIK leaves these structures relatively undisturbed—except at the flap margin—by virtue of a stromal-epithelial flap. This difference in the degree of central epithelial trauma is a major factor in the clinical and histological differences noted after LASIK and PRK. Differences in the level of peripheral epithelial injury, depending on side-cut energies, is also likely an important determinant of the difference in healing between LASIK performed with a femtosecond laser compared to LASIK performed with a microkeratome (Netto MV and Wilson SE, unpublished data, 2005).
Specifically, after PRK disruption of the epithelial basement membrane over the central cornea amplifies the wound healing response and accounts for higher rates of regression and haze. In a rabbit model, keratocyte apoptosis, keratocyte proliferation and myofibroblast generation are significantly greater after PRK for high myopia (−9D) than after LASIK for equivalent myopia (Mohan et al., 2003; Wilson, 2002). In LASIK, keratocyte apoptosis and proliferation are observed immediately anterior and posterior to the lamellar interface. In PRK, however, keratocyte apoptosis localizes to the anterior stroma, while the posterior and peripheral stroma is dominated by keratocyte proliferation (Mohan et al., 2003). It is feasible that the increased postoperative load born by the residual stroma signals a proliferative keratocyte response aimed at increasing structural resistance to this stress. Failure on the part of the posterior keratocytes to generate sufficient resistance to stress relaxation and viscoelastic creep could be a contributing factor in ectasia (Comaish and Lawless, 2002; Dupps, 2005). A concerning decline in keratocyte density in the flap and anterior sub-ablation zone has been noted on confocal microscopy after LASIK, but the clinical significance of this finding remains unclear (Erie et al., 2004).
Refractive regression is a major challenge after PRK for myopia, hyperopia and astigmatism, especially for high levels of correction, and is both more common and more pronounced than after LASIK (Dausch et al., 1996; Jackson et al., 1998; Kim et al., 1997). The source of regression is attributed to differential changes in the thickness of the cornea due to a combination of stromal remodeling and epithelial hyperplasia. These processes predominate in regions of greater tissue removal, and the refractive effect is a relative “undoing” of the initial correction. The relative contributions of the stroma and the epithelium in regression have been debated and appear to be a function of postoperative time, type of refractive surgery, whether treatment was directed at hyperopia or myopia and other factors (Lohmann and Guell, 1998; Moller-Pedersen et al., 2000; Park and Kim, 1999; Reinstein et al., 2005). Newer anterior segment imaging technologies such as very high frequency ultrasound and optical coherence tomography (OCT) allow differentiation of epithelial, stromal and residual stromal thicknesses and will be useful tools for studying these processes in more detail. Enhancement surgery for apparent residual refractive error prior to the 3 to 6 month postoperative visit is generally avoided because of the possibility of ultimately overcorrecting a patient with slowly-resolving epithelial hyperplasia.
In LASIK, the distance of the ablation bed, and resulting stromal cellular responses, from the epithelium and absence of epithelial basement membrane disruption favor a more moderate healing response. However, cases involving very thin flaps or microkeratome-induced abrasions are likely to respond similarly to PRK with a higher incidence of regression and stromal haze (Mohan et al., 2003). Although haze is much more common after PRK for high myopia than in LASIK or PRK for low myopia, it is considered clinically significant in only about 0.5% to 3% (Kapadia and Wilson, 2000). It is common to see a transitory insignificant haze lasting 1 to 3 months, but late-onset haze presenting 2 or more months after surgery is more likely to be clinically significant and often associated with severe regression. Furthermore, direct implantation or ingrowth of epithelium into the lamellar interface provides a local source of epithelial cytokines and can result in interface haze, regression and diffuse lamellar keratitis (DLK) (Wilson et al., 2003a). DLK is a diffuse, noninfectious inflammatory infiltrate that can occur after LASIK at the level of the flap-residual stromal interface. DLK, with its associated inflammatory cells and up-regulation of PDGF and chemotactic factors, can in turn stimulate increased wound healing and refractive regression (Wilson and Ambrosio, 2002). Many cases of clinically significant haze improve without intervention—even after 1 postoperative year (Netto et al., 2005b).
The intensity of the corneal response is clearly related to the magnitude of attempted treatment. Thus, the cellular responses noted above are more pronounced after PRK for high myopia than after PRK for low myopia (−4.5D) (Wilson, 2002). Similarly, clinical regression has been shown to be more pronounced after PRK for corrections greater than −6D (Kim et al., 1997). One hypothesis for this effect relates to the increased depth of stromal disruption and differences in the distribution and behavior of keratocytes in the posterior stroma (Wilson et al., 1992). However, stromal irregularity is also a powerful stimulant of myofibroblast generation and haze (Netto et al., 2006). A relationship between haze formation after PRK and induced stromal surface irregularity has recently been demonstrated (Netto et al., 2006), and PTK-smoothing with methylcellulose was shown experimentally to be effective for reducing irregularity as well as myofibroblast density and haze. Stromal surface irregularity is a function of the treatment magnitude and may result in aberrant basement membrane regeneration, increased keratocyte exposure to TGF-beta and increased numbers of myofibroblasts. This increased cellular response correlates with stromal haze.
3.3. Wound healing after femtosecond flap creation in LASIK
Wound healing responses may be more pronounced after femtosecond flap creation than after mechanical microkeratome procedures. Interface irregularity may be higher after femtosecond flap creation due to the explosive cavitation process associated with plasma formation (Netto et al., 2005a; Solomon et al., 2004), and increases in DLK and flap-edge DLK have been noted (Binder, 2004). Rabbit studies corroborate a more pronounced inflammatory response after femtosecond flap creation (Kim et al., 2006; Netto et al., 2005a). The femtosecond delivery pattern may trigger more extensive release of proinflammatory cytokines due to a wider path of epithelial disruption, larger gutters (Netto et al., 2005a), and slight delays in epithelialization relative to microkeratome-assisted surgery. Employing lower side-cut angles to reduce the path length through the epithelium, lower side-cut and bed energies and increased frequency of postoperative topical corticosteroids may reduce the incidence of DLK. Flap adhesion strength may be higher after femtosecond-assisted surgery due to enhanced peripheral epithelial injury and inflammation (Kim et al., 2006). Associated reductions in flap displacement rates and increased difficulty of late flap lifts for enhancement surgery appear to be noted clinically, but have yet to be established in controlled studies.
3.4. Wound healing after alternative surface ablation procedures
Newer approaches to surface ablation that employ alcohol-assisted (LASEK) or microkeratome-based epithelial flap creation (epi-LASIK) (Pallikaris et al., 2003) seek to circumvent some of the wound healing challenges of PRK. By preserving an epithelial flap that can be repositioned atop the ablated anterior stroma, these techniques attempt to reduce stromal exposure to epithelium- and tear-derived cytokines with the goal of minimizing postoperative pain, myofibroblast generation and haze formation. The use of ethanol to facilitate separation of an epithelial flap may introduce variability in the ablation rate due to tissue dehydration and can also increase surface cytokine levels due to epithelial injury. One prospective, randomized contralateral eye study (Pirouzian et al., 2004) comparing re-epithelialization dynamics and pain after PRK and LASEK demonstrated smaller epithelial defects at day 1 following LASEK but larger defects at day 3 than after PRK with no detectable advantage in patient comfort. A human organ-culture model comparing PRK and LASEK also suggests a significant delay in epithelial healing in LASEK with a corresponding delay in keratocyte regeneration (Rajan et al., 2005).
3.5. Modulation of wound healing in refractive surgery
Although postoperative topical corticosteroids are routinely used after refractive surgery procedures and may in some individual eyes help refractive regression and haze, prolonged use after PRK has been discouraged by some due to evidence that any efficacy depends on continued administration (Corbett et al., 1995). In rabbits, the strength of healed keratotomy wounds is lower than normal after topical steroid use and higher than normal with NSAID use (McCarey et al., 1995). The naturally-occurring antimetabolite mitomycin-C (MMC) induces keratocyte and myofibroblast apoptosis and is used routinely by many for prevention of haze in PRK for high myopia (Talamo et al., 1991). Some have also demonstrated efficacy in reversing PRK-induced haze and regression (Vigo et al., 2003). Controversy remains over the possibility of long-term implications of MMC-mediated keratocyte depletion (Netto et al, 2006b), particularly since postoperative keratocyte density is decreased even without MMC (Erie et al., 2004). Because of this concern, there is a movement toward decreasing the concentration (from 0.02% to 0.002%) and duration of exposure when MMC is used. Whenever MMC is used, changes in wound healing often necessitate nomogram modifications to optimize refractive outcome.
Amniotic membrane patching has shown promise in rabbits for haze prevention after PRK through a proposed inhibition of TGF-beta action (Wang et al., 2001), and one clinical study has demonstrated shortened epithelial healing times and lower incidence of haze after LASEK when an inferior limbal strip of amniotic membrane was placed at the time of surgery (Lee et al., 2004). Transplantation of tissue-engineered epithelial cell sheets cultured from autologous limbal biopsy specimens have been shown in rabbits to provide immediate epithelialization, as well as decreased haze, keratocyte apoptosis and alpha-SMA relative to controls (Hayashida et al., 2006). Despite promising results, questions regarding the cost effectiveness and patient acceptance of amniotic membrane or epithelial transplantation in this setting remain. Pharmacologic therapies directed at specific modulators such as the TGF-beta isoforms continue to be explored (Wilson et al., 2001a), and gene therapy will provide an exciting means of transiently expressing genes of interest for investigating and potentially controlling the processes of regeneration and fibrosis (Netto et al., 2005b).
4. The interface of wound healing and biomechanics
The cornea undergoes significant structural and biological alterations after refractive surgery, and the relationship between these processes has been studied in postmortem tissue. A histopathological study in LASIK flaps of organ donors found a relationship between wound maturity and resistance to flap distraction (lifting) forces (Schmack et al., 2005). Flap cohesive strength was maximal at the flap margins, was associated with hypercellular fibrotic scars and increased as a function of postoperative time. Flap-edge cohesion was only 28% that of normal specimens, however, and was adversely affected by the presence of epithelial ingrowth. The central interface was characterized by hypocellular primitive scar and presented far less cohesive strength. The contribution of flap cohesion to the overall biomechanical stability of the cornea is not known but is far more likely to be a factor in late flap dislocation than in ectasia. Although in vivo flap cohesion is likely to be higher due to active endothelial transport (Bissen-Miyajima et al., 2004), the relative paucity of cohesive strength in the central interface might preserve a low-resistance migration pathway necessary for late-onset cases of DLK (Netto et al., 2005b). Similarly, epithelial ingrowth may be associated with regression, haze and DLK not only because of its proximity to the interface but also because flap edge cohesion is notably impaired in its presence.
Given that one evolutionary goal of healing is restoration of mechanical integrity (Wilson, 2002), mechanisms must exist by which keratocytes or their derivatives “sense” local changes in stress or strain and then respond with an appropriate (or in disease, inappropriate) series of actions for remodeling such areas to reduce or redistribute the mechanical stimulus. These are dynamic processes and are best studied in living models. Myofibroblasts are distinguished from keratocytes by a larger number of stress fibers and adhesion complexes (Mar et al., 2001), and these contractile units reflect the myofibroblast’s role as a biomechanical effector of stromal matrix stabilization. The matrix-deforming interactions of corneal fibroblasts and a fibrillar collagen substrate have been directly observed and quantified in culture (Petroll et al., 2004), and such models, when combined with advanced structural and functional imaging modalities, provide a promising vehicle for continued investigation of the mechanical consequences of cell-cell and cell-matrix signaling during wound repair and refractive regression. These healing effects can ultimately be incorporated into mathematical models as a viscoelastic consideration. As our understanding of these processes improves, so will our ability to offer rational interventions for further improving the predictability of refractive surgery and minimizing its complications.
Acknowledgements
We are grateful to Cynthia Roberts, Ph.D. and Todd Doehring, Ph.D. for their contributions to Figure 2 and Figure 3, respectively, and to Rajiv Mohan, Ph.D, Renato Ambrosio, Jr., MD, and Marcelo Netto, MD for work in generating components of Figure 4.
Supported in part by US Public Health Service grants EY010056 and EY015638 from the National Eye Institute and HD049091 from the National Institute of Child Health and Human Development, Multidisciplinary Clinical Research Career Development Programs Grant, National Institutes of Health, Bethesda, MD.
Footnotes
Proprietary interest statement: The authors have no proprietary or financial interest in relation to this manuscript.
References
- Andreassen TT, Simonsen AH, Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res. 1980;31:435–441. doi: 10.1016/s0014-4835(80)80027-3. [DOI] [PubMed] [Google Scholar]
- Bhawan J, Majno G. The myofibroblast. Possible derivation from macrophages in xanthogranuloma. Am J Dermatopathol. 1989;11:255–258. doi: 10.1097/00000372-198906000-00010. [DOI] [PubMed] [Google Scholar]
- Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg. 2004;30:26–32. doi: 10.1016/S0886-3350(03)00578-9. [DOI] [PubMed] [Google Scholar]
- Bissen-Miyajima H, Nakamura K, Kaido M, Shimmura S, Tsubota K. Role of the endothelial pump in flap adhesion after laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:1989–1992. doi: 10.1016/j.jcrs.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Brandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS) Ophthalmology. 2001;108:1779–1788. doi: 10.1016/s0161-6420(01)00760-6. [DOI] [PubMed] [Google Scholar]
- Bryant MR, McDonnell PJ. Constitutive laws for biomechanical modeling of refractive surgery. J Biomech Eng. 1996;118:473–481. doi: 10.1115/1.2796033. [DOI] [PubMed] [Google Scholar]
- Bryant MRFD, Campos M, McDonnell PJ. Finite element analysis of corneal topographic changes after excimer laser phototherapeutic keratectomy. Invest Ophthalmol Vis Sci. 1993;31:804. [Google Scholar]
- Buzard KA. Introduction to biomechanics of the cornea. Refract Corneal Surg. 1992;8:127–138. [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RY, Wilson SE. Hyperopic laser in situ keratomileusis: primary and secondary treatments are safe and effective. Cornea. 2001;20:388–393. doi: 10.1097/00003226-200105000-00010. [DOI] [PubMed] [Google Scholar]
- Comaish IF, Lawless MA. Progressive post-LASIK keratectasia: biomechanical instability or chronic disease process? J Cataract Refract Surg. 2002;28:2206–2213. doi: 10.1016/s0886-3350(02)01698-x. [DOI] [PubMed] [Google Scholar]
- Corbett MC, O'Brart DP, Marshall J. Do topical corticosteroids have a role following excimer laser photorefractive keratectomy? J Refract Surg. 1995;11:380–387. doi: 10.3928/1081-597X-19950901-15. [DOI] [PubMed] [Google Scholar]
- Dada T, Vajpayee RB, Gupta V, Sharma N, Dada VK. Microkeratome-induced reduction of astigmatism after penetrating keratoplasty. Am J Ophthalmol. 2001;131:507–508. doi: 10.1016/s0002-9394(00)00828-x. [DOI] [PubMed] [Google Scholar]
- Damji KF, Muni RH, Munger RM. Influence of corneal variables on accuracy of intraocular pressure measurement. J Glaucoma. 2003;12:69–80. doi: 10.1097/00061198-200302000-00015. [DOI] [PubMed] [Google Scholar]
- Dausch DG, Klein RJ, Schroder E, Niemczyk S. Photorefractive keratectomy for hyperopic and mixed astigmatism. J Refract Surg. 1996;12:684–692. doi: 10.3928/1081-597X-19960901-09. [DOI] [PubMed] [Google Scholar]
- Dupps WJ, Jr, Roberts C. Effect of acute biomechanical changes on corneal curvature after photokeratectomy. J Refract Surg. 2001;17:658–669. doi: 10.3928/1081-597X-20011101-05. [DOI] [PubMed] [Google Scholar]
- Dupps WJ., Jr Biomechanical modeling of corneal ectasia. J Refract Surg. 2005;21:186–190. doi: 10.3928/1081-597X-20050301-15. [DOI] [PubMed] [Google Scholar]
- Edmund C. Corneal topography and elasticity in normal and keratoconic eyes. A methodological study concerning the pathogenesis of keratoconus. Acta Ophthalmol Suppl. 1989;193:1–36. [PubMed] [Google Scholar]
- Ehlers N. Studies on the hydration of the cornea with special reference to the acid hydration. Acta Ophthalmol (Copenh) 1966;44:924–931. doi: 10.1111/j.1755-3768.1966.tb05524.x. [DOI] [PubMed] [Google Scholar]
- Erie JC, Nau CB, McLaren JW, Hodge DO, Bourne WM. Long-term keratocyte deficits in the corneal stroma after LASIK. Ophthalmology. 2004;111:1356–1361. doi: 10.1016/j.ophtha.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Fagerholm P, Fitzsimmons TD, Orndahl M, Ohman L, Tengroth B. Phototherapeutic keratectomy: long-term results in 166 eyes. Refract Corneal Surg. 1993;9:S76–S81. [PubMed] [Google Scholar]
- Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- Ford M, Dupps WJ, Huprikar N, Lin R, Rollins AM. OCT Elastography by pressure-induced optical feature flow. Proc SPIE. 2006 In press. [Google Scholar]
- Friedenwald JS. Contribution to the theory and practice of tonometry. Am J Ophthalmol. 1937;20:985–1024. [Google Scholar]
- Gherghel D, Hosking SL, Mantry S, Banerjee S, Naroo SA, Shah S. Corneal pachymetry in normal and keratoconic eyes: Orbscan II versus ultrasound. J Cataract Refract Surg. 2004;30:1272–1277. doi: 10.1016/j.jcrs.2003.11.049. [DOI] [PubMed] [Google Scholar]
- Gilbert ML, Roth AS, Friedlander MH. Corneal flattening by shallow circular trephination in human eye bank eyes. Refract Corneal Surg. 1990;6:113–116. [PubMed] [Google Scholar]
- Grabner G, Eilmsteiner R, Steindl C, Ruckhofer J, Mattioli R, Husinsky W. Dynamic corneal imaging. J Cataract Refract Surg. 2005;31:163–174. doi: 10.1016/j.jcrs.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Grzybowski DM, Roberts CJ, Mahmoud AM, Chang JS., Jr Model for nonectatic increase in posterior corneal elevation after ablative procedures. J Cataract Refract Surg. 2005;31:72–81. doi: 10.1016/j.jcrs.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Guell JL, Velasco F, Roberts C, Sisquella MT, Mahmoud A. Corneal flap thickness and topography changes induced by flap creation during laser in situ keratomileusis. J Cataract Refract Surg. 2005;31:115–119. doi: 10.1016/j.jcrs.2004.09.045. [DOI] [PubMed] [Google Scholar]
- Guirao A. Theoretical elastic response of the cornea to refractive surgery: risk factors for keratectasia. J Refract Surg. 2005;21:176–185. doi: 10.3928/1081-597X-20050301-14. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida Y, Nishida K, Yamato M, Yang J, Sugiyama H, Watanabe K, Hori Y, Maeda N, Kikuchi A, Okano T, Tano Y. Transplantation of tissue-engineered epithelial cell sheets after excimer laser photoablation reduces postoperative corneal haze. Invest Ophthalmol Vis Sci. 2006;47:552–557. doi: 10.1167/iovs.05-0995. [DOI] [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–283. [PubMed] [Google Scholar]
- Hjortdal JO, Jensen PK. In vitro measurement of corneal strain, thickness, and curvature using digital image processing. Acta Ophthalmol Scand. 1995;73:5–11. doi: 10.1111/j.1600-0420.1995.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Jackson WB, Casson E, Hodge WG, Mintsioulis G, Agapitos PJ. Laser vision correction for low hyperopia. An 18-month assessment of safety and efficacy. Ophthalmology. 1998;105:1727–1738. doi: 10.1016/S0161-6420(98)99045-5. discussion 1737–1728. [DOI] [PubMed] [Google Scholar]
- Jaycock PD, Lobo L, Ibrahim J, Tyrer J, Marshall J. Interferometric technique to measure biomechanical changes in the cornea induced by refractive surgery. J Cataract Refract Surg. 2005;31:175–184. doi: 10.1016/j.jcrs.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for 'corneal crystallins'. J Cell Sci. 1999;112(Pt 5):613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Jue B, Maurice DM. The mechanical properties of the rabbit and human cornea. J Biomech. 1986;19:847–853. doi: 10.1016/0021-9290(86)90135-1. [DOI] [PubMed] [Google Scholar]
- Kapadia MS, Wilson SE. One-year results of PRK in low and moderate myopia: fewer than 0.5% of eyes lose two or more lines of vision. Cornea. 2000;19:180–184. doi: 10.1097/00003226-200003000-00011. [DOI] [PubMed] [Google Scholar]
- Katsube N, Wang R, Okuma E, Roberts C. Biomechanical response of the cornea to phototherapeutic keratectomy when treated as a fluid-filled porous material. J Refract Surg. 2002;18:S593–S597. doi: 10.3928/1081-597X-20020901-19. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Bachmann LM, Thiel MA. Intraocular pressure measurements using dynamic contour tonometry after laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2003;44:3790–3794. doi: 10.1167/iovs.02-0946. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim MS, Hahn TW, Lee YC, Sah WJ, Park CK. Five years results of photorefractive keratectomy for myopia. J Cataract Refract Surg. 1997;23:731–735. doi: 10.1016/s0886-3350(97)80282-9. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim MJ, Kim TI, Choi HJ, Pak JH, Tchah H. A femtosecond laser creates a stronger flap than a mechanical microkeratome. Invest Ophthalmol Vis Sci. 2006;47:599–604. doi: 10.1167/iovs.05-0458. [DOI] [PubMed] [Google Scholar]
- Klyce SD, Dohlman CH, Tolpin DW. In vivo determination of corneal swelling pressure. Exp Eye Res. 1971;11:220–229. doi: 10.1016/s0014-4835(71)80026-x. [DOI] [PubMed] [Google Scholar]
- Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Investigative Ophthalmology and Visual Science. 1991;32:2244–2258. [PubMed] [Google Scholar]
- Lee HK, Kim JK, Kim SS, Kim EK, Kim KO, Lee IS, Seong GJ. Effect of amniotic membrane after laser-assisted subepithelial keratectomy on epithelial healing: clinical and refractive outcomes. J Cataract Refract Surg. 2004;30:334–340. doi: 10.1016/S0886-3350(03)00575-3. [DOI] [PubMed] [Google Scholar]
- Lindstrom RL, Hardten DR, Houtman DM, Witte B, Preschel N, Chu YR, Samuelson TW, Linebarger EJ. Six-month results of hyperopic and astigmatic LASIK in eyes with primary and secondary hyperopia. Trans Am Ophthalmol Soc. 1999;97:241–255. discussion 255–260. [PMC free article] [PubMed] [Google Scholar]
- Litwin KL, Moreira H, Ohadi C, McDonnell PJ. Changes in corneal curvature at different excimer laser ablative depths. Am J Ophthalmol. 1991;111:382–384. doi: 10.1016/s0002-9394(14)72335-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Roberts CJ. An ultrasound propagation model for characterizing biomechanical properties of ocular tissue. Second International Conference on the Ultrasonic Measurement and Imaging of Tissue Elasticity; Corpus Christi, Texas. 2003. p. 97. [Google Scholar]
- Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005;31:146–155. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Lohmann CP, Guell JL. Regression after LASIK for the treatment of myopia: the role of the corneal epithelium. Semin Ophthalmol. 1998;13:79–82. doi: 10.3109/08820539809059822. [DOI] [PubMed] [Google Scholar]
- Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Mar PK, Roy P, Yin HL, Cavanagh HD, Jester JV. Stress fiber formation is required for matrix reorganization in a corneal myofibroblast cell line. Exp Eye Res. 2001;72:455–466. doi: 10.1006/exer.2000.0967. [DOI] [PubMed] [Google Scholar]
- Maurice DM. The cornea and sclera. In: Davson H, editor. The Eye. Orlando, FL: Academic Press; 1984. pp. 1–158. [Google Scholar]
- McCarey BE, Napalkov JA, Pippen PA, Koester JM, al Reaves T. Corneal wound healing strength with topical antiinflammatory drugs. Cornea. 1995;14:290–294. doi: 10.1097/00003226-199505000-00010. [DOI] [PubMed] [Google Scholar]
- Meek KM, Newton RH. Organization of collagen fibrils in the corneal stroma in relation to mechanical properties and surgical practice. J Refract Surg. 1999;15:695–699. doi: 10.3928/1081-597X-19991101-18. [DOI] [PubMed] [Google Scholar]
- Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, Bron AJ. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46:1948–1956. doi: 10.1167/iovs.04-1253. [DOI] [PubMed] [Google Scholar]
- Mishima S, Hedbys BO. Physiology of the cornea. Int Ophthalmol Clin. 1968;8:527–560. [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–1245. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- Mularoni A, Laffi GL, Bassein L, Tassinari G. Two-step LASIK with topography-guided ablation to correct astigmatism after penetrating keratoplasty. J Refract Surg. 2006;22:67–74. doi: 10.3928/1081-597X-20060101-14. [DOI] [PubMed] [Google Scholar]
- Munger R, Dohadwala AA, Hodge WG, Jackson WB, Mintsioulis G, Damji KF. Changes in measured intraocular pressure after hyperopic photorefractive keratectomy. J Cataract Refract Surg. 2001;27:1254–1262. doi: 10.1016/s0886-3350(01)00971-3. [DOI] [PubMed] [Google Scholar]
- Munnerlyn CR, Koons SJ, Marshall J. Photorefractive keratectomy: a technique for laser refractive surgery. J.Cataract Refract.Surg. 1988;14:46–52. doi: 10.1016/s0886-3350(88)80063-4. [DOI] [PubMed] [Google Scholar]
- Nawa Y, Masuda K, Ueda T, Hara Y, Uozato H. Evaluation of apparent ectasia of the posterior surface of the cornea after keratorefractive surgery. J Cataract Refract Surg. 2005;31:571–573. doi: 10.1016/j.jcrs.2004.05.050. [DOI] [PubMed] [Google Scholar]
- Nelson ML, Brady S, Mader TH, White LJ, Parmley VC, Winkle RK. Refractive changes caused by hypoxia after laser in situ keratomileusis surgery. Ophthalmology. 2001;108:542–544. doi: 10.1016/s0161-6420(00)00592-3. [DOI] [PubMed] [Google Scholar]
- Netto MV, Wilson SE. Corneal wound healing relevance to wavefront guided laser treatments. Ophthalmol Clin North Am. 2004;17:225–231. doi: 10.1016/j.ohc.2004.03.002. vii. [DOI] [PubMed] [Google Scholar]
- Netto MV, Dupps WJ, Jr, Mohan RR, Sinha S, Rayborn M, Krueger RR, Wilson SE. Corneal morphology and wound healing response following flap creation with the femtosecond laser. Annual Meeting of the American Society of Cataract and Refractive Surgery; Washington DC. 2005a. [Google Scholar]
- Netto MV, Mohan RR, Ambrosio R, Jr, Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005b;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2005c doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006a;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, proliferation, haze, and keratocyte density. J. Ref. Surg. 2006b doi: 10.3928/1081-597x-20060601-08. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brart DP, Corbett MC, Verma S, Heacock G, Oliver KM, Lohmann CP, Kerr Muir MG, Marshall J. Effects of ablation diameter, depth, and edge contour on the outcome of photorefractive keratectomy. J Refract Surg. 1996;12:50–60. doi: 10.3928/1081-597X-19960101-12. [DOI] [PubMed] [Google Scholar]
- Pallikaris IG, Kymionis GD, Panagopoulou SI, Siganos CS, Theodorakis MA, Pallikaris AI. Induced optical aberrations following formation of a laser in situ keratomileusis flap. J Cataract Refract Surg. 2002;28:1737–1741. doi: 10.1016/s0886-3350(02)01507-9. [DOI] [PubMed] [Google Scholar]
- Pallikaris IG, Katsanevaki VJ, Kalyvianaki MI, Naoumidi II. Advances in subepithelial excimer refractive surgery techniques: Epi-LASIK. Curr Opin Ophthalmol. 2003;14:207–212. doi: 10.1097/00055735-200308000-00007. [DOI] [PubMed] [Google Scholar]
- Pallikaris IG, Kymionis GD, Ginis HS, Kounis GA, Tsilimbaris MK. Ocular rigidity in living human eyes. Invest Ophthalmol Vis Sci. 2005;46:409–414. doi: 10.1167/iovs.04-0162. [DOI] [PubMed] [Google Scholar]
- Park CK, Kim JH. Comparison of wound healing after photorefractive keratectomy and laser in situ keratomileusis in rabbits. J Cataract Refract Surg. 1999;25:842–850. doi: 10.1016/s0886-3350(99)00047-4. [DOI] [PubMed] [Google Scholar]
- Petroll WM, Cavanagh HD, Jester JV. Dynamic three-dimensional visualization of collagen matrix remodeling and cytoskeletal organization in living corneal fibroblasts. Scanning. 2004;26:1–10. doi: 10.1002/sca.4950260102. [DOI] [PubMed] [Google Scholar]
- Pinsky PM, Datye DV. A microstructurally-based finite element model of the incised human cornea. J Biomech. 1991;24:907–922. doi: 10.1016/0021-9290(91)90169-n. [DOI] [PubMed] [Google Scholar]
- Pirouzian A, Thornton JA, Ngo S. A randomized prospective clinical trial comparing laser subepithelial keratomileusis and photorefractive keratectomy. Arch Ophthalmol. 2004;122:11–16. doi: 10.1001/archopht.122.1.11. [DOI] [PubMed] [Google Scholar]
- Polack FM. Morphology of the cornea. I. Study with silver stains. Am J Ophthalmol. 1961;51:1051–1056. doi: 10.1016/0002-9394(61)91794-9. [DOI] [PubMed] [Google Scholar]
- Porter J, MacRae S, Yoon G, Roberts C, Cox IG, Williams DR. Separate effects of the microkeratome incision and laser ablation on the eye's wave aberration. Am J Ophthalmol. 2003;136:327–337. doi: 10.1016/s0002-9394(03)00222-8. [DOI] [PubMed] [Google Scholar]
- Rajan MS, Watters W, Patmore A, Marshall J. In vitro human corneal model to investigate stromal epithelial interactions following refractive surgery. J Cataract Refract Surg. 2005;31:1789–1801. doi: 10.1016/j.jcrs.2005.02.047. [DOI] [PubMed] [Google Scholar]
- Randleman JB, Russell B, Ward MA, Thompson KP, Stulting RD. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267–275. doi: 10.1016/S0161-6420(02)01727-X. [DOI] [PubMed] [Google Scholar]
- Reinstein DZ, Ameline B, Puech M, Montefiore G, Laroche L. VHF digital ultrasound three-dimensional scanning in the diagnosis of myopic regression after corneal refractive surgery. J Refract Surg. 2005;21:480–484. doi: 10.3928/1081-597X-20050901-10. [DOI] [PubMed] [Google Scholar]
- Roberts C. The cornea is not a piece of plastic. J Refract Surg. 2000;16:407–413. doi: 10.3928/1081-597X-20000701-03. [DOI] [PubMed] [Google Scholar]
- Roberts C. Biomechanics of the cornea and wavefront-guided laser refractive surgery. J Refract Surg. 2002;18:S589–S592. doi: 10.3928/1081-597X-20020901-18. [DOI] [PubMed] [Google Scholar]
- Schmack I, Dawson DG, McCarey BE, Waring GO, 3rd, Grossniklaus HE, Edelhauser HF. Cohesive tensile strength of human LASIK wounds with histologic, ultrastructural, and clinical correlations. J Refract Surg. 2005;21:433–445. doi: 10.3928/1081-597X-20050901-04. [DOI] [PubMed] [Google Scholar]
- Seiler T, Matallana M, Sendler S, Bende T. Does Bowman's layer determine the biomechanical properties of the cornea? Refract Corneal Surg. 1992;8:139–142. [PubMed] [Google Scholar]
- Seiler T, Quurke AW. Iatrogenic keratectasia after LASIK in a case of forme fruste keratoconus. J Cataract Refract Surg. 1998;24:1007–1009. doi: 10.1016/s0886-3350(98)80057-6. [DOI] [PubMed] [Google Scholar]
- Siganos CS, Kymionis GD, Astyrakakis N, Pallikaris IG. Management of corneal ectasia after laser in situ keratomileusis with INTACS. J Refract Surg. 2002;18:43–46. doi: 10.3928/1081-597X-20020101-06. [DOI] [PubMed] [Google Scholar]
- Smolek MK, McCarey BE. Interlamellar adhesive strength in human eyebank corneas. Investigative Ophthalmology and Visual Science. 1990;31:1087–1095. [PubMed] [Google Scholar]
- Smolek MK. Interlamellar cohesive strength in the vertical meridian of human eye bank corneas. Investigative Ophthalmology and Visual Science. 1993;34:2962–2969. [PubMed] [Google Scholar]
- Smolek MK, Klyce SD. Is keratoconus a true ectasia? An evaluation of corneal surface area. Arch Ophthalmol. 2000;118:1179–1186. doi: 10.1001/archopht.118.9.1179. [DOI] [PubMed] [Google Scholar]
- Solomon R, Donnenfeld E, Perry HD, Solomon K. Scanning electron microscopy ultrastructural comparison of femtosecond laser vs microkeratome lamellar keratectomy. Annual Meeting of the American Academy of Ophthalmology; New Orleans, LA. 2004. [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Saito J, Yanai R, Yamada N, Chikama T, Seki K, Nishida T. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003;22:113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Talamo JH, Gollamudi S, Green WR, De La Cruz Z, Filatov V, Stark WJ. Modulation of corneal wound healing after excimer laser keratomileusis using topical mitomycin C and steroids. Arch Ophthalmol. 1991;109:1141–1146. doi: 10.1001/archopht.1991.01080080101040. [DOI] [PubMed] [Google Scholar]
- Tervo T, Vesaluoma M, Bennett GL, Schwall R, Helena M, Liang Q, Wilson SE. Tear hepatocyte growth factor (HGF) availability increases markedly after excimer laser surface ablation. Exp Eye Res. 1997;64:501–504. doi: 10.1006/exer.1996.0226. [DOI] [PubMed] [Google Scholar]
- Tuominen IS, Tervo TM, Teppo AM, Valle TU, Gronhagen-Riska C, Vesaluoma MH. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp Eye Res. 2001;72:631–641. doi: 10.1006/exer.2001.0999. [DOI] [PubMed] [Google Scholar]
- Vigo L, Scandola E, Carones F. Scraping and mitomycin C to treat haze and regression after photorefractive keratectomy for myopia. J Refract Surg. 2003;19:449–454. doi: 10.3928/1081-597X-20030701-12. [DOI] [PubMed] [Google Scholar]
- Vinciguerra P, Munoz MI, Camesasca FI, Grizzi F, Roberts C. Long-term follow-up of ultrathin corneas after surface retreatment with phototherapeutic keratectomy. J Cataract Refract Surg. 2005;31:82–87. doi: 10.1016/j.jcrs.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Waheed S, Chalita MR, Xu M, Krueger RR. Flap-induced and laser-induced ocular aberrations in a two-step LASIK procedure. J Refract Surg. 2005;21:346–352. doi: 10.3928/1081-597X-20050701-08. [DOI] [PubMed] [Google Scholar]
- Wang H, Prendiville PL, McDonnell PJ, Chang WV. An ultrasonic technique for the measurement of the elastic moduli of human cornea. J Biomech. 1996;29:1633–1636. [PubMed] [Google Scholar]
- Wang MX, Gray TB, Park WC, Prabhasawat P, Culbertson W, Forster R, Hanna K, Tseng SC. Reduction in corneal haze and apoptosis by amniotic membrane matrix in excimer laser photoablation in rabbits. J Cataract Refract Surg. 2001;27:310–319. doi: 10.1016/s0886-3350(00)00467-3. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Lloyd SA, He YG. EGF, basic FGF, and TGF beta-1 messenger RNA production in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1987–1995. [PubMed] [Google Scholar]
- Wilson SE, Liang Q, Kim WJ. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Invest Ophthalmol Vis Sci. 1999a;40:2185–2190. [PubMed] [Google Scholar]
- Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999b;18:293–309. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Hong JW. Bowman's layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea. 2000;19:417–420. doi: 10.1097/00003226-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Hong JW, Lee JS, Choi R, Mohan RR. The wound healing response after laser in situ keratomileusis and photorefractive keratectomy: elusive control of biological variability and effect on custom laser vision correction. Arch Ophthalmol. 2001a;119:889–896. doi: 10.1001/archopht.119.6.889. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrosio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001b;20:625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2002;100:411–433. [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Ambrosio R., Jr Sporadic diffuse lamellar keratitis (DLK) after LASIK. Cornea. 2002;21:560–563. doi: 10.1097/00003226-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Hutcheon AE, Mohan RR, Ambrosio R, Zieske JD, Hong J, Lee J. Effect of ectopic epithelial tissue within the stroma on keratocyte apoptosis, mitosis, and myofibroblast transformation. Exp Eye Res. 2003a;76:193–201. doi: 10.1016/s0014-4835(02)00277-4. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Netto M, Ambrosio R., Jr Corneal cells: chatty in development, homeostasis, wound healing, and disease. Am J Ophthalmol. 2003b;136:530–536. doi: 10.1016/s0002-9394(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- Woo SL, Kobayashi AS, Lawrence C, Schlegel WA. Mathematical model of the corneo-scleral shell as applied to intraocular pressure-volume relations and applanation tonometry. Ann Biomed Eng. 1972;1:87–98. doi: 10.1007/BF02363420. [DOI] [PubMed] [Google Scholar]