Abstract
Diabetic retinopathy is a chronic low-grade inflammatory disease; however, the mechanisms remain elusive. In the present study, we demonstrated that endoplasmic reticulum (ER) stress was activated in the retina in animal models of diabetes and oxygen-induced retinopathy (OIR). Induction of ER stress by tunicamycin resulted in significantly increased expression of inflammatory molecules in the retina. Inhibition of ER stress by chemical chaperone 4-phenyl butyric acid ameliorated inflammation in cultured human retinal endothelial cells exposed to hypoxia, and in the retinas of diabetic and OIR mice. These findings indicate that ER stress is a potential mediator of retinal inflammation in diabetic retinopathy.
Keywords: ER stress, inflammation, diabetic retinopathy, oxygen-induced retinopathy, human retinal endothelial cells
Diabetic retinopathy is the leading cause of vision impairment in adults in Western world, and this predicament is set to worsen due to global epidemic of diabetes[1]. Several inter-related pathways, such as oxidative stress, polyol pathway, and PKC activation, have been shown to contribute to diabetes-induced retinal damages[1]. In addition, diabetic retinopathy is recently recognized as a chronic low-grade inflammatory disease[2]. We and others reported that inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF) are significantly up-regulated in the retina and correlated with vascular leakage in animal models of diabetes and oxygen-induced retinopathy (OIR)[2–4]. The levels of VEGF and TNF-α are also increased in the vitreous from diabetic patients with retinopathy[5,6]. Inhibition of the expressions or blockade of the activities of VEGF and TNF-α suppresses blood-retinal barrier (BRB) breakdown and retinal vascular leakage in diabetic animals, indicating that a critical role of inflammation in diabetic retinopathy [7,8]. However, the mechanisms by which diabetes elicits inflammatory response remain elusive.
Endoplasmic reticulum (ER) is the primary intracellular compartment responsible for protein biosynthesis and folding. It is also envisioned as the earliest signal transducing site, responding to various cellular stressors, such as hypoxia and oxidative stress [9–11]. ER stress as a result of accumulation of unfolded or misfolded proteins in the ER leads to the activation of three ER-localized transmembrane proteins, including inositol-requiring enzyme 1α (IRE1α), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6), which in turn initiate unfolded protein response (UPR). While transient and low grade ER stress can be overcome by the UPR, persistent and severe ER stress results in cell apoptosis and also causes inflammatory gene expression [12–14]. In epithelial and mesenchyme-derived cells, TNF-α expression is up-regulated by ER stress inducers thapsigargin or tunicamycin, and deficiency of IRE1α significantly decreased ER stress-induced TNF-α activity[13]. In human aorta endothelial cells, selective siRNA targeting of the activating transcription factor 4 (ATF4), an effector of the PERK UPR arm, attenuate the expression of interleukin 8 (IL-8), IL-6 and monocyte chemoattractant protein-1 (MCP-1) induced by oxidized lipids[15]. Blockade of ATF4 expression or activity also mitigates VEGF expression in various types of cells exposed to different stimuli, such as homocysteine, oxidants, and growth factors[16–18]. These results suggest a possible role of ER stress in the regulation of inflammatory response.
In the present study, we demonstrated, for the first time, that ER stress is implicated in diabetic retinopathy and in oxygen-induced ischemic retinopathy. Using ER stress inducer tunicymycin and chemical chaperone 4-phenyl butyric acid (PBA), we further demonstrated that ER stress is a potential mediator of diabetic-induced inflammation in retinal endothelial cells and in the retina.
MATERIAL AND METHODS
Animals
C57BL/6J and Akita mice were purchased from the Jackson Laboratory (Bar Harbor, MI). Care, use and treatment of all animals in this study were in strict agreement with the Statement for the Use of Animals in Ophthalmic and Vision Research from the Association for Research in Vision and Ophthalmology and with the guidelines set forth by the University of Oklahoma.
Mouse model of oxygen-induced retinopathy (OIR)
OIR mouse model was established as described previously[4,19]. Briefly, newborn mice at postnatal day 7 (P7) were randomly assigned to experimental or control groups. Mice in experimental groups were exposed to hyperoxia (75% O2) for 5 days and then returned to normoxia (room air), whilst control groups were maintained constantly in room air.
Cell culture
Primary human retinal microvascular endothelial cells (HREC) were obtained from Cell Systems Inc. (Kirkland, WA) and cultured in DMEM supplied with 10% fetal bovine serum, 1% heparin, 1% ITS, 1% Antibiotics, and 1‰ ECGS as described previously[4]. Cells with passages of 4–8 were used in the experiments.
Periocular injection and retina preparation
Periocular injection was performed as described previously[20]. Briefly, mice were anesthetized with ketamine and xylazine, and a 30-gauge needle was used to inject 20 μl of desired reagent into the posterior tenon’s capsule in the inferior temporal quadrant of the eyeball under an operating microscope. Mice were euthanized at different time points after treatment as indicated. Retinas were carefully dissected under an operating microscope as described [4,20,21], flash frozen with liquid nitrogen and stored at − 80°C for RNA and protein analysis.
Real-time reverse transcription (RT)-PCR
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer’s protocol. Real-time RT-PCR was performed using the iSript cDNA Synthesis Kit and SYBR® Green PCR Master Mix (Bio-Rad Laboratories, Hercules, CA) as described (41). The mRNA levels of target genes were normalized by 18s ribosomal RNA levels. Primers specific for glucose-regulated protein 78 (GRP78) (forward, 5′-TCATCGGACGCACTTGGAA-3′; reverse, 5′-CAACCACCTTGAATGGCAAGA -3′) [22] was used in the experiments.
Western blot analysis
Retinas and cells were lysed in RIPA buffer with protease inhibitor cocktail, PMSF and sodium orthovanadate (Santa Cruz Biotechnology, Santa Cruz, CA). Protein concentration was quantified by BCA protein assay (Pierce Biotechnology, Inc., Rockford, IL). Fifty μg of protein were resolved by SDS-PAGE and then blotted with specific antibodies: anti-phospho-IRE1α (Abcam, Cambridge, MA), anti-XBP1 (Santa Cruz Biotechnology, CA), anti-phospho-eIF2α (Cell Signalling Technology, Boston, MA), anti-ATF4 (Santa Cruz Biotechnology, CA), anti-GRP78 (Abcam, Cambridge, MA), anti-VEGF (Santa Cruz Biotechnology, CA) and anti-TNF-α (Abcam, Cambridge, MA) antibodies. The same membrane was stripped and reblotted with an anti-β-actin antibody (Abcam) as loading control.
Immunohistochemistry
Immunohistochemistry was performed on paraffin sections (5 μm) as described previously[23]. Briefly, retinal sections were incubated with a rabbit anti-GRP78 antibody (1:500). After extensive washes, the sections were incubated with a Dako EnVision™+ Single Reagents, a horseradish peroxidase (HRP)–labeled conjugated with secondary antibody (Dako, Carpinteria, CA), and then developed with 3,3′ diaminobenzidine (Sigma, St. Louis, MO) as a chromogen.
Immunofluorescence study in cell culture
GRP78 expression and subcellular location in cultured HREC were determined by immunofluorescence staining as described previously[4]. Briefly, HREC were seeded and grown to 80% confluence on 4-chamber slides (Nalge Nunc International Corp., Naperville, IL). After quiescence for 12 h, cells were exposed to hypoxia for 16 h. After fixation with 3.7% formaldehyde for 10 min and permeabilization, cells were incubated with primary antibody at 4 °C overnight followed secondary antibody for 1 h. The slides were visualized and photographed under a fluorescent microscope (Olympus, Hamburg, Germany).
Statistical analysis
The quantitative data were presented as mean ± SD. Statistical analyses were performed using one-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison test. Statistical differences were considered significant at a P value of less than 0.05.
RESULTS
Increased VEGF and TNF-α expression in the retina of Akita mice
The Akita mouse is a genetic model of type 1 diabetes. Previous studies from us and others showed that inflammatory cytokines VEGF and TNF-α are major mediators of retinal vascular permeability in STZ-induced diabetic animals[4,7]. Thus, we determined the expression of VEGF and TNF-α in the retinas of Akita mice. The results showed that the protein levels of VEGF and TNF-α were increased by 5.45-fold and 2.4-fold, respectively, in the retinas of Akita mice after 12 weeks of hyperglycemia (Fig. 1A, 1B). These results, in keeping with previous studies[4,7,24], indicate a role of inflammation in retinal damage in Akita mice..
Figure 1. Over-expression of pro-inflammatory factors and Up-regulation of ER stress markers in the retinas of Akita mice.
A). Western blot analysis of TNF-α and VEGF in the retinas of Akita mice. The membrane was reblotted with β-actin antibody as loading control. B). Retinal levels of TNF-α and VEGF were quantified by densitometry from 4 individual animals (mean ± SD, n = 4). C). mRNA level of GRP78 in the retina determined by real-time RT-PCR and normalized by 18S (mean ± SD, n = 7 in control group and n = 4 in Akita group). D). Representative images of GRP 78 expression in the retinas from 4 Akita mice and 5 littermate controls. Note more intensive signal of GRP 78 (brown color) in the inner retina of Akita mice (C-b) when compared to control (C-a). Magnification: 200x. ONL: outer nuclear layer; INL: inner nuclear layer; GCL: ganglion cell layer. E). Western blot analysis of phospho-IRE1α, phospho-eIF2α and ATF4 in the retinas of Akita mice. E). Retinal levels of phospho-IRE1α, phospho-eIF2α and ATF4 were quantified by densitometry (mean ± SD, n = 8). * p<0.05 and **p<0.01.
Up-regulation of GRP 78 and activation of IRE1α and eIF2α in the retina of Akita mice
Glucose-regulated protein 78 (GRP78) is a prominent ER-resident chaperon, which binds to the three ER stress sensors, but more stably with misfolded or unfolded proteins[25]. Thus, uprgulation of GRP78 is one of the most commonly used markers of ER stress. We determined the expression of GRP78 in the Akita retina by real-time RT-PCR and by immunohistochemistry. The results showed that GRP78 mRNA was significantly increased in the retina of Akita mice (Fig. 1C). In keeping, the protein level of GRP78 was also increased in the retina of Akita mice, with more intensive signals in the inner retina (Fig. 1D-b). It is notable that GRP78 was constantly expressed at a modest level in the cytoplasm of cells in the inner nuclear layer and ganglion cell layer, and the inner segment of photoreceptors in the non-diabetic control animals (Fig. 1D-a), suggesting GRP78 may plays an important role in maintaining the normal ER function in retinal cells.
We next examined the state of activation of IRE1α and PERK, two major transmembrane transducers sensing ER stress. The results showed that the phosphorylation of IRE1α at Ser724 was significantly up-regulated, indicating an activation of IRE1α in the retinas of Akita mice, when compared with age-matched non-diabetic controls (Fig. 1E, 1F). Moreover, robust splicing of XBP1 mRNA by activated IRE1α was observed in these retinas (not shown). In parallel, phosphorylation of eIF2α was significantly increased in the retinas of Akita mice, indicating increased PERK activity (Fig. 1E, 1F). Moreover, expression of ATF4, a downstream effector of phosphorylation of eIF2 was significantly increased (Fig. 1E, 1F). These findings together indicate that ER stress is activated in diabetic retinopathy.
Activation of ER stress and increased inflammation in OIR
OIR is a widely used animal model for ischemic retinal diseases, such as retinopathy of prematurity (ROP) and proliferative diabetic retinopathy (PDR) [4,19,26]. We have previously shown that inflammatory cytokines, including VEGF and TNF-α, are significantly upregulated in the retinas of OIR mice at P16, which contribute to subsequent retinal vascular leakage and neovascularization in the retina [4,27]. Here we further evaluate the implication of ER stress in retinal inflammation in OIR. The results showed that in OIR mice at P15, expression of GRP78 was significantly increased in the retina at both the RNA level (Fig. 2A) and the protein level (Fig. 2B), when compared with non-oxygen-treated littermate controls. In keeping, phosphorylation of eIF2α and the expression of ATF4 were also significantly upregulated in the retinas of OIR mice (Fig. 2C, 2D), suggesting that ER stress is enhanced in the OIR retina.
Figure 2. Activation of ER stress and inflammation in the retina of OIR mice.
A). mRNA level of GRP78 in the retina of OIR mice determined by real-time RT-PCR and normalized by 18S (mean ± SD, n = 5). B). Protein level of GRP78 determined by Western blot analysis. Lower panel: densitometry results (mean ± SD, n = 8). C). Western blot analysis of phospho-eIF2α and ATF4 in the retina. D). Retinal levels of phospho-eIF2α and ATF4 were quantified by densitometry (mean ± SD, n=8). **p<0.01.
Up-regulation of inflammatory cytokines in the retina by ER stress inducer tunicamycin
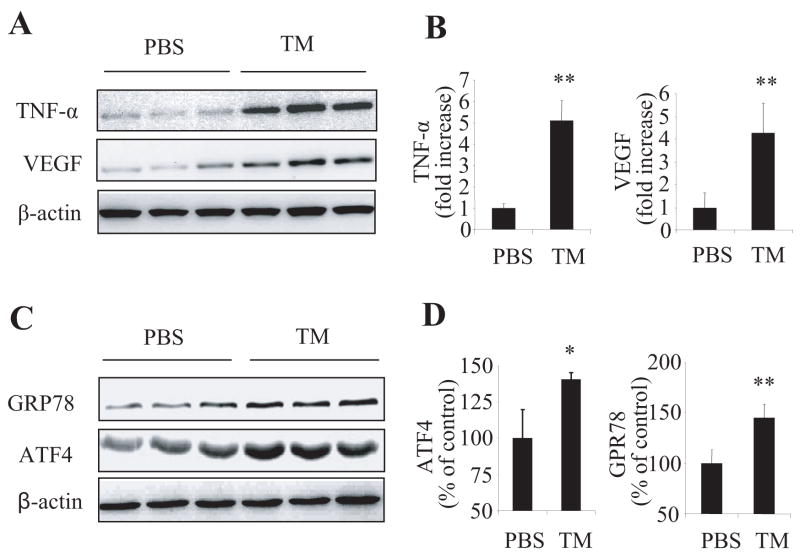
To further confirm the causative role of ER stress in retinal inflammation, we used a common ER stress inducer tunicamycin to determine whether ER stress is sufficient to induce retinal inflammation in vivo. Periocular injection, a non-invasive local delivery route, was employed to minimize the side effects of intravitreal injection on retinal inflammatory status. Twenty-four hours after tunicymycin (10μg/eye) treatment, the expression of TNF-α and VEGF was robustly increased in the retina by 5.1-fold and 4.3-fold respectively, when compared to that in the contralateral eyes receiving vehicle treatment (Fig. 3A). Activation of ER stress was confirmed by increased expression of ER markers, such as GRP78 and ATF4 (Fig. 3B). Periocular injection of a low dose (1μg/eye) of tunicamycin also induced a significant increase of TNF-α and VEGF expression in the retina (not shown).
Figure 3. Up-regulation of pro-inflammatory factors in the retina by tumicamycin.
Adult C57BL/6J mice received periocular injection of tunicamycin in one eye and PBS as control in the contralateral eye. Twenty four hours after injection, retinas were dissected and expression of pro-inflammatory factors (TNF-α and VEGF) (A, B) and ER stress markers (GRP78 and ATF4) (C, D) were determined by Western blot analysis and quantified by densitometry (mean ± SD, n = 4). * p<0.05 and **p<0.01.
Inhibition of ER stress by chemical chaperone ameliorates hypoxia-induced inflammation in retinal vascular endothelial cells
Hypoxia is an important feature of diabetic retinopathy and a potent inducer of inflammation in the retina [4,28]. Our previous studies showed that exposure of retinal vascular endothelial cells to hypoxia causes significant increase of expression of inflammatory cytokines, including VEGF and TNF-α [4]. Thus, here we examined whether hypoxia induces inflammatory cytokine overexpression via ER stress in cultured HREC. Exposure of HRECs to hypoxia (2% O2) for 16 h significantly increased GRP78 expression (Fig. 4A), in parallel with increased phosphorylation of IRE1α and eIF2α, and expression of ATF4 (Fig. 4B, 4C), indicating an activation of ER stress by hypoxia. Hypoxia also significantly upregualted VEGF and TNF-α expression as expected (Fig. 4D). Pre-treatment of cells with PBA attenuated hypoxia-induced VEGF and TNF-α over-expression (Fig. 4D), in parallel with a decrease in ER stress markers (Fig. 4B, 4C). These results suggest that ER stress is, at least in part, responsible for hypoxia-induced inflammation in retinal endothelial cells.
Figure 4. Inhibition of ER stress by PBA ameliorated hypoxia-induced VEGF and TNF-α expression in HREC.
A). HREC were treated with hypoxia (2% O2) for 16h and GPR78 expression was determined by immunostaining. A-a: control; A-b: hypoxia. B–E). HREC were pre-treated with PBA at the doses as indicated for 8h and then exposed to hypoxia (2% O2) for 16h. B–C). Expression of phospho-IRE1α, phospho-eIF2α, and ATF4 was determined by Western blot analysis and quantified by densitometry (mean ± SD, n= 3) D–E). Expression of VEGF and TNF-α was measured by Western blot analysis and quantified by densitometry (mean ± SD, n = 3). The values statistically different from control were indicated as ** p<0.01; from hypoxia indicated as † p<0.05, ‡ p<0.01.
Local or systemic administration of PBA mitigated VEGF expression in the retina of Akita or OIR mice
We further determined the effects of PBA on VEGF expression in the retina of Akita and OIR mice. Previous studies showed that systemic administration of PBA restored blood glucose homeostasis in diabetic animal models. Thus, PBA (0.4 μmol/eye) was delivered by periocular injection and same amount of vehicle was injected into the counterlateral eye as control in the same Akita mouse to avoid possible interference of blood glucose on retinal inflammation. The treatment was given in the Akita mice after hyperglycemia onset, twice a week, for 6 weeks. The OIR mice received daily intraperitoneal injection of PBA (40 mg/kg body weight) from P7 to P14. The expression of VEGF in the retina was measured by Western blot analysis. The results showed that retinal VEGF expression was markedly decreased by PBA treatment in both the Akita mice (Fig. 5A) and the OIR mice (Fig. 5B).
Figure 5. Local or systemic administration of PBA mitigated VEGF expression in the retina of Akita or OIR mice.
A). PBA (0.4 μmol/eye) was injected periocularly into one eye and same amount of vehicle was injected into the counterlateral eye as control in the Akita mice, twice a week, for 6 weeks. Expression of VEGF in the retina was measured by Western blot analysis and quantified by densitometry (mean ± SD, n = 4). B). OIR mice received intraperitoneal injection of PBA (40 mg/kg/body weight/day) or PBS from P7 to P14. Retinal VEGF expression was measured by Western blot analysis at P15 (mean ± SD, n = 5). * p<0.05 and ** p<0.01.
DISCUSSION
ER stress has been implicated in the pathogenesis of a broad range of diseases involving accumulation of unfolded or misfolded proteins in the ER [9,10,25]. Interestingly, recent evidence suggests that ER stress is also a possible cause of inflammation [14,15,29]. Several studies demonstrated that ER stress upregulated expression of inflammatory cytokines, such as TNF-α, in various cultured cell lines [13,15,16]. In the present study, we demonstrated that ER stress is elevated in the retina of animal models of diabetes and OIR, two major diseases causing BRB breakdown and retinal vascular leakage. Furthermore, we showed that induction of ER stress by tunicamycin is sufficient to elicit retinal inflammatory gene expression, and moreover, inhibition of ER stress by chemical chaperone PBA significantly attenuated inflammatory cytokine VEGF and TNF-α expression in cultured primary human retinal endothelial cells and in the retina of Akita mice and OIR mice. These results provide the first evidence that ER stress contributes to retinal inflammation in diabetic retinopathy.
The Akita mouse model is a commonly used genetic model of type 1 diabetes. Several features of non-proliferative diabetic retinopathy have been observed in the retinas of Akita mice, including vascular leakage and formation of acellular capillaries [24]. In keeping, the expression of VEGF and TNF-α were significantly increased in the Akita retinas (Fig. 1). Increased expression of VEGF and TNF-α was also observed in the retinas with OIR (Fig. 2), a well-accepted model for proliferative diabetic retinopathy, which develops retinal ischemia and consequent aberrant new vessel growth in the retina. Interestingly, we found that multiple ER stress markers, including GRP78, phospho-IRE1α, and phosphor-eIF2α were significantly up-regulated in the retina of both animal models. Although the mechanisms remain elusive, we speculate that hypoxia is a possible cause of ER stress in the retina of diabetic and OIR mice. It is well accepted that intraretinal hypoxia is an important pathophysiological feature of diabetic retinopathy[28,30]. Supplement of inspired oxygen to patients with chronic diabetic macular edema significantly reduced retinal thickness, and the discontinuation of oxygen therapy caused increased thickening of the macula, suggesting that retinal hypoxia is involved in the development and maintenance of diabetic macular edema[31]. Remarkable intraretinal hypoxia has also been observed in OIR mice at P12 (not shown). In addition, there is overwhelming evidence showing that hypoxia induces expression of inflammatory cytokines, such as VEGF and TNF-α, which mediate endothelial cell injury and blood-retinal barrier breakdown in diabetic retinopathy[4,32,33]. Our results demonstrated that exposure of cultured primary human retinal endothelial cells to hypoxia induced significantly increased expression of GRP78, and phosphorylation of IRE1α and eIF2α (Fig. 4), suggesting a potent effect of hypoxia on inducing ER stress in retinal endothelial cells. Moreover, attenuation of ER stress by PBA significantly mitigated VEGF and TNF-α expression induced by hypoxia, indicating that ER stress is, at least in part, responsible for hypoxia-induced inflammatory cytokine expression in retinal endothelial cells.
In addition to hypoxia, a number of deleterious factors, such as oxidant generation, advanced glycation end product formation, growth factor overexpression, contribute to endothelial cell dysfunction in diabetes [32–35]. Among these factors, oxidative stress is another potential cause of ER stress in the diabetic retinas. Several studies demonstrated that various oxidants or oxidatively modified lipids induce ER stress in cultured endothelial cells and neurons [29,36,37]. Moreover, accumulation of misfolded proteins in the ER results in generation of reactive oxygen species[38], and deletion of C/EBP homologus protein (CHOP), a downstream effector of PERK-eIF-ATF4 pathway, significantly reduced oxidative stress and pancreatic β cell apoptosis[11]. These studies suggest that oxidative stress is closely associated with ER stress. We found that retinal expression of 3-nitrotyrosine (3-NT) is markedly increased in Akita mice (not shown), indicating enhanced production of peroxynitrite, a potent oxidant generated from the reaction of superoxide and nitric oxide in the diabetic retina. Significantly increased NADPH activity and lipid hydroperoxide production was also observed in the retina of OIR mice[39,40]. Thus, it would be great interest to pursue if and how oxidative stress interacts with ER stress and the UPR in diabetic retinopathy in future studies.
Taken together, our study demonstrated that ER stress is activated in the retina and retinal endothelial cells under diabetic and hypoxic conditions. At least two UPR branches are involved in ER stress response and may contribute to inflammation in diabetic retinopathy. Further studies are warranted to investigate which UPR pathway(s) is responsible for the regulation of inflammatory response in diabetic retinopathy.
Supplementary Material
Acknowledgments
The authors thank Dr. Jian-xing Ma for helpful discussion and the Diabetes COBRE Histology Core Facility at OUHSC for the assistance in immunohistochemical study. This study was supported by NIH grant P20RR024215, JDRF grants 5-2007-793 and 18-2007-860, and a research award from OCAST. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Abbreviations
- ATF
activating transcription factor
- eIF
eukaryotic initiation factor
- ER
endoplasmic reticulum
- GRP 78
glucose regulated protein 78
- HREC
human retinal endothelial cells
- IRE1α
inositol-requiring enzyme 1α
- PBA
4-phenyl butyric acid
- PERK
PKR-like ER kinase
- OIR
oxygen-induced retinopathy
- TNF-α
tumor necrosis factor-α
- UPR
unfolded protein response
- VEGF
vascular endothelial growth factor
- XBP1
X-box binding protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frank RN. Diabetic retinopathy New England. Journal of Medicine. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 2.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joussen AM, et al. Suppression of diabetic retinopathy with angiopoietin-1.[comment] American Journal of Pathology. 2002;160:1683–93. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma J-x. Pigment epithelium-derived factor (PEDF) is an endogenous anti-inflammatory factor. FASEB J. 2006;20:323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 5.Mitamura Y, Takeuchi S, Matsuda A, Tagawa Y, Mizue Y, Nishihira J. Monocyte chemotactic protein-1 in the vitreous of patients with proliferative diabetic retinopathy. Ophthalmologica. 2001;215:415–418. doi: 10.1159/000050900. [DOI] [PubMed] [Google Scholar]
- 6.Ogata N, Nishikawa M, Nishimura T, Mitsuma Y, Matsumura M. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. American Journal of Ophthalmology. 2002;134:348–53. doi: 10.1016/s0002-9394(02)01568-4. [DOI] [PubMed] [Google Scholar]
- 7.Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB Journal. 2002;16:438–40. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 8.Ishida S, et al. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44:2155–2162. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- 9.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 10.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gargalovic PS, et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 13.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–62. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargalovic PS, et al. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc Natl Acad Sci U S A. 2006;103:12741–12746. doi: 10.1073/pnas.0605457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, Townes TM, Abcouwer SF. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279:14844–14852. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- 17.Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 2005;280:20331–20339. doi: 10.1074/jbc.M411275200. [DOI] [PubMed] [Google Scholar]
- 18.Malabanan KP, Kanellakis P, Bobik A, Khachigian LM. Activation transcription factor-4 induced by fibroblast growth factor-2 regulates vascular endothelial growth factor-A transcription in vascular smooth muscle cells and mediates intimal thickening in rat arteries following balloon injury. Circ Res. 2008;103:378–87. doi: 10.1161/CIRCRESAHA.107.168682. [DOI] [PubMed] [Google Scholar]
- 19.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 20.Zhang SX, Sima J, Wang JJ, Shao C, Fant J, Ma JX. Systemic and periocular deliveries of plasminogen kringle 5 reduce vascular leakage in rat models of oxygen-induced retinopathy and diabetes. Curr Eye Res. 2005;30:681–689. doi: 10.1080/02713680590934102. [DOI] [PubMed] [Google Scholar]
- 21.Zhang SX, Sima J, Shao C, Fant J, Chen Y, Rohrer B, Gao G, Ma J-x. Plasminogen kringle 5 reduces vascular leakage in the retina of rat model of the oxygen-induced retinopathy and diabetes. Diabitologia. 2004;47:124–131. doi: 10.1007/s00125-003-1276-4. [DOI] [PubMed] [Google Scholar]
- 22.Tirosh B, Iwakoshi NN, Glimcher LH, Ploegh HL. Rapid turnover of unspliced Xbp-1 as a factor that modulates the unfolded protein response. J Biol Chem. 2006;281:5852–5860. doi: 10.1074/jbc.M509061200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang SX, Wang JJ, Gao G, Parke K, Ma JX. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37:1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- 24.Barber AJ, et al. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–8. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 25.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 27.Zhang SX, Ma JX, Sima J, Chen Y, Hu MS, Ottlecz A, Lambrou GN. Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am J Pathol. 2005;166:313–321. doi: 10.1016/S0002-9440(10)62255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, Stitt AW. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci. 2006;47:5561–5568. doi: 10.1167/iovs.06-0647. [DOI] [PubMed] [Google Scholar]
- 29.Sanson M, et al. Oxidized Low-Density Lipoprotein Triggers Endoplasmic Reticulum Stress in Vascular Cells. Prevention by Oxygen-Regulated Protein 150 Expression. Circ Res. 2008 Dec 23; doi: 10.1161/CIRCRESAHA.108.183749. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Linsenmeier RA, Braun RD, McRipley MA, Padnick LB, Ahmed J, Hatchell DL, McLeod DS, Lutty GA. Retinal hypoxia in long-term diabetic cats. Investigative Ophthalmology & Visual Science. 1998;39:1647–57. [PubMed] [Google Scholar]
- 31.Nguyen QD, Shah SM, Van Anden E, Sung JU, Vitale S, Campochiaro PA. Supplemental oxygen improves diabetic macular edema: a pilot study. Invest Ophthalmol Vis Sci. 2004;45:617–624. doi: 10.1167/iovs.03-0557. [DOI] [PubMed] [Google Scholar]
- 32.Zhang SX, Ma JX. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog Retin Eye Res. 2007;26:1–37. doi: 10.1016/j.preteyeres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Braun L, Kardon T, Reisz-Porszasz ZS, Banhegyi G, Mandl J. The regulation of the induction of vascular endothelial growth factor at the onset of diabetes in spontaneously diabetic rats. Life Sci. 2001;69:2533–42. doi: 10.1016/s0024-3205(01)01327-3. [DOI] [PubMed] [Google Scholar]
- 34.Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116:73–79. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 35.Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007:43603. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhoták S, Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- 37.Lange PS, Chavez JC, Pinto JT, Coppola G, Sun CW, Townes TM, Geschwind DH, Ratan RR. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J Exp Med. 2008;205:1227–1242. doi: 10.1084/jem.20071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity . Mol Vis. 2007;13:840–53. [PMC free article] [PubMed] [Google Scholar]
- 40.Saito Y, Uppal A, Byfield G, Budd S, Geisen P, Hartnett ME, Saito Y, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008;49:1591–1598. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.