Abstract
Implicit learning is thought to underlie the acquisition of many skills including reading. Previous research has shown that some forms of implicit learning are reduced in individuals with dyslexia (e.g., sequence learning) whereas other forms are spared (e.g., spatial context learning). However, it has been proposed that dyslexia-related motor dysfunction may have contributed to the implicit sequence learning deficits reported earlier. To assess implicit sequence learning in the absence of a motor sequence, 16 young adults diagnosed with dyslexia (20.6 ± 1.5 years) and 18 healthy controls (20.8 ± 2.0 years) completed a triplet frequency learning task (TRIP) that involved learning a sequential regularity in which the location of certain events followed a repeating pattern but motor responses did not. Participants also completed the spatial contextual cueing task (SCCT), which involved learning a spatial regularity in which the location of distractors in some visual arrays predicted the target location. In addition, neuropsychological tests of real-word and pseudo-word reading were administered. TRIP task analyses revealed no between-group differences in pattern learning, but a positive correlation between individual learning scores and reading ability indicated that poor readers learned less well than did good readers. Thus, earlier reports of reduced implicit sequence learning in dyslexics cannot be entirely accounted for by motor sequencing deficits. No significant correlations or group differences in learning were found for SCCT. These findings offer additional evidence for a link between poor reading and impaired implicit sequence learning.
Keywords: implicit learning, sequence learning, spatial context learning, dyslexia, reading ability
INTRODUCTION
Developmental dyslexia is characterized by low reading achievement despite normal intelligence and ample education or learning opportunity (DSM-IV-TR, 2000). The reading weakness is typically attributed to phonological processing deficits whereby individuals with dyslexia have difficulty learning associations between how words appear in print (graphemes) and how they sound (phonemes) (Bradley & Bryant, 1983; Ramus et al., 2003; Snowling, 2001). However, this explanation does not account for the wide range of sensory, cognitive, and motor deficits also observed in dyslexia (Habib, 2000; Ramus et al., 2003).
An alternative theory is that deficits associated with dyslexia are due to underlying impairments in skill learning (e.g., Nicolson & Fawcett, 1990; Rudel, 1985). This view is in line with dyslexia technically being classified as a learning disorder (DSM-IV-TR, 2000). Skills, such as reading, can be acquired through both explicit and implicit processes. For example, grapheme-phoneme associations are initially learned through explicit memorization, but implicit rule-based decoding skill evolves through repeated exposures to regularly formed words (Gombert, 2003; Uhry & Clark, 2005). Research has shown that explicit, effortful forms of skill learning are spared in poor readers and dyslexics (Sperling, Lu, & Manis, 2004; Vicari et al., 2003, Exp 2) whereas studies of implicit learning have revealed a mixed pattern of spared (Howard et al., 2006; Kelly, Griffiths, & Frith, 2002; Russeler, Gerth, & Munte, 2006) and impaired (Howard et al., 2006; Sperling et al., 2004; Vicari et al., 2005) learning.
Implicit learning occurs in a wide range of tasks that reveal non-conscious, unintentional sensitivity to regularities among stimuli (Seger, 1994). Studies have shown that these tasks not only differ in the nature of the regularity present but also in the underlying neural systems they engage (Forkstam & Petersson, 2005; Stadler & Frensch, 1997). For example, implicit sequence learning tasks examine the learning of sequential regularities. In the serial reaction time task (SRTT) (Nissen & Bullemer, 1987), participants respond faster and more accurately to visual stimuli that follow a repeating sequence of locations versus stimuli that occur at randomly determined locations. On the other hand, implicit spatial context learning tasks investigate how spatial regularities are learned. In the spatial contextual cueing task (SCCT) (Chun & Jiang, 1998), search performance is better for visual arrays in which the configuration of distractors predicts the location of a target compared to non-predictive arrays. Sequence learning tasks such as the SRTT have been shown to rely on a frontal-striatal-cerebellar network whereas spatial context learning in the SCCT is mediated by medial temporal lobe structures, specifically the hippocampus and/or parahippocampal cortex (Chun & Phelps, 1999; Greene et al., 2007; Prull, Gabrieli, & Bunge, 2000).
One study by Howard, and colleagues (2006) found a dissociation between these two forms of implicit learning in the same group of young adults with dyslexia. Sequence learning was measured with a modified version of the SRTT, the alternating serial reaction time task (ASRT) (Howard & Howard, 1997), in which stimuli that follow a repeating sequence of locations (pattern) alternate with randomly determined stimuli (random). Analyses showed that the dyslexic group learned significantly less than age-matched controls on the ASRT task as determined by faster and more accurate responses to pattern versus random stimuli. But on the SCCT, there was no group difference in spatial context learning. In addition, correlations between reading ability and implicit learning revealed that poor reading ability was associated with reduced sequence learning, but preserved spatial context learning. These outcomes are consistent with data indicating that differences in brain function associated with dyslexia overlap with the neural systems involved in implicit sequence learning but not with those involved in implicit spatial context learning. That is, compared to controls, individuals with dyslexia show reduced activation in inferior frontal and left temporal-parietal areas during language-based tasks (Collins & Rourke, 2003; Habib, 2000). Other imaging studies find that dyslexics have abnormal structure and function of the cerebellum (Eckert et al., 2003; Finch, Nicolson, & Fawcett, 2002; Menghini et al., 2006; Nicolson et al., 1999; Rae et al., 2002) and striatum (Brown et al., 2001). Together these results suggest that frontal-striatal-cerebellar regions are affected in dyslexia. In contrast, abnormalities in medial temporal lobe structures are not known to be characteristic of dyslexia.
Implicit sequence learning deficits, like those observed by Howard and colleagues in 2006, have been reported in multiple studies of children and young adults with dyslexia (Menghini et al., 2006; c.f. Russeler et al., 2006; Stoodley, Harrison, & Stein, 2006; Vicari et al., 2005; Waber et al., 2003). However, all of these studies used tasks in which a motor sequence was present. This is potentially important because there is evidence that dyslexic individuals have problems executing sequential motor movements. For example, deficient planning and timing of the sequential motor plans involved in speech production have been proposed to underlie dyslexia-related difficulties in repeating series of syllables and reading complex phrases (Catts, 1989; Wolff, Cohen, & Drake, 1984; Wolff, Michel, & Ovrut, 1990a). Non-linguistic tasks also reveal motor sequencing problems in dyslexia. For instance, poor readers perform worse on finger tapping tasks when they require alternating movements between hands versus repeating them with a single finger (Wolff et al., 1990b), and dyslexic children demonstrate inferior coordination of movements during copying tasks (Denckla, 1985). Therefore, the question arises as to whether such motor sequencing deficits may have been the sole source of impairment reported in the earlier implicit sequence learning studies.
The present study was designed to assess implicit sequence learning in the absence of motor sequencing in young adults with and without dyslexia using a variation of the ASRT that contained no motor sequence (Howard et al., submitted; Howard et al., 2004). The same individuals completed the SCCT, which also does not require participants to use motor sequencing. In addition to examining group differences, correlation analyses were performed using measures of the two types of implicit learning and reading ability because previous studies have indicated that continuous measures of reading skill may be more sensitive than the dichotomous group variable (Conlon, Sanders, & Zapart, 2004; Howard et al., 2006; Shaywitz et al., 1992). It was expected that if earlier reports of reduced implicit sequence learning in poor readers and individuals with dyslexia were solely due to motor sequencing deficits, then there should be no group differences on the non-motor sequence learning task and no relationship between non-motor sequence learning and reading ability. However, if the deficits were due at least in part to a more general sequencing deficit, then the dyslexic group should learn less than the non-dyslexic group in the present task, and poor reading should be associated with less learning. For spatial context learning, it was expected that there would be no group differences in learning and no relationship between measures of learning and reading ability, indicating preserved spatial context learning in young adult poor readers.
METHODS
Participants
Participants were 16 individuals with dyslexia and 18 non-dyslexic controls. Inclusion criteria for all participants were being an undergraduate student at the Catholic University of America between the ages of 18 and 25 years. Participants in the dyslexic group met criteria for a diagnosis of dyslexia according to the Catholic University Disability Support Services Office, which was based on current and comprehensive neuropsychological evaluations and interviews with qualified professionals. Individuals were excluded from the dyslexic group if they also met criteria for dysgraphia, or had an unspecified learning disorder. Participants in the control group had no history of learning disability. Table 1 presents demographic and neuropsychological characterizations for each group. Participants received either payment or course credit and gave informed consent for experimental procedures approved by The Catholic University of America Institutional Review Board.
Table 1.
Demographics and neuropsychological test results
| Dyslexic (n=16) | Control (n=18) | t | |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Age | 20.6 ± 1.5 | 20.8 ± 2.0 | ns |
| Male/Female | 4/12 | 5/13 | |
| NEUROPSYCHOLOGICAL TESTS | |||
| WASI Two-Subtest IQ SS | 109.8 ± 10.5 | 115.6 ± 9.1 | ns |
| WJ-III Word Identification SS | 92.0 ± 8.2 | 103.5 ± 6.8 | 4.1** |
| WJ-III Word Attack SS | 87.1 ± 10.0 | 98.8 ± 10.7 | 3.6* |
| WJ-III Spelling SS | 93.4 ± 10.4 | 110.2 ± 7.4 | 5.4** |
| RAN average speed | 28.3 ± 3.7 | 23.9 ± 3.9 | −3.4* |
| RAN average errors | 1.8 ± 4.1 | 0.9 ± 0.7 | ns |
| WAIS-III Digit Symbol coding | 69.4 ± 16.0 | 85.1 ± 23.3 | 2.3* |
| WAIS-III Digit Symbol pairing | 14.8 ± 2.8 | 14.8 ± 3.2 | ns |
| WAIS-III Digit Symbol recall | 7.7 ± 1.4 | 7.8 ± 1.1 | ns |
| WAIS-III Digit Span forward | 9.8 ± 2.1 | 11.7 ± 2.1 | 2.7* |
| WAIS-III Digit Span backward | 6.6 ± 2.5 | 8.1 ± 2.1 | ns |
| Auditory Consonant Trigrams | 5.4 ± 2.9 | 8.1 ± 1.8 | 3.2** |
Notes. All scores are given as mean ± SD based on raw data, except where standard scores are noted. Independent sample t tests show group effects
p < .05
p < .001, ns = not significant.
WASI = Wechsler Abbreviated Scale of Intelligence; WJ-III = Woodcock-Johnson, 3rd edition; SS = age-adjusted standard score with a mean of 100 and standard deviation of 15; RAN = Rapid Automatized Naming; and WAIS-III = Wechsler Adult Intelligence Scale, 3rd edition.
General procedure
Participants completed 1-hour testing sessions on each of three separate days. On the first day, they signed an informed consent form and then completed the triplet task (TRIP). The spatial contextual cueing task (SCCT) was completed on the second day. Neuropsychological tests were administered on the third day. The three testing sessions were completed within a one week period for all participants, except one dyslexic whose testing spanned 12 weeks.
Triplets frequency learning task (TRIP)
For the TRIP task, participants viewed four black outlined open circles presented in a horizontal row on a 15 in. (38 cm) Apple iMac computer monitor. For purposes of description below, the four stimulus locations are referred to as 1, 2, 3, and 4; where 1 is the leftmost position and 4 is the rightmost position. However, numbers never appeared on the screen. Each trial, referred to as a triplet, was comprised of three discrete events, in which two circles filled in red, one after the other, and then one circle filled in green. Participants were instructed to respond to the location of the green event using the middle and index fingers of each hand to press the corresponding buttons (“z”, “x”, “/” and “.” on the keyboard). Each of the three events was presented for 120 ms with a 150 ms inter-stimulus interval. The green event remained on the screen until the correct response was made, and 650 ms later the next trial began. Participants completed 30, 50-trial blocks. They were encouraged to take short breaks after each block.
Like the ASRT (Howard et al., 2006), the TRIP task contained a second order sequential structure in which the location of every other stimulus follows a repeating pattern. As a result of this sequential regularity in the ASRT task, stimulus location on trial n predicts the location of trial n+2. For example, in the sequence 1r2r3r4r, numbers represent the predictable, alternating trials that follow the repeating pattern; with 1 through 4 denoting the location of the filled in circle, and the letter “r” referring to trials where the stimulus could occur at any of the four locations. The same sequential regularity occurred in the TRIP task, with the location of the first event within a triplet predicting the location of the third event. For example, in the triplet 1r2, where 1 and “r” refer to red events and 2 refers to the green event, the first red circle at location 1 predicts that the green circle will occur at location 2, with the location of the second red event being randomly determined. Thus, the triplet 1r2 is consistent with the repeating pattern 1r2r3r4r. In the ASRT, runs of three consecutive trials (i.e., triplets) that were consistent with the pattern (high frequency triplets) occurred 75% of the time, and pattern-inconsistent triplets (low frequency triplets) occurred 25% of the time. However, in this TRIP task, high frequency and low frequency triplets occurred 90% and 10% of the time, respectively.
Participants received feedback at the end of each block that was designed to direct responding to 92% accuracy. The feedback consisted of mean reaction time and mean accuracy for a given block, plus a statement prompting them to “focus more on accuracy” if their mean accuracy was below 90% or to “focus more on speed” if their mean accuracy was above 94%.
A recognition task and an interview were used to assess explicit knowledge of the sequential regularity. In a 24-trial recognition task (Howard et al., 2004), all possible combinations of triplet events were presented with the circles filled in black, not red and green. For each triplet, participants were instructed to indicate whether that combination of events occurred “frequently” or “infrequently” during the task by pressing one of two buttons. A post-experiment interview was also administered. Questions ranged from general inquiries about strategies used to improve performance and the frequency of events occurring at each location to more specific questions about the relationships between the red and green events, asking participants to describe the regularity if they noticed one.
Spatial contextual cueing task (SCCT)
Stimuli and procedures for the SCCT used here are identical to those described previously (Howard et al., 2006). Participants viewed visual arrays on a 15 in. (38 cm) Apple iMac computer monitor. Arrays were comprised of 11 distractors (the letter L rotated by 0, 90, 180, or 270 degrees) and one target (horizontal letter T) that were white against a gray background. Distractors were made more similar to the target by offsetting the L legs by 3 pixels (Chun & Phelps, 1999, Exp 2). On each trial, a fixation dot appeared for one second, followed by an array. Participants were instructed to search the array for the target and respond as quickly and accurately as possible by pressing ‘z’ if the tail of the horizontal T was pointing left or ‘/’ if it was pointing right, using their left and right index fingers, respectively. Auditory feedback after each response indicated correct (short beep) and incorrect (long tone) responses. If responses did not occur within six seconds, the trial ended with a long tone indicating an incorrect response, and the next trial began. Participants completed a practice block of 24 novel arrays, and then 30, 24-trial test blocks. They were encouraged to take short breaks after each block.
Twelve arrays repeated across all test blocks. In these familiar arrays, the locations of the distractors predicted the location of the target but not its orientation. Therefore, this regularity could not be used to predict the correct response. The remaining 12 arrays for each block were novel across the experiment. Within each block, presentation of familiar and novel arrays was randomized.
A recognition task and an interview were used to assess explicit knowledge of the spatial regularity. In a 24-trial recognition task (Chun & Jiang, 2003) the screen was divided into four quadrants by short lines placed at the midpoints of each side of the screen. The 12 familiar arrays from the test blocks and 12 novel arrays were presented with a distractor in place of the target location. For each array, participants were instructed to indicate which quadrant the target would most likely have occurred in by pressing one of four buttons on the keyboard. A post-experiment interview was also administered with questions ranging from general inquiries about the task and material to more specific questions asking participants if they noticed that certain displays repeated across trials.
Neuropsychological testing
A battery of neuropsychological tests was administered to characterize the cognitive profiles of each group. Some tests were later correlated with measures of implicit learning. An intelligence quotient (IQ) was calculated using two subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999): the Vocabulary subtest that involved defining orally presented words, and the Matrix Reasoning subtest that entailed identifying the missing piece of an abstract visual display. Real-word and pseudo-word reading were assessed with the Woodcock-Johnson (WJ-III) (Woodcock, McGrew, & Mather, 2001) Word Identification and Word Attack subtests, respectively, in which participants read aloud lists of English words or pronounceable nonsense words. The WJ-III Spelling subtest measured participants’ ability to write orally presented words. Rapid Automatized Naming (RAN) (Denckla & Rudel, 1974) assessed the speed of naming separate series of colors, objects, letters, and numbers. The Wechsler Adult Intelligence Scale (WAIS-III) (Wechsler, 1997) Digit Symbol Coding subtest measured hand-eye coordination and processing speed by giving participants two minutes to fill-in the symbols that corresponded to a series of digits. Cued-recall of the digit-symbol pairs and free recall of the symbols were assessed with the WAIS-III Digit Symbol Pairing and Recall subtests, respectively. Working memory was measured with WAIS-III Digit Span Forward and Backward, and Consonant Trigrams (Peterson & Peterson, 1959). The digit span task involved repeating progressively longer strings of numbers in the same (forward) or reverse (backward) order they were presented, and Consonant Trigrams required that participants recall three consonants after short intervals during which they performed a distracting counting task.
RESULTS
Demographics and neuropsychological data
Demographic information and neuropsychological test results are presented in Table 1. The dyslexic and control groups were matched on age and gender. Neuropsychological tests revealed a pattern characteristic of dyslexia in that both groups were of normal intelligence, but the dyslexic group performed significantly worse than the control group on measures of real-word and pseudo-word reading (WJ-III Word Identification and Word Attack subtests, respectively), spelling (WJ-III Spelling), naming speed (RAN average speed of the four stimulus sets), hand-eye coordination and processing speed (WAIS-III Digit Symbol Coding), and working memory (WAIS-III Digit Span Forward and Consonant Trigrams).
Implicit Learning Data: Group analyses
Implicit learning was examined in each task using separate repeated-measures ANOVAs for reaction time and accuracy measures. For each block, median reaction times on correct trials and mean accuracy scores were calculated for high frequency and low frequency triplets in the TRIP task and for familiar and novel arrays in the SCCT. A variable of epoch was then created by taking the mean of these scores across groups of five blocks.
Implicit sequence learning (TRIP)
To assess potential group differences in implicit sequence learning, group (dyslexic, control) X triplet type (high frequency, low frequency) X epoch (1–6) mixed design ANOVAs were conducted separately for reaction time and accuracy measures (see Figure 1).
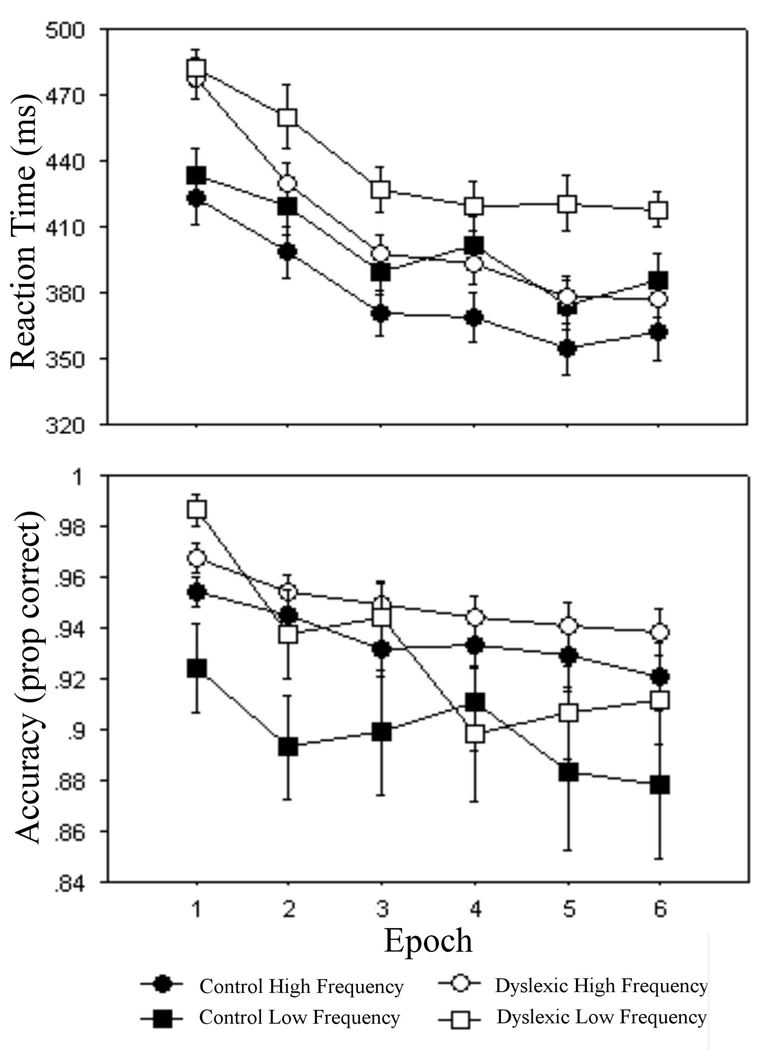
Figure 1.
For the TRIP task, responses to high frequency (circle) and low frequency (square) triplets for the control (closed) and dyslexic (open) groups on mean of median reaction time (ms) and mean accuracy (proportion correct) measures.
For reaction time, the dyslexic group (423.4 ± 51.8 ms) responded significantly slower than the control group (390.1 ± 54.6 ms), F(1, 32) = 5.9, p < .03. A main effect of epoch, F(5, 160) = 67.7, p < .001, and a group X epoch interaction, F(5, 160) = 2.5, p < .04, showed that overall speed increased more across epochs for the dyslexic group compared to controls. Sequence learning was revealed by significant effects of triplet type, F(1, 32) = 112.9, p < .001, and triplet type X epoch, F(5, 160) = 4.4, p < .001, with faster responses to high frequency (393.4 ± 54.0 ms) versus low frequency (418.1 ± 54.8 ms) triplets, a difference that increased across epochs. Group X triplet type and group X triplet type X epoch interactions were not significant, p’s > .10, indicating that we did not detect group differences in sequence learning.
For accuracy, the dyslexic group (94.0 ± 5.9 %) did not differ from the control group (91.7 ± 8.0 %), p > .15, showing that as intended, the feedback provided after every block successfully matched the groups on overall accuracy. A significant main effect of epoch, F(5, 160) = 7.2, p < .001, showed that accuracy decreased across epochs, which is typical in sequence learning tasks as participants make increasingly more errors on pattern-inconsistent trials as they learn the regularity. Sequence learning was seen as a main effect of triplet type, F(1, 32) = 13.6, p < .001, with more accurate responses to high frequency (94.2 ± 4.0 %) versus low frequency (91.4 ± 9.2 %) triplets. In line with the reaction time results, group differences in sequence learning were not observed in either the group X triplet type or group X triplet type X epoch interactions, p’s > .21.
Implicit spatial context learning (SCCT)
To assess implicit spatial context learning, group (dyslexic, control) X array type (familiar, novel) X epoch (1–6) mixed design ANOVAs were conducted for both behavioral measures (see Figure 2). One control participant was not included in this analysis because the data were lost due to a computer error.
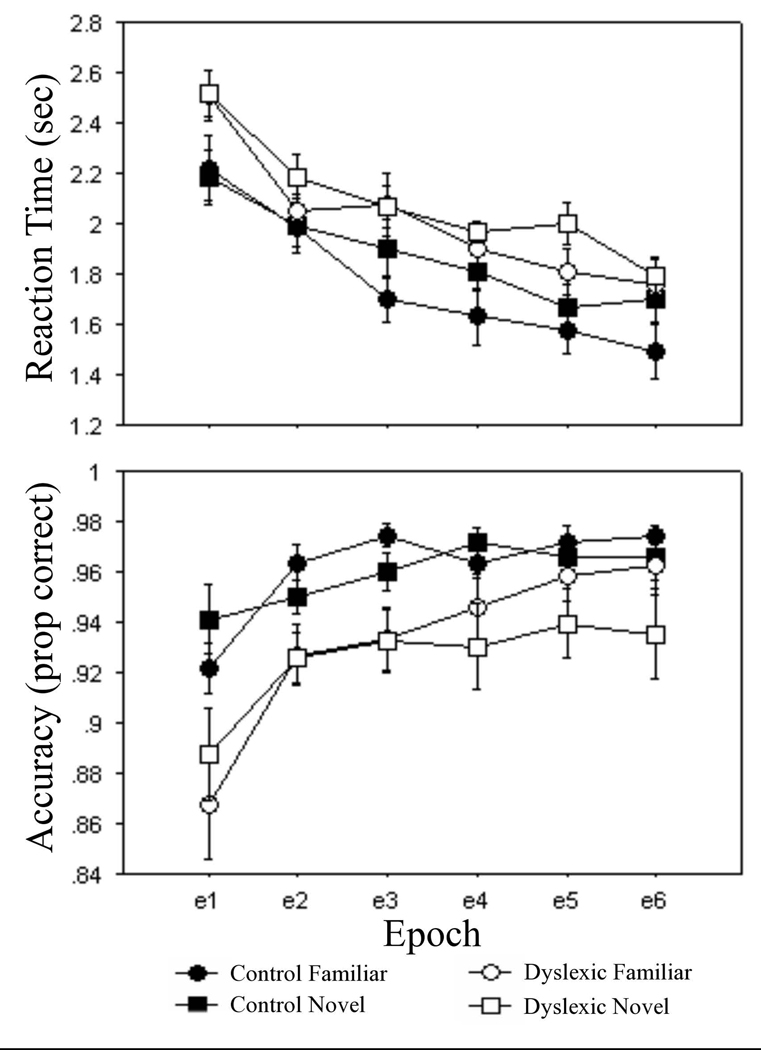
Figure 2.
For the SCCT, responses to familiar (circle) and novel (square) arrays for the control (closed) and dyslexic (open) groups on mean of median reaction time (sec) and mean accuracy (proportion correct) measures.
For reaction time, responses were significantly slower in the dyslexic group (2.1 ± 0.4 sec) compared to the control group (1.8 ± 0.5 sec), F(1, 31) = 4.8, p < .04. There was a significant main effect of epoch, F(5, 155) = 64.8, p < .001, with responses speeding up across epochs. Spatial context learning was revealed by faster responses to familiar arrays (1.9 ± 0.5 sec) compared to novel arrays (2.0 ± 0.4 sec), F(1, 31) = 4.3, p < .05. There were no significant group differences in learning (group X array type, p > .68; group X array type X epoch, p > .06). However, the marginal three-way interaction was followed up with separate array type X epoch ANOVAs for each group that revealed significant learning in the control group (array type, F(1, 16) = 5.1, p < .04; array type X epoch, F(5, 80) = 2.9, p < .03) but not in the dyslexic group (p’s > .34).
For accuracy, the dyslexic group (92.9 ± 6.2 %) made significantly more errors than the control group (96.0 ± 3.5 %), F(1, 31) = 6.9, p < .02. A main effect of epoch, F(5, 155) = 22.1, p < .001, showed that accuracy increased across epochs. The array type effect did not attain significance, p > .19, but spatial context learning was seen with a significant array type X epoch interaction, F(5, 155) = 3.3, p < .01. Group X array type and group X array type X epoch interactions were not significant, p’s > .29, indicating that there were no group differences in learning.
Correlations between implicit learning and reading ability
As indicated earlier, it is important to conduct correlations between individual measures of reading ability and implicit learning because these analyses may better capture relationships with reading skill, especially when there is a broad range of reading ability within both groups, and an overlap between the groups. Learning scores for each behavioral measure were calculated for each participant by taking the difference, in the final epoch, between high frequency versus low frequency triplets for the TRIP task and familiar versus novel arrays for the SCCT. Real-word and pseudo-word reading were measured for each participant using age-adjusted standard scores from the WJ-III Word Identification and Word Attack subtests.
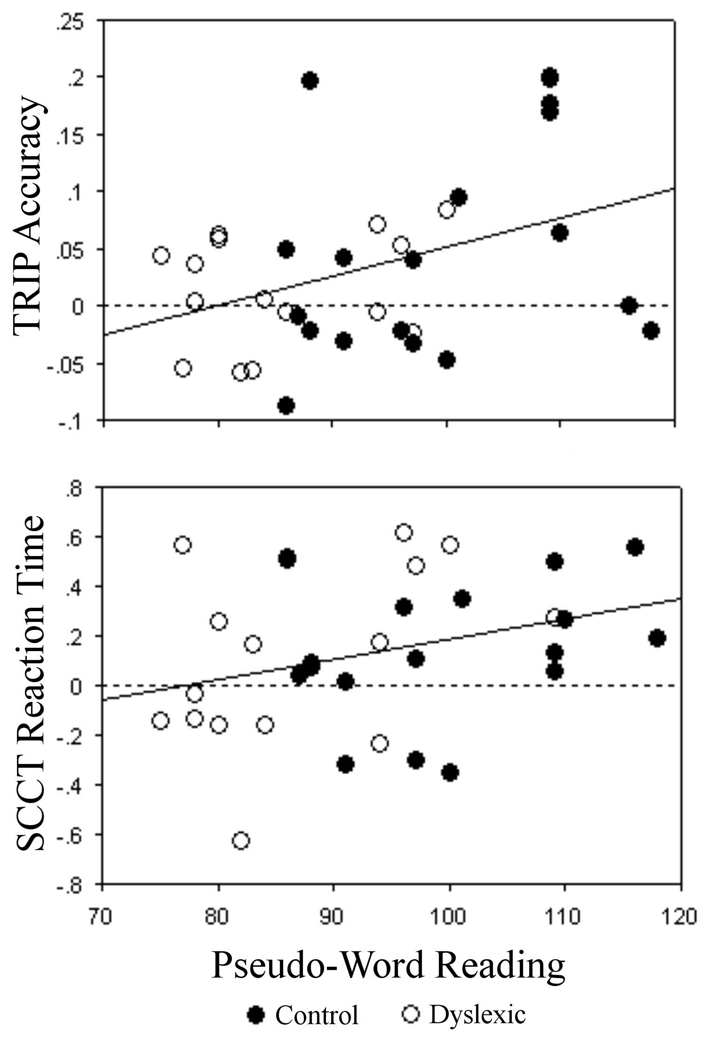
A significant positive correlation was observed for the TRIP accuracy learning score and pseudo-word reading, r = +.38, p < .03. Thus, although there were no group differences in sequence learning, this correlation shows that poor reading is associated with reduced sequence learning. There was also a non-significant trend, r = +.31, p < .09, for poor pseudo-word reading to be associated with lower SCCT reaction time learning scores, which is in line with the group analyses that revealed spatial context learning on the reaction time measure in the control group but not the dyslexic group. Scatter plots for these correlations are presented in Figure 3. Correlations between implicit learning scores and real-word reading were in the same direction as above, but they did not attain significance, p’s > .40.
Figure 3.
Scatter plots separately comparing learning scores for the TRIP task (accuracy) and SCCT (reaction time) to the measure of pseudo-word reading (age-scaled score) for the control (closed circle) and dyslexic (open circle) groups.
Correlations between implicit learning and rapid naming
Because of the similarities between sequential processing in implicit sequence learning and rapid naming (e.g., sequential eye movements, processing sequentially presented stimuli, and executing sequential motor responses), correlations were also conducted between TRIP learning scores and measures of average speed and average errors from the four stimulus sets of the RAN. In line with previous studies that show poor reading is associated with worse performance on the RAN (Denckla & Rudel, 1976; Wolf, Bowers, & Biddle, 2000), a significant negative correlation was seen between pseudo-word reading and average RAN speed, r = −.44, p < .01. More importantly, results revealed a significant negative correlation between RAN speed and the TRIP accuracy learning score, r = −.39, p < .03, indicating that individuals who performed more slowly on the rapid naming task also learned less on the TRIP task.
Measures of Implicitness
Following the TRIP task, participants completed a recognition task in which they judged the frequency of all combinations of triplet events. Mean recognition ratings were calculated for each participant as the proportion of times high frequency and low frequency triplets were reported as having occurred “frequently” during the task. A group (dyslexic, control) X triplet type (high frequency, low frequency) mixed design ANOVA showed no significant effects (p’s > .14), indicating that high frequency (.42 ± .14) and low frequency (.45 ± .08) triplets were rated as occurring equally often in both the dyslexic and control groups. Similarly, analyses of the post-experiment interview did not reveal any explicit awareness. Three dyslexic and six control participants indicated that they noticed a relationship between either of the two red events and the third green event, but no individual accurately described the nature of the regularity.
After the SCCT, participants completed a recognition task that involved viewing arrays in which the targets were replaced by distractors and indicated in which quadrant the target would most likely have occurred. Mean accuracy scores were calculated for each participant for familiar and novel arrays. A group (dyslexic, control) X array type (familiar, novel) mixed design ANOVA revealed no significant effects (p’s > .08). That is, target quadrants were recognized equally often for familiar and novel arrays for both groups. Thus, as for the TRIP task, analyses of the post-experiment interviews did not reveal any explicit awareness of the spatial regularity. Eight dyslexic and five control participants indicated that they noticed certain displays repeated across trials. However, none of them reported explicitly trying to memorize the displays to facilitate performance.
Taken together, recognition tests and post-experiment interviews for both the TRIP and SCCT indicated that no participant had explicit awareness of either regularity even though they responded faster and/or more accurately to high frequency versus low frequency triplets and familiar versus novel arrays.
DISCUSSION
This study examined the relationship between two forms of implicit learning (sequence learning and spatial context learning) and reading ability in young adults with and without dyslexia. Implicit sequence learning was assessed in the absence of motor sequencing using the TRIP task to determine whether motor sequencing deficits could be the sole source of sequence learning impairment previously seen in poor readers and individuals with dyslexia (Howard et al., 2006; Menghini et al., 2006; Stoodley et al., 2006; Vicari et al., 2005). In contrast to previous studies that used motor sequence learning tasks, between-group analyses revealed no group differences in learning. However, in line with those studies, pseudo-word reading was positively correlated with the TRIP accuracy learning score, indicating that poor readers had sequence learning deficits even when there was no motor sequence to be learned. Implicit spatial context learning, measured with the SCCT task, revealed no group differences in learning as previously reported (Howard et al., 2006), and there were no significant correlations between spatial context learning and reading ability. Unlike some previous studies that could not rule out explicit learning, results observed here must be due to implicit learning because there was no evidence of explicit awareness in either the TRIP or SCCT task, as assessed via recognition tests and verbal reports. The findings are discussed in more detail below.
Implicit sequence learning
A significant positive correlation between pseudo-word reading and TRIP learning on the accuracy measure was found in the current study. The analogous correlation was also observed in a study by Howard and colleagues (2006), using the ASRT learning task. This suggests that poor (pseudo-word) readers are relatively worse at sequence learning whether or not a motor sequence is present, because the ASRT task contained a sequence of motor responses but the TRIP task does not. Thus, earlier reports of sequence learning deficits (Howard et al., 2006; Menghini et al., 2006; Stoodley et al., 2006; Vicari et al., 2005) cannot be explained by motor sequencing deficits alone. Instead, implicit sequence learning deficits in poor readers are likely due, at least in part, to difficulty learning other forms of sequential information that are present in both the TRIP and ASRT tasks, such as the perceptual sequence of stimuli and/or the sequence of eye movements to fixate the target stimuli (Goschke, 1998; Mayr, 1996; Seger, 1997).
In fact, there is existing evidence that poor readers have perceptual sequence learning deficits. For example, one study found significantly less learning in dyslexics versus controls using a task that contained a perceptual sequence but not motor response or eye movement sequences (Vicari et al., 2003). Perceptual sequence learning deficits are predicted by the temporal processing theory, which states that individuals with dyslexia are poorer at integrating sequential sensory information, especially when stimuli are presented rapidly (Habib, 2000). This theory developed from work showing that children and young adults with dyslexia have difficulty on tasks that involve processing rapid sequences of auditory (Helenius, Uutela, & Hari, 1999; Tallal, 1980) and visual (Conlon et al., 2004; Eden et al., 1995) stimuli. Other explanations have been proposed for the perceptual sequencing deficits in dyslexia, including deficient sensory processing in the magnocellular pathway (Stein, 2001), delayed attention shifting (Hari & Renvall, 2001), and limited perceptual memory (Ben-Yehudah et al., 2001). According to the latter explanation, individuals with dyslexia have a limited capacity to retain perceptual traces and compare them across short intervals. In the TRIP task, learning involves retaining perceptual traces across the three events within a trial, binding these events into a triplet, and then comparing triplets across trials. Thus, an inability to retain and compare perceptual traces could manifest as implicit sequence learning deficits in poor readers.
It could also be argued that difficulty learning the sequence of eye movements to fixate the targets contributes to sequence learning deficits in poor readers. However, support for this view is hard to establish because there are conflicting results from examinations of oculomotor control in poor readers. Some studies report that individuals with dyslexia make more eye movements and have difficulty maintaining fixation compared to controls (Biscaldi, Gezeck, & Stuhr, 1998; Eden et al., 1994; Pavlidis, 1981) whereas others find no group differences (Black et al., 1984; Olson, Kliegl, & Davidson, 1983; Stanley, Smith, & Howell, 1983). If present, abnormal eye movements in poor readers would affect their ability to fixate the targets during sequence learning tasks, which may influence their perception of the sequential stimuli, and ultimately affect their sequence learning performance. In either case, the TRIP task does not allow us to separate perceptual and eye movement sequencing deficits in poor readers
Sequencing deficits in poor readers have also been observed in analyses of the rapid automatized naming task (RAN). Although the RAN is usually thought of as a measure of phonological retrieval (Wagner & Torgesen, 1987) or processing speed (Wolf & Bowers, 1999), the nature of the task suggests that sequential processing is also involved. Stimuli for the RAN include separate cards that display series of colored squares, objects, letters, and numbers. Participants are instructed to name the items on each card out loud as fast as possible. Thus, successful completion of the task involves making sequential eye movements to fixate the items, processing the perceptual sequence of stimuli, and then executing the speech-related sequence of motor movements. Results from both group analyses and correlations between pseudo-word reading and RAN average speed replicated previous findings of reduced rapid naming ability in poor readers (Denckla & Rudel, 1976; Wolf et al., 2000). Interestingly, slow naming was significantly associated with less learning on the TRIP accuracy learning score, supporting the notion that both RAN and TRIP tasks reflect a sequencing deficit in poor readers.
The relationship between implicit sequence learning, reading ability, and speech production makes sense from an evolutionary perspective. According to this view, language skills such as speech and reading may have evolved from similar but more basic processes like sequence learning. Speech production involves sequential motor movements and is therefore thought to have adapted from neural networks involved in motor sequencing (Lieberman, 2002). Similarly, reading, an even more recent sequential skill than speech, may have evolved from more primitive sequencing functions (Dehaene, 2004). This perspective is strengthened by the fact that these three processes rely on similar neural structures including frontal regions, cortical motor areas, the striatum, and the cerebellum (Bohland & Guenther, 2006; Fiez & Petersen, 1998; Lieberman, 2002; Riecker et al., 2005; Wildgruber, Ackerman, & Grodd, 2001).
When dyslexic and control groups were combined, both the present TRIP study and a previous study that employed the ASRT (Howard et al., 2006) found positive correlations between reading ability (pseudo-word reading) and implicit sequence learning. However, unlike the previous study, the current study did not find significant group differences in learning. Interpreting the between-group effects is complicated because, in addition to differences in the presence (ASRT) or absence (TRIP) of motor sequencing, the TRIP and ASRT tasks differ in ways that do not allow for direct comparison across tasks. For example, although both tasks had the same structural complexity (i.e., event n+2 is predicted by event n), the ratio of high frequency triplets to low frequency triplets was higher in the TRIP task (9:1) compared to the ASRT task (3:1), perhaps making the TRIP task easier to learn. The TRIP task also isolated the triplets from the sequence by presenting each triplet as a discrete trial, whereas triplets in the ASRT task occur in a continuous overlapping stream. Nonetheless, because correlation analyses revealed similar effects for reading ability across studies, it suggests that, as previously indicated by others (Conlon et al., 2004; Howard et al., 2006; Shaywitz et al., 1992), our understanding of this heterogeneous disorder might be clarified if future research includes correlation analyses that treat reading ability as a continuous variable in addition to group analyses that treat reading skill as a dichotomous variable based on diagnoses of dyslexia.
Implicit spatial context learning
Results for implicit spatial context learning in this study were only partially consistent with our predictions based on an earlier examination of spatial context learning in young adults with dyslexia (Howard et al., 2006). As expected, there were no significant group differences in learning on the SCCT. However, there was a trend for a positive correlation between pseudo-word reading and SCCT learning on the reaction time measure, whereas a negative correlation had been reported previously (Howard et al., 2006). Direct comparisons of the results from these two studies is complicated because in the present study, but not in the earlier study, the dyslexic group was significantly less accurate than the control group. In addition, none of the group comparisons or correlations between reading ability and SCCT learning in the present study is significant, revealing only marginal trends. Thus, results from both studies suggest that implicit spatial context learning is preserved in poor readers.
Future considerations
Additional research is necessary to clarify the impaired and spared implicit learning abilities in poor readers and individuals diagnosed with dyslexia. Deficits have been reported for sequence learning (Howard et al., 2006; Menghini et al., 2006; Stoodley et al., 2006; Vicari et al., 2005), mirror drawing (Vicari et al., 2005), and categorical learning (Sperling et al., 2004). However, other forms of implicit learning, such as spatial context learning (Howard et al., 2006) and some types of artificial grammar learning (Pothos & Kirk, 2004; Russeler et al., 2006), appear to be relatively spared. This information may be relevant to training programs that could improve reading fluency in dyslexia by either targeting underlying deficits in implicit learning or by capitalizing on intact implicit learning processes.
Acknowledgments
This research was funded by grant R37 AG15450 from the National Institute on Aging/National Institutes of Health. The authors want to thank Carly Shoupe, Elizabeth Bonner, Tara Kraft, Richard Garlipp, and Lauren Mays for their help with data collection. Preliminary findings from this project were presented at the 25th Rodin Remediation Academy Conference in Washington, DC in October 2006.
REFERENCES
- Ben-Yehudah G, Sackett E, Malchi-Ginzberg L, Ahissar M. Impaired temporal contrast sensitivity in dyslexics is specific to retain-and-compare paradigms. Brain. 2001;124:1381–1395. doi: 10.1093/brain/124.7.1381. [DOI] [PubMed] [Google Scholar]
- Biscaldi M, Gezeck S, Stuhr V. Poor saccadic control correlates with dyslexia. Neuropsychologia. 1998;36(11):1189–1202. doi: 10.1016/s0028-3932(97)00170-x. [DOI] [PubMed] [Google Scholar]
- Black JL, Collins DW, De Roach JN, Zubrick S. A detailed study of sequential saccadic eye movements for normal- and poor-reading children. Perceptual and Motor Skills. 1984;59(2):423–434. doi: 10.2466/pms.1984.59.2.423. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32(2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Bradley L, Bryant PE. Categorizing sounds and learning to read- a causal connection. Nature. 1983;301:419–421. [Google Scholar]
- Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL. Preliminary evidence of widespread morphological variations in the brain in dyslexics. Neurology. 2001;56(6):781–783. doi: 10.1212/wnl.56.6.781. [DOI] [PubMed] [Google Scholar]
- Catts HW. Speech production deficits in developmental dyslexia. Journal of Speech and Hearing Disorders. 1989;54(3):422–428. doi: 10.1044/jshd.5403.422. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Contextual cueing: Implicit learning and memory of visual context guides spatial attention. Cognitive Psychology. 1998;36:28–71. doi: 10.1006/cogp.1998.0681. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Implicit, long-term spatial contextual memory. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2003;29(2):224–234. doi: 10.1037/0278-7393.29.2.224. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnestic subjects with hippocampal damage. Nature Neuroscience. 1999;2(9):844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Collins DW, Rourke BP. Learning-disabled brains: A review of the literature. Journal of Clinical and Experimental Neuropsychology. 2003;25(7):1011–1034. doi: 10.1076/jcen.25.7.1011.16487. [DOI] [PubMed] [Google Scholar]
- Conlon E, Sanders M, Zapart S. Temporal processing in poor adult readers. Neuropsychologia. 2004;42:142–157. doi: 10.1016/j.neuropsychologia.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Evolution of human cortical circuits for reading and arithmetic: The "neuronal recycling" hypothesis. In: Dehaene S, Duhamel JR, Hauser M, Rizzolatti G, editors. From monkey brain to human brain. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- Denckla MB. Motor coordination in dyslexic children: Theoretical and clinical implications. In: Duffy FH, Geschwind N, editors. Dyslexia: A neuroscientific approach to clinical evaluation. Boston, MA: Little, Brown and Company; 1985. pp. 187–195. [Google Scholar]
- Denckla MB, Rudel RD. Rapid automatized naming (R.A.N.): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976;14:471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Rapid "automatized" naming of pictured objects, colors, letters, and numbers by normal children. Cortex. 1974;10:186–202. doi: 10.1016/s0010-9452(74)80009-2. [DOI] [PubMed] [Google Scholar]
- DSM-IV-TR. Diagnostic and statistical manual of mental disorders, text revision. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain. 2003;126(2):482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Eden GF, Stein JF, Wood HM, Wood FB. Differences in eye movements and reading problems in dyslexic and normal children. Vision Research. 1994;34(10):1345–1358. doi: 10.1016/0042-6989(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Eden GF, Stein JF, Wood HM, Wood FB. Temporal and spatial processing in reading disabled and normal children. Cortex. 1995;31:451–468. doi: 10.1016/s0010-9452(13)80059-7. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences, USA. 1998;95(3):914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AJ, Nicolson RI, Fawcett AJ. Evidence for a neuroanatomical difference within the olivo-cerebellar pathway of adults with dyslexia. Cortex. 2002;38(4):529–539. doi: 10.1016/s0010-9452(08)70021-2. [DOI] [PubMed] [Google Scholar]
- Forkstam C, Petersson KM. Towards an explicit account of implicit learning. Current Opinions in Neurology. 2005;18:435–441. doi: 10.1097/01.wco.0000171951.82995.c4. [DOI] [PubMed] [Google Scholar]
- Gombert JE. Implicit and explicit learning to read: Implication as for subtypes of dyslexia. Current Psychology Letters: Behavior, Brain & Cognition. 2003;10(1) [Google Scholar]
- Goschke T. Implicit learning of perceptual and motor sequences. In: Stadler MA, Frensch PA, editors. Handbook of implicit learning. Thousand Oaks, CA: Sage Publications, Inc; 1998. [Google Scholar]
- Greene AJ, Gross WL, Elsinger CL, Rao SM. Hippocampal differentiation without recognition: An fMRI analysis of the contextual cueing task. Learning and Memory. 2007;14(8):548–554. doi: 10.1101/lm.609807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib M. The neurological basis of developmental dyslexia. An overview and working hypothesis. Brain. 2000;123:2373–2399. doi: 10.1093/brain/123.12.2373. [DOI] [PubMed] [Google Scholar]
- Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. TRENDS in Cognitive Sciences. 2001;5(12):525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- Helenius P, Uutela K, Hari R. Auditory stream segregation in dyslexic adults. Brain. 1999;122(5):907–913. doi: 10.1093/brain/122.5.907. [DOI] [PubMed] [Google Scholar]
- Howard JH, Jr, Howard DV. Age differences in implicit learning of higher order dependencies in serial patterns. Psychology and Aging. 1997;12:634–656. doi: 10.1037//0882-7974.12.4.634. [DOI] [PubMed] [Google Scholar]
- Howard JHJ, Howard DV, Dennis NA, Kelly AJ. Implicit learning of predictive relationships in three-event visual sequences by young and old adults. doi: 10.1037/a0012797. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JHJ, Howard DV, Japikse KC, Eden GF. Dyslexics are impaired on implicit higher-order sequence learning but not on implicit spatial context learning. Neuropsychologia. 2006;44(7):1131–1144. doi: 10.1016/j.neuropsychologia.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Howard JHJ, Kelly AJ, Dennis NA, Vaidya CJ, Barr RF, Howard DV. Age-related deficits in implicit learning of higher-order sequential structure in the absence of motor sequencing; Paper presented at the Cognitive Aging Conference; Atlanta, GA. 2004. [Google Scholar]
- Kelly SW, Griffiths S, Frith U. Evidence for implicit sequence learning in dyslexia. Dyslexia. 2002;8(1):43–52. doi: 10.1002/dys.208. [DOI] [PubMed] [Google Scholar]
- Lieberman P. On the nature and evolution of the neural bases of human language. American Journal of Physical Anthropology. 2002;119(S35):36–62. doi: 10.1002/ajpa.10171. [DOI] [PubMed] [Google Scholar]
- Mayr U. Spatial attention and implicit sequence learning: Evidence for independent learning of spatial and nonspatial sequences. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1996;22:350–364. doi: 10.1037//0278-7393.22.2.350. [DOI] [PubMed] [Google Scholar]
- Menghini D, Hagberg GE, Caltagirone C, Petrosini L, Vicari S. Implicit learning deficits in dyslexic adults: An fMRI study. Neuroimage. 2006;33:1218–1226. doi: 10.1016/j.neuroimage.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ. Automaticity: A new framework for dyslexia research? Cognition. 1990;30:159–182. doi: 10.1016/0010-0277(90)90013-a. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, Brooks DJ. Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. Lancet. 1999;15(353):1662–1667. doi: 10.1016/S0140-6736(98)09165-X. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Olson RK, Kliegl R, Davidson BJ. Dyslexic and normal readers' eye movements. Journal of Experimental Psychology: Human Perception and Performance. 1983;9(5):816–825. doi: 10.1037//0096-1523.9.5.816. [DOI] [PubMed] [Google Scholar]
- Pavlidis GT. Do eye movements hold the key to dyslexia? Neuropsychologia. 1981;19(1):58–64. doi: 10.1016/0028-3932(81)90044-0. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Peterson MJ. Short-term retention of individual verbal items. Journal of Experimental Psychology. 1959;58:193–198. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- Pothos EM, Kirk J. Investigating learning deficits associated with dyslexia. Dyslexia. 2004;10:61–76. doi: 10.1002/dys.266. [DOI] [PubMed] [Google Scholar]
- Prull MW, Gabrieli JD, Bunge SA. Age-related changes in memory: A cognitive neuroscience perspective. In: Craik FI, Salthouse TA, editors. The handbook of aging and cognition. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. pp. 91–153. [Google Scholar]
- Rae C, Harasty JA, Dzendrowskyj TE, Talcott JB, Simpson JM, Blamire AM, et al. Cerebellar morphology in developmental dyslexia. Neuropsychologia. 2002;40(8):1285–1292. doi: 10.1016/s0028-3932(01)00216-0. [DOI] [PubMed] [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, et al. Theories of developmental dyslexia: Insights from a multiple case study of dyslexic adults. Brain. 2003;126:841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, et al. Fmri reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;644:700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Rudel RD. The definition of dyslexia: Language and motor deficits. In: Duffy FH, Geschwind N, editors. Dyslexia: A neuroscientific approach to clinical evaluation. Boston, MA: Little Brown; 1985. [Google Scholar]
- Russeler J, Gerth I, Munte TF. Implicit learning is intact in adult developmental dyslexic readers: Evidence from the serial reaction time task and artificial grammar learning. Journal of Clinical and Experimental Neuropsychology. 2006;28(5):808–827. doi: 10.1080/13803390591001007. [DOI] [PubMed] [Google Scholar]
- Seger CA. Implicit learning. Psychological Bulletin. 1994;115(2):163–196. doi: 10.1037/0033-2909.115.2.163. [DOI] [PubMed] [Google Scholar]
- Seger CA. Two forms of sequential implicit learning. Consciousness and Cognition. 1997;6(1):108–131. doi: 10.1006/ccog.1996.0285. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Escobar MD, Shaywitz BA, Fletcher JM, Makuch R. Evidence that dyslexia may represent the lower tail of a normal distribution of reading ability. The New England Journal of Medicine. 1992;326:145–150. doi: 10.1056/NEJM199201163260301. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. From language to reading and dyslexia. Dyslexia. 2001;7:37–46. doi: 10.1002/dys.185. [DOI] [PubMed] [Google Scholar]
- Sperling AJ, Lu ZL, Manis FR. Slower implicit categorical learning in adult poor readers. Annals of Dyslexia. 2004;54(2):281–303. doi: 10.1007/s11881-004-0014-z. [DOI] [PubMed] [Google Scholar]
- Stadler MA, Frensch PA, editors. Handbook of implicit learning. Thousand Oaks, CA: Sage Publications, Inc; 1997. [Google Scholar]
- Stanley G, Smith GA, Howell EA. Eye movements and sequential tracking in dyslexic and control children. British Journal of Psychology. 1983;74:181–187. doi: 10.1111/j.2044-8295.1983.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7(1):12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Harrison EP, Stein JF. Implicit motor learning deficits in dyslexic adults. Neuropsychologia. 2006;44(5):795–798. doi: 10.1016/j.neuropsychologia.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain and Language. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Uhry JK, Clark DB. Dyslexia theory and practice of instruction. 3rd ed. Baltimore, MD: York Press; 2005. [Google Scholar]
- Vicari S, Finzi A, Menghini D, Marotta L, Baldi S, Petrosini L. Do children with developmental dyslexia have an implicit learning deficit? Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:1392–1397. doi: 10.1136/jnnp.2004.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Marotta L, Menghini D, Molinari M, Petrosini L. Implicit learning deficit in children with developmental dyslexia. Neuropsychologia. 2003;41:108–114. doi: 10.1016/s0028-3932(02)00082-9. [DOI] [PubMed] [Google Scholar]
- Waber DP, Marcus DJ, Forbes PW, Bellinger DC, Weiler MD, Sorensen LG, et al. Motor sequence learning and reading ability: Is poor reading associated with sequencing deficits? Journal of Experimental Child Psychology. 2003;84(4):338–354. doi: 10.1016/s0022-0965(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skill. Psychological Bulletin. 1987;101(2):192–212. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wildgruber D, Ackerman H, Grodd W. Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: Effect of syllable repetition rate evaluated by fMRI. Neuroimage. 2001;13(1):101–109. doi: 10.1006/nimg.2000.0672. [DOI] [PubMed] [Google Scholar]
- Wolf M, Bowers PG. The double-deficit hypothesis for the developmental dyslexias. Journal of Educational Psychology. 1999;91:415–438. [Google Scholar]
- Wolf M, Bowers PG, Biddle K. Naming-speed processes, timing, and reading: A conceptual review. Journal of Learning Disabilities. 2000;33(4):387–407. doi: 10.1177/002221940003300409. [DOI] [PubMed] [Google Scholar]
- Wolff PH, Cohen C, Drake C. Impaired motor timing control in specific reading retardation. Neuropsychologia. 1984;22(5):587–600. doi: 10.1016/0028-3932(84)90023-x. [DOI] [PubMed] [Google Scholar]
- Wolff PH, Michel GF, Ovrut M. The timing of syllable repetitions in developmental dyslexia. Journal of Speech and Hearing Research. 1990a;33:281–289. doi: 10.1044/jshr.3302.281. [DOI] [PubMed] [Google Scholar]
- Wolff PH, Michel GF, Ovrut M, Drake C. Rate and timing precision of motor coordination in developmental dyslexia. Developmental Psychology. 1990b;26(3):349–359. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III tests of achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]