Abstract
Tuberous Sclerosis Complex is a multisystem disorder exhibiting a wide range of manifestations characterized by tumour-like lesions called hamartomas in the brain, skin, eyes, heart, lungs and kidneys. Tuberous Sclerosis Complex is genetically determined with an autosomal dominant inheritance and is caused by inactivating mutations in either the TSC1 or TSC2 genes. TSC1/2 genes play a fundamental role in the regulation of phosphoinositide 3-kinase (PI3K) signalling pathway, inhibiting the mammalian target of rapamycin (mTOR) through activation of the GTPase activity of Rheb. Mutations in TSC1/2 genes impair the inhibitory function of the hamartin/tuberin complex, leading to phosphorylation of the downstream effectors of mTOR, p70 S6 kinase (S6K), ribosomal protein S6 and the elongation factor binding protein 4E-BP1, resulting in uncontrolled cell growth and tumourigenesis.
Despite recent promising genetic, diagnostic, and therapeutic advances in Tuberous Sclerosis Complex, continuing research in all aspects of this complex disease will be pivotal to decrease its associated morbidity and mortality. In this review we will discuss and analyse all the important findings in the molecular pathogenesis of Tuberous Sclerosis Complex, focusing on genetics and the molecular mechanisms that define this multisystemic disorder.
Key Words: Tuberous sclerosis, tuberin, hamartin, mutations, genetics, multifactorial disease, germ-line mosaicism, rapamycin.
INTRODUCTION
Tuberous Sclerosis Complex (TSC) is a dominantly inherited disease of high penetrance, characterized pathologically by the presence of hamartomas (tumour-like lesions) in multiple organ systems. Well known clinical manifestations include epilepsy, learning difficulties, behavioural problems, and skin lesions. Many patients have renal lesions, usually angiomyolipomas; cysts, polycystic renal disease, and renal carcinoma can also occur. Approximately one in 8,000 adults and one in 6,000 newborns are affected by TSC. Although TSC is often inherited, new mutations have been implicated in up to 75% of all cases. Males and females are equally likely to have TSC and the chance of passing it on to offspring is 50%.
Identification of the genes causing the condition and study of their protein products has shed light on the pathogenesis of the disease and provided valuable new information about signalling pathways regulating protein synthesis and cell growth. There is now the exciting possibility of drug therapy for some of the manifestations of the disease.
This review highlights the most significant concepts in the genetics and the molecular biology of TSC with emphasis on new advances in the knowledge of its pathophysiological mechanisms, the contribute given by animal models, and the role of rapamycin in TSC pharmacological therapy.
CLINICAL OVERVIEW OF TUBEROUS SCLEROSIS COMPLEX
The clinical features of TSC have been reviewed in detail [1, 2]. Multisystem involvement in TSC results in a wide range of manifestations [3, 4].
Neurological Phenotype
The commonest presentation is with seizures in infancy or early childhood, particularly infantile spasms. Partial and generalized seizures, atonic seizures (drop attacks) and myoclonic seizures also occur with the pattern of seizures evolving through childhood. A population-based study estimated that around 80% of children with TSC have epilepsy [5] and a prevalence of mental retardation of 44% which in two thirds of cases was profound (IQ<21). There is a strong association between mental retardation and epilepsy so that significant learning disability is very rare in patients with no history of seizures [5,6]. Risk factors for mental retardation include onset of seizures before 12 months of age, poor epilepsy control and infantile spasms. Infantile spasms develop in approximately one third of TSC patients and may be the initial symptom in almost 70% of affected infants that come to medical attention [7]. The majority of patients with TSC and infantile spasms unfortunately go on to develop other seizure types. Behaviour problems are common in TSC, particularly autism, autistic spectrum disorders, attention deficit hyperactivity disorder and sleep disturbance in children [8].
The common brain lesions in TSC are tubers in the cerebral cortex and subependymal nodules (SEN) along the lateral walls of the lateral ventricles [3]. Histologically, tubers are disorganized areas of cortex lacking the normal laminated architecture. Large cells resembling astrocytes but positive for both glial and neuronal markers are a conspicuous feature. SEN comprise a mixture of vascular stroma and astrocytic-like cells, some of which are large resembling those in tubers. Tubers may be associated with underlying white matter abnormalities such as migration lines [9]. Individuals with mental retardation tend to have more tubers than those with normal intelligence [6, 9] and it has been suggested that autism spectrum disorder is particularly associated with tubers in the temporal lobes [10]. SEN usually remain dormant throughout life, but they can increase in size, developing into a subependymal giant cell astrocytoma (SEGA) that occurs in 6–14% of TSC cases with the peak incidence in later childhood and adolescence.
Renal Lesions
The renal manifestations of TSC include angiomyolipomas (AMLs), simple cysts, polycystic kidney disease, and renal-cell carcinoma. [11] These lesions likely arise in infancy or early childhood, increasing in size and number with age [12]. After neurologic complications, renal involvement is the second most common cause of morbidity and mortality in TSC [11]. AMLs are found in as many as 80% of TSC patients but are typically asymptomatic [11,12]. AML belongs to the family of perivascular epithelioid cell tumours and is typically composed of blood vessels, adipose tissue, and smooth muscle–like cells.
Renal cysts are also very common in TSC occurring in 17% of children and as many as 47% of adults. Like AMLs, they are frequently multiple and bilateral. However, renal cysts are more likely to become symptomatic than AMLs [11,12]. Polycystic kidney disease may also occur. It is a more severe, distinct entity with innumerable cysts that enlarge, replace renal parenchyma, and cause renal insufficiency and hypertension typically at an early age.
Cardiac Lesions
Cardiac rhabdomyomas are benign tumours which are the most common childhood tumour involving the heart [3]. Cardiac rhabdomyomas are detected in ~60% of TSC patients and are often the first clinical manifestation of TSC [13]. Serial observations have demonstrated that the majority of these lesions become less prominent over time, with some disappearing altogether as assessed by ultrasound, so that surgical resection is performed only when they cause life-threatening complications. Pathologically, rhabdomyoma cells are aberrant glycogen-filled myocytes. Following routine histologic processing, loss of the glycogen leads to a distinctive appearance referred to as spider cells due to the radial arrangement of residual sarcoplasm extending out from the nucleus.
Dermatological Phenotypes
Several types of skin lesion can occur in TSC [3]. Hypopigmented macules on the trunk and limbs are usually present at birth or become apparent during infancy. They can take any shape but are classically pointed at one end and rounded at the other resembling an ash leaf. Similar lesions on the scalp are associated with hypopigmented hair (poliosis). By 5 years of age, most children are developing angiofibromas on the face in the form of multiple flesh coloured or red papules. These typically occur over the nose, nasolabial folds, cheeks and chin. Fibrous plaques can develop on the forehead. Shagreen patches, which are raised brown or flesh coloured connective tissue naevi, often appear on the lower back during childhood. In adolescents and adults, ungual fibromas in the form of pink or red nodules on the finger and toe nails are a common finding. Gingival fibromas also occur. Some adults with TSC develop ‘confetti-like’ hypopigmentation on the limbs.
Pulmonary Manifestations
Lymphangiomyomatosis (LAM) is a rare disorder of unknown aetiology caused by proliferation of atypical smooth muscle cells in the peribronchial, perivascular, and perilymphatic tissues of the lung [14]. LAM occurs almost exclusively in young women, typically presenting between 30 to 35 years of age. Rare cases of LAM have been reported in men, but the occurrence of LAM in men is controversial. LAM may occur sporadically but is also closely associated with TSC and it may affect up to 26% of female patients. Clinically, LAM is a progressive disorder of the lung, causing dyspnea, spontaneous pneumothorax, hemoptysis, cough, chylothorax cor pulmonale, and chest pain, eventually leading to progressive respiratory failure and death.
Histopathologically, LAM causes diffuse cystic destruction of the tissues of the lung by abnormal spindle-shaped, closely packed smooth-muscle cells. Multifocal, micronodular pneumocyte hyperplasia and clear-cell lung tumours have also been found in TSC patients. Renal AMLs are present in 50% of individuals with sporadic LAM and it has been hypothesized that LAM is the result of metastatic spread of benign AML smooth-muscle cells [14].
Ophthalmologic Manifestations
The most common ocular findings in TSC are retinal hamartomas, appearing in 40% to 50% of patients [1, 15]. Their incidence increases with age. In the vast majority of TSC patients, they remain clinically stable and asymptomatic [16].
GENETICS OF TUBEROUS SCLEROSIS COMPLEX
The prevalence of TSC is estimated at 1/10,000, and about two-thirds of the cases are sporadic with no family history due to new mutational events [17]; the mutation rate has been estimated at 2.5×10−5/gamete [18]. TSC is due to inactivating mutations in either of two genes, TSC1 (on chromosome 9q34) or TSC2 (on chromosome 16p13.3). These mutations comprise the usual mix of nonsense, missense, insertion and deletion mutations, involving nearly all of the exons of TSC1 and TSC2 (Table 1). Although clinical expression of TSC varies greatly, in its classic form, there is 100% penetrance. Mutational studies of TSC patients have demonstrated that mutations in TSC2 are about five times more common than mutations in TSC1 in the sporadic TSC population, whereas the ratio is 1:1 in large families with multiple generations affected. Correspondingly, TSC1 disease is milder than TSC2 disease in multiple respects, which appears to be due to a reduced rate of second hit events [19-21]. Recently, several families have been described in which there are unusually mild manifestations of TSC, with most ‘affecteds’ not meeting diagnostic criteria, segregating with missense mutations in TSC2 [22].
Table 1.
Number of Mutation Reported for Tuberous Sclerosis Complex Patients
| Mutation | TSC1 | Percentage | TSC2 | Percentage |
|---|---|---|---|---|
| Substitutions | 97 | 36.3% | 380 | 49.4% |
| Insertions | 10 | 3.7% | 36 | 4.7% |
| Deletion | 117 | 43.8% | 265 | 34.5% |
| Duplication | 41 | 15.4% | 80 | 10.4% |
| Insertion/deletions | 2 | 0.8% | 8 | 1.0% |
| Total | 267 | 769 |
For many years, the prevailing model has been that the hamartomas of TSC develop through a two-hit mechanism in which there is complete loss of expression of functional TSC1 or TSC2, supported by findings of loss of heterozygosity (LOH) in TSC tumour samples [23-26]. However, the rate of LOH appears to vary according to lesion type, and LOH may not occur in all SEGAs and cortical tubers [24, 26]. On the other hand, recent evidence suggests that both tuber giant cells and SEGA cells have similar immunophenotypes, and SEGAs commonly sustain two-hit inactivation of either TSC1 or TSC2.
Structure of Hamartin (TSC1) and Tuberin (TSC2)
The TSC genes TSC1 and TSC2 were first identified by positional cloning strategies. They encode previously unknown proteins, termed hamartin and tuberin respectively, that form a functional complex.
The TSC1 gene consists of 23 exons, of which the last 21 contain coding sequence and the second is alternatively spliced [27]. Maximal promoter activity is present in a 587-bp region + 77 to -510 bp with respect to the transcription start site (TSS) in the TSC1 upstream region. Interestingly, this region contains no consensus TATA box or CAAT box. However, a 521-bp fragment surrounding the TSS exhibits the characteristics of a CpG island which overlaps with the promoter region. Putative binding sites for several known transcription factors, namely Sp1, E2F, CdxA, GATA, c-Ets, HSF2, Ik2, USF and SRY are found in the upstream region [28].
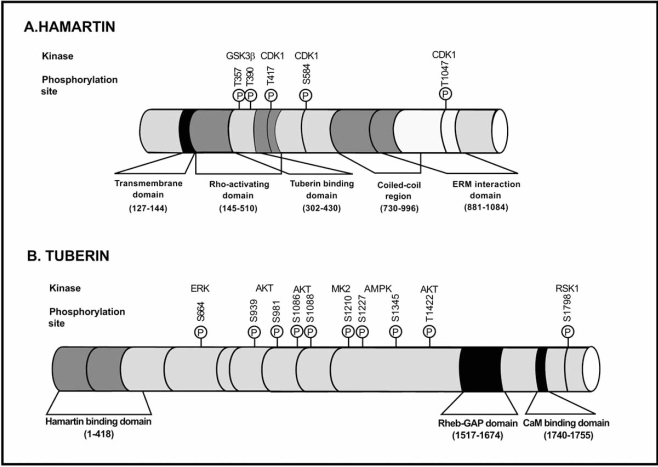
Hamartin, the predicted product, comprised 1164 amino acids (130 kDa). It had no significant homology to tuberin or other known vertebrate proteins but did have significant homology to a Schizosaccharomyces pombe predicted protein [29]. Hamartin is ubiquitously expressed [27], and contains a putative transmembrane domain at amino acids 127–144 and a coiled coil domain (CCD) spanning amino acids 719–998 [30]. The amino acid residues 145–510 of hamartin contain the function for activation of Rho GTPase, and amino acid residues 881–1084 interact with the N-terminal of the ezrin–radixin–moezin (ERM) family of actin-binding proteins (Fig. 1) [31, 32]. These ubiquitously expressed proteins crosslink cortical actin filaments to the plasma membrane, organising the cytoskeleton and acting as substrates for the tyrosine kinase of the epidermal growth factor receptor. Hamartin has also been shown to interact with neurofilament-L [33].
Fig. (1). Biochemical structure of hamartin and tuberin.
In this figure are rappresented the regulatory phosphorylation sites and respective kinases responsible for their phosphorylation.
The TSC2 gene has 41 small exons spanning 45 kb of genomic DNA and encodes a 5.5 kb mRNA. Exons 25, 26 and 31 are subject to alternative splicing [17]. The encoded protein tuberin has a full-length isoform of 1807 amino acids (198 kDa). A region spanning residues 1517–1674 and encoded by exons 34–38 has significant homology to the GTPase-activiating proteins (‘GAPs’) human rap1 GAP and murine Spa1 (Fig. 1) [30,34]. However, only modest GAP activity of tuberin for rap1 and rab5 has been demonstrated biochemically.
TSC2 contains a calmodulin-binding domain and an oestrogen-receptor-a-binding domain [35].
The gene for polycystic kidney disease, PKD1, is located immediately centromeric to TSC2, accounting for the occurrence of both conditions in families with large rearrangements (contiguous gene syndromes) [36]. The C-terminal domain within the TSC2 protein was recognized as being homologous to other GAP domains when it was cloned in 1993 [30].
TSC1 and TSC2 regions responsible for heterodimerisation have been identified [37]. The region of hamartin known to span the interacting domain with tuberin is within the amino acids 302–430, and the first 418 amino acids of tuberin contain the binding site for hamartin. Hamartin stabilizes tuberin by inhibiting its interaction with the HERC1 ubiquitin ligase [38]. Proteasome-mediated degradation of tuberin has been shown to be induced via its binding to the E6 oncoprotein of the human papillomavirus (HPV16 E6). HPV16 E6-induced degradation of tuberin leads to activation of S6K [39]. Binding of FIP200 to hamartin was suggested to disrupt the TSC protein complex formation [40]. Another hamartin binding protein has also been implicated in the regulation of the TSC tumour suppressor complex. TBC7 was reported to enhance ubiquitination and degradation of hamartin [41].
The two chaperone proteins, HSCP-70 and HSP70-1, bind to tuberin. Chaperone proteins recognise and bind misfolded proteins. Increased interactions of both chaperone proteins with the disease causing tuberin mutant R611Q have been reported [42]. The hamartin/tuberin heterodimeric complex formation provides a tentative explanation for the similar disease phenotype in TSC patients with mutations in either of the two TSC genes.
Hamartin and tuberin are coexpressed in cells of several organs, such as kidney, brain, lung, and pancreas. Hamartin has been reported to interact with Plk1 and to be localized to the centrosome. Cdk1 phosphorylates hamartin at several sites, of which the phosphorylation at T310 regulates its interaction with Plk1 [43].
Tuberin has been described to be localised to the cytosol and the membrane fraction within the cytoplasm and to the nucleus. Akt-mediated phosphorylation of tuberin has been demonstrated to regulate both, the translocation of tuberin from the membrane to cytosol and the nuclear/cytoplasmic localisation of tuberin [44].
Mutations and Polymorphisms
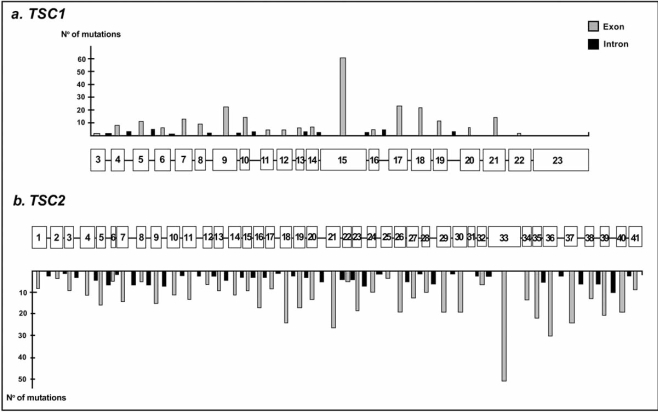
The mutation spectra of the TSC genes are very heterogeneous and no hotspots for mutations have been reported. Indeed, more than 200 TSC1 and 700 TSC2 unique allelic variants have been reported [20, 21, 45-48]. There are many mutations in each gene that are seen recurrently, but no single mutation accounts for more than about 1% of all TSC patients. Despite complete penetrance of the disease, phenotypic variability can make the determination of disease status difficult among family members of affected individuals.
The major proportions in both genes are subtle mutations composed of nonsense mutations, small deletions and insertions, splice site changes, and, for TSC2, additionally missense mutations. All these mutations are distributed over the entire regions of both genes (Fig. 2).
Fig. (2).
Mutation spectra of TSC1 and TSC2.
Among the large number of TSC mutations identified as disease causing, only a few affect splicing regulatory sites. The frequency and nature of TSC splice site mutations reported so far are in agreement with these overall findings: in TSC1 they represent 8% and in TSC2 they comprise 12% of the total number of identified mutations. Most changes were merely assumed to influence the mature transcripts, especially when they affect highly conserved sequences at exon/intron junctions, while few are confirmed on the transcript or the protein level. Mayer et al. [49] performed an RNA based screening of the entire coding regions of both TSC genes applying the protein truncation test (PTT) and identified a high proportion of unusual splicing abnormalities affecting the TSC2 gene. Two cases exhibited different splice acceptor mutations in intron 9 (IVS9315GCA and IVS933CCG) both accompanied by exon 10 skipping and simultaneous usage of a cryptic splice acceptor in exon 10. Another splice acceptor mutation (IVS38318ACG) destroyed the putative polypyrimidine structure in intron 38 and resulted in simultaneous intron retention and usage of a downstream cryptic splice acceptor in exon 39. Another patient bore a CCT transition in intron 8 (IVS8+281CCT) activating a splice donor site and resulting in the inclusion of a newly recognised exon in the mRNA followed by a premature stop.
Nellist et al. [50] showed that pathogenic tuberin amino-acid substitutions disrupt the tuberin–hamartin complex and subsequently have investigated how these mutations affect the role of tuberin in the control of signal transduction through mTOR. The R611Q, R611W, A614D, C696Y and V769E substitutions [51] disrupted the tuberin–hamartin interaction, and prevented the phosphorylation of tuberin by PKB, the inhibition of S6 and S6K phosphorylation, and the stimulation of Rheb GTPase activity, cause TSC because they result in major conformational changes to tuberin. The 609insS and F615S amino-acid changes play a similar effect, but do not completely inactivate tuberin.
The N525S and K599M substitutions inhibited the phosphorylation of S6K and S6, and increased the GTPase activity of Rheb. The K599M substitution is a de novo mutation [46] and has been shown to reduce the tuberin–dependent inhibition of phosphorylation of overexpressed 4E-BP1 [52]. However, this effect is weak compared to the V769E tuberin variant [53].
Recently, Nellist et al. [54] found that deletion of isoleucine at amino acid residue 820 of TSC2 and the TSC2 L1511H, C244R and Y598H amino acid substitutions are sufficient to cause TSC. The TSC2 R1772C, T993M, S132C, F143L and A196T substitutions are rare polymorphisms that do not inhibit TSC1–TSC2 function, and do not cause TSC.
Previous reports have identified a mutation consisting of a 34 bp deletion affecting portions of exon 38 and the adjacent intron 38 of TSC2. Roberts et al. [55] found this genetic variation in 4 of 800 TSC patients. In every case, the variant was present in one unaffected parent of the sporadically affected child. They excluded the possibility of mosaicism in the parents with this variant and conclude that this deletion is a rare polymorphism that does not cause TSC, but may be a modifier of the TSC phenotype.
Major genes for TSC and autosomal dominant polycystic kidney disease (PKD), TSC2 and PKD1, respectively, lie adjacent to each other at chromosome 16p3.3, suggesting a role for PKD1 in the etiology of renal cystic disease in TSC.
Features of TSC and autosomal dominant PKD have been observed in patients with a TSC2-PKD1 contiguous gene syndrome. In these patients a large part of the adjacent TSC2 and PKD1 genes has been deleted on one chromosome. In a study described by Sampson et al. [56] 17 of 22 patients with such a deletion have been diagnosed with a very severe form of PKD, already manifesting within the first year of life. A possible explanation for this severe phenotype is a functional link between the TSC2 protein and polycystin-1 in protein sorting as described by Kleymenova et al. [57]. The frequency of genomic deletions involving only the PKD1-gene is low [58]. Ariyurek et al. [58] observed 4 deletions in 125 patients. In another study a 5-kb deletion (region intron 34-3'UTR) and a 2-kb deletion (region intron 30-34) were found upon analysis in a set of 167 patients [59]. Furthermore, a 3-kb deletion was reported in the region intron 1-exon 5, identified using long-range PCR performed on 24 patients [60]. Likely, patients with a deletion that disrupts the PKD1 and TSC2 genes are usually identified as TSC patients. Mosaicism for deletions involving TSC2 and PKD1 was a frequent phenomenon and was associated with preserved renal function in some cases. Among mosaics, disease severity did not correlate with the frequency of the mutant allele in lymphocytes; the level of mosaicism in renal tissue is likely to be more important. Five of the 27 unrelated patients studied by Sampson et al. [56] had multiple cysts in both kidneys, but no detectable disruption of PKD1. All were identified, through ultrasound screening, as having renal cystic disease. Large rearrangements of TSC2 were defined in 3 of these patients.
The high degree of variability of TSC clinical manifestations, including those among related and unrelated patients with the same mutation [19], suggests the possibility that modifier genes influence disease severity.
Because IFN-γ has been shown to be a useful mediator of tumour regression in animal models of kidney tumours [61, 62] and because there is a known high-expressing allele of IFN-γ in humans, Dabora et al. [63] examined the relationship between the IFN-γ genotype and the severity of renal disease in TSC patients who had TSC2 mutations; they found an association between IFN-γ allele 2 and the absence of kidney AMLs in TSC2 patients.
This finding suggests that IFN-γ allele 2 may be a genetic modifier that reduces kidney AMLs development or growth. Because allele 2 has been shown to be associated with a higher level of IFN-γ expression in mitogen-stimulated mononuclear cells in vitro [64], it is plausible that this association is due to a reduction in kidney AMLs development in the presence of higher levels of IFN-γ.
The enzyme 8-oxoguanine glycosylase 1 (OGG1) repairs 8-oxo-2-deoxyguanosine residue (8-oxodG) an oxidatively damaged promutagenic base. Genetic variations in OGG1 gene have been shown to modulate DNA repair capacity and are related risk of tumour development. Habib et al. [65] tried to determine whether genetic variants in OGG1 play a role in susceptibility to AML in TSC patients. They identified showed the presence of significant association between the Ser326Cys polymorphism of OGG1 and AML. Moreover, they also assessed the presence of oxidative DNA damage in kidney sections by immunostaining for 8-oxodG. 8-OxodG staining was highly abundant in kidney AML tissue from TSC patients compared to weak staining in uninvolved tissue from the same TSC patients or normal kidney from healthy subjects. Taken together, these findings suggest that OGG1 Ser326Cys variant of may confer risk for development of AMLs by increasing oxidative DNA damage.
Following the observation of a TSC patient with a de novo reciprocal translocation t(3;12) (p26.3;q23.3), Fahsold et al. [66] have undertaken a linkage study in 15 TSC families using polymorphic DNA markers neighbouring the chromosome breakpoints. Significant lod scores have been obtained for markers D12S7 (Zmax = 2.34, theta = 0.14) and PAH (phenylalanine hydroxylase) (Zmax = 4.34, theta = 0.0). In multipoint linkage analysis, the peak lod score was 4.56 at the PAH gene locus. These data suggest the existence of a third gene locus for TSC (TSC3) on chromosome 12q22-24.1. Dysfunctions of phenylalanine hydroxylase pathway might be involved in the pathogenesis of TSC.
Germ-Line Mosaicism Hypothesis
Germ-line mosaicism has been demonstrated in both common and uncommon genetic disorders [67]. Because most germ-line mutations are likely to be mitotic in origin and because the mutation rate multiplied by the number of mitoses necessary to form the gametes is 11 [68], germ-line mosaicism would be expected to occur to some degree in all genetic disorders. Empiric recurrence risks of specific diseases can be estimated, but risks for individual families depend on the percentage of affected gametes in the germ line of the parent with mosaicism.
Fifteen families with two or more affected children and apparently unaffected parents have been reported and are thought to illustrate examples of germ-line mosaicism [69, 70]. Yates et al. [71] have proved germ-line mosaicism for TSC2 in one family, by molecular analysis.
Unaffected parents who have had a child affected with TSC usually are given a low (1%) recurrence risk. Evidence that germ-line mosaicism is not an uncommon phenomenon would increase the estimated risk in cases of sporadic TSC and, thus, would have implications for genetic counselling.
Rose et al. [72] found germ-line mosaicism in five families with mutations in the TSC2 gene and in one family with the causative mutation in the TSC1 gene.
MOLECULAR BIOLOGY OF TUBEROUS SCLEROSIS COMPLEX
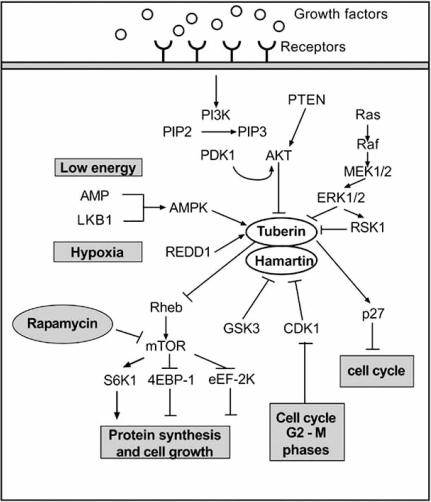
Led by seminal studies in Drosophila, the TSC1/TSC2 complex has been positioned in an ancestrally conserved signalling pathway that regulates cell growth. TSC1/TSC2 receives inputs from at least three major signalling pathways in the form of kinase-mediated phosphorylation events that regulate its function as a GAP protein [73]: the PI3K-Akt pathway, the ERK1/2-RSK1 pathway and the LKB1-AMPK pathway.
TSC1/TSC2 functions as a GAP towards Rheb, which is a major regulator of the mammalian target of rapamycin (mTOR). In the absence of either TSC1 or TSC2, high levels of Rheb-GTP lead to constitutive activation of mTOR–raptor signalling, thereby leading to enhanced and deregulated protein synthesis and cell growth (Fig. 3).
Fig. (3). Tuberous sclerosis complex signalling.
Figure showing signalling pathways involved in the regulation of TSC complex controlling mammalian target of rapamycin (mTOR) activity.
PI3K=phosphatidylinositol 3-kinase. PIP2=phosphatidyl-inositol (4,5) biphosphate. PIP3= phosphatidyl-inositol (3,4,5) triphosphate. PDK1= phosphoinositide-dependent protein kinase 1. PTEN= Phosphatase and tensin homolog. AKT=Protein kinase B. REDD-1=DNA-damage inducible transcript 4 protein. RSK-1=ribosomal protein S6 kinase alpha-1. LKB1=Serine/threonine-protein kinase 11. ERK=extracellular signal-related kinase. Rheb=Ras homologue enriched in brain. S6K1=ribosomal protein S6 kinase beta-1. 4E-BP1=eukaryotic translation initiation factor 4E-binding protein 1. eEF-2K=elongation factor 2 kinase. CDK1= Cyclin dependent Kinase 1.
TSC Upstream Pathways
The pathways signalling through TSC1–2 have multiple separate phosphorylation sites. Sites identified so far include those for GSK3β (glycogen synthase kinase 3β) [74] and CDK1 (cyclin dependent kinase 1) [75] on TSC1 and those for ERK1/2 (extracellular signal-regulated kinase 1 and 2) and RSK1 (p90 ribosomal protein S6 kinase 1) [76-78], MK2 [79], Akt (protein kinase B) [80], AMPK (adenosine monophosphate-activated protein kinase) [81] and GSK3β [82] on TSC2.
Inoki et al. [82] suggested a possible function of TSC2/mTOR signalling in tumourigenesis caused by dysfunction of the Wnt pathway and a mechanism by which Wnt stimulates protein synthesis and cell growth. This possibility is given by the fact that Wnt stimulates the mTOR signalling pathway via inhibiting GSK3 phosphorylation of TSC2.
The phosphorylation of tuberin by Akt and MK2 promotes the binding of tuberin with 14-3-3 proteins. 14-3-3 proteins are members of a group of proteins that specifically interact with phosphorylated proteins, facilitating the phosphorylation-dependent control of protein activity [83]. Detection of a ternary complex of tuberin, hamartin and 14-3-3 suggests that the tuberin-14-3-3 interaction is compatible with tuberin-hamartin binding and that 14-3-3 proteins interact with the tuberin-hamartin complex [84, 85]. A possible function of the interaction between 14-3-3 proteins and phosphorylated tuberin is to inhibit the formation of tuberin-hamartin complex, in order to decrease the stability of tuberin and release of the activated mTOR [84].
Recently, Yasui et al. [86] identified NADE (p75NTR-associated cell death executor) as a novel interactor protein with hamartin. NADE has been shown to mediate NGF-induced apoptosis in neuronal cells through the interaction with p75NTR [87, 88]. Hamartin binds to NADE with its coiled coil domain domain. Down-regulation of hamartin with TSC1 siRNA led to failure of NGF-induced apoptosis in PC12h cells suggesting that the association of hamartin with NADE is involved in neuronal cell death, which could explain why hamartoma cells are not eliminated in TSC. Current data supports a model that, apart from receptor tyrosine kinases, G protein-coupled receptors are also able to regulate tuberin activity [89]. Direct evidence has shown that G i/o - and G q -coupled receptors can regulate tuberin phosphorylation in a PI3K-dependent or –independent manner, while G 12/13 may have a dual role in regulating tuberin.
A recent study has demonstrated that the forkhead transcription factor FoxO is capable of binding to tuberin [90]. FoxO binds to an adjacent region near the GAP domain, thus inhibiting the GAP activity towards Rheb.
TSC Downstream Pathways
The best understood output pathway is from the GAP domain of TSC2, although other downstream pathways are also likely.
Phosphorylation of several sites on TSC1–2 stimulates the GAP function of TSC2, whereas phosphorylation of other sites inhibits it [37, 73, 91, 92]. Inhibition of the GAP function of TSC2 shifts the balance of its substrate Rheb to the Rheb-GTP form, which activates the mTOR protein and ultimately leads to phosphorylation of ribosomal protein S6 and 4E-BP1 resulting in increased protein synthesis and cell proliferation. TSC1–2 is also proposed to function in cell-cycle control by regulating the cyclin-dependent kinase inhibitor p27 [93]. p27 protein levels are regulated through ubiquitin-dependent degradation [94].
Skp2 is the F-box protein, which together with other proteins forms an SCF-type E3 ubiquitin ligase complex, whose task is to target p27 for degradation by the proteasome [95, 96]. Neither tuberin nor hamartin are in a complex with Skp2 and tuberin does not affect Skp2 protein levels, and the SCFSkp2 ubiquitin ligase does not regulate tuberin stability. However, binding of tuberin to p27 sequesters p27 from Skp2 accompanied by a stabilization of the p27 interaction with cdk2, and hence, Skp2-induced p27 degradation and cell cycle progression is abolished by tuberin’s protective binding to p27. The observed binding of the tumour suppressor protein tuberin to the tumour suppressor protein p27 provides a molecular explanation for the effects of the TSC genes on p27 protein stability [97]. Through Rac and Rho, TSC1 and TSC2 have a signalling role in the development and maintenance of the actin cytoskeleton [31, 32].
Ozcan et al. [98] found that loss of TSC1 or TSC2 in cell lines and mouse or human tumours caused endoplasmic reticulum (ER) stress and activated the unfolded protein response. The resulting ER stress played a significant role in the mTOR-mediated negative feedback inhibition of insulin action and increased the vulnerability to apoptosis
Tuberin downregulates the DNA repair enzyme 8-oxoguanine DNA-glycosylase (OGG1) with important functional consequences, compromising the ability of cells to repair damaged DNA resulting in the accumulation of the mutagenic oxidized DNA, 8-oxo-dG. OGG1 localizes with tuberin preferentially in kidney cortex. Loss of tuberin is accompanied by the loss of OGG1 contributing to tumourgenesis [99].
ANIMAL MODELS OF TUBEROUS SCLEROSIS
Mouse and Drosophila models carrying mutant TSC1 and TSC2 alleles have been reported, as has a naturally occurring TSC2 mutant rat, the ‘Eker’ rat. These models have provided a valuable resource for investigating the functions of TSC1 and TSC2.
Eker Rat
First described in the 1950s, the Eker rat strain contains a germline inactivation of one allele of the gene encoding TSC2 and has served as an animal model for hereditary renal cell carcinoma [100, 101]. Following the identification of TSC1 and TSC2 as genes associated with human disease, murine models lacking TSC1 or TSC2 were generated by gene targeting [102].
In these models, homozygous TSC1 or TSC2 mutants die at an embryonic stage, whereas heterozygous carriers are predisposed to tumour formation. These studies confirmed the tumour suppressor function of TSC1 and TSC2 as inferred from human genetic analysis of the TSC.
In particular, the Eker rat carries an inactivating retrotransposon insertion mutation in exon 30 of the TSC2 gene [103]. It develops bilateral multifocal solid and cystic renal adenomas and extra-renal pathology, including uterine leiomyoma, splenic haemangioma, pituitary adenoma and SEN hamartomas in the brain [104]. The phenotype is transmitted as an autosomal dominant trait with embryonic lethality in the homozygote at days 10–12 with disrupted neuroepithelial growth and development [101]. Allelic loss and intragenic mutation have been demonstrated in tumours in the Eker rat [105] and transgenic expression of TSC2 in the Eker rat and Eker tumour cell lines support its tumour-suppressor function [100].
TSC1- and TSC2-Knockout Mice
Mice with targeted disruption of TSC1 or TSC2 have been generated and express a phenotype similar to the Eker rat [102]. Multiple renal cystadenomas develop and lead to renal cell carcinoma in approx. 5–15% of mice by 18–24 months. Although there is considerable inter-strain variability in tumour development this appears to be more rapid in TSC2 than TSC1 heterozygotes. Histologically benign hepatic haemangiomas and histologically malignant but non-metastasizing haemangiosarcomas of the limbs and tail also occur. Lung adenomas have also been observed, but seem to have limited growth potential. Homozygotes die in midgestation (E10.5– E12.5) and exhibit growth failure, hepatic hypoplasia, cardiac hypertrophy and anaemia.
Drosophila
The identification of TSC1 and TSC2 homologues in Drosophila coincided with an exciting burst of studies that implicate a role for the insulin signalling pathway in the control of cell growth in Drosophila and mammals. Cell growth – the process in which cells accumulate mass – is a distinct process, which should be distinguished from cell proliferation, because cell proliferation per se does not necessarily drive cell growth [106].
The initial implication of insulin signalling in the control of cell size in Drosophila was demonstrated by the fact that PI3K (p110) and IRS (chico) are required cell-autonomously to promotes cell growth [107, 108]; subsequently, various studies showed the involvement of other components of the insulin pathway in cell growth, including Akt [109], PTEN [110], PDK1 [111] and S6K [112]. Collectively, these studies demonstrate that downregulation of the insulin pathway leads to decreased cell size, whereas upregulation of this pathway results in the opposite phenotype. A unique feature of the Drosophila system is that, in this organism, it is possible to monitor cell growth in ‘mosaic’ animals containing genetically mutant cells in an otherwise wildtype genetic background; this allows an investigation of whether a given gene is required cell-autonomously or non cell-autonomously for cell growth.
Homozygous mutations of the TSC2 gene are lethal to Drosophila during larval development. However, TSC2−/− clones generated in specific tissues exhibit the ‘gigas’ phenotype characterized by increased cell and organ size with generally normal differentiation and morphology [113]. Mutagenesis screens revealed that TSC1 mutations also cause a gigas-like phenotype [114, 115].
Schizosaccharomyces Pombe
Extensive homology search fails to identify TSC1 and TSC2 homologues in the worm Caenorhabditis elegans or the budding yeast Saccharomyces Cerevisiae [116]. At present, it is unclear whether this reflects a true absence of such molecules or functional homologues of TSC1 and TSC2, in these organisms, have diverged beyond recognition by simple homology search. By contrast, the fission yeast Schizosaccharomyces pombe contains possible homologues of TSC1 and TSC2 [116]. Similar to their mammalian counterparts, the TSC1 and TSC2 homologues of S. pombe form a protein complex. Deletion of the TSC1 or TSC2 genes in this organism results in similar defects in the localization of amino acid permease, nutrient uptake and conjugation. Although studies of TSC1 and TSC2 homologues in S. pombe are at an early stage, because of the simplicity of this organism and the power of genetic analysis, this system clearly has tremendous potential as a genetic model for understanding the molecular mechanisms of TSC1 and TSC2 functions.
RAPAMYCIN: MORE THAN A PROMISE
Rapamycin, also referred to as Sirolimus, has powerful antiproliferative and immunosuppressant activity [117]. Since 1999, it has been United States Food and Drug Administration approved for the prophylaxis of organ rejection in patients older than 13 years. Rapamycin, discovered in 1965 from soil samples on Easter Island, known to its indigenous population as Rapa Nui, was isolated from Streptomyces hygroscopicus [117]. It was found to be active against several strains of yeast and fungi. However, no activity was observed against gram-positive or gram-negative bacteria.
Rapamycin and Tuberous Sclerosis Complex
Studies on sirolimus activity have shown that it binds to its intracellular receptor FKBP12 (FK506-binding protein 12), a member of the family of FK506-binding proteins. It has also been demonstrated that the binding of sirolimus to FKBP12 is required for the inhibitory effect of sirolimus on mTOR function [118]. It has been shown that the sirolimus/FKBP12 complex, but not FK506/FKBP12 complex, binds with very high affinity to mTOR. At the same time, FK506 competitively inhibits the effects of sirolimus that are mediated by mTOR. Unlike most kinase inhibitors, sirolimus does not completely inhibit the kinase activity of mTOR [119]. Instead, sirolimus/FKBP12 complex activity causes derepression of specific protein phosphatases, which leads to dephosphorylation of mTOR downstream effectors like S6K1 or 4E-BP1. Therefore, Rapamycin, increasing levels of unphosphorylated S6K, causes inhibition of translation, and produces cell cycle arrest and the possibility of reduced hamartoma formation or regression [120].
In addition, many of the skin, brain, and kidney hamartomas seen in TSC, including cutaneous angiofibromas and LAM, are vascular and contain an endothelial component.
Sirolimus decreased VEGF production, which may also be beneficial in these vascular tumours because of its antiangiogenic effects [117, 120]. Oral sirolimus therapy can also induce regression of astrocytomas associated with TSC. Five patients with TSC and astrocytomas were treated with oral sirolimus at standard immunosuppressive doses (serum levels 5-15 ng/mL) from 2.5 to 20 months and showed astrocytoma regression [121]. An open-label clinical trial demonstrated that AMLs in TSC patients regressed somewhat during sirolimus therapy but tended to increase in volume after the therapy was stopped [122]. Rauktys et al. [123] have also shown that topical administration of rapamycin is an effective treatment for TSC-related tumours in a mouse model demonstrating that transdermal delivery of rapamycin is feasible and topical rapamycin should be further investigated as a novel treatment approach for TSC skin disease such as facial angiofibromas.
If one can establish the prenatal diagnosis of TSC and begin using sirolimus early, it may be possible to prevent the development of TSC manifestations, similar to the situation with early diagnosed and treated phenylketonuria [124]. However, the utility of mTOR inhibitors in treating epilepsy in TSC has not been investigated in either clinical trials or animal models. Zeng et al. [125] recently demonstrated that rapamycin has strong efficacy for preventing seizures and prolonging survival in Tsc1GFAPCKO mice, a mouse model of TSC with conditional inactivation of the Tsc1 gene in glial fibrillary acidic protein (GFAP)–positive cells (Tsc1GFAPCKO mice), which develops progressive epilepsy, encephalopathy, and premature death, as well as cellular and molecular brain abnormalities likely contributing to epileptogenesis.
CONCLUSION/FUTURE DIRECTIONS
Significant progress has been made in the understanding of the genetic and pathogenic aspects of this serious and multi-system disorder. A multidisciplinary approach is essential for an early, accurate diagnosis and proper management of affected individuals. The genetic basis of TSC is now well understood and genetic testing is available for the majority of families, but better methods need to be developed for rapid and reliable identification of pathogenic mutations. Key priorities for future research include a better understanding of functional relationship between TSC1 and TSC2 and their pathways and a gained insight into the relationships between genotypes and phenotypes.
The delineation of the TSC biochemical signalling pathways suggest strategies for developing targeted therapies including mTOR inhibition, which are being evaluated in clinical trials.
REFERENCES
- 1.Curatolo P. London: Mac Keith Press; 2003. Tuberous Sclerosis Complex: From Basic Science to Clinical Phenotypes. [Google Scholar]
- 2.Narayanan V. Tuberous sclerosis complex: Genetics to pathogenesis. Pediatr. Neurol. 2003;29:404–9. doi: 10.1016/j.pediatrneurol.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Gomez MR, Sampson JR, Whittemore VH. Tuberous Sclerosis Complex. Oxford: Oxford University Press; 1999. [Google Scholar]
- 4.Curatolo P, Verdecchia M, Bombardieri R. Tuberous sclerosis complex: a review of neurological aspects. Eur. J. Pediatr. Neurol. 2002;6:15–23. doi: 10.1053/ejpn.2001.0538. [DOI] [PubMed] [Google Scholar]
- 5.Joinson C, O’Callaghan FJ, Osborne JP, Martyn C, Harris T, Bolton PF. Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychol. Med. 2003;33:335–44. doi: 10.1017/s0033291702007092. [DOI] [PubMed] [Google Scholar]
- 6.Curatolo P, Seri S, Verdecchia M, Bombardieri R. Infantile spasms in tuberous sclerosis complex. Brain Dev. 2001;23:502–7. doi: 10.1016/s0387-7604(01)00300-x. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima K, Inoue Y, Fujiwara T, Yagi K. Long-term follow-up study of West syndrome associated with tuberous sclerosis. Brain Dev. 2001;23:698–704. doi: 10.1016/s0387-7604(01)00275-3. [DOI] [PubMed] [Google Scholar]
- 8.Gillberg IC, Gillberg C, Ahlsen G. Autistic behaviour and attention deficits in tuberous sclerosis: a population-based study. Dev. Med. Child. Neurol. 1994;36:50–6. doi: 10.1111/j.1469-8749.1994.tb11765.x. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd CW, Houser OW, Gomez MR. MR findings in tuberous sclerosis complex and correlation with seizure development and mental impairment. Am. J. Neuroradiol. 1995;16:149–55. [PMC free article] [PubMed] [Google Scholar]
- 10.Bolton PF, Park RJ, Higgins JN, Griffiths PD, Pickles A. Neuroepileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain. 2002;125:1247–55. doi: 10.1093/brain/awf124. [DOI] [PubMed] [Google Scholar]
- 11.O’Callaghan FJ, Noakes MJ, Martyns CN, Osborne JP. An epidemiological study of renal pathology in tuberous sclerosis complex. BJU Int. 2004;94:853–7. doi: 10.1111/j.1464-410X.2004.05046.x. [DOI] [PubMed] [Google Scholar]
- 12.Casper KA, Donnelly LF, Chen B, Bissler JJ. Tuberous sclerosis complex: renal imaging findings. Radiology. 2002;225:451–6. doi: 10.1148/radiol.2252011584. [DOI] [PubMed] [Google Scholar]
- 13.Watson GH. Cardiac rhabdomyomas in tuberous sclerosis. Ann. NY Acad. Sci. 1991;615:50–7. doi: 10.1111/j.1749-6632.1991.tb37747.x. [DOI] [PubMed] [Google Scholar]
- 14.Chorianopoulos D, Stratakos G. Lymphangioleiomyomatosis and Tuberous Sclerosis Complex. Lung. 2008 doi: 10.1007/s00408-008-9087-5. [DOI] [PubMed] [Google Scholar]
- 15.Rowley SA, O’Callaghan FJ, Osborne JP. Ophthalmic manifestations of tuberous sclerosis: a population based study. Br. J. Ophthalmol. 2001;85:420–3. doi: 10.1136/bjo.85.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmer-Galler IE, Robertson DM. Long-term observation of retinal lesions in tuberous sclerosis. Am. J. Ophthalmol. 1995;119:318–24. doi: 10.1016/s0002-9394(14)71174-2. [DOI] [PubMed] [Google Scholar]
- 17.Cheadle JP, Reeve MP, Sampson JR, Kwiatkowski DJ. Molecular genetic advances in tuberous sclerosis. Hum. Genet. 2000;107:97–114. doi: 10.1007/s004390000348. [DOI] [PubMed] [Google Scholar]
- 18.Sampson JR, Scahill SJ, Stephenson JB, Mann L, Connor JM. Genetic aspects of tuberous sclerosis in the west of Scotland. J. Med. Genet. 1989;26:28–31. doi: 10.1136/jmg.26.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, Kasprzyk-Obara J, Domanska-Pakiela D, Kwiatkowski DJ. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am. J. Hum. Genet. 2001;68:64–80. doi: 10.1086/316951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones AC, Shyamsundar MM, Thomas MW, Maynard J, Idziaszczyk S, Tomkins S, Sampson JR, Cheadle JP. Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am. J. Hum. Genet. 1999;64:1305–15. doi: 10.1086/302381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sancak O, Nellist M, Goedbloed M, Elfferich P, Wouters C, Maat-Kievit A, Zonnenberg B, Verhoef S, Halley D, van den Ouweland A. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype-phenotype correlations and comparison of diagnostic DNA techniques in tuberous sclerosis complex. Eur. J. Hum. Genet. 2005;13:731–41. doi: 10.1038/sj.ejhg.5201402. [DOI] [PubMed] [Google Scholar]
- 22.Au KS, Hebert AA, Roach ES, Northrup H. Complete inactivation of the TSC2 gene leads to formation of hamartomas. Am. J. Hum. Genet. 1999;65:1790–5. doi: 10.1086/302648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green AJ, Smith M, Yates JR. Loss of heterozygosity on chromosome 16p13.3 in hamartomas from tuberous sclerosis patients. Nat. Genet. 1994;6:193–6. doi: 10.1038/ng0294-193. [DOI] [PubMed] [Google Scholar]
- 24.Henske EP, Scheithauer BW, Short MP, Wollmann R, Nahmias J, Hornigold N, van Slegtenhorst M, Welsh CT, Kwiatkowski DJ. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am. J. Hum. Genet. 1996;59:400–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Niida Y, Stemmer-Rachamimov AO, Logrip M, Tapon D, Perez R, Kwiatkowski DJ, Sims K, MacCollin M, Louis DN, Ramesh V. Survey of somatic mutations in tuberous sclerosis complex (TSC) hamartomas suggests different genetic mechanisms for pathogenesis of TSC lesions. Am. J. Hum. Genet. 2001;69:493–503. doi: 10.1086/321972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han S, Santos TM, Puga A, Roy J, Thiele EA, McCollin M, Stemmer-Rachamimov A, Ramesh V. Phosphorylation of tuberin as a novel mechanism for somatic inactivation of the tuberous sclerosis complex proteins in brain lesions. Cancer. Res. 2004;64:812–6. doi: 10.1158/0008-5472.can-03-3277. [DOI] [PubMed] [Google Scholar]
- 27.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Hal-ley D, Young J, Burley M, Jeremiah S, Woodward K, Nah-mias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL, Sepp T, Ali JB, Ward S, Green AJ, Yates JR, Kwiatkowska J, Henske EP, Short MP, Haines JH, Jozwiak S, Kwiatkowski DJ. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–8. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- 28.Ali M, Girimaji SC, Kumar A. Identification of a core promoter and a novel isoform of the human TSC1 gene transcript and structural comparison with mouse homolog. Gene. 2003;320:145–154. doi: 10.1016/s0378-1119(03)00821-7. [DOI] [PubMed] [Google Scholar]
- 29.Sampson JR. TSC1 and TSC2: genes that are mutated in the human genetic disorder tuberous sclerosis. Biochem. Soc. Trans. 2003;31:592–6. doi: 10.1042/bst0310592. [DOI] [PubMed] [Google Scholar]
- 30.The European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–15. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- 31.Lamb RF, Roy C, Diefenbach TJ, Vinters HV, Johnson MW, Jay DG, Hall A. The TSC1 tumor suppressor hamartin regulates cell adhesion through ERM proteins and the GTPase Rho. Nat. Cell. Biol. 2000;2:281–7. doi: 10.1038/35010550. [DOI] [PubMed] [Google Scholar]
- 32.Goncharova E, Goncharov D, Noonan D, Krymskaya VP. TSC2 modulates actin cytoskelton and focal adhesion through TSC1-binding domain and the Rac1 GTPase. J. Cell. Biol. 2004;167:1171–82. doi: 10.1083/jcb.200405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haddad LA, Smith N, Bowser M, Niida Y, Murthy V, Gon-zalez-Agosti C, Ramesh V. The TSC1 tumor suppressor hamartin interacts with neurofilament-L and possibly functions as a novel integrator of the neuronal cytoskelton. J. Biol. Chem. 2002;277:44180–6. doi: 10.1074/jbc.M207211200. [DOI] [PubMed] [Google Scholar]
- 34.Maheshwar MM, Cheadle JP, Jones AC, Myring J, Fryer AE, Harris PC, Sampson JR. The GAP-related domain of tuberin, the product of the TSC2 gene, is a target for missense mutations in tuberous sclerosis. Hum. Mol. Genet. 1997;6:1991–6. doi: 10.1093/hmg/6.11.1991. [DOI] [PubMed] [Google Scholar]
- 35.York B, Lou D, Panettieri RA Jr, Krymskaya VP, Vanaman TC, Noonan DJ. Cross-talk between tuberin, calmodulin, and estrogen signalling pathways. FASEB J. 2005;19:1202–4. doi: 10.1096/fj.04-3142fje. [DOI] [PubMed] [Google Scholar]
- 36.Brook-Carter PT, Peral B, Ward CJ, Thompson P, Hughes J, Maheshwar MM, Nellist M, Gamble V, Harris PC, Sampson JR. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease—a contiguous gene syndrome. Nat. Genet. 1994;8:328–32. doi: 10.1038/ng1294-328. [DOI] [PubMed] [Google Scholar]
- 37.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N. Engl. J. Med. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 38.Chong-Kopera H, Inoki K, Li Y, Zhu T, Garcia-Gonzalo FR, Luis Rosa J, Guan KL. TSC1 stabilizes TSC2 by inhibiting the interaction between TSC2 and the HERC1 ubiquitin ligase. J. Biol. Chem. 2006;281:8313–6. doi: 10.1074/jbc.C500451200. [DOI] [PubMed] [Google Scholar]
- 39.Lu Z, Hu X, Li Y, Zheng L, Zhou Y, Jiang H, Ning T, Basang Z, Zhang C, Ke Y. Human papillomavirus 16 E6 oncoprotein interferences with insulin signaling pathway by binding to tuberin. J. Biol. Chem. 2004;279:35664–70. doi: 10.1074/jbc.M403385200. [DOI] [PubMed] [Google Scholar]
- 40.Gan B, Melkoumian ZK, Wu X, Guan KL, Guan JL. Identification of FIP200 interaction with the TSC1-TSC2 complex and its role in regulation of cell size control. J. Cell. Biol. 2005;170:379–89. doi: 10.1083/jcb.200411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakashima A, Yoshino K, Miyamoto T, Eguchi S, Oshiro N, Kikkawa U, Yonezawa K. Identification of TBC7 having TBC domain as a novel binding protein to TSC1-TSC2 complex. Biochem. Biophys. Res. Commun. 2007;361:218–23. doi: 10.1016/j.bbrc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Nellist M, Burgers PC, van den Ouweland AM, Halley DJ, Luider TM. Phosphorylation and binding partner analysis of the TSC1-TSC2 complex. Biochem. Biophys. Res. Commun. 2005;333:818–26. doi: 10.1016/j.bbrc.2005.05.175. [DOI] [PubMed] [Google Scholar]
- 43.Astrinidis A, Senapedis W, Henske EP. Hamartin, the tuberous sclerosis complex 1 gene product, interacts with polo-like kinase 1 in a phosphorylation-dependent manner. Hum. Mol. Genet. 2006;15:287–97. doi: 10.1093/hmg/ddi444. [DOI] [PubMed] [Google Scholar]
- 44.Rosner M, Hanneder M, Siegel N, Valli A, Hengstschläger M. The tuberous sclerosis gene products hamartin and tuberin are multifunctional proteins with a wide spectrum of interacting partners. Mutat. Res. 2008;658:234–46. doi: 10.1016/j.mrrev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Jones AC, Daniells CE, Snell RG, Tachataki M, Idziaszczyk SA, Krawczak M, Sampson JR, Cheadle JP. Molecular genetic and phenotypic analysis reveals difference between TSC1 and TSC2 associated familial and sporadic tuberous sclerosis. Hum. Mol. Genet. 1997;6:2155–61. doi: 10.1093/hmg/6.12.2155. [DOI] [PubMed] [Google Scholar]
- 46.Niida Y, Lawrence-Smith N, Banwell A, Hammer E, Lewis J, Beauchamp RL, Sims K, Ramesh V, Ozelius L. Analysis of both TSC1 and TSC2 for germline mutations in 126 urelated patients with tuberous sclerosis. Hum. Mutat. 1999;14:412–22. doi: 10.1002/(SICI)1098-1004(199911)14:5<412::AID-HUMU7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 47.van Slegtenhorst M, Verhoef S, Tempelaars A, Bakker L, Wang Q, Wessels M, Bakker R, Nellist M, Lindhout D, Hal-ley D, van den Ouweland A. Mutational spectrum of TSC1 gene in a cohort of 225 tuberous sclerosis complex patients: no evidence for genotype-phenotype correlation. J. Med. Genet. 1999;36:285–9. [PMC free article] [PubMed] [Google Scholar]
- 48.http://chromium.liacs.nl/lovd/index.php?select_db=TSC1 or _db=TSC2
- 49.Mayer K, Ballhausen W, Leistner W, Rott H. Three novel types of splicing aberrations in the tuberous sclerosis TSC2 gene caused by mutations apart from splice consensus sequences. Biochim. Biophys. Acta. 2000;1502:495–507. doi: 10.1016/s0925-4439(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 50.Nellist M, Verhaaf B, Goedbloed MA, Reuser AJ, van den Ouweland AM, Halley DJ. TSC2 missense mutations inhibit tuberin phosphorylation and prevent formation of the tuberin-hamartin complex. Hum. Mol. Genet. 2001;10:2889–98. doi: 10.1093/hmg/10.25.2889. [DOI] [PubMed] [Google Scholar]
- 51.Nellist M, Sancak O, Goedbloed MA, Rohe C, van Netten D, Mayer K, Tucker-Williams A, van den Ouweland AM, Halley DJ. Distinct effects of single amino-acid changes to tuberin on the function of the tuberin-hamartin complex. Eur. J. Hum. Genet. 2005;13:59–68. doi: 10.1038/sj.ejhg.5201276. [DOI] [PubMed] [Google Scholar]
- 52.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc. Natl. Acad. Sci. USA. 2002;99:13571–6. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase (PI3K)/Akt-dependent and -independent phosphorylation of tuberin. J. Biol. Chem. 2003;278:37288–96. doi: 10.1074/jbc.M303257200. [DOI] [PubMed] [Google Scholar]
- 54.Nellist M, Sancak O, Goedbloed M, Adriaans A, Wessels M, Maat-Kievit A, Baars M, Dommering C, van den Ouweland A, Halley D. Functional characterisation of the TSC1-TSC2 complex to assess multiple TSC2 variants identified in single families affected by tuberous sclerosis complex. BMC Med. Genet. 2008;9:10. doi: 10.1186/1471-2350-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts PS, Ramesh V, Dabora S, Kwiatkowski DJ. A 34 bp deletion within TSC2 is a rare polymorphism, not a pathogenic mutation. Ann. Hum. Genet. 2003;67:495–503. doi: 10.1046/j.1529-8817.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 56.Sampson JR, Maheshwar MM, Aspinwall R, Thompson P, Cheadle JP, Ravine D, Roy S, Haan E, Bernstein J, Harris PC. Renal cystic disease in tuberous sclerosis: Role of the polycystic kidney disease 1 gene. Am. J. Hum. Genet. 1997;61:843–51. doi: 10.1086/514888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleymenova E, Ibraghimov-Beskrovnaya O, Kugoh H, Everitt J, Xu H, Kiguchi K, Landes G, Harris P, Walzer C. Tuberin-dependent membrane localization of polycystin-1: a functional link between polycystic kidney disease and the TSC2 tumor suppressor gene. Mol. Cell. 2001;7:823–32. doi: 10.1016/s1097-2765(01)00226-x. [DOI] [PubMed] [Google Scholar]
- 58.Ariyurek Y, Lantinga-van Leeuwen I, Spruit L, Ravine D, Breuning MH, Peters DJ. Large Deletions in the Polycystic Kidney Disease 1 (PKD1) Gene. Hum. Mutat. 2004;23:99. doi: 10.1002/humu.9208. [DOI] [PubMed] [Google Scholar]
- 59.The European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–94. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 60.Thomas R, Mc Connell R, Whittacker J, Kirkpatrick P, Bradley J, Sandford R. Identification of mutations in the repeated part of the autosomal dominant polycystic kidney disease type 1 gene, PKD1, by long-range PCR. Am. J. Hum. Genet. 1999;65:39–49. doi: 10.1086/302460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JK, Sayers TJ, Brooks AD, Back TC, Young HA, Komschlies KL, Wipsgginton JM, Wiltrout RH. IFN-g-dependent delay of in vivo tumor progression by Fas overexpression on murine renal cancer cells. J. Immunol. 2000;164:231–9. doi: 10.4049/jimmunol.164.1.231. [DOI] [PubMed] [Google Scholar]
- 62.Becker C, Pohla H, Frankenberger B, Schüler T, Assenmacher M, Schendel DJ, Blankenstein T. Adoptive tumor therapy with T lymphocytes enriched through an IFN-g capture assay. Nat. Med. 2001;7:1159–62. doi: 10.1038/nm1001-1159. [DOI] [PubMed] [Google Scholar]
- 63.Dabora SL, Roberts P, Nieto A, Perez R, Jozwiak S, Franz D, Bissler J, Thiele EA, Sims K, Kwiatkowski DJ. Association between a high-expressing interferon-γ allele and a lower frequency of kidney angiomyolipomas in TSC2 Patients. Am. J. Hum. Genet. 2002;71:750–8. doi: 10.1086/342718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pravica V, Asderakis A, Perrey C, Hajeer A, Sinnott PJ, Hutchinson IV. In vitro production of IFN-γ correlates with CA repeat polymorphism in the human IFN-γ gene. Eur. J. Immunogenet. 1999;26:1–3. doi: 10.1046/j.1365-2370.1999.00122.x. [DOI] [PubMed] [Google Scholar]
- 65.Habib SL, Danial E, Nath S, Schneider J, Jenkinson CP, Duggirala R, Abboud HE, Thameem F. Genetic polymorphisms in OGG1 and their association with angiomyolipoma, a benign kidney tumor in patients with tuberous sclerosis. Cancer. Biol. Ther. 2007;8:7. doi: 10.4161/cbt.7.1.5120. [DOI] [PubMed] [Google Scholar]
- 66.Fahsold R, Rott HD, Lorenz P. A third gene locus for tuberous sclerosis is closely linked to the phenylalanine hydroxylase gene locus. Hum. Genet. 1991;88:85–90. doi: 10.1007/BF00204934. [DOI] [PubMed] [Google Scholar]
- 67.Hall JG. Somatic mosaicism: observations related to clinical genetics. Am. J. Hum. Genet. 1988;43:355–63. [PMC free article] [PubMed] [Google Scholar]
- 68.van der Meulen MA, van der Meulen MJ, te Meerman GT. Recurrence risk for germinalmosaics revisited. J. Med. Genet. 1995;32:102–4. doi: 10.1136/jmg.32.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Northrup H, Wheless JW, Bertin TK, Lewis RA. Variability of expression in tuberous sclerosis. J. Med. Genet. 1993;30:41–3. doi: 10.1136/jmg.30.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruggieri M, Carbonare C, Magro G, Magone N, Grasso S, Tine A, Pavone L, Gomez MR. Tuberous sclerosis complex: neonatal deaths in three of four children of consanguineous, non-expressing parents. J. Med. Genet. 1997;34:256–260. doi: 10.1136/jmg.34.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yates JRW, van Bakel I, Sepp T, Payne SJ, Webb DW, Nevin NC, Green AJ. Female germline mosaicism in tuberous sclerosis confirmed by molecular genetic analysis. Hum. Mol. Genet. 1997;6:2265–9. doi: 10.1093/hmg/6.13.2265. [DOI] [PubMed] [Google Scholar]
- 72.Rose VM, Au KS, Pollom G, Roach ES, Prashner HR, Northrup H. Germ-line mosaicism in tuberous sclerosis: how common? Am. J. Hum. Genet. 1999;64:986–92. doi: 10.1086/302322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum. Mol. Genet. 2005;14:251–8. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- 74.Mak BC, Kenerson HL, Aicher LD, Barnes EA, Yeung RS. Aberrant b-Catenin Signaling in Tuberous Sclerosis. Am. J. Pathol. 2005;167:107–16. doi: 10.1016/s0002-9440(10)62958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Astrinidis A, Senapedis W, Coleman TR, Henske EP. Cell cycle-regulated phosphorylation of hamartin, the product of the tuberous sclerosis complex 1 gene, by cyclin-dependent kinase 1/cyclin B. J. Biol. Chem. 2003;278:51372–9. doi: 10.1074/jbc.M303956200. [DOI] [PubMed] [Google Scholar]
- 76.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk: implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 77.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. USA. 2004;101:13489–94. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ballif BA, Roux PP, Gerber SA, MacKeigan JP, Blenis J, Gygi SP. Quantitative phosphorylation profiling of the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets, the tuberous sclerosis tumor suppressors. Proc. Natl. Acad. Sci. USA. 2005;102:667–72. doi: 10.1073/pnas.0409143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, Inoki K, Vacratsis P, Guan KL. The p38 and MK2 kinase cascade phosphorylates tuberin, the tuberous sclerosis 2 gene product, and enhances its interaction with 14-3-3. J. Biol. Chem. 2003;278:13663–71. doi: 10.1074/jbc.M300862200. [DOI] [PubMed] [Google Scholar]
- 80.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol. Cell. 2002;10:151–62. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 81.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 82.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 Integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 83.Yaffe MB, Cantley LC. Signal transduction. Grabbing phosphoproteins. Nature. 1999;402:30–1. doi: 10.1038/46925. [DOI] [PubMed] [Google Scholar]
- 84.Nellist M, Goedbloed MA, Halley DJ. Regulation of tuberous sclerosis complex (TSC) function by 14-3-3 proteins. Biochem. Soc. Trans. 2003;31:587–91. doi: 10.1042/bst0310587. [DOI] [PubMed] [Google Scholar]
- 85.Shumway SD, Li Y, Xiong Y. 14-3-3 binds to and negatively regulates the tuberous sclerosis complex 2 (TSC2) tumor suppressor gene product, tuberin. J. Biol. Chem. 2003;278:2089–92. doi: 10.1074/jbc.C200499200. [DOI] [PubMed] [Google Scholar]
- 86.Yasui S, Tsuzaki K, Ninomiya H, Floricel F, Asano Y, Maki H, Takamura A, Nanba E, Higaki K, Ohno K. The TSC1 gene product hamartin interacts with NADE. Mol. Cell. Neurosci. 2007;35:100–8. doi: 10.1016/j.mcn.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 87.Mukai J, Hachiya T, Shoji-Hoshino S, Kimura MT, Nadano D, Suvanto P, Hanaoka T, Li Y, Irie S, Greene LA, Sato TA. NADE, a p75NTR-associated cell death executor, is involved in signal transduction mediated by the common neurotrophin receptor p75NTR. J. Biol. Chem. 2000;275:17566–70. doi: 10.1074/jbc.C000140200. [DOI] [PubMed] [Google Scholar]
- 88.Mukai J, Shiji S, Kimura MT, Okubo S, Sano H, Suvanto P, Li Y, Irie S, Sato TA. Structure-function analysis of NADE. J. Biol. Chem. 2002;227:13973–82. doi: 10.1074/jbc.M106342200. [DOI] [PubMed] [Google Scholar]
- 89.Wu EHT, Wu KHH, Wong YH. Tuberin: a stimulus-regulated tumor suppressor protein controlled by a diverse array of receptor tyrosine kinases and G protein-coupled receptors. Neurosignals. 2006;15:217–27. doi: 10.1159/000101333. [DOI] [PubMed] [Google Scholar]
- 90.Cao Y, Kamioka Y, Yokoi N, Kobayashi T, Hino O, Ono-dera M, Mochizuki N, Nakae J. Interaction of FOXO1 and TSC2 induces insulin resistance through activation of the mammalian target of rapamycin/p70 S6K pathway. J. Biol. Chem. 2006;281:40242–51. doi: 10.1074/jbc.M608116200. [DOI] [PubMed] [Google Scholar]
- 91.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat. Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 92.Tee AR, Blenis J. mTOR, translational control and human disease. Semin. Cell. Dev. Biol. 2005;16:29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 93.Rosner M, Freilinger A, Hengstschläger M. Akt regulates nuclear/cytoplasmic localization of tuberin. Oncogene. 2007;26:521–31. doi: 10.1038/sj.onc.1209812. [DOI] [PubMed] [Google Scholar]
- 94.Rosner M, Freilinger A, Hengstschläger M. The tuberous sclerosis genes and regulation of the cyclin-dependent kinase inhibitor p27. Mutat. Res. 2006;613:10–6. doi: 10.1016/j.mrrev.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 95.Carrano AC, Etan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell. Biol. 1999;1:193–9. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 96.Sutterlüty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Müller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell. Biol. 1999;1:207–14. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 97.Rosner M, Hengstschläger M. Tuberin binds p27 and negatively regulates its interaction with the SCF component Skp2. J. Biol. Chem. 2004;279:48707–15. doi: 10.1074/jbc.M405528200. [DOI] [PubMed] [Google Scholar]
- 98.Ozcan U, Ozcan L, Yilmaz E, Düvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell. 2008;29:541–51. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Habib SL, Simone S, Barnes JJ, Abboud HE. Tuberin haploinsufficiency is associated with the loss of OGG1 in rat kidney tumors. Mol. Cancer. 2008;7:10. doi: 10.1186/1476-4598-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kobayashi T, Mitani H, Takahashi R, Hirabayashi M, Ueda M, Tamura H, Hino O. Transgenic rescue from embryonic lethality and renal carcinogenesis in the Eker rat model by introduction of a wild-type Tsc2 gene. Proc. Natl. Acad. Sci. USA. 1997;94:3990–3. doi: 10.1073/pnas.94.8.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rennebeck G, Kleymenova EV, Anderson R, Yeung RS, Artzt K, Walker CL. Loss of function of the tuberous sclerosis 2 tumor suppressor gene results in embryonic lethality characterized by disrupted neuroepithelial growth and development. Proc. Natl. Acad. Sci. USA. 1998;95:15629–34. doi: 10.1073/pnas.95.26.15629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sexdependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum. Mol. Genet. 2002;11:525–34. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 103.Kobayashi T, Hirayama Y, Kobayashi E, Kubo Y, Hino O. A germline insertion in the tuberous sclerosis (Tsc2) gene gives rise to the Eker rat model of dominantly inherited cancer. Nat. Genet. 1995;9:70–4. doi: 10.1038/ng0195-70. [DOI] [PubMed] [Google Scholar]
- 104.Yeung RS, Katsetos CD, Klein-Szanto A. Subependymal astrocytic hamartomas in the Eker rat model of tuberous sclerosis. Am. J. Pathol. 1997;151:1477–86. [PMC free article] [PubMed] [Google Scholar]
- 105.Kobayashi T, Urakami S, Hirayama Y, Yamamoto T, Nishizawa M, Takahara T, Kubo Y, Hino O. Intragenic Tsc2 somatic mutations as Knudson's second hit in spontaneous and chemically induced renal carcinomas in the Eker rat model. Jpn. J. Cancer Res. 1997;88:254–61. doi: 10.1111/j.1349-7006.1997.tb00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–93. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- 107.Leevers SJ, Weinkove D, MacDougall LK, Hafen E, Water-field MD. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 1996;15:6584–94. [PMC free article] [PubMed] [Google Scholar]
- 108.Böhni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–75. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- 109.Verdu J, Buratovich MA, Wilder EL, Birnbaum MJ. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat. Cell. Biol. 1999;1:500–6. doi: 10.1038/70293. [DOI] [PubMed] [Google Scholar]
- 110.Gao X, Neufeld TP, Pan D. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev. Biol. 2000;221:404–18. doi: 10.1006/dbio.2000.9680. [DOI] [PubMed] [Google Scholar]
- 111.Cho KS, Lee JH, Kim S, Kim D, Koh H, Lee J, Kim C, Kim J, Chung J. Drosophila phosphoinositide-dependent kinase-1 regulates apoptosis and growth via the phosphoinositide 3-kinase-dependent signaling pathway. Proc. Natl. Acad. Sci. USA. 2001;98:6144–9. doi: 10.1073/pnas.101596998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285:2126–9. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- 113.Ito N, Rubin GM. Gigas, a Drosophila homolog of tuberous sclerosis gene product-2, regulates the cell cycle. Cell. 1999;96:529–39. doi: 10.1016/s0092-8674(00)80657-1. [DOI] [PubMed] [Google Scholar]
- 114.Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes. Dev. 2001;15:1383–92. doi: 10.1101/gad.901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–55. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 116.Matsumoto S, Bandyopadhyay A, Kwiatkowski DJ, Maitra U, Matsumoto T. Role of the Tsc1-Tsc2 complex in signalling and transport across the cell membrane in the fission yeast Schizosaccharomyces pombe. Genetics. 2002;161:1053–63. doi: 10.1093/genetics/161.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jozwiak J, Jozwiak S, Oldak M. Molecular activity of sirolimus and its possible applications in tuberous sclerosis treatment. Med. Res. Rev. 2006;26:160–80. doi: 10.1002/med.20049. [DOI] [PubMed] [Google Scholar]
- 118.Bierer BE, Mattila PS, Standaert RF, Herzenberg LA, Bu-rakoff SJ, Crabtree G, Schreiber SL. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc. Natl. Acad. Sci. USA. 1990;87:9231–5. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Edinger AL, Linardic CM, Chiang GG, Thompson CB, Abraham RT. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;63:8451–60. [PubMed] [Google Scholar]
- 120.Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–58. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 121.Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethura-man G, Dinopoulos A, Thomas G, Crone KR. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann. Neurol. 2006;59:490–8. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 122.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, Salisbury S, Franz DN. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rauktys A, Lee N, Lee L, Dabora SL. Topical rapamycin inhibits tuberous sclerosis tumor growth in a nude mouse model. BMC Dermatol. 2008;8:1. doi: 10.1186/1471-5945-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schwartz RA, Fernandez G, Kotulska K, Jozwiak S. Tuberous sclerosis complex: advances in diagnosis, genetics and management. J. Am. Acad. Dermatol. 2007;57:189–202. doi: 10.1016/j.jaad.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 125.Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann. Neurol. 2008;63:444–53. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]