Abstract
Estrogen-related receptor (ERRα) plays a critical role in basal and cAMP-induced expression of the human surfactant protein-A (SP-A) gene in lung type II cells through direct binding to an ERR response element (ERRE, 5′-TGACCTTA-3′) within its 5′-flanking region. Furthermore, protein kinase A (PKA) up-regulates ERRα activation of the hSP-A promoter. In the present study, using cultured human fetal lung type II cells, we observed that cAMP enhanced ERRα phosphorylation and nuclear expression levels. cAMP/PKA stimulation of ERRα activation of the SP-A promoter was blocked by the PKA inhibitor, H89, whereas the MAPK P38 inhibitor, SB203580, and the MAPK kinase inhibitor, PD98059, had negligible to modest effects. This suggests that cAMP acts selectively through PKA to increase ERRα transcriptional activity. Of several coactivators tested, steroid receptor coactivator 2 (SRC-2) had the most pronounced effect to increase ERRα transcriptional activity at the SP-A promoter; this was enhanced by cotransfection with PKA catalytic subunit (PKAcat). Interestingly, SRC-2, ERRα, and PKAcat in type II cell nuclear extracts interacted at the ERRE; this was enhanced by cAMP and inhibited by H89. cAMP increased in vivo binding of PKAcat and SRC-2 to the ERRE genomic region in lung type II cells. In mutagenesis studies, three serines (S87, S114, and S277) were found to be critical for PKA and SRC-2 induction of ERRα transcriptional activity. Collectively, these findings indicate that cAMP/PKA signaling enhances ERRα phosphorylation and nuclear localization, recruitment to the SP-A promoter, and interaction with PKAcat and SRC-2, resulting in the up-regulation of SP-A gene transcription.
Cyclic AMP upregulation of SP-A promoter activity in lung type II cells is mediated by PKA-induced expression, phosphorylation and DNA binding of ERRα.
Surfactant protein A (SP-A), the major protein of the lipoprotein surfactant, is a C-type lectin that plays an important role in innate immunity within the lung alveolus (see Ref. 1 for review). SP-A gene transcription is initiated in fetal lung after approximately 80% of gestation is completed and reaches maximal levels just before birth (2). Our findings suggest that SP-A secreted from the fetal lung during late gestation may serve as a signal for the initiation of labor (3). SP-A gene expression is essentially lung specific (4), occurs primarily in alveolar type II cells (5,6,7), and is up-regulated by cAMP and IL-1 and inhibited by glucocorticoids (8,9,10,11) in human fetal lung type II cells; cAMP and IL-1 stimulation of SP-A expression is prevented when the cells are cultured in a hypoxic environment (12,13,14). The human genome contains two highly similar SP-A genes, SP-A1 and SP-A2 (15,16). In studies using midgestation human fetal lung explants, hSP-A2 was found to be far more responsive to the inductive effects of cAMP analogs than hSP-A1 (17,18); thus, our studies to define the mechanisms for cAMP regulation of SP-A expression have focused on the gene encoding hSP-A2.
In studies using transgenic mice (19,20) and transfected type II cells (21,22,23,24,25), we found that as little as approximately 300 bp of SP-A 5′-flanking sequence mediates lung cell-specific, developmental, and cAMP-regulated expression. This region contains four response elements that are highly conserved in the SP-A genes of various species (26). These include an element that binds orphan nuclear receptor estrogen-related receptor (ERR)α at −240 bp (ERRE) (27), a thyroid transcription factor (TTF)-1 binding element (TBE) at −170 bp, which binds TTF-1/Nkx2.1 and nuclear factor-κB (NF-κB) (14), an E-box at −87 bp, which binds the basic helix-loop-helix-leucine zipper transcription factors, upstream stimulatory factor 1 (28) and upstream stimulatory factor 2 (29), and a GT box at −60 bp, which binds Sp1 (24). In type II cell transfection studies using reporter constructs containing 5′-flanking sequences from the rabbit (21,22,29,30), human (14,23,24,27,31), and baboon (31) SP-A genes, we found that the ERRE, TBE, E-box, and the GT-box each serve essential roles in basal and cAMP induction of SP-A promoter activity. Mutation in any one of these elements markedly reduces or abolishes cAMP induction of SP-A expression. This suggests that this genomic region serves as an enhanceosome and that basal and cAMP induction of SP-A2 promoter activity are mediated by the cooperative interaction of transcription factors bound to each of these response elements (26). We previously observed that cAMP acts through protein kinase A (PKA) to increase TTF-1 phosphorylation (31,32) and binding to TBE (31) and enhances TTF-1 interaction with coactivators CREB-binding protein (CBP) and steroid receptor coactivator (SRC)-1 to further increase transcriptional activity (33).
ERRα is an orphan member of the nuclear receptor family that appears to play an important role in the regulation of lipid homeostasis and energy metabolism (34). We recently found that ERRα−/− mice manifested decreased SP-A expression compared with wild-type (wt) and heterozygous littermates (27). Moreover, ERRα overexpression in lung type II cells enhanced cAMP induction of endogenous hSP-A expression, and cotransfection of PKAcat enhanced ERRα stimulation of hSP-A2 promoter activity (27). In recent studies using transgenic mice carrying fusion genes comprised of various amounts of 5′-flanking sequence from the hSP-A2 gene fused to hGH, as reporter, we observed that as little as 175 bp of hSP-A2 5′-flanking DNA promoted appropriate developmental and lung cell-specific expression. However, the 175-bp genomic region (which lacks the ERRE) was insufficient to mediate cAMP regulation of SP-A promoter activity (20). Collectively, these findings suggest that ERRα is an important mediator of SP-A gene expression and its induction by cAMP.
ERRα does not have a natural known ligand; however, its activity appears to be regulated by growth factor-signaling pathways. ERRα homodimerization, DNA binding, and transcriptional activation have been reported to be regulated by phosphorylation (35,36). In breast cancer cells, epidermal growth factor (EGF/ErbB1) enhanced ERRα phosphorylation and DNA-binding activity (35). These effects of EGF were suggested to be mediated by protein kinase Cδ (PKCδ), which selectively catalyzed phosphorylation of the ERRα DNA-binding domain in vitro (35). PKCδ also enhanced ERRα-mediated transcriptional activation of the pS2 promoter in MCF-7 breast cancer cells (35). Overexpression of ErbB2/HER-2/neu in MCF-7 cells has also been found to increase ERRα transcriptional activity (36). Furthermore, ERRα was phosphorylated in vitro by signaling components of the ErbB2 pathway, MAPK and Akt, whereas ERRα transcriptional activity in breast cancer cells was abrogated by specific inhibitors of ErbB2 and of MAPK kinase (MEK)/MAPK and PI3K/Akt signaling pathways (36). It was suggested that the ErbB2 status of a cell and the resulting phosphorylation of specific residues in ERRα may be required for the specific recruitment of coactivator glucocorticoid receptor-interacting protein (GRIP)-1/SRC-2/transcriptional intermediary factor 2/nuclear receptor coactivator 2 (36).
In light of the importance of cAMP and PKA in the regulation of SP-A gene transcription, in the present study, we further explored the role of PKA in cAMP-mediated activation of ERRα transcriptional activity. We observed that PKA enhanced ERRα transcriptional activity at the hSP-A2 promoter. This induction was specifically blocked by the PKA inhibitor, H89, but only modestly inhibited by the MEK inhibitor, PD98059, and unaffected by the P38/MAPK inhibitor, SB20580, suggesting a direct effect of PKA in ERRα activation. Importantly, cAMP enhanced binding of the PKA catalytic subunit (PKAcat), the coactivator, SRC-2, and ERRα at the SP-A ERRE. Furthermore SRC-2 potently enhanced ERRα transcriptional activation of the hSP-A2 promoter in a PKA-dependent manner. Our findings suggest that ERRα may be an important mediator of cAMP/PKA induction of SP-A promoter activity in lung type II cells.
Results
ERRα expression and nuclear localization are enhanced by cAMP in lung type II cells
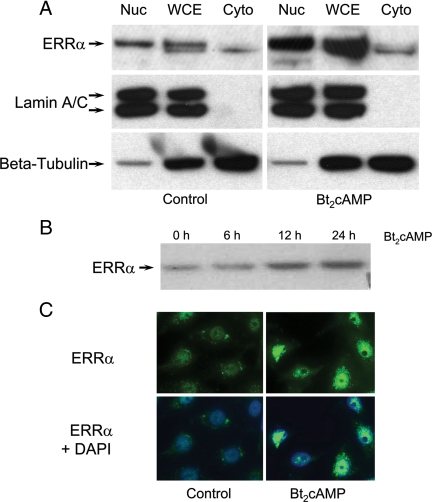
Previously, we observed that the ERRE at −240 bp is essential for cAMP induction of hSP-A expression (23). ERRα overexpression enhanced cAMP stimulation of endogenous hSP-A expression, and cotransfection of PKA catalytic subunit increased ERRα stimulation of hSP-A promoter activity in transfected lung adenocarcinoma cells (27). To investigate the mechanisms for the inductive effects of cAMP on ERRα transcriptional activity, effects of dibutyryl-cAMP (Bt2cAMP) (1 mm) on ERRα expression and subcellular localization were analyzed in human type II cells by immunoblotting and immunocytochemistry, respectively (Fig. 1). After 24 h of culture in the presence of Bt2cAMP, ERRα levels were markedly increased in whole-cell lysates and in isolated nuclear fractions (Fig. 1A, upper panel). Reprobing of the blots with antibodies to lamin A/C, a nuclear marker, indicates that there was no nuclear protein contamination of the cytoplasmic fractions (Fig. 1A, middle panel). The stimulatory effects of Bt2cAMP on nuclear levels of ERRα were evident after 12 h of cAMP treatment (Fig. 1B). The inductive effects of Bt2cAMP on nuclear levels of ERRα also were evident by immunostaining of type II cells after 24 h of treatment (Fig. 1C). In this immunocytochemical study, type II cells were cultured in the absence or presence of Bt2cAMP in the presence of the nuclear export inhibitor leptomycin to block ERRα re-entry into the cytoplasm. These findings suggest that ERRα expression and nuclear localization are enhanced by cAMP.
Figure 1.
Bt2cAMP treatment increases ERRα expression and nuclear localization in lung type II cells. A, Lung type II cells were cultured in the absence (left upper panels) or presence (right upper panels) of Bt2cAMP (1 mm) for 24 h. ERRα levels in whole-cell extracts (WCE) and in cytoplasmic (Cyto) and nuclear (Nuc) fractions were analyzed by immunoblotting for ERRα, lamin A/C, and β-tubulin. Shown is a representative experiment that was repeated three times with similar observations. B, Lung type II cells were cultured in the presence of Bt2cAMP for 0, 6 h, 12 h, or 24 h. ERRα expression levels in nuclei were analyzed by immunoblotting for ERRα. Shown is a representative experiment that was repeated three times with similar observations. C, Lung type II cells were treated with the nuclear export inhibitor leptomycin B (40 nm) for 1 h, followed by incubation with vehicle or 1 mm Bt2cAMP for 24 h. Cells were fixed and analyzed for ERRα localization by immunofluoresence. DAPI was used for cell nuclear staining.
ERRα stimulation of hSP-A2 promoter activity and nuclear localization is selectively blocked by the PKA inhibitor H89
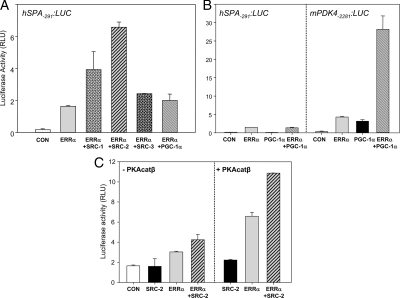
In previous studies, we observed that ERRα transcriptional activity was potentiated by PKA (27). To determine whether this effect of PKA was direct, or mediated via PKA activation of the MAPK signaling pathway (37), A549 lung adenocarcinoma cells were transfected with a hSP-A-291:LUC reporter construct containing the ERRE, and cotransfected with or without an ERRα expression vector, PKA catalytic β-subunit (PKAcatβ), and the appropriate empty vectors. After transfection, the cells were cultured for an additional 24 h in the absence or presence of the PKA inhibitor, H89 (10 μm), the p38 MAPK inhibitor, SB203580 (5 μm), or the MEK inhibitor, PD98059 (50 μm). As we reported previously (27), overexpression of ERRα increased hSP-A2 promoter activity, as compared with the empty vector control. Although cotransfected PKAcatβ had no effect on its own, it synergistically enhanced ERRα stimulation of hSP-A2 promoter activity (Fig. 2A). The PKA inhibitor H89 abrogated the stimulatory effects of ERRα and of ERRα + PKAcatβ on hSP-A2 promoter activity (Fig. 2A). By contrast, SB203580 had no apparent effect on ERRα transcriptional activity in the absence or presence of cotransfection of PKAcatβ, whereas PD98059 caused only modest inhibition (Fig. 2B). As shown in Fig. 2C, H89 inhibited cAMP stimulation of ERRα nuclear levels, whereas PD98059 had no apparent effect. These findings suggest that PKA primarily acts directly to enhance ERRα activation of the hSP-A2 promoter.
Figure 2.
PKA stimulates ERRα activation of the hSP-A2 promoter and increases nuclear levels of ERRα in type II cells. A549 cells were cotransfected with an hSP-A-291:LUC reporter construct containing the ERRE, in the absence or presence of expression vectors for ERRα ± PKA-β catalytic subunit (PKAcatβ). A Renilla luciferase expression plasmid (phRL-TK) was cotransfected as internal control to correct for transfection efficiency. Twenty-four hours after transfection, cells were treated for an additional 24 h either with H89 (10 μm) (panel A), SB203580 (5 μm), or PD98059 (50 μm) (panel B). Cells were harvested, and cell lysates were assayed for firefly luciferase and Renilla luciferase activities. Data are presented as the mean ± sem of triplicate samples from a representative experiment that was repeated three times. RLU, Relative light units. C, Human fetal lung type II cells were cultured in the absence or presence of Bt2cAMP in the absence or presence of H89 or PD98059 for 24 h. Cells were fixed and analyzed for ERRα localization by immunofluoresence. DAPI was used for cell nuclear staining. CON, Control.
SRC-2 is a potent coactivator of ERRα transcriptional activity and potentiates PKA activation of ERRα transcriptional activity at the hSP-A2 promoter
Several putative cofactors have been suggested to be important for ERRα transcription activity, including the p160 family member, SRC-2/GRIP1 (36,38) and PPARγ coactivator (PGC)-1α (39,40). To further define the transcriptional mechanisms for ERRα activation of hSP-A2 expression, we analyzed the effects of a number of candidate cofactors in A549 cells cotransfected with the hSP-A-291:LUC reporter and ERRα and PKAcatβ expression vectors. As can be seen in Fig. 3A, cotransfection of SRC-1 and -2 enhanced ERRα stimulation of hSP-A2 promoter activity by 2- and 3.5-fold, respectively, whereas SRC-3/pCIP/ACTR/AIB1 and PGC-1α expression vectors had little or no effect. The lack of effect of PGC-1α was surprising, in light of its reported action to markedly enhance ERRα transcriptional activation via two estrogen response elements upstream of the alcohol dehydrogenase minimal promoter (39) and via an ERRE within the mouse pyruvate dehydrogenase kinase 4 (mPDK4) promoter (40). We therefore carried out parallel cotransfections in lung A549 adenocarcinoma cells of ERRα and PGC-1α expression vectors and either hSP-A-291:LUC or mPDK4-2281:LUC (gift of Dr. Daniel P. Kelly, Burnham Institute, Orlando, FL) reporter constructs. Interestingly, whereas PGC-1α markedly coactivated ERRα stimulation of the mPDK4 promoter activity, it had no effect on ERRα up-regulation of hSP-A2 promoter activity in the A549 cells (Fig. 3B). These findings suggest that promoter context plays an important role in coregulator-specific effects.
Figure 3.
SRC-2 is the most potent coactivator of ERRα transcriptional activity and enhances PKA activation of ERRα transcriptional activity at the hSP-A promoter. A, A549 human lung adenocarcinoma cells were cotransfected with an hSP-A-291:LUC reporter construct together with an expression vector encoding ERRα in the absence or presence of expression vectors for coactivators SRC-1, SRC-2, SRC-3, or PGC-1α. The phRL-TK expression plasmid was cotransfected to correct for transfection efficiency. Cells were harvested after 48 h, and cell lysates were assayed for firefly luciferase and Renilla luciferase activities. Data are presented as the mean ± sem of triplicate samples from a representative experiment that was repeated three times with similar observations. B, A549 cells were cotransfected either with hSP-A-291:LUC or mPDK4-2281:LUC reporter constructs, and expression vectors for ERRα and PGC-1α, added alone or in combination. The phRL-TK expression plasmid was cotransfected to correct for transfection efficiency. Cells were harvested after 48 h, and cell lysates were assayed for firefly luciferase and Renilla luciferase activities. Data are the mean ± sem of triplicate samples from a representative experiment repeated twice with similar observations. C, A549 cells were cotransfected with a hSP-A-291:LUC reporter construct in the absence or presence of expression vectors for ERRα, SRC-2, and PKAcatβ, in various combinations. phRL-TK expression plasmid was cotransfected to correct for transfection efficiency. Cells were harvested after 48 h, and lysates were assayed for firefly luciferase and Renilla luciferase activities. Data are presented as the mean ± sem of triplicate samples from a representative experiment that was repeated three times with similar observations. RLU, Relative light units; CON, control.
To examine the role of PKA on SRC-2 and ERRα enhancement of hSP-A2 promoter activity, A549 cells were cotransfected with hSP-A-291:LUC and expression vectors for ERRα, SRC-2, and PKAcatβ in various combinations; the corresponding empty vectors were cotransfected as controls, where appropriate. As can be seen, the effect of SRC-2 to increase ERRα transcriptional activity was further enhanced by cotransfection of PKAcat (Fig. 3C). These results suggest that PKAcat and SRC-2 act in a cooperative manner to increase ERRα transcriptional activity at the hSP-A2 promoter.
PKAcat and SRC-2 interaction with ERRα in lung type II cells is enhanced by cAMP
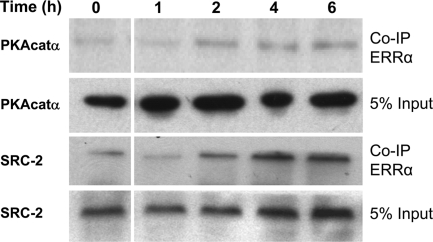
To examine the possible interactions of ERRα, PKA, and SRC-2 in lung type II cells and the effects of cAMP treatment, type II cells were incubated with recombinant adenoviruses expressing FLAG-tagged ERRα for 3 h. After viral infection, the type II cells were cultured in control, serum-free medium for 12 h, followed by incubation with Bt2cAMP for 0–6 h. Nuclear proteins were isolated from the cells at the various time points, and FLAG-tagged ERRα proteins were isolated using agarose-conjugated mouse anti-FLAG antibodies. The FLAG-tagged ERRα was eluted from the beads using 3×FLAG peptide, fractionated by SDS-PAGE and subjected to immunoblotting using anti-SRC-2 or PKAcat antibodies. As can be seen in the immunoblot in Fig. 4, both PKAcat and SRC-2 were present in a complex with ERRα; PKAcat and SRC-2 interactions with ERRα were enhanced by cAMP treatment in a time-dependent manner. These findings suggest that ERRα, SRC-2, and PKAcat form a complex in type II cells that is enhanced by cAMP.
Figure 4.
Bt2cAMP treatment increases the interaction of PKAcatα and SRC-2 with ERRα in lung type II cells. Lung type II cells were infected with recombinant adenovirus encoding Flag-tagged ERRα and cultured in the presence of Bt2cAMP (1 mm) for 0–6 h. Cell lysates were prepared at each time point and subjected to immunoprecipitation using an anti-Flag antibody. The immunoprecipitates were resolved on a 4–12% NUPAGE gradient gel and transferred to nitrocellulose. The association of the PKAcatα and SRC-2 with ERRα was analyzed by immunoblotting. Shown is a representative experiment that was repeated twice with similar observations. Co-IP, Coimmunoprecipitation.
cAMP enhances ERRα, PKAcat, and SRC-2 binding to the ERRE
Recent findings suggest that kinases can physically associate with promoters of actively transcribed genes (41). Having observed that PKAcatα associates with ERRα, we used a DNA affinity precipitation assay (DAPA) to determine whether a complex of ERRα, SRC-2, and PKAcat binds to the ERRE and whether binding activity is altered by cAMP in human fetal lung type II cells. Nuclear extracts from type II cells treated with or without cAMP and the PKA inhibitor H89 for 6 h were incubated with the annealed biotinylated ERRE from the hSP-A2 gene or with ERREmut oligonucleotides; the binding of endogenous ERRα, SRC-2, and PKAcatα was analyzed by immunoblotting. As can be seen in Fig. 5A, ERRα, PKAcatα, and SRC-2 bound to the wt ERRE, but not to the mutated response element. Furthermore, binding of ERRα (Fig. 5B), PKAcatα (Fig. 5C), and SRC-2 (Fig. 5D) was enhanced by cAMP treatment, whereas cAMP-induced binding activity was inhibited by H89. H89 treatment also reduced binding of ERRα and PKAcatα in nuclear extracts of type II cells cultured in the absence of Bt2cAMP. As can be seen, nuclear levels of ERRα, PKAcatα, and SRC-2 were unaffected after 6 h of cAMP treatment.
Figure 5.
Binding of endogenous ERRα, PKAcatα, and SRC-2 to the hSP-A2 ERRE in type II cell nuclear extracts is enhanced by cAMP and inhibited by H89. Nuclear extracts from type II cells that had been cultured with or without Bt2cAMP for 6 h were incubated with biotin-labeled oligonucleotides containing the wt (ERRE) or mutated ERRE (ERREmut) and captured using Tetralink Tetrameric Avidin Resin. The products were resolved on 4–10% gradient SDS-PAGE gels. Immunoblotting was performed using ERRα, PKAcatα, and SRC-2 antibodies (panel A). Nuclear extracts from type II cells that had been cultured for 6 h ± Bt2cAMP ± H89 were incubated with the annealed biotinylated hSP-A2 ERRE; binding of endogenous ERRα (panel B), PKAcatα (panel C), and SRC-2 (panel D) was analyzed by immunoblotting. Shown are representative experiments that were repeated two to four times with similar observations. CON, Control.
cAMP increases in vivo binding of PKA and SRC-2 to the hSP-A2 genomic region containing the ERRE in human fetal lung type II cells
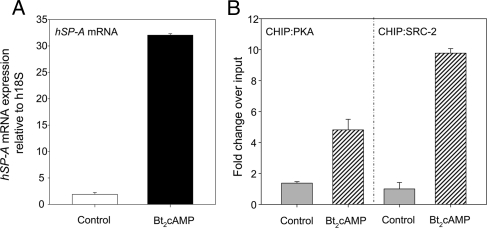
To determine whether cAMP alters binding of SRC-2 and PKAβ to the ERRE in vivo, we carried out chromatin immunoprecipitation (ChIP) assays using human fetal lung type II cells. The cells were cultured for 24 h ± Bt2cAMP. ChIP assays were then carried out using quantitative PCR with primers that amplified a 125-bp region containing the ERRE. As can be seen in Fig. 6, treatment of type II cells with Bt2cAMP caused a pronounced increase in recruitment of PKA and SRC-2 to the hSP-A2 ERRE-containing region, indicating that PKA and SRC-2 binding to the hSP-A2 promoter is enhanced by cAMP treatment of type II cells.
Figure 6.
cAMP induction of SP-A expression is associated with increased in vivo binding of PKA and SRC-2 to the ERRE region of the hSP-A2 promoter. Human fetal lung type II cells were cultured for 24 h in the absence or presence of Bt2cAMP (1 mm). A, RNA was isolated from some of the dishes of cells for analysis of SP-A mRNA using quantitative RT-PCR. B, ChIP was used to analyze in vivo binding of PKAcatβ and SRC-2 to a 125-bp genomic region of the hSP-A2 promoter containing the ERRE using qPCR. Data are expressed as arbitrary units. The bars represent the means ± sem of data from triplicate samples.
cAMP treatment increases ERRα phosphorylation in human fetal lung type II cells
In light of stimulatory effects of cAMP/PKA on ERRα nuclear localization, transcriptional activity, and association with SRC-2 at the ERRE, we next sought to determine whether cAMP enhances ERRα phosphorylation. When whole-cell lysates, nuclear extracts, and cytoplasmic fractions of type II cells cultured in the presence of Bt2cAMP for 24 h were analyzed by immunoblotting, it was evident that ERRα migrated as two bands in the whole-cell lysate; the nuclear extract was enriched in the slower migrating immunoreactive band, whereas the cytoplasmic fraction was enriched in the more rapidly migrating band (Fig. 7A). To determine whether these differences in migration could be due to phosphorylation, type II cell nuclear and cytoplasmic extracts were incubated for 10 min with or without λ-phosphatase. As can be seen, although the λ-phosphatase had no effect on migration of the cytoplasmic ERRα, the apparent molecular weight of nuclear ERRα protein was reduced to that of the cytoplasmic species (Fig. 7A).
Figure 7.
Bt2cAMP increases ERRα phosphorylation. A, Human fetal lung type II cells were incubated in the absence or presence of Bt2cAMP for 24 h. Whole-cell lysates (WCE), as well as nuclear extracts (NE) and cytoplasmic fractions (Cyto) were resolved on 4–12% NUPAGE gradient SDS gels (Invitrogen) and analyzed by immunoblotting using ERRα antibodies (upper panel). Lung type II cell nuclear (NE, 10 μg) and cytoplasmic (Cyto, 30 μg) proteins were treated with vehicle (−) or with λ-phosphatase (400 U) at 30 C for 10 min, resolved on a 4–12% NUPAGE gradient SDS gel, and analyzed by immunoblotting for ERRα (lower panel). B, Lung type II cells were infected with a recombinant adenovirus encoding Flag-tagged ERRα, and then treated with Bt2cAMP (1 mm) for 0–6 h. Cell lysates were prepared, subjected to immunoprecipitation using anti-Flag antibody, and resolved on a 4–12% NUPAGE gradient SDS gel. The gels were stained for phosphorylated ERRα using the Pro-Q diamond phosphospecific fluorescent dye (upper panel) and for total protein using the SYPRO Ruby fluorescent dye (lower panel) and analyzed by fluorescence imaging. Shown are representative experiments repeated two to four times with similar observations.
These observations suggest that nuclear ERRα is phosphorylated in lung type II cells, whereas the cytoplasmic protein is not.
We next analyzed temporal effects of cAMP on ERRα phosphorylation in primary type II cells infected with recombinant adenoviruses expressing FLAG-tagged ERRα. The infected cells were treated with Bt2cAMP for 1–6 h, which represent times where ERRα expression showed no apparent changes in response to cAMP treatment (Figs. 1B and 5B). Nuclear proteins were isolated from cells collected at the indicated time points, immunoprecipitations were performed using an anti-FLAG antibody, and the proteins were separated on gradient SDS-PAGE gels. The presence of phosphorylated proteins was assessed by phosphostaining, and total ERRα was analyzed by protein staining using SYPRQ fluorescent dye. As can be seen in Fig. 7B (upper panel), phosphorylation of ERRα increased in response to cAMP treatment in a time-dependent manner with a marked increase observed after 4 h. By contrast, levels of total immunoprecipitated ERRα (lower panel) remained relatively unaltered by Bt2cAMP treatment during the 6-h incubation period. These findings suggest that cAMP causes enhanced phosphorylation of ERRα in the cultured type II cells; this temporally precedes the effect of cAMP to increase ERRα expression and nuclear localization.
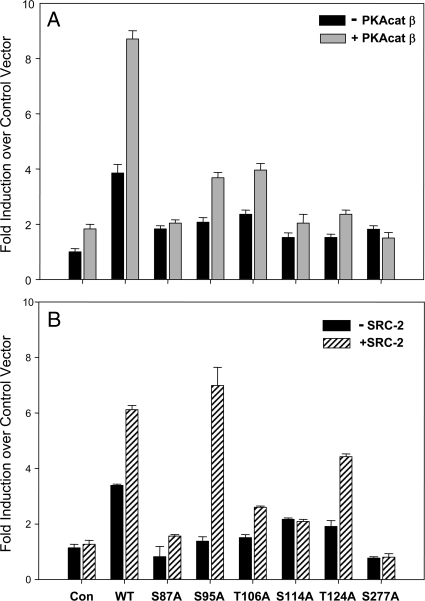
Several Ser/Thr residues required for PKA-dependent ERRα transcriptional activity
Putative serine or threonine phosphorylation sites in ERRα were individually mutated to alanine in pSG-ERRα expression plasmid. Either wt or mutant ERRαs were cotransfected into A549 cells with the hSP-A-291: LUC reporter construct containing the ERRE in the absence or presence of cotransfected PKAcatβ (Fig. 8A) or SRC-2 (Fig. 8B) expression vectors. The S87A, S114A, and S277A mutants manifested a complete loss of both PKA- and SRC-2-induced transcriptional activity (Fig. 8, A and B). Expression levels of the ERRα mutant proteins were similar to those of wt (data not shown). Serines 87 and 114 are in the DNA-binding domain, whereas S277 is within the ligand-binding/activation function 2 domain. Surprisingly, when S87, S114, and S277 were mutated to negatively charged glutamic acid, there also was found to be a loss of basal and PKA-mediated transcriptional activity (data not shown).
Figure 8.
Ser/Thr residues required for PKA-dependent ERRα transcriptional activity. Putative Ser and Thr phosphorylation sites throughout ERRα were mutated to Ala. Expression vectors containing either wt ERRα or one of these mutations were cotransfected with the hSP-A-291:LUC reporter ± an expression vector for PKAcatβ (panel A) or SRC-2 (panel B). phRL-TK expression plasmid was cotransfected to correct for transfection efficiency. Cells were harvested after 48 h, and lysates were assayed for firefly luciferase and Renilla luciferase activities. Data are presented as the mean ± sem of triplicate samples from a representative experiment repeated three times with similar observations. Con, Control.
Discussion
cAMP enhances the rate of type II cell differentiation in cultured midgestation human fetal lung explants (42) and is required for maintenance of the differentiated phenotype in fetal lung type II cells (43). Furthermore, endogenously produced prostaglandins that increase cAMP formation play an important role in promoting type II cell differentiation and inducing hSP-A2 expression in the cultured human fetal lung tissue (44). As mentioned above, cAMP stimulation of SP-A expression is mediated by transcription factors that bind to a number of conserved response elements within a 300-bp region upstream of the hSP-A2 gene transcription start site; mutation of any one element abrogates the inductive effects of cAMP (26). One of these essential elements, the ERRE, binds the orphan nuclear receptor, ERRα (27). Importantly, the capacity of ERRα to increase hSP-A2 promoter activity is potentiated by cAMP and by PKA (27).
In the present study, ERRα protein was found to be present both in cytoplasmic and nuclear fractions of cultured lung type II cells; nuclear ERRα levels were increased by cAMP treatment. By immunohistochemical analysis of type II cells treated with the nuclear export inhibitor, leptomycin B, cAMP stimulation of nuclear ERRα accumulation was found to be even more pronounced. These findings suggest that cAMP enhances ERRα expression and nuclear localization in lung type II cells. In previous studies, we found that nuclear localization of TTF-1, together with cofactors SRC-1 and CBP/p300, was increased in the human fetal lung explants in association with type II cell differentiation; this was further enhanced by cAMP (33). Similarly, NF-κB activation by proinflammatory cytokines is associated with increased nuclear translocation and binding of NF-κB p50 and p65 to target gene promoters (45); phosphorylation of p65 by PKA increases its transcriptional activity by enhancing p65 association with CBP (46). We previously observed that cAMP and IL-1 stimulated binding of endogenous NF-κB and TTF-1 proteins to the TBE region of the hSP-A2 promoter in human fetal lung cells (13). Thus, increased nuclear translocation of transcription factors and enhanced interaction with cofactors may provide one of several mechanisms by which cAMP stimulates SP-A expression.
PKA is the primary effector of cAMP regulation of cellular function. In addition to its direct actions to catalyze phosphorylation of transcription factors, enzymes, and other cellular proteins, PKA also serves as a modulator of several other signaling pathways, including MAPK (47). cAMP/PKA can activate the MAPKs ERK1/2 via phosphorylation of RAP1 and B-Raf or inhibit MAPK signaling via phosphorylation of RAF-1 (47,48). To determine whether PKA activates ERRα transcriptional activity at the hSP-A2 promoter directly or via activation of other signaling pathways, A549 cells cotransfected with a hSP-A-291:LUC reporter and expression vectors for ERRα and PKA were cultured in the absence or presence of the several signaling inhibitors. Our finding that PKA activation of ERRα transcriptional activity at the hSP-A2 promoter was markedly reduced by the PKA inhibitor H89 (49), but largely unaffected by the p38 MAPK inhibitor SB203580 (50) and only modestly diminished by the MEK inhibitor PD98059 (51), suggests that MAPK/ERK, or MAPK/JNK pathways are not the primary mediators of cAMP/PKA up-regulation of ERRα transcriptional activity in lung type II cells.
ERRα has been suggested to constitutively activate transcription; no natural ligand(s) for ERRα has been identified (38,52,53). Moreover, structural studies of ERRα have revealed a transcriptionally active conformation in the absence of a ligand (54). Rather, ERRα transcriptional activity has been observed to be mainly up-regulated by interaction with cofactors, including PGC-1α and PGC-1β (55,56,57), p160 family members, SRC-1, SRC-2, and SRC-3 (36,38,58) and inhibited by nuclear receptor corepressor-interacting protein 140 (RIP-140) (59). Using a candidate approach, we found that SRC-2/GRIP1 served as a potent coactivator of ERRα at the ERRE of the hSP-A2 promoter. This is consistent with findings of other studies in which SRC-2 was found to be a strong coactivator of ERRα transcriptional activity on ERE- and ERRE-containing promoter constructs (36,38,60). We also observed that SRC-2 acted synergistically with PKA to enhance ERRα transcriptional activity, suggesting that a PKA-mediated mechanism may facilitate SRC-2-ERRα interaction and recruitment to its response element in DNA. Although PGC-1α has been reported to function as a strong coactivator of ERRα in certain systems (39,40,55,56,57), we found in the present study that PGC-1α failed to stimulate ERRα transcriptional activity at the hSP-A2 promoter in lung adenocarcinoma cells. Our finding that PGC-1α markedly enhanced ERRα activation of a mPDK4 promoter construct in the lung adenocarcinoma cells suggests that gene context is of great importance in coregulator selectivity. cAMP/PKA also has been reported to promote transcriptional activation of the orphan nuclear receptor Nur77 via effects to enhance Nur77 homodimerization and increase DNA binding and recruitment of SRC-2 (61).
Phosphorylation is known to impact transcription factors at the levels of nuclear translocation, DNA binding, interaction with coregulators, and transcriptional activity (62). ERRα has been reported to exist as a phosphoprotein (35,36,63); enhanced ERRα phosphorylation in breast cancer cells was found to be associated with increased transcriptional activity (36). Notably, ERRα transcriptional activity was correlated with Erb2/HER-2/neu expression in BT-474 breast cancer cells and was inhibited when the cells were treated with the ErbB2 inhibitor, trastuzumab, the EGF receptor tyrosine kinase inhibitor, gefitinib, as well as inhibitors of MEK/MAPK PI3K/Akt signaling pathways (36). Several kinases were shown to mediate ERRα phosphorylation. For example, MAPKs and Akts phosphorylated ERRα in vitro (36). Barry and Giguere (35) reported that EGF treatment of breast cancer cells stimulated ERRα phosphorylation, DNA binding, and transcriptional activity. They observed that protein kinase Cδ, which is activated by EGF and phorbol esters, increased ERRα phosphorylation within its DNA-binding domain in vitro and enhanced in vitro DNA binding activity. Data also were presented to indicate that phosphorylation increased ERRα homodimerization and interaction with PGC-1α at a consensus ERRE (35). On the other hand, Vu et al. (64) reported that serines 19 and 22 within the AF-1 domain of ERRα serve as major sites of in vivo phosphorylation in breast cancer cells; mutation of S19A enhanced ERRα response to the coactivator SRC-2/GRIP1.
In the present study, we obtained evidence that cAMP increased ERRα phosphorylation in lung type II cells. ERRα phospho-staining was increased by cAMP treatment within 4–6 h, a time when no apparent changes in ERRα expression levels were observed. cAMP, acting through PKA, also enhanced ERRα interaction with SRC-2 in human fetal lung type II cells, both within nuclear extracts and bound to the ERRE. Moreover, the PKA inhibitor H89 diminished the cAMP-induced interaction of ERRα and SRC-2 with the ERRE in lung type II cells. Collectively, our findings suggest that cAMP/PKA may increase ERRα phosphorylation, which in turn leads to its enhanced nuclear localization and interaction with SRC-2 at the ERRE. ChIP studies further indicated that cAMP treatment enhanced binding of endogenous PKA and SRC-2 to the ERRE in human fetal lung type II cells.
In light of the potential importance of ERRα phosphorylation on its transcriptional activation by cAMP/PKA, we mutated Ser and Thr residues throughout the protein corresponding to consensus sites for PKA, PKC, and MAPK phosphorylation to Ala or Glu. Transcriptional activity of each of the mutants were compared with wt when overexpressed in lung adenocarcinoma cells cotransfected with an SP-A-291:LUC expression vector, with or without cotransfection of expression vectors for PKA or SRC-2. A profound decline in ERRα transcriptional activity, as well as PKA and SRC-2 activation, was observed when S87, S114, and S277 were mutated to Ala, suggesting the importance of these putative phosphorylation sites in transcriptional activation. However, similar decreases in transcriptional activity were found when these serines were mutated to the phosphomimetic, glutamic acid, suggesting that impaired transcriptional activity of the mutants was not solely due to the loss of a negative charge imposed by phosphorylation. In light of these findings, it is possible that these serine residues are required for structural and/or functional integrity of ERRα or that phosphorylation of these residues is actually necessary for DNA binding and/or interaction with coregulators. Notably, in studies of the role of NF-κB p50 phosphorylation in its DNA-binding activity, it was observed that phosphorylation of Ser337 played a critical role. Substitution of the negatively charged aspartic acid for serine at that position impaired DNA binding to the same extent as substitution of an alanine, whereas substitution of a threonine maintained DNA binding activity (65). It was suggested that phosphorylation of Ser337 facilitated DNA binding by causing a conformational change in the hinge region of the protein (65). As opposed to previous findings in breast cancer cells (64), mutation of Ser 19 and 22 of ERRα had no effect on transcriptional activity or on PKA or SRC-2-mediated induction (Liu, D., and C. R. Mendelson, unpublished observations).
We also must consider the possibility that PKA may play a more important role in facilitating the phosphorylation of coregulatory proteins and enhancing their interaction with ERRα at the ERRE. Phosphorylation of SRC-2 by the MAPK pathway has been suggested to increase steroid receptor-mediated transcription (66,67). Notably, it has recently been reported that cAMP/PKA enhanced expression of the ERα target gene, pS2, by causing a rapid recruitment of SRC-2 to the pS2 promoter (68). Furthermore, PKA caused a redistribution and colocalization of estrogen receptor and SRC-2 to subnuclear foci in cotransfected COS-1 cells (68). SRC-1 transcriptional activity also is regulated by cAMP/PKA. cAMP-induced phosphorylation of SRC-1 by Erk1/2 was found to enhance its cooperative, functional interaction with coactivator CBP (69).
In this study, we observed that the PKA catalytic subunit was physically associated with ERRα and SRC-2 in lung type II cells, both within nuclear extracts and bound to the ERRE; these interactions were enhanced by cAMP and inhibited by H89. Such findings suggest that recruitment of PKA to the complex of proteins bound to the ERRE may enhance phosphorylation of coregulators and other proteins that modulate chromatin structure. Interestingly, in addition to its association with regulatory subunits, the PKA catalytic subunit (PKAcat) was reported to be maintained in an inactive state in the cytoplasm as a complex with IκB. Signals that cause degradation of IκB resulted in the activation of PKAcat, which in turn catalyzed phosphorylation of NF-κB p65 (70) and, potentially, other regulatory proteins. PKA also stimulates phosphorylation, DNA binding, and transcriptional activity of NF-κB p65 and p50 (46,70). Phosphorylation of p65 enhances its binding to coactivator (CBP) (46), whereas p50 interacts with steroid receptor coactivator-1 (SRC-1) (71).
Recent studies suggest that kinases might serve dual functions in transcriptional regulation: a structural role, by mediating crucial protein-protein interactions within various transcription complexes, and an enzymatic role, by modifying target proteins in such complexes to alter their activity (41,72). In this regard, progesterone treatment of breast cancer cells resulted in a rapid activation of Erk and Msk protein kinases, their recruitment together with phosphorylated progesterone receptor to the mouse mammary tumor virus promoter and phosphorylation of histone H3-S10, with subsequent dissociation of heterochromatin protein 1 (HP1) γ and binding of RNA polymerase II and chromatin remodeling complexes (73). We recently reported that cAMP treatment of lung type II cells stimulated recruitment of IκB kinase α (IKKα) to the TBE with an associated increase in local phosphorylation of histone H3-S10, a known target of IKKα, and dissociation of HP1α (11). The findings presented in the current study suggest that PKA also may be recruited to the hSP-A2 promoter in a complex with ERRα.
In conclusion, we propose that cAMP acting via PKA may enhance ERRα activation of hSP-A2 expression in lung type II cells by facilitating ERRα nuclear localization. Within the nucleus, ERRα and PKAcat interact at the ERRE as part of a transcriptional complex that also includes coactivator SRC-2. Association of PKAcat with SRC-2 may further enhance SRC-2 activity and binding to the ERRE, as well as recruitment and functional interaction with other transcription factors and coregulators that make up the hSP-A2 enhanceosome.
Materials and Methods
Recombinant plasmids, adenoviruses, and antibodies
The hSP-A-291:LUC fusion gene was constructed as described previously (27). Human ERRα was excised from the expression plasmid CMV/ERR (provided by Dr. Tim Willson, GlaxoSmithKline, Research Triangle Park, NC) and subcloned into the pSG5 expression vector (Stratagene, La Jolla, CA) between BamHI and BglII restriction sites. pCR3.1-pSRC-1, pCR3.1-SRC-2, and pCR3.1-SRC-3 expression vectors were kindly provided by Dr. Bert W. O’Malley (Baylor College of Medicine, Houston, TX). Mouse PGC-1α expression vector was provided by Dr. Bruce M. Spiegelman (Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA). Mouse PDK4. Luc.2281 and pcDNA3.1-PGC1α were provided by Dr. Daniel P. Kelly (Burnham Institute, Orlando, FL). An expression vector for PKA catalytic subunit. RSV/PKA-cat-β was provided by Dr. Richard A. Maurer, Oregon Health Sciences University (Portland, OR). Recombinant adenoviruses containing Flag-tagged ERRα were generated, as described previously (27). Briefly, ERRα cDNA was subcloned into p3XFLAG-CMV10 vector (Sigma-Aldrich, St. Louis, MO). The flag-tagged ERRα was subsequently inserted into the pShuttle vector (Stratagene) to generate pShuttle-FLAG-ERRα. Recombinant adenoviral particles containing the pShuttle-FLAG-ERRα fusion gene were then generated by cotransformation of electrocompetent BJ5183 bacteria with these fusion constructs and pAdEasy-1. PacI-digested recombinant adenoviral fusion constructs were then transfected into human embryonic kidney (HEK) 293 cells for adenoviral packaging and propagation. Viral DNAs were analyzed by restriction endonuclease digestion, PCR, and DNA sequencing to confirm the presence of the fusion genes. The recombinant adenoviruses were titered in human embryonic kidney (HEK) 293 cells at least three times to determine the number of infectious viral particles (plaque-forming units).
Antibodies used in these studies were from the following sources: anti-PKAcatα (Cell Signaling Technology, Beverly, MA; catalog no. 4782); anti-SRC-2 (BD Biosciences, Palo Alto, CA; catalog no. 610984); anti-FLAG M2 (Sigma-Aldrich; catalog no. F1804).
Culture of human fetal lung explants and isolation and culture of lung type II cells and lung adenocarcinoma cells
Midgestation human fetal lung tissues were obtained from Advanced Bioscience Resources (Alameda, CA) in accordance with the Donors Anatomical Gift Act of the State of Texas. The protocols were approved by the Human Research Review Committee of the University of Texas Southwestern Medical Center at Dallas. Human fetal type II pneumonocytes were isolated and cultured as described in detail previously (43). Briefly, the fetal lung tissues were minced and rinsed in serum-free Waymouth’s MB752/1 medium (Invitrogen, Carlsbad, CA). Lung explants were placed on lens paper supported by stainless steel grids in 35-mm sterile dishes containing 0.5 ml serum-free Waymouth’s medium containing Bt2cAMP (1 mm) (Roche Molecular Biochemicals, Indianapolis, IN) for 3–5 d to enrich the population of differentiated type II cells. Cells were dispersed from the explants by digestion with collagenases type I (0.5 mg/ml; Sigma Chemical Co., St. Louis, MO) and type IA (0.5 mg/ml; Sigma) for approximately 15 min. The resulting cell suspension was depleted of fibroblasts by incubation with diethylaminoethyl-dextran (250 μg/ml) for 30 min at 37 C, followed by centrifugation at 400 × g for 5 min. The cell pellet was resuspended in Waymouth’s MB752/1 medium containing 10% (vol/vol) fetal bovine serum (FBS; Gemini Bio-Products, Woodland, CA), plated onto 60-mm tissue culture dishes or Thermanox coverslips (Nunc, Naperville, IL) coated with extracellular matrix prepared from Madin-Darby canine kidney cells (CRL 6253; American Type Culture Collection, Manassas, VA; 2–5 × 106 cells/60-mm dish) (43) and incubated overnight. Cells were then washed twice with medium to eliminate dead and nonadherent cells and cultured in Waymouth’s medium without FBS. The plating density of the cells after overnight incubation was approximately 50–60%.
The human lung adenocarcinoma cell line A549 (ATCC CCL 185) was maintained in Waymouth’s MB752/1 medium containing FBS (10% vol/vol).
Transient transfections
Before transfection, lung A549 cells were plated onto 35-mm tissue culture dishes and grown to logarithmic phase at 50–80% confluence. After washing with PBS, the cells were transfected with hSP-A-291:LUC (1 μg), with or without cotransfection of expression vectors for ERRα (0.3 μg), PKA β-catalytic subunit (RSV/PKAcatβ, 0.2 μg). phRL-TK (0.2 μg, Renilla luciferase; Promega Corp., Madison, WI) was cotransfected as a control for transfection efficiency, and each experimental condition was assayed in triplicate. Plasmid DNA for each transfection was incubated with Superfect (QIAGEN, Inc., Valencia, CA) in Waymouth’s MB752/1 medium without serum before being added to the cells. The cells were incubated with the Superfect/DNA mixture for 4 h at 37 C before washing in culture medium. After 24 h, transfected cells were treated with or without the PKA inhibitor, H89 (10 μm; Calbiochem), the p38 MAPK inhibitor, SB203580 (5 μm; Calbiochem), or the MEK inhibitor, PD98059 (50 μm, Calbiochem) for 24 h, and the cell lysates were collected and assayed for luciferase activity by use of the Dual-Glo luciferase assay kit (Promega), using a 7715 Microplate luminometer (Cambridge Technology, Cambridge, UK).
In cotransfection experiments to analyze the efficacy of coactivators, cultured A549 cells were transfected with hSP-A-291:LUC (0.8 μg), with or without expression vectors (0.3 μg) for ERRα (pSG5-ERRα), SRC-1 (pCR3.1-pSRC-1), SRC-2 (pCR3.1-SRC-2), SRC-3 (pCR3.1-SRC-3), or PGC-1α (pcDNA3.1-PGC1α) (0.2 μg), as well as appropriate amounts of empty vectors, and phRL-TK (0.2 μg) as a control for transfection efficiency. To compare effects of PGC-1α overexpression on mPDK4 and hSP-A2 promoter activities, A549 cells were cotransfected with mPDK4. Luc.2281 (0.8 μg) or hSP-A-291:LUC (0.8 μg), with or without ERRα (0.3 μg), and PGC-1α (0.2 μg) expression vectors and the appropriate amounts of empty vectors. phRL-TK (0.2 μg) was cotransfected as a control for transfection efficiency. The cell lysates were collected 48 h after transfection and assayed for firefly luciferase and Renilla luciferase activities, as described above.
Mutagenesis of more than 30 serines and threonines to alanine residues within ERRα; analysis of effects on transcriptional activity
The hERRα sequence was analyzed for consensus sequences for PKA, PKC, and MAPK phosphorylation. More than 30 potential Ser/Thr phosphorylation sites throughout the sequence were individually mutated to Ala or Glu using a QuikChange II Site-Directed Mutagenesis Kit (Stratagene), according to the instructions of the manufacturer. The ERRα mutants were subcloned into the pSG5 expression vector (Stratagene), described above, and cotransfected with hSP-A-291:LUC into A549 cells with or without expression vectors for PKAcatβ or SRC-2, described above.
Quantitative RT-PCR
Total RNA was isolated from type II cells cultured ± Bt2cAMP by the one-step method (TRIzol; Invitrogen). RNA was treated with deoxyribonuclease to remove any contaminating DNA, and 4 μg were reversed transcribed using random primers and Superscript II RNase H reverse transcriptase (Invitrogen). Validated primer sets directed against hSP-A mRNA (forward: 5′-GGG CAG TGG AAT GAC AGG AA-3′; reverse: 5′-CTG AAG GCC AGA CAG GAT CC-3′) along with the constitutively expressed 18S rRNA (forward: 5′-ACC GCA GCT AGG AAT AAT GGA-3′; reverse: 5′-GCC TCA GTT CCG AAA ACC A-3′) were used for quantitative PCR amplification. The ABI Prism 7700 detection system (PE Applied Biosystems, Foster City, CA) was employed using the DNA binding dye SYBR green (PE Applied Biosystems) for the quantitative detection of PCR products as previously described (11). Data were normalized by analyzing the ratio of the target cDNA concentrations to that of 18S rRNA.
Phosphatase treatment and immunoblotting
Whole-cell extracts were prepared by three cycles of freezing and thawing in lysis buffer [50 mm Tris-HCl (pH 7.5),150 mm NaCl, 0.5% Triton X-100, 1 mm EDTA, 1 mm dithiothreitol (DTT)], containing a protease inhibitor cocktail (Roche), and the Set I phosphatase inhibitor cocktail (diluted 1:100, Calbiochem). Nuclear and cytoplasmic extracts were prepared as before (27). Nuclear extracts (10 μg) or cytoplasmic fractions (30 μg) were incubated with 400 U of λ-phosphatase (New England Biolabs, Inc., Beverly, MA) in a 10-μl reaction volume at 30 C for 10 min. Proteins were fractionated in gradient polyacrylamide gels (Invitrogen) and transferred onto Hybond-P (Amersham Biosciences, Piscataway, NJ). Blots were probed first by using rabbit antibody for hERRα (provided by Patricia J. Willy, Exelixis, Inc., San Francisco, CA) or mouse antibody for hERRα (provided by Dr. Donald P. McDonnell, Duke University Medical Center, Durham, NC) and then with horseradish peroxidase-conjugated goat antirabbit IgG (1:10,000) or horseradish peroxidase-conjugated goat antimouse IgG (1:10,000) (Amersham Biosciences) as the secondary antibody. Immunoreactive bands were visualized by using an Enhanced Chemiluminescence System according to the manufacturer’s recommendations (Amersham Biosciences).
Immunoprecipitation, phosphostaining, and coimmunoprecipitation
Human fetal lung type II cells were infected with FLAG-ERRα adenoviral expression vector (m.o.i = 1) for 3 h. The medium was then aspirated and replaced with fresh medium in the absence or presence of Bt2cAMP (1 mm) for up to 6 h. After washing twice with cold PBS, whole cell lysates and nuclear extracts were prepared, as described above. FLAG-tagged ERRα protein complexes were immunoprecipitated using agarose-conjugated mouse anti-FLAG antibodies (Sigma-Aldrich; catalog no. A2220). Briefly, 50 μg of nuclear proteins were incubated with 25 μl FLAG-M2 beads in immunoprecipitation buffer (50 mm Tris-HCl, pH 7.5; 150 mm NaCl; 1 mm EDTA; 1 mm DTT; Set I phosphatase inhibitor cocktail and protease inhibitor cocktail, as above) for 4 h. The beads were pelleted and sequentially washed twice with low-salt wash buffer (50 mm Tris-HCl, pH 7.5; 150 mm NaCl; 0.1% Triton X-100; 1 mm EDTA; 1 mm DTT), twice with high -salt wash buffer (50 mm Tris-HCl, pH 7.5; 0.5 m NaCl; 0.1% Triton X-100; 1 mm EDTA; 1 mm DTT), twice more with low-salt wash buffer, and then eluted with 30 μl of 3×FLAG peptide (catalog no. F4799; Sigma-Aldrich) for 30 min. The eluted proteins were denatured with 1× SDS loading buffer, boiled for 5 min, fractionated on gradient polyacrylamide gels, and subjected to immunoblotting, as above, using ERRα, PKAcatα, or SRC-2 antibodies, or phosphostaining. Phosphoproteins were visualized in the gel by incubation with Pro-Q Diamond phospho-specific fluorescent dye (Invitrogen Molecular Probes), according to the manufacturer’s instructions. The gels were fixed in 50% methanol-10% acetic acid overnight, washed with three changes of deionized distilled water for 10 min per wash, followed by incubation in Pro-Q Diamond phosphoprotein gel stain for 90 min and destaining with three successive washes of 20% acetonitrile in 50 mm sodium acetate (pH 4.0) for 30 min each, and two final washes with deionized distilled water. Total protein staining was performed using SYPRO Ruby dye (Invitrogen Molecular Probes). The gel was then washed with destaining buffer and visualized by Typhoon 9200 Variable Mode Imager (Amersham Pharmacia Biotech, Piscataway, NJ).
Indirect immunofluorescence microscopy
Type II cells were cultured on glass chamber slides and treated with Bt2cAMP (1 mm), or in control medium for 24 h. Cells were washed twice with ice-cold PBS, fixed in 3% paraformaldehyde in PBS for 15 min at room temperature, washed again, and permeabilized in 0.2% Triton X-100 in PBS for 5 min, followed by incubation with 1% BSA in PBS for 1 h. The cells were then incubated with monoclonal anti-ERRα antibody in PBS/BSA overnight at 4 C. Cells were washed four times with PBS, followed by incubation with fluorescein-conjugated goat antimouse IgG (10 μg/ml in PBS/BSA) for 1 h at room temperature. The slides were additionally washed four times with PBS, and the coverslips were mounted using 4′,6-diamidino-2-phenylindole (DAPI) Gel/Mount aqueous mounting medium (Fisher Scientific, Pittsburgh, PA). The immunoreactive proteins were visualized under a fluorescent microscope (Nikon Eclipse 90i fluorescence differential interference contrast-enabled microscope; Nikon, Melville, NY).
DAPA
DAPA was performed using a modification of the method of Wu (74). Briefly, human fetal lung type II cells were harvested from 100-mm dishes. Cells were collected into a 15-ml conical tube and centrifuged at 550 × g for 5 min. The supernatant was removed, and the pellet was transferred to a 1.5-ml microcentrifuge tube and resuspended in two packed cell volumes of buffer A (10 mm HEPES, pH 7.9; 1.5 mm MgCl2; 10 mm KCl), containing proteinase and phosphatase inhibitors (Calbiochem) and kept on ice for 10 min. After the addition of Nonidet P-40 (final concentration, 0.5%), the tube was vortexed briefly and centrifuged at 12,000 × g for 30 sec. The pellet was then resuspended in two thirds packed cell volumes of buffer C (20 mm HEPES, pH 7.9; 1.5 mm MgCl2; 420 mm NaCl; 0.2 mm EDTA; 2.5% glycerol) with inhibitors and stirred for 1 h. After centrifugation at 10,400 × g for 20 min, the supernatant was dialyzed in protein-DNA binding buffer (10 mm HEPES, pH 7.9; 1.5 mm MgCl2; 70 mm KCl; 2.5% glycerol) for 2 h. Protein concentrations were measured using the BCA method (Bio-Rad Laboratories, Hercules, CA). Nuclear proteins (100 μg) were incubated with the following annealed biotinylated oligonucleotides:
ERRE sense: (5′-biotin-GCAGAGTGGGTGACCTTAGCCAGT-3′), ERRE antisense (5′-biotin-CACTGGCTAAGGTCACCCACTCTG-3′); ERREmut sense: (5′-biotin-GCAGAGTGGGTTCTAGAAGCCAGT-3′), ERREmut antisense: (5′-biotin-CACTGGCTTCTAGAACCCACTCTG-3′) on ice for 2 h. Tetralink Tetrameric Avidin Resin (40 μl, Promega) was then added, and the reaction was rocked gently for 2 h. After centrifugation at 550 × g for 1 min, the pellet was washed with buffer D (10 mm HEPES, pH 7.9; 1.5 mm MgCl2; 70 mm KCl) three times and suspended in 40 μl of 1× SDS sample loading buffer. The products were resolved on 4–10% gradient SDS-PAGE gels. Immunoblotting was performed using ERRα, SRC-2, and PKAcatα antibodies, as described above.
ChIP
ChIP was carried out as described previously (13). Briefly, confluent 100-mm dishes of type II cells cultured for 24 h ± Bt2cAMP were placed in 0.4% formalin and incubated on an orbital shaker for 10 min at room temperature. Cross-linking was terminated by addition of glycine (0.125 m, final concentration). Cells were washed with ice-cold 1× PBS, collected, lysed, and sonicated on ice to produce sheared soluble chromatin. The lysates were diluted with ChiP dilution buffer (Upstate Biotechnology, Inc., Lake Placid, NY; no. 17-295). Aliquots of diluted lysates were combined ± antibodies against PKAcatβ (Cell Signaling no. 4782), SRC2 (Upstate; no. 06-986), or with nonimmune IgG and incubated at 4 C overnight. Immune complexes were collected on protein A/G agarose and eluted. Cross-linking of immunoprecipitated chromatin complexes and of input controls was reversed by heating at 65 C for 4 h, followed by proteinase K (Invitrogen) treatment. DNA was purified and analyzed by quantitative PCR using forward and reverse primers that amplified a 125-bp sequence from the hSP-A2 genomic region containing the ERRE:
Forward: (5′-AAC CAG GAA CCC AGT GGA GC-3′); reverse: (5′-CTC ACT GGC TAA GGT CAC CCA-3′). qPCR results, expressed as fold-change over input, were calculated using ΔΔCt method.
Acknowledgments
We thank Ms. Margaret Smith for the expert isolation of human fetal type II cells.
Footnotes
This work was supported by National Institutes of Health Grant R37 HL050022 (to C.R.M.).
Disclosure Summary: None of the authors has anything to declare regarding potential conflicts of interest.
First Published Online March 5, 2009
Abbreviations: Bt2cAMP, Dibutyryl-cAMP; CBP, CREB-binding protein; ChIP, chromatin immunoprecipitation; DAPA, DNA affinity precipitation assay; DAPI, 4′,6-diamidino-2-phenylindole; DTT, dithiothreitol; EGF, epidermal growth factor; ERR, estrogen-related receptor; FBS, fetal bovine serum; GRIP, glucocorticoid receptor-interacting protein; MEK, MAPK kinase; NF-κB, nuclear factor-κB; PGC, PPARγ coactivator; PKA, protein kinase A; PKAcat, PKA catalytic subunit; PKC, protein kinase C; SDS, sodium dodecyl sulfate; SP-A, surfactant protein A; SRC, steroid receptor coactivator; TBE, TTF-1 binding element; TTF, thyroid transcription factor; wt, wild type.
References
- Wright JR 2005 Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68 [DOI] [PubMed] [Google Scholar]
- Mendelson CR, Chen C, Boggaram V, Zacharias C, Snyder JM 1986 Regulation of the synthesis of the major surfactant apoprotein in fetal rabbit lung tissue. J Biol Chem 261:9938–9943 [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Mendelson CR 2004 Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA 101:4978–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggaram V, Qing K, Mendelson CR 1988 The major apoprotein of rabbit pulmonary surfactant. Elucidation of primary sequence and cyclic AMP and developmental regulation. J Biol Chem 263:2939–2947 [PubMed] [Google Scholar]
- Phelps DS, Floros J 1988 Localization of surfactant protein synthesis in human lung by in situ hybridization. Am Rev Respir Dis 137:939–942 [DOI] [PubMed] [Google Scholar]
- Auten RL, Watkins RH, Shapiro DL, Horowitz S 1990 Surfactant apoprotein A (SP-A) is synthesized in airway cells. Am J Respir Cell Mol Biol 3:491–496 [DOI] [PubMed] [Google Scholar]
- Wohlford-Lenane CL, Snyder JM 1992 Localization of surfactant-associated proteins SP-A and SP-B mRNA in rabbit fetal lung tissue by in situ hybridization. Am J Respir Cell Mol Biol 7:335–343 [DOI] [PubMed] [Google Scholar]
- Odom MJ, Snyder JM, Boggaram V, Mendelson CR 1988 Glucocorticoid regulation of the major surfactant associated protein (SP-A) and its messenger ribonucleic acid and of morphological development of human fetal lung in vitro. Endocrinology 123:1712–1720 [DOI] [PubMed] [Google Scholar]
- Iannuzzi DM, Ertsey R, Ballard PL 1993 Biphasic glucocorticoid regulation of pulmonary SP-A: characterization of inhibitory process. Am J Physiol Lung Cell Mol Physiol 264:L236–L244 [DOI] [PubMed] [Google Scholar]
- Alcorn JL, Islam KN, Young PP, Mendelson CR 2004 Glucocorticoid inhibition of SP-A gene expression in lung type II cells is mediated via the TTF-1-binding element. Am J Physiol Lung Cell Mol Physiol 286:L767–L776 [DOI] [PubMed] [Google Scholar]
- Islam KN, Mendelson CR 2008 Glucocorticoid/glucocorticoid receptor inhibition of surfactant protein-A (SP-A) gene expression in lung type II cells is mediated by repressive changes in histone modification at the SP-A promoter. Mol Endocrinol 22:585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acarregui MJ, Snyder JM, Mendelson CR 1993 Oxygen modulates the differentiation of human fetal lung in vitro and its responsiveness to cAMP. Am J Physiol Lung Cell Mol Physiol 264:L465–L474 [DOI] [PubMed] [Google Scholar]
- Islam KN, Mendelson CR 2006 Permissive effects of oxygen on cyclic AMP and interleukin-1 stimulation of surfactant protein A gene expression are mediated by epigenetic mechanisms. Mol Cell Biol 26:2901–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam KN, Mendelson CR 2002 Potential role of nuclear factor κB and reactive oxygen species in cAMP and cytokine regulation of surfactant protein-A gene expression in lung type II cells. Mol Endocrinol 16:1428–1440 [DOI] [PubMed] [Google Scholar]
- McCormick SM, Boggaram V, Mendelson CR 1994 Characterization of mRNA transcripts and organization of human SP-A1 and SP-A2 genes. Am J Physiol Lung Cell Mol Physiol 266:L354–L366 [DOI] [PubMed] [Google Scholar]
- Katyal SL, Singh G, Locker J 1992 Characterization of a second human pulmonary surfactant-associated protein SP-A gene. Am J Respir Cell Mol Biol 6:446–452 [DOI] [PubMed] [Google Scholar]
- McCormick SM, Mendelson CR 1994 Human SP-A1 and SP-A2 genes are differentially regulated during development and by cAMP and glucocorticoids. Am J Physiol Lung Cell Mol Physiol 266:L367–L374 [DOI] [PubMed] [Google Scholar]
- Kumar AR, Snyder JM 1998 Differential regulation of SP-A1 and SP-A2 genes by cAMP, glucocorticoids, and insulin. Am J Physiol Lung Cell Mol Physiol 274:L177–L185 [DOI] [PubMed] [Google Scholar]
- Alcorn JL, Hammer RE, Graves KR, Smith ME, Maika SD, Michael LF, Gao E, Wang Y, Mendelson CR 1999 Analysis of genomic regions involved in regulation of the rabbit surfactant protein A gene in transgenic mice. Am J Physiol Lung Cell Mol Physiol 277:L349–L361 [DOI] [PubMed] [Google Scholar]
- Liu D, Yi M, Smith M, Mendelson CR 2008 Critical role of the TTF-1 response element in temporal, spatial and hormonal regulation of human surfactant protein-A2 promoter activity in transgenic mice. Am J Physiol Lung Cell Mol Physiol 295:L264–L271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcorn JL, Gao E, Chen Q, Smith ME, Gerard RD, Mendelson CR 1993 Genomic elements involved in transcriptional regulation of the rabbit surfactant protein-A gene. Mol Endocrinol 7:1072–1085 [DOI] [PubMed] [Google Scholar]
- Michael LF, Alcorn JL, Gao E, Mendelson CR 1996 Characterization of the cyclic adenosine 3′,5′-monophosphate response element of the rabbit surfactant protein-A gene: evidence for transactivators distinct from CREB/ATF family members. Mol Endocrinol 10:159–170 [DOI] [PubMed] [Google Scholar]
- Young PP, Mendelson CR 1996 A CRE-like element plays an essential role in cAMP regulation of human SP-A2 gene in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 271:L287–L299 [DOI] [PubMed] [Google Scholar]
- Young PP, Mendelson CR 1997 A GT box element is essential for basal and cyclic adenosine 3′,5′-monophosphate regulation of the human surfactant protein A2 gene in alveolar type II cells: evidence for the binding of lung nuclear factors distinct from Sp1. Mol Endocrinol 11:1082–1093 [DOI] [PubMed] [Google Scholar]
- Li J, Gao E, Seidner SR, Mendelson CR 1998 Differential regulation of baboon SP-A1 and SP-A2 genes: structural and functional analysis of 5′-flanking DNA. Am J Physiol Lung Cell Mol Physiol 275:L1078–L1088 [DOI] [PubMed] [Google Scholar]
- Mendelson CR, Gao E, Li J, Young PP, Michael LF, Alcorn JL 1998 Regulation of expression of surfactant protein-A. Biochim Biophys Acta 1408:132–149 [DOI] [PubMed] [Google Scholar]
- Liu D, Hinshelwood MM, Giguère V, Mendelson CR 2006 Estrogen related receptor-α enhances surfactant protein-A gene expression in fetal lung type II cells. Endocrinology 147:5187–5195 [DOI] [PubMed] [Google Scholar]
- Gao E, Wang Y, Alcorn JL, Mendelson CR 1997 The basic helix-loop-helix-zipper transcription factor USF1 regulates expression of the surfactant protein-A gene. J Biol Chem 272:23398–23406 [DOI] [PubMed] [Google Scholar]
- Gao E, Wang Y, Alcorn JL, Mendelson CR 2003 Transcription factor USF2 is developmentally regulated in fetal lung and acts together with USF1 to induce SP-A gene expression. Am J Physiol Lung Cell Mol Physiol 284:L1027–L1036 [DOI] [PubMed] [Google Scholar]
- Gao E, Alcorn JL, Mendelson CR 1993 Identification of enhancers in the 5′-flanking region of the rabbit surfactant protein A (SP-A) gene and characterization of their binding proteins. J Biol Chem 268:19697–19709 [PubMed] [Google Scholar]
- Li J, Gao E, Mendelson CR 1998 Cyclic AMP-responsive expression of the surfactant protein-A gene is mediated by increased DNA binding and transcriptional activity of thyroid transcription factor-1. J Biol Chem 273:4592–4600 [DOI] [PubMed] [Google Scholar]
- Yan C, Whitsett JA 1997 Protein kinase A activation of the surfactant protein B gene is mediated by phosphorylation of thyroid transcription factor 1. J Biol Chem 272:17327–17332 [DOI] [PubMed] [Google Scholar]
- Yi M, Tong GX, Murry B, Mendelson CR 2002 Role of CBP/p300 and SRC-1 in transcriptional regulation of the pulmonary surfactant protein-A (SP-A) gene by thyroid transcription factor-1 (TTF-1). J Biol Chem 277:2997–3005 [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguère V 2003 Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor α. Mol Cell Biol 23:7947–7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JB, Giguère V 2005 Epidermal growth factor-induced signaling in breast cancer cells results in selective target gene activation by orphan nuclear receptor estrogen-related receptor α. Cancer Res 65:6120–6129 [DOI] [PubMed] [Google Scholar]
- Ariazi EA, Kraus RJ, Farrell ML, Jordan VC, Mertz JE 2007 Estrogen-related receptor α1 transcriptional activities are regulated in part via the ErbB2/HER2 signaling pathway. Mol Cancer Res 5:71–85 [DOI] [PubMed] [Google Scholar]
- Stork PJ, Schmitt JM 2002 Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258–266 [DOI] [PubMed] [Google Scholar]
- Xie W, Hong H, Yang NN, Lin RJ, Simon CM, Stallcup MR, Evans RM 1999 Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2. Mol Endocrinol 13:2151–2162 [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A 2003 The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor α (ERRα). J Biol Chem 278:9013–9018 [DOI] [PubMed] [Google Scholar]
- Wende AR, Huss JM, Schaeffer PJ, Giguère V, Kelly DP 2005 PGC-1α coactivates PDK4 gene expression via the orphan nuclear receptor ERRα: a mechanism for transcriptional control of muscle glucose metabolism. Mol Cell Biol 25:10684–10694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CW, Davis RJ 2006 Proteins kinases: chromatin-associated enzymes? Cell 127:887–890 [DOI] [PubMed] [Google Scholar]
- Odom MJ, Snyder JM, Mendelson CR 1987 Adenosine 3′,5′-monophosphate analogs and β-adrenergic agonists induce the synthesis of the major surfactant apoprotein in human fetal lung in vitro. Endocrinology 121:1155–1163 [DOI] [PubMed] [Google Scholar]
- Alcorn JL, Smith ME, Smith JF, Margraf LR, Mendelson CR 1997 Primary cell culture of human type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am J Respir Cell Mol Biol 17:672–682 [DOI] [PubMed] [Google Scholar]
- Acarregui MJ, Snyder JM, Mitchell MD, Mendelson CR 1990 Prostaglandins regulate surfactant protein A (SP-A) gene expression in human fetal lung in vitro. Endocrinology 127:1105–1113 [DOI] [PubMed] [Google Scholar]
- Baldwin Jr AS 1996 The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol 14:649–683 [DOI] [PubMed] [Google Scholar]
- Zhong H, Voll RE, Ghosh S 1998 Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1:661–671 [DOI] [PubMed] [Google Scholar]
- Gerits N, Kostenko S, Shiryaev A, Johannessen M, Moens U 2008 Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: comradeship and hostility. Cell Signal 20:1592–1607 [DOI] [PubMed] [Google Scholar]
- Fimia GM, Sassone-Corsi P 2001 Cyclic AMP signalling. J Cell Sci 114:1971–1972 [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H 1990 Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem 265:5267–5272 [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC 1995 SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett 364:229–233 [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR 1995 A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA 92:7686–7689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard S, Dwyer MA, McDonnell DP 2007 Definition of the molecular basis for estrogen receptor-related receptor-α-cofactor interactions. Mol Endocrinol 21:62–76 [DOI] [PubMed] [Google Scholar]
- Tremblay AM, Giguère V 2007 The NR3B subgroup: an ovERRview. Nucl Recept Signal 5:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Filipuzzi I, Schilb A, Riou V, Graham A, Strauss A, Geiser M, Fournier B 2004 Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor α (ERRα): crystal structure of ERRα ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1α. J Biol Chem 279:49330–49337 [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A 2004 The estrogen-related receptor α (ERRα) functions in PPARγ coactivator 1α (PGC-1α)-induced mitochondrial biogenesis. Proc Natl Acad Sci USA 101:6472–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi N, Ikeda K, Horie-Inoue K, Yagi K, Okazaki Y, Inoue S 2007 Estrogen-related receptor α modulates the expression of adipogenesis-related genes during adipocyte differentiation. Biochem Biophys Res Commun 358:813–818 [DOI] [PubMed] [Google Scholar]
- Akter MH, Yamaguchi T, Hirose F, Osumi T 2008 Perilipin, a critical regulator of fat storage and breakdown, is a target gene of estrogen receptor-related receptor α. Biochem Biophys Res Commun 368:563–568 [DOI] [PubMed] [Google Scholar]
- Lu D, Kiriyama Y, Lee KY, Giguère V 2001 Transcriptional regulation of the estrogen-inducible pS2 breast cancer marker gene by the ERR family of orphan nuclear receptors. Cancer Res 61:6755–6761 [PubMed] [Google Scholar]
- Augereau P, Badia E, Carascossa S, Castet A, Fritsch S, Harmand PO, Jalaguier S, Cavailles V 2006 The nuclear receptor transcriptional coregulator RIP140. Nucl Recept Signal 4(e024):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Teng CT 2000 Estrogen receptor-related receptor α 1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem 275:20837–20846 [DOI] [PubMed] [Google Scholar]
- Maira M, Martens C, Batsché E, Gauthier Y, Drouin J 2003 Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Mol Cell Biol 23:763–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KH, Montminy M 2005 Can you hear me now? Regulating transcriptional activators by phosphorylation. Sci STKE 2005(pe44):1–5 [DOI] [PubMed] [Google Scholar]
- Sladek R, Bader JA, Giguère V 1997 The orphan nuclear receptor estrogen-related receptor α is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol 17:5400–5409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu EH, Kraus RJ, Mertz JE 2007 Phosphorylation-dependent sumoylation of estrogen-related receptor α1. Biochemistry 46:9795–9804 [DOI] [PubMed] [Google Scholar]
- Hou S, Guan H, Ricciardi RP 2003 Phosphorylation of serine 337 of NF-κB p50 is critical for DNA binding. J Biol Chem 278:45994–45998 [DOI] [PubMed] [Google Scholar]
- Gregory CW, Fei X, Ponguta LA, He B, Bill HM, French FS, Wilson EM 2004 Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem 279:7119–7130 [DOI] [PubMed] [Google Scholar]
- Lopez GN, Turck CW, Schaufele F, Stallcup MR, Kushner PJ 2001 Growth factors signal to steroid receptors through mitogen-activated protein kinase regulation of p160 coactivator activity. J Biol Chem 276:22177–22182 [DOI] [PubMed] [Google Scholar]
- Fenne IS, Hoang T, Hauglid M, Sagen JV, Lien EA, Mellgren G 2008 Recruitment of coactivator GRIP1 to an estrogen receptor transcription complex is regulated by the cAMP-dependent protein kinase. Endocrinology 149:4336–4345 [DOI] [PubMed] [Google Scholar]
- Rowan BG, Garrison N, Weigel NL, O'Malley BW 2000 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol Cell Biol 20:8720–8730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S 1997 The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413–424 [DOI] [PubMed] [Google Scholar]
- Naumann M, Scheidereit C 1994 Activation of NF-κB in vivo is regulated by multiple phosphorylations. EMBO J 13:4597–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds JW, Mahadevan LC 2006 Cell signaling. Protein kinases seek close encounters with active genes. Science 313:449–451 [DOI] [PubMed] [Google Scholar]
- Vicent GP, Ballaré C, Nacht AS, Clausell J, Subtil-Rodríguez A, Quiles I, Jordan A, Beato M 2006 Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell 24:367–381 [DOI] [PubMed] [Google Scholar]
- Wu KK 2006 Analysis of protein-DNA binding by streptavidin-agarose pulldown. Methods Mol Biol 338:281–290 [DOI] [PubMed] [Google Scholar]