Abstract
Type 2 diabetes results from pancreatic ß-cell failure in the setting of insulin resistance. This model of disease progression has received recent support from the results of genome-wide association studies that identify genes potentially regulating ß-cell growth and function as type 2 diabetes susceptibility loci. Normal ß-cell compensation for an increased insulin demand includes both enhanced insulin-secretory capacity and an expansion of morphological ß-cell mass, due largely to changes in the balance between ß-cell proliferation and apoptosis. Recent years have brought significant progress in the understanding of both extrinsic signals stimulating ß-cell growth as well as mediators intrinsic to the ß-cell that regulate the compensatory response. Here, we review the current knowledge of mechanisms underlying adaptive expansion of ß-cell mass, focusing on lessons learned from experimental models of physiologically occurring insulin-resistant states including diet-induced obesity and pregnancy, and highlighting the potential importance of interorgan cross talk. The identification of critical mediators of islet compensation may direct the development of future therapeutic strategies to enhance the response of ß-cells to insulin resistance.
This review addresses the molecular mechanisms underlying compensatory expansion of ß-cell mass in response to insulin resistance, the failure of which leads to diabetes.
Diabetes affects more than 23 million individuals in the United States, and well over 150 million worldwide, with complications involving multiple organ systems, making it a formidable medical and public health issue. Perhaps even more astounding, it is estimated that more than 50 million American adults have impaired fasting glucose or impaired glucose tolerance (prediabetes), predisposing them to the development of overt disease. In adults, type 2 diabetes represents 90–95% of diagnosed cases and is associated with multiple factors including older age, obesity, and family history, with an underlying complex genetics. Insulin resistance has long been considered the hallmark of type 2 diabetes, but the natural history of diabetes depends, in large part, on the adaptation of pancreatic ß-cells to meet the increased demand for insulin that results from insulin resistance. In fact, ß-cell failure has been described as the primary determinant of whether an insulin-resistant individual will progress to diabetes (1).
The process of normal ß-cell compensation for insulin resistance comprises a combination of increased insulin-secretory capacity and an expansion of ß-cell mass. Indeed, morphological analyses of pancreatic tissue at autopsy have found increased ß-cell volume in obese nondiabetic patients, implying postnatal plasticity of ß-cell mass in humans (2,3). In contrast, patients with type 2 diabetes have a significant reduction in islet volume due to increased ß-cell apoptosis, correlating a deficit in ß-cell mass with the presence of overt disease (3). Moreover, further evaluation of obese patients (including nondiabetic, prediabetic, and diabetic subsets) has revealed a negative correlation between blood glucose levels and ß-cell volume below a certain threshold of ß-cell number, again strengthening the association between reduced ß-cell mass and disease severity (4). These observations suggest that therapeutic strategies designed to stimulate expansion of ß-cell mass or prevent loss of ß-cells may have success in preventing, slowing, or even reversing the progression to type 2 diabetes.
Animal models of insulin resistance have also demonstrated the plasticity of ß-cell mass, providing experimental systems in which to identify the extracellular signals and molecular mechanisms that underlie the compensatory response. These include genetically induced insulin resistance as well as more physiologically relevant models, including diet-induced obesity and pregnancy, both of which result in significant expansion of ß-cell mass in vivo (5,6,7). A recent study in rats further demonstrated that ß-cell mass (as well as pulsatile insulin secretion) adaptively increases with age-related decreases in insulin sensitivity (8). A 96-h glucose infusion induces ß-cell replication in rodents, directly demonstrating the proliferative potential of adult ß-cells and suggesting that either hyperglycemia itself, or perhaps the resulting hyperinsulinemia, acts as a key mitogenic stimulus (discussed in detail below) (9,10,11). Moreover, animal models of type 2 diabetes such as the Zucker diabetic fatty rat and the human islet amyloid polypeptide-transgenic mouse recapitulate the progressive decline in ß-cell mass due to increased apoptosis that accompanies the development of the disease in humans (12,13). Thus, animal models of insulin resistance encompass a range of etiologies that can be used to better understand the extrinsic and intrinsic mediators of morphological ß-cell compensation in systems that have the potential to translate to human physiology.
Extrinsic Stimuli for Adaptive Expansion of ß-Cell Mass
A physiological adaptive expansion of ß-cell mass can occur via a net increase in the generation of new ß-cells, a net decrease in ß-cell death, or an increase in ß-cell size. In theory, new ß-cells can arise from replication of existing cells or from neogenesis, that is, differentiation from a more immature precursor, but the relevance of neogenesis to postnatal expansion of ß-cell mass has been hotly debated. Although relatively recent lineage-tracing studies in mice have suggested that proliferation of existing ß-cells serves as the major source of newly formed ß-cells in adults, sporadic examples of either neogenesis or directed transdifferentiation (the conversion of one differentiated cell type into another) have continued to emerge, leaving open the possibility that replication may not be the sole contributor to new adult ß-cells in vivo (14,15,16,17). Regardless, given that ß-cell proliferation and apoptosis appear to play dominant roles in age-related and adaptive ß-cell turnover in humans, this review will focus on those processes and not address the interesting questions of neogenesis or hypertrophy (3,18).
Glucose vs. insulin
It has long been appreciated that glucose can stimulate ß-cell proliferation in glucose infusion models. This effect, however, could be due either to hyperglycemia itself or to the secondary hyperinsulinemia. Recently, several attempts have been made to determine the relative importance of glucose vs. insulin as a ß-cell trophic factor. Compelling evidence for the critical role of insulin in the maintenance and expansion of ß-cell mass comes largely from genetic approaches that disrupt insulin or IGF-I signaling in mouse models. One of the earliest of these systems involved ß-cell-specific deletion of the insulin receptor (IR) using two loxP-flanked IR alleles combined with a rat insulin II promoter-driven Cre recombinase. These IR knockout (ßIRKO) mice exhibit a progressive impairment in glucose tolerance accompanied by defects in acute-phase insulin secretion (19). The additional finding of reduced ß-cell mass in both diabetic and nondiabetic subsets of ßIRKO mice implies the specific importance of insulin signaling in regulation of ß-cell mass, even in the absence of hyperglycemia (20). In contrast, although IGF-I receptor (IGF1R) homozygous null mutations in mice result in reduced islet area during late embryonic development and P0.5, as assessed just before neonatal lethality (21), targeted deletion in the ß-cell using rat insulin II promoter-driven Cre recombinase (ßIGF1RKO) has no effect on ß-cell mass or insulin content, suggesting a dominant role for IR-mediated insulin/IGF-I signaling in stimulation of postnatal ß-cell growth (22). Consistent with this conclusion, transgenic overexpression of human IGF-I under the control of the widely expressed mouse metallothionein I promoter also fails to alter ß-cell mass (23). Studies in compound ß-cell-specific IR and IGF1R null/heterozygote mouse models further support a primary role for the IR, although double ßIRKO/ßIGF1RKO mice do display accelerated development of diabetes with ß-cell mass reduction evident by 2 wk postnatally, an age when ß-cell mass is normal in ßIRKO mice, suggesting either a context-specific role for IGF1R signaling in ß-cell mass regulation or, possibly, an additional role for glucose toxicity imposed by the defect in IGF1R signaling (24). Most relevant to the pathogenesis of diabetes, normal compensatory ß-cell hyperplasia is completely absent in ßIRKO mice in the context of two models of insulin resistance, the genetically induced liver-specific IRKO (LIRKO) mouse and the environmentally induced high-fat diet (HFD)-fed mouse (25).
Genetic disruption of various components of the insulin/IGF-I signaling pathways downstream of the receptors also results in alterations in ß-cell mass. For example, global deficiency of the receptor docking protein insulin receptor substrate (IRS)-2, but not IRS-1, results in reduced ß-cell mass neonatally, before the onset of peripheral insulin resistance, as well as a failure of ß-cell mass to expand in response to the subsequent decrease in insulin sensitivity (26). Further down the pathway, ß-cell-specific deletion of 3-phosphoinositide-dependent protein kinase 1 (PDK-1), a serine/threonine kinase that phosphorylates Akt, results in diabetes associated with reduced islet size and density (27), whereas transgenic mice overexpressing a constitutively active myristoylated Akt1 in the ß-cell have increased ß-cell mass (28,29). Finally, increased activation of targets normally inhibited by Akt downstream of insulin signaling [e.g. Foxo1, glycogen synthase kinase (GSK)ß] and deletion of those normally activated by Akt (e.g. S6K) all negatively impact ß-cell mass, demonstrating that ß-cell growth is sensitive to perturbations in insulin signaling at multiple levels (30,31,32). This large body of genetic in vivo evidence for a key role of insulin in stimulating the expansion of ß-cell mass has received additional support from ex vivo studies of cultured islets (33,34). Overall, these results demonstrate a role for insulin signaling in ß-cell growth and suggest that the increased circulating insulin that normally accompanies elevated glucose levels may be primarily responsible for expansion of ß-cell mass in response to hyperglycemia.
However, a few notable observations counter this idea and suggest that glucose itself, rather than insulin, presents a critical stimulus for islet growth. More than a decade ago, mice deficient for both nonallelic insulin genes, Ins1 and Ins2, were generated and, not surprisingly, these mice exhibited fetal growth retardation and neonatal lethality associated with diabetic ketoacidosis (35). Surprisingly, however, islet area was increased nearly 1.5-fold in these insulin-deficient pups, a pattern that was present even in adult Ins1−/− or Ins2−/− single mutant mice and compound heterozygotes (35,36,37). Although these results cannot exclude potential contributions of IGF-mediated signaling via the IR, they demonstrate that insulin itself is not required for expansion of ß-cell mass, at least not during embryogenesis. Additional evidence dissociating insulin from ß-cell growth comes from studies on the effects of solid insulinoma transplant in rats in which sc implantation of an insulin-producing tumor causes hypoglycemia and a reduction in endogenous ß-cell volume in the recipient animal (38,39). More recently, Terauchi et al. (40) have shown that mice haploinsufficient for the gene encoding the ß-cell glucose sensor glucokinase (Gck) fail to expand their ß-cell mass in response to HFD-induced insulin resistance, specifically implicating glucose metabolism in the stimulation of compensatory islet hyperplasia. Finally, in vitro systems have demonstrated that 24-h exposure to increasing glucose concentrations induces proliferation of INS-1 cells and significantly reduces the rate of apoptosis in Min6 cells and cultured islets, although these effects could still depend upon glucose-stimulated insulin secretion (41,42).
Although the relative importance of glucose vs. insulin can be debated in light of the contributions described above, in reality their roles may not be separable. For example, glucose and IGF-I trigger several common downstream signaling events in INS-1 cells, including phosphorylation of Irs2, MAPK, and p70S6K, and IGF-I stimulates proliferation of these cells only in the presence of physiological levels of glucose (42). In Min6 cells, the antiapoptotic effects of glucose require Akt, and high glucose induces Foxo1 phosphorylation and nuclear exclusion, dependent on both phosphatidylinositol 3-kinase and the IR (41,43). It is also interesting to note that microarray analyses of gene expression in islets isolated from HFD-fed Gck+/− mice compared with HFD-fed wild-type controls revealed down-regulation of Irs2, Igf1r, and PDK-1, further suggesting cross talk between glucose metabolism and insulin signaling (40). Indeed, transgenic overexpression of Irs2 in the ß-cells of Gck+/− mice was sufficient to rescue the impaired glucose tolerance and ß-cell proliferation defect in the setting of diet-induced insulin resistance, placing glucose upstream of Irs2 expression, potentially via cAMP-mediated phosphorylation of cAMP response element-binding protein at serine 133 (40). Thus, glucose-stimulated events in the ß-cell feed into the classical insulin-signaling cascade, blurring the distinction between the two individual stimuli and increasing the difficulty of assigning relative importance to each; however, it is clear that the normally co-occurring combination of glucose and insulin can present a powerful stimulus for ß-cell growth in the setting of an increased insulin demand.
Signals from other organs
Whereas the significance of glucose and insulin as key ß-cell trophic factors during insulin resistance has been appreciated for some time, so has the necessary existence of other circulating factors capable of stimulating postnatal expansion of ß-cell mass (Fig. 1). This is implied, for example, by the observation that enhanced ß-cell proliferation precedes the onset of hyperglycemia in ob/ob and db/db mice (44,45). Furthermore, islet grafts transplanted from wild-type donors into prehyperglycemic IR+/−/IRS-1+/− mice display increased graft volume with a 3-fold increase in intragraft ß-cell proliferation, strongly suggesting the presence of circulating factors other than glucose that can trigger ß-cell growth in insulin-resistant states (46). The past few years have brought tremendous strides in our understanding of the cross talk between ß-cells and other tissues and have uncovered signals from some unexpected sources, including fat, muscle, and even bone. An elegant example of interorgan communication has recently been described in which hepatic ERK activity transmits a proproliferative signal to the ß-cell via the peripheral and central nervous system, and appropriate compensatory expansion of ß-cell mass in the setting of insulin resistance requires that this metabolic relay pathway be intact (47). Table 1 contains a comprehensive list of additional secreted factors that may play critical roles in the regulation of ß-cell mass, including pituitary-derived (e.g. prolactin and GH) and gut-derived [e.g. glucagon-like peptide (GLP)-1 and gastrin] hormones, whereas some particularly novel advances are described in detail below.
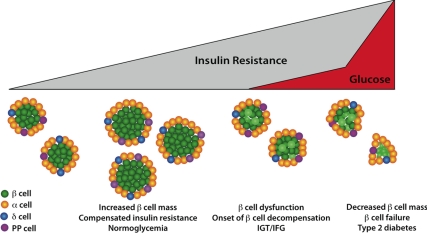
Figure 1.
Dynamics of ß-cell mass during the progression of insulin resistance to diabetes. In the setting of insulin resistance, the pancreatic islet insulin-secreting ß-cells respond, in part, via a compensatory increase in ß-cell mass, elevating plasma insulin levels to maintain normoglycemia. Changes in both ß-cell proliferation and survival play important roles in this adaptive expansion of ß-cell mass and in the reduction in mass that is associated with progressive ß-cell dysfunction, eventually leading to type 2 diabetes. These cellular processes are regulated by extracellular signals from a number of tissues, as described in detail in the text. IGT, Impaired glucose tolerance; IFG, impaired fasting glucose; PP, pancreatic polypeptide.
Table 1.
Potential Signals Regulating Adaptive Expansion of ß-Cell Mass
| Relevant source(s) | Effect on ß-cell mass (basal) | Effect on compensatory ß-cell mass expansion | References | |
|---|---|---|---|---|
| Leptin | Adipose | In vivo: Pdx1 (or RIP)-Cre-mediated ß-cell ObR deletion results in 2- to 3-fold ↑ ß-cell mass | HFD: Pdx1-Cre-mediated ß-cell ObR deletion results in ∼30% ↓ in islet area | 56,57,58 |
| Ex vivo: long-term leptin exposure triggers apoptosis in human islets | ||||
| Resistin | Adipose (rodents); macrophages (humans) | In vivo: long-term ICV resistin ↑ ß-cell mass and proliferation in rats | Not tested | 50 |
| Wnts | Adipose | Ex vivo: exposure to fat-cell-conditioned medium ↑ proliferation of INS-1 cells and mouse islets; effect inhibited by Wnt antagonist | Not tested | 61 |
| Osteocalcin | Bone (osteoblasts) | In vivo: ↓ ß-cell mass & proliferation in Ocn−/−; low-dose osteocalcin ↑ wild-type islet proliferation | HFD: osteocalcin improves glucose tolerance in wild-type mice; effect on ß-cell mass not tested | 70,71 |
| Ex vivo: exogenous osteocalcin induces cyclinD2 and cdk4 in mouse islets | ||||
| GLP-1 and GIP | Gut (L-cells/K-cells) | In vivo: Exendin-4 ↑ ß-cell mass and proliferation in mice | HFD: GLP-1R/GIPR double KO mice have ↓ ß-cell mass expansion/proliferation on HFD; however, 8-wk Ex-4 administration has no effect on ß-cell mass in HFD-fed mice | 130,131,132,133,134,135,136 |
| Ex vivo: Exendin-4 induces proliferation of cultured ß-cells; GLP-1 ↓ high-glucose/ palmitate-induced apoptosis of INS-1 cells and human islets | ||||
| db/db mice: 2-wk Ex-4 treatment of prediabetic mice ↑ ß-cell mass via ↑ proliferation and ↓ apoptosis | ||||
| ZDF rats: short-term continuous GLP-1 infusion ↑ ß-cell mass via ↑ proliferation and ↓ apoptosis | ||||
| Gastrin | Gut | In vivo: ↑ ß-cell proliferation near gastrinomas in humans; gastrin−/− mice show no defect in ß-cell mass | Not tested | 137,138 |
| Prolactin | Pituitary | In vivo: PRL-R−/− mice have ↓ ß-cell mass | Not formally tested but strongly implicated in pregnancy-induced ß-cell hyperplasia | 139,140 |
| Ex vivo: ↑ proliferation of mouse, rat, human islets | ||||
| In vivo: GH-receptor−/− mice have ↓ ß-cell mass and ↓ proliferation | HFD: HFD-fed GHR−/− mice have normal ß-cell mass expansion relative to chow diet, but lower absolute ß-cell mass | 139,141,142 | ||
| GH | Pituitary | In vitro: ↑ proliferation of ß-cells | ||
| Placental lactogen | Placenta | In vivo: RIP-mPL1 transgenic mice ↑ ß-cell proliferation, islet size, and islet area; Double mPL1/PTHrP transgenic mice have ↑ ß-cell proliferation/mass and are resistant to STZ-induced ß-cell apoptosis | Not formally tested but strongly implicated in pregnancy-induced ß-cell hyperplasia | 143,144 |
| Ex vivo: ↑ proliferation of ß-cells | ||||
| PTH-related protein (PTHrP) | Ubiquitous, including islet | Ex vivo: ↓ apoptosis in cultured islets | Not tested | 144,145,146 |
| In vivo: RIP-PTHrP transgenic mice have ↑ ß-cell mass, likely due to ↓ apoptosis | ||||
| Hepatocyte growth factor (HGF) | Mesenchyme; endothelium; adipose (humans) | In vivo: RIP-HGF transgenic mice have ↑ islet area and ß-cell proliferation; however, RIP-Cre-mediated ß-cell deletion of c-met (HGF receptor) has no effect on ß-cell mass | Not tested, although ↑ HGF levels in islet endothelium correlates with ↑ ß-cell proliferation in pregnant rats | 147,148 |
| Melanin-concentrating hormone (MCH) | Hypothalamus; islet | In vivo: MCH-overexpressing transgenic mice have 2-fold ↑ in islet size associated with ↑ insulin resistance | HFD: MCH knockout mice fail to expand ß-cell mass on HFD; increased MCH receptor expression in islets from HF-fed mice | 149,150 |
ICV, Intracerebroventricular; GIP, glucose-dependent insulinotropic polypeptide; KO, knockout; STZ, streptozocin; ZDF, Zucker diabetic fatty; ↑, increased; ↓, decreased.
Adipose-derived signals
Insulin resistance resulting from HFD-induced obesity is associated with a particular milieu of circulating factors in the plasma, any of which could signal to the islet to trigger an appropriate compensatory response. An attractive candidate source of signals to the ß-cell is the adipocyte itself. Indeed, the role of adipose tissue as a bona fide endocrine organ rather than simply an energy storage depot is now well appreciated, and plasma levels of adipocyte-secreted hormones such as leptin and resistin are elevated in obesity, at least in mice (48,49). Although recognized initially for their effects on the brain and peripheral tissues, a better understanding of the interactions between these two factors and the ß-cell has been emerging.
Some effects of leptin and resistin on ß-cell mass and function may occur indirectly via their hypothalamic actions (50); however, leptin at least may be capable of signaling directly to the islet. Leptin receptors are expressed on pancreatic ß-cells, and exogenous leptin inhibits insulin production and secretion from human islets (51,52,53,54,55). Additionally, long-term exposure to leptin triggers apoptosis in cultured human islets, again suggesting a direct action on the ß-cell (56). Indeed, two different mouse models of conditional leptin receptor (ObR) inactivation have uncovered a role for leptin in directly regulating ß-cell mass, although some discrepancies remain regarding the precise mechanisms. In one model, a rat insulin promoter (RIP)-Cre transgene with ß-cell and hypothalamic specificity was used to drive expression of a dysfunctional leptin receptor. Mice homozygous for the inactive receptor exhibited fasting hypoglycemia with hyperinsulinemia, consistent with leptin’s proposed inhibitory effect on basal insulin secretion, although, surprisingly, glucose tolerance and glucose-stimulated insulin secretion were also impaired (57). Islet area was expanded almost 3-fold, suggesting a negative effect of leptin on ß-cell mass, but the increased fat mass and decreased insulin sensitivity manifest in these RIP-Cre;ObRloxP/loxP mice complicates this interpretation (57). In contrast, deletion of the ObR gene in the ß-cell using a Pdx1-Cre recombinase, which does not exhibit hypothalamic expression, resulted in improved glucose tolerance and enhanced insulin secretion (58). A similar 2-fold increase in ß-cell mass in the absence of insulin resistance or increased body weight was noted, strengthening the argument that leptin suppresses ß-cell expansion (58). In the setting of HFD-induced obesity, however, Pdx1-Cre-driven ß-cell loss of the leptin receptor worsened glucose tolerance, impairing both insulin secretion and compensatory expansion of ß-cell mass (58). Therefore, although the function of leptin may be context dependent, the high circulating leptin levels accompanying increased adiposity could play a role in normal ß-cell adaptation for insulin resistance.
Another emerging class of potential mediators of this adipo-insular axis are adipose-derived Wnts. Receptors for Wnt ligands are expressed in ß-cells, and Wnts have been shown to induce ß-cell proliferation (59,60). A recent study demonstrated that treatment of INS-1 cells or mouse islets with human-derived fat cell-conditioned medium stimulated proliferation and insulin secretion in a manner inhibited by the addition of a soluble Wnt antagonist (61). Thus, in theory, adipose-derived Wnts might directly promote both functional and morphological ß-cell compensation during obesity-associated insulin resistance, although this has yet to be experimentally tested. This possibility is particularly intriguing in light of the recent interest in TCF7L2, a gene encoding an effector of the Wnt signaling pathway in the ß-cell, mutations in which have been linked to type 2 diabetes (discussed below) (62,63,64).
Muscle-derived signals
Type 2 diabetes and, in fact, prediabetes have been associated with physiological changes in skeletal muscle, including reduced expression of the transcriptional coactivator PPARγ coactivator 1α (PGC1α) (65,66). Muscle-specific deletion of PGC1α, however, causes impaired glucose tolerance in mice fed a HFD not via a decrease in peripheral insulin sensitivity but rather via impaired islet function (67). A proposed mediator of this muscle-islet cross talk is the cytokine IL-6, expression of which is increased in muscle-specific PGC1α knockout mice and which can inhibit glucose-stimulated insulin secretion in isolated islets (67). Therefore, this so-called “myokine” might be a critical component of the proinflammatory plasma milieu associated with type 2 diabetes that modulates islet function in this setting (68). Intriguingly, a recent study revealed direct action of IL-6 on the expansion of islet α-cell mass via effects on α-cell proliferation and apoptosis both in culture and in vivo (69). Further, insulin-secretory defects were observed in IL-6 knockout mice fed a HFD, thereby uncovering a role for IL-6 in functional ß-cell compensation for insulin resistance, potentially dependent upon its direct role in the α-cell (69).
Bone-derived signals
Perhaps the most unexpected recently identified source of signals to the ß-cell is the skeletal system. In 2007, Lee et al. (70) generated mice with mutations in the osteoblast-specific gene Esp encoding the OST-PTP protein tyrosine phosphatase and observed metabolic consequences. In particular, the mutant mice exhibited hypoglycemia due to hyperinsulinemia, enhanced glucose-stimulated insulin secretion, and increased ß-cell proliferation and mass (70). This study identified the osteoblast-secreted hormone osteocalcin as a likely mediator of these bone-derived effects on the ß-cell, because osteocalcin null mice exhibit an opposite phenotype, including decreased ß-cell mass (70). Subsequently, osteocalcin has been shown to have direct effects on the expression of insulin and the cell cycle mediators cyclin D2 and cyclin-dependent kinase (CDK)4 (addressed in more detail in the next section) in cultured islets and Min6 cells (71). Moreover, osteocalcin administration protects mice from HFD-induced glucose intolerance via its effects on both ß-cells and adipocytes, although its effects on ß-cell mass in this context were not assessed (71). Thus, this novel osteo-insular cross talk may provide another promising target for enhancing islet compensation for insulin resistance.
Molecular Mechanisms in the ß-Cell
In addition to identifying extrinsic factors that can influence ß-cell mass and function in insulin-resistant states, enhancing islet compensation requires an understanding of the processes intrinsic to the ß-cell that mediate its response to an increased demand for insulin. Importantly, distinct extrinsic signals may converge upon common pathways within the ß-cell. Recent lessons from experimental models of insulin resistance as well as clues from human genome-wide association studies are discussed below.
Proliferative pathways and cell cycle mediators
Although basal postnatal ß-cell replication rates appear to be very low (72), increased proliferation comprises a major part of compensatory expansion of ß-cell mass in animal models of insulin resistance, implicating cell cycle components as potential mediators of this process (73). For example, the cyclinD/CDK4 complex is a key regulator of the G1/S transition that acts via phosphorylation-mediated inactivation of the tumor suppressor retinoblastoma (Rb) protein. Global deletion of CDK4 in mice results in diabetes associated with reduced islet area whereas global knock-in or ß-cell-specific transgenic expression of an overactive CDK4 (CDK4R24C) causes islet hyperplasia (74,75). RIP-driven transgenic overexpression of CDK4R24C rescues hyperglycemia in db/db mice, likely due to the resulting nearly 14-fold increase in ß-cell area (76). Mice homozygous null for cyclin D2, the predominant D-type cyclin in murine islets, also exhibit impaired postnatal ß-cell proliferation and, although the requirement of D-cyclins in the islet response to insulin resistance has not been formally tested, they are downstream of various proproliferative signaling pathways implicated in adaptive expansion, including the Janus family of tyrosine kinases/signal transducer and activator of transcription (JAK/STAT) and Gsα-triggered cascades (77,78,79,80). Further support for the importance of this cyclinD/CDK4-mediated G1/S transition in morphological ß-cell compensation for insulin resistance comes from genetic analyses of CDK4 regulators. Deletion of the gene encoding the CDK4 inhibitor p27Kip1 (Cdkn1b) in mice improves hyperglycemia in two different genetic models of insulin-resistant diabetes, the Irs2−/− and Lepr−/− mice, by enhancing ß-cell proliferation and mass (81). Conversely, overexpression of p27 driven by the RIP causes hyperglycemia with reduced ß-cell mass, although use of an inducible transgene suggests that the critical window occurs only during embryonic development and early postnatal life, times when ß-cell proliferation rates are normally high (81,82). Homozygous deletion of Skp2, a substrate adaptor for a ubiquitin ligase complex that binds to and targets p27 for proteasomal degradation, limits compensatory expansion of ß-cell mass in response to HFD-feeding, apparently due to a proliferative defect (83). Meanwhile, transcriptional inhibition of p27 via down-regulation of the tumor suppressor gene menin (Men1) has also recently been shown to be a critical event in ß-cell hyperplasia during pregnancy (84,85). Thus, altogether, there is convincing evidence for the critical role of cyclinD/CDK4-mediated cell cycle regulation, particularly involving p27, in promoting adaptive ß-cell growth, suggesting that some of these factors may be fruitful therapeutic targets to enhance ß-cell compensation. However, it is worth noting here the complexity of cell cycle control, coordinated by multiple cyclins, CDKs, and other regulatory proteins, such that the result of a given manipulation can sometimes be difficult to predict. For example, homozygous deletion of p21, another important CDK inhibitor, or even Rb itself, has no effect on ß-cell mass or proliferation in vivo (86,87).
Several intracellular signaling pathways have been shown to promote expansion of ß-cell mass by enhancing ß-cell replication. For example, the proproliferative effects of GH, prolactin, and placental lactogen depend upon the Jak2/Stat5 cascade, ultimately resulting in Stat5 nuclear translocation, and adaptive ß-cell hyperplasia during pregnancy may involve direct activation of cyclin D2 expression by activated Stat5 (88,89,90). Impaired ß-cell proliferation accompanied by reduced cyclin D2 expression is also a feature of mice deficient for Gsα, underscoring a critical role for G protein-coupled receptor-signaling pathway components in islet hyperplasia, at least under unstressed conditions (79).
Additionally, multiple effectors of the insulin/IGF-signaling pathway mediate adaptive ß-cell proliferation in genetic and diet-induced models of insulin resistance. Although the role of Akt itself has not been formally tested in these models, perturbations in signaling components both upstream (IR, Irs2) and downstream (Foxo1, GSK3ß) of Akt have been shown to affect compensatory ß-cell hyperplasia (20,25,40). For example, constitutively nuclear (active) Foxo1 limits expansion of ß-cell mass in response to genetically induced insulin resistance via a proliferative defect whereas, conversely, Foxo1 haploinsufficiency partially rescues ß-cell mass in the context of Irs2 deficiency (30,91). Heterozygous or homozygous deletion of GSK3ß, another Akt target normally inhibited downstream of insulin as well as other trophic signals including Wnts, glucose-dependent insulinotropic polypeptide (GIP) and GLP-1, improves compensatory ß-cell hyperplasia in genetic models of insulin resistance (92). Furthermore, transgenic overexpression of overactive GSK3ß in vivo or pharmacological inhibition of GSK3ß activity in vitro reduces or promotes ß-cell proliferation, respectively, although a clear mechanistic link to specific cell cycle components remains to be identified (31,93). Finally, although the ß-cell mass deficit described in mice homozygous null for S6K1, an Akt target positively regulated by insulin signaling, is due to a defect in ß-cell size rather than proliferation, recent evidence has implicated the S6K1 activator mTOR in ß-cell hyperplasia (32,94). In this study, mice with ß-cell-specific ablation of Tsc2, itself a downstream target of Akt and a negative regulator of mTOR, exhibit increased ß-cell proliferation and mass, although thus far these animals have not been studied in an insulin-resistant context (94).
A number of transcription factors may lie downstream and mediate the proproliferative effects of the signaling pathways mentioned above but specific roles for transcription factors in compensatory expansion of ß-cell mass have been proven for only a few. For example, ß-cell-specific deletion of hepatocyte nuclear factor 4α [the gene underlying maturity onset diabetes of the young (MODY)1] in female mice prevents ß-cell hyperplasia during pregnancy, reducing the replication rate by approximately 60% relative to controls (95). Haploinsufficiency of another MODY gene Pdx1 (MODY4, Ipf1) limits compensatory expansion of ß-cell mass in the IR+/−/IRS-1+/− and LIRKO genetic models of insulin resistance, although this effect was not observed in the Glut4+/− insulin-resistant model (96,97). Low levels of Sox6 have been found in islets of both HFD-fed wild-type and chow-fed ob/ob mice, suggesting a role for down-regulation of this transcription factor in normal islet compensation (98). Indeed, Sox6 interacts with ß-catenin to negatively regulate the cyclin D1 promoter (99). Finally, targeted ablation of the nuclear hormone receptor peroxisomal proliferator-activated receptor (PPAR)γ in the ß-cell has a positive effect on ß-cell proliferation and islet mass in mice fed normal chow while resulting in impaired expansion of ß-cell mass in the setting of HFD-induced obesity (100).
Apoptotic pathways and ß-cell survival
Although often difficult to quantify directly, loss of ß-cells via apoptosis contributes significantly to the deficit in ß-cell mass associated with the development of type 2 diabetes (3). Various animal models have substantiated the critical role of maintaining (or perhaps enhancing) ß-cell survival in the setting of insulin resistance. For example, RIP-Cre mediated homozygous deletion of caspase 8, a key component of the extrinsic pathway of apoptosis, improves glucose tolerance in HFD-fed mice via, at least in part, a significant increase in ß-cell survival and islet area, without affecting proliferation (101). This extrinsic pathway is responsive to extracellular cues such as Fas or cytokines like IL-1ß, which has been proposed to contribute to ß-cell glucotoxicity (102). Indeed, it has recently been demonstrated that IL-1 receptor antagonism decreases ß-cell apoptosis in mice fed a HFD, further suggesting that proapoptotic signaling via the extrinsic pathway contributes to failure of ß-cell compensation for insulin resistance (103). The role of the intrinsic pathway of apoptosis is revealed by the phenotype of mice deficient in the proapoptotic Bcl-2 family member BAD (104). BAD null mice exhibit improved glucose tolerance and increased ß-cell mass in the context of HFD-feeding (104). Unexpectedly, BAD also mediates functional ß-cell compensation via its direct interaction with the glycolytic enzyme glucokinase that is required for normal nutrient-metabolic coupling (104). Of note, although BAD is a critical regulator of the mitochondrial pathways of cell death that sense intracellular stresses, it is also downstream of extracellular-responsive factors like Akt and p70S6K (105,106).
An increasingly strong mechanistic link has recently been emerging between endoplasmic reticulum (ER) stress and ß-cell apoptosis and failure in the setting of insulin resistance. Autopsy sections of patients with type 2, but not type 1, diabetes revealed ß-cell expression of the ER stress marker Chop (107), and mouse models of genetically induced insulin resistance have also been associated with increased islet expression of markers of ER stress and the unfolded protein response (108,109). Mice with a mutation, eIF2α, in a critical component of the unfolded protein response, are more susceptible to diabetes when fed a HFD, a phenotype that is rescued in the setting of additional absence of Chop, an effector of ER-stress-induced apoptosis (110,111). Homozygous deletion of Chop also reduces ß-cell apoptosis (and increases proliferation) in the Lepr−/− mouse model (111). This process is particularly relevant to the pathogenesis of type 2 diabetes because, in addition to the stress on the ß-cell protein folding/secretory machinery imposed by an increased demand for insulin output, islet amyloid deposition, which characterizes human type 2 diabetes, independently stimulates ER stress-induced apoptosis (107). Finally, although the molecular mechanisms remain unclear, ER stress has been shown to trigger a protective autophagic response in eukaryotic cells (112). In support of a role for autophagy in islet compensation for insulin resistance, recent data demonstrate that ß-cell-specific deletion of the autophagy gene Atg7 worsens hyperglycemia in HFD-fed mice, in part due to increased ß-cell apoptosis (113).
Some of the signaling pathway and transcription factor components described above that regulate ß-cell proliferation also affect cell survival in the setting of insulin resistance, including GSK3ß, Irs2, and TOR (26,92,114). GLP-1 exerts its cytoprotective effects on the ß-cell, at least in part, via modulation of the ER stress-induced translational regulator Atf4 (109). Recent work has shown that Foxo1 mediates ER stress- and fatty acid-induced ß-cell death in vitro (115). Additionally, PPARγ agonism by the thiazolidinedione rosiglitazone decreases islet amyloid polypeptide-induced apoptosis in cultured human islets and enhances ß-cell mass in the OLETF rat model of type 2 diabetes (116,117). Given the critical role of apoptosis in ß-cell failure during the progression to type 2 diabetes and our increasing understanding of the mechanisms underlying this loss of ß-cells, therapeutic strategies to enhance ß-cell survival are likely to be fruitful in improving the course of the disease.
Lessons from genome-wide association studies
Much of the data concerning the molecular mechanisms underlying adaptive postnatal expansion of ß-cell mass has been derived from experimental models of physiological insulin resistance; however, recent advances in human genetics now present the opportunity to generate mechanistic hypotheses directly from patients with type 2 diabetes. Our understanding of genetic predisposition has made tremendously rapid progress during the past year and a half with the results of several collaborative genome-wide association studies (recently reviewed in Ref. 118). Strikingly, the majority of genes linked to type 2 diabetes are likely involved in ß-cell function, underscoring the critical role of islet failure in the progression from insulin resistance to overt diabetes (63,64,119,120,121). Notably, the most consistently associated genes include transcription factors downstream of Wnt signaling (TCF7L2, HHEX), PPARγ, cell cycle regulators (CDKAL1, CDKN2A/B, CDC123), the potassium channel KCNJ11, and the zinc transporter SLC30A8 (118). These results suggest an important role for insulin secretion, Wnt signaling, and ß-cell proliferation in islet compensation, and these clues have already directed new lines of investigation in experimental models. In particular, TCF7L2 has garnered considerable interest and, although its effects on insulin secretion remain debatable, small interfering RNA-mediated TCF7L2 knockdown in cultured human islets reduces ß-cell proliferation and increases apoptosis, implying a role for this factor in regulating ß-cell mass (122,123). In support of this, another recent study suggests that TCF7L2 mediates the proproliferative effects of GLP-1 (124). These exciting genome-wide association results provide insight into specific factors critical for islet compensation in humans and, more broadly, reinforce the importance of the ß-cell as a therapeutic target for type 2 diabetes.
Conclusions
Recent advances in our understanding of both extracellular and intracellular mediators of adaptive expansion of ß-cell mass raise hope for the development of new therapeutic strategies to enhance islet compensation to delay, prevent, or even reverse the onset of type 2 diabetes; however, critical issues remain to be addressed regarding feasibility and the translatability of findings in rodent models to humans.
For example, will it be possible to enhance the proliferative potential (and/or curb loss of ß-cells) in the setting of hyperglycemia and ß-cell glucotoxicity or is the damage to the islet irreparable? Two elegant mouse models of regulated ß-cell destruction in vivo have suggested that recovery of ß-cell mass after a major insult is indeed an attainable goal. In one model, RIP-driven tamoxifen-inducible expression of the protooncogene c-Myc in the ß-cell was shown to stimulate ß-cell apoptosis, resulting in near-total ß-cell ablation and the development of diabetes by 6–10 d of continuous tamoxifen administration (125). Strikingly, however, subsequent withdrawal of tamoxifen resulted in substantial regeneration of islet mass accompanied by reversal of hyperglycemia (125). Further characterization of this ß-cell recovery confirmed complete normalization of ß-cell mass and architecture approximately 90 d after tamoxifen withdrawal and suggested that increased proliferation of both ductal and islet cells contributed to this restoration of islet area (126). In another model of inducible ß-cell destruction, in this case driven by doxycycline-regulated expression of diphtheria toxin, removal of doxycycline after a 7-d administration allowed total recovery of ß-cell mass and normalization of hyperglycemia (15). Using a lineage-tracing strategy, this study concluded that proliferation of remaining ß-cells constituted the major source of new ß-cells (15). Altogether, these results reveal the capacity of ß-cell mass to recover, even in the setting of severe hyperglycemia, raising the hope for therapeutically reversing ß-cell failure in type 2 diabetic patients. The critical mass, as well as functional health, of remaining ß-cells required to allow recovery still remains unknown and will remain so for humans until technical advances permit the imaging of human islet mass in vivo.
There may exist physiological barriers to robust stimulation of expansion of ß-cell mass that must be considered and overcome. Teta et al. (127) recently proposed, based upon in vivo nucleotide labeling as well as mathematical modeling of cell turnover, that adult ß-cells possess a replication refractory period during which further mitosis is somehow inhibited. Interestingly, this period appears to be regulated, decreasing in length after partial pancreatectomy, presumably to allow faster recovery of islet mass (127). Therefore, there is hope that this barrier, if it translates to human islet physiology, may indeed be surmountable with increased understanding of the specific regulatory mechanisms involved.
Finally, some lessons learned from animal models may have limited analogy to human physiology. For example, despite its critical role in regulation of ß-cell mass in mouse islets, CDK4 is not expressed in human islets (128). Although this observation raises obvious concerns regarding the therapeutic relevance of data generated in mouse models, it is important to note that adenovirally mediated coexpression of CDK4 and cyclin D1 is still capable of stimulating proliferation of cultured human islets (129). Additionally, human islets do express CDK6, a homolog of CDK4 that may represent another potential target to enhance the proliferative capacity of the ß-cell (128). As we learn more and more about the extrinsic signals and intrinsic mediators of the ß-cell response to insulin resistance and consider new possibilities for therapeutic intervention, the challenges ahead will include identifying those that are most applicable, feasible, and, ultimately, effective for the treatment of type 2 diabetes.
Acknowledgments
We thank Cynthia Khoo for artistic support for Fig. 1.
Footnotes
This work was supported by National Institutes of Health Grants DK49210 and DK0-68157 (to D.A.S.) and T32-GM08216 (to M.M.S.).
Disclosure Summary: M.S. has nothing to declare. D.S. is a coinventor on U.S. Patent PCT/US99/18099. D.S. received an honorarium from Merck Research Laboratories.
First Published Online February 5, 2009
Abbreviations: CDK, Cyclin-dependent kinase; ER, endoplasmic reticulum; GLP, glucagon-like peptide; GSK, glycogen synthase kinase; HFD, high-fat diet; IGF1R, IGF-I receptor; IR, insulin receptor; IRKO, IR knockout; IRS, insulin receptor substrate; JAK/STAT, Janus family of tyrosine kinases/signal transducer and activator of transcription; MODY, maturity onset diabetes of the young; ObR, leptin receptor; PGC1α, PPARγ coactivator 1α; PDK1, 3-phosphoinositide-dependent protein kinase 1; PPAR, peroxisomal proliferator-activated receptor; RIP, rat insulin promoter.
References
- Prentki M, Nolan CJ 2006 Islet β cell failure in type 2 diabetes. J Clin Invest 116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU 1985 Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 4:110–125 [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC 2003 β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52:102–110 [DOI] [PubMed] [Google Scholar]
- Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC 2006 Relationship between β-cell mass and fasting blood glucose concentration in humans. Diabetes Care 29:717–718 [DOI] [PubMed] [Google Scholar]
- Brüning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR 1997 Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88:561–572 [DOI] [PubMed] [Google Scholar]
- Hull RL, Kodama K, Utzschneider KM, Carr DB, Prigeon RL, Kahn SE 2005 Dietary-fat-induced obesity in mice results in β cell hyperplasia but not increased insulin release: evidence for specificity of impaired β cell adaptation. Diabetologia 48:1350–1358 [DOI] [PubMed] [Google Scholar]
- Parsons JA, Brelje TC, Sorenson RL 1992 Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 130:1459–1466 [DOI] [PubMed] [Google Scholar]
- Matveyenko AV, Veldhuis JD, Butler PC 2008 Adaptations in pulsatile insulin secretion, hepatic insulin clearance and β-cell mass to age-related insulin resistance in the rat. Am J Physiol Endocrinol Metab 295:E832–E841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S, Deery D, Leahy JL, Weir GC 1989 Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes 38:49–53 [DOI] [PubMed] [Google Scholar]
- Steil GM, Trivedi N, Jonas JC, Hasenkamp WM, Sharma A, Bonner-Weir S, Weir GC 2001 Adaptation of β-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab 280:E788–E796 [DOI] [PubMed] [Google Scholar]
- Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O'Donnell CP, Garcia-Ocaña A 2007 Glucose infusion in mice: a new model to induce β-cell replication. Diabetes 56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky KS 1998 Role of apoptosis in failure of β-cell mass compensation for insulin resistance and β-cell defects in the male Zucker diabetic fatty rat. Diabetes 47:358–364 [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Soeller WC, Butler PC 2003 Increased β-cell apoptosis prevents adaptive increase in β-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52:2304–2314 [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA 2004 Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429:41–46 [DOI] [PubMed] [Google Scholar]
- Nir T, Melton DA, Dor Y 2007 Recovery from diabetes in mice by β cell regeneration. J Clin Invest 117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H 2008 β Cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132:197–207 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA 2008 In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC 2008 β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR 1999 Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96:329–339 [DOI] [PubMed] [Google Scholar]
- Otani K, Kulkarni RN, Baldwin AC, Krutzfeldt J, Ueki K, Stoffel M, Kahn CR, Polonsky KS 2004 Reduced β-cell mass and altered glucose sensing impair insulin-secretory function in βIRKO mice. Am J Physiol Endocrinol Metab 286:E41–E49 [DOI] [PubMed] [Google Scholar]
- Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF 1999 Irs-2 coordinates Igf-1 receptor-mediated β-cell development and peripheral insulin signalling. Nat Genet 23:32–40 [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Holzenberger M, Shih DQ, Ozcan U, Stoffel M, Magnuson MA, Kahn CR 2002 β-Cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter β-cell mass. Nat Genet 31:111–115 [DOI] [PubMed] [Google Scholar]
- Robertson K, Lu Y, De Jesus K, Li B, Su Q, Lund PK, Liu JL 2008 A general and islet cell-enriched overexpression of IGF-I results in normal islet cell growth, hypoglycemia, and significant resistance to experimental diabetes. Am J Physiol Endocrinol Metab 294:E928–E938 [DOI] [PubMed] [Google Scholar]
- Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, Peters JL, Shackman JG, Zhang M, Artner I, Satin LS, Stein R, Holzenberger M, Kennedy RT, Kahn CR, Kulkarni RN 2006 Total insulin and IGF-I resistance in pancreatic β cells causes overt diabetes. Nat Genet 38:583–588 [DOI] [PubMed] [Google Scholar]
- Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN 2007 Insulin receptors in β-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci USA 104:8977–8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF 1998 Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904 [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Kido Y, Uchida T, Asahara S, Shigeyama Y, Matsuda T, Takeda A, Tsuchihashi D, Nishizawa A, Ogawa W, Fujimoto Y, Okamura H, Arden KC, Herrera PL, Noda T, Kasuga M 2006 Ablation of PDK1 in pancreatic β cells induces diabetes as a result of loss of β cell mass. Nat Genet 38:589–593 [DOI] [PubMed] [Google Scholar]
- Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ 2001 Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat Med 7:1133–1137 [DOI] [PubMed] [Google Scholar]
- Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA 2001 Islet β cell expression of constitutively active Akt1/PKB α induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest 108:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Hribal ML, Lin HV, Bennett WR, Ward A, Accili D 2006 Role of the forkhead protein FoxO1 in β cell compensation to insulin resistance. J Clin Invest 116:775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Tanabe K, Bernal-Mizrachi E, Permutt MA 2008 Mice with β cell overexpression of glycogen synthase kinase-3β have reduced β cell mass and proliferation. Diabetologia 51:623–631 [DOI] [PubMed] [Google Scholar]
- Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G 2000 Hypoinsulinaemia, glucose intolerance and diminished β-cell size in S6K1-deficient mice. Nature 408:994–997 [DOI] [PubMed] [Google Scholar]
- Johnson JD, Bernal-Mizrachi E, Alejandro EU, Han Z, Kalynyak TB, Li H, Beith JL, Gross J, Warnock GL, Townsend RR, Permutt MA, Polonsky KS 2006 Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci USA 103:19575–19580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beith JL, Alejandro EU, Johnson JD 2008 Insulin stimulates primary β-cell proliferation via Raf-1 kinase. Endocrinology 149:2251–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvillié B, Cordonnier N, Deltour L, Dandoy-Dron F, Itier JM, Monthioux E, Jami J, Joshi RL, Bucchini D 1997 Phenotypic alterations in insulin-deficient mutant mice. Proc Natl Acad Sci USA 94:5137–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux L, Desbois P, Lamotte L, Duvillie B, Cordonnier N, Jackerott M, Jami J, Bucchini D, Joshi RL 2001 Compensatory responses in mice carrying a null mutation for Ins1 or Ins2. Diabetes 50(Suppl 1):S150–S153 [DOI] [PubMed] [Google Scholar]
- Duvillié B, Currie C, Chrones T, Bucchini D, Jami J, Joshi RL, Hill DJ 2002 Increased islet cell proliferation, decreased apoptosis, and greater vascularization leading to β-cell hyperplasia in mutant mice lacking insulin. Endocrinology 143:1530–1537 [DOI] [PubMed] [Google Scholar]
- Chick WL, Warren S, Chute RN, Like AA, Lauris V, Kitchen KC 1977 A transplantable insulinoma in the rat. Proc Natl Acad Sci USA 74:628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaura C, Chen L, Appel M, Alam T, Inman L, Hughes SD, Milburn JL, Unger RH, Newgard CB 1991 Expression of reg/PSP, a pancreatic exocrine gene: relationship to changes in islet β-cell mass. Mol Endocrinol 5:226–234 [DOI] [PubMed] [Google Scholar]
- Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T 2007 Glucokinase and IRS-2 are required for compensatory β cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest 117:246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Bernal-Mizrachi E, Ohsugi M, Permutt MA 2002 Glucose promotes pancreatic islet β-cell survival through a PI 3-kinase/Akt-signaling pathway. Am J Physiol Endocrinol Metab 283:E784–E793 [DOI] [PubMed] [Google Scholar]
- Hügl SR, White MF, Rhodes CJ 1998 Insulin-like growth factor I (IGF-I)-stimulated pancreatic β-cell growth is glucose-dependent. Synergistic activation of insulin receptor substrate-mediated signal transduction pathways by glucose and IGF-I in INS-1 cells. J Biol Chem 273:17771–17779 [DOI] [PubMed] [Google Scholar]
- Martinez SC, Cras-Méneur C, Bernal-Mizrachi E, Permutt MA 2006 Glucose regulates Foxo1 through insulin receptor signaling in the pancreatic islet β-cell. Diabetes 55:1581–1591 [DOI] [PubMed] [Google Scholar]
- Chick WL, Like AA 1970 Studies in the diabetic mutant mouse. 3. Physiological factors associated with alterations in β cell proliferation. Diabetologia 6:243–251 [DOI] [PubMed] [Google Scholar]
- Edvell A, Lindström P 1999 Initiation of increased pancreatic islet growth in young normoglycemic mice (Umea +/?). Endocrinology 140:778–783 [DOI] [PubMed] [Google Scholar]
- Flier SN, Kulkarni RN, Kahn CR 2001 Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci USA 98:7475–7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai J, Katagiri H, Yamada T, Ishigaki Y, Suzuki T, Kudo H, Uno K, Hasegawa Y, Gao J, Kaneko K, Ishihara H, Niijima A, Nakazato M, Asano T, Minokoshi Y, Oka Y 2008 Regulation of pancreatic β cell mass by neuronal signals from the liver. Science 322:1250–1254 [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM 1995 Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161 [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA 2001 The hormone resistin links obesity to diabetes. Nature 409:307–312 [DOI] [PubMed] [Google Scholar]
- Park S, Hong SM, Sung SR, Jung HK 2008 Long-term effects of central leptin and resistin on body weight, insulin resistance, and β-cell function and mass by the modulation of hypothalamic leptin and insulin signaling. Endocrinology 149:445–454 [DOI] [PubMed] [Google Scholar]
- Emilsson V, Liu YL, Cawthorne MA, Morton NM, Davenport M 1997 Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes 46:313–316 [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, Heller RS, Habener JF 1996 Leptin receptors expressed on pancreatic β-cells. Biochem Biophys Res Commun 224:522–527 [DOI] [PubMed] [Google Scholar]
- Fehmann HC, Berghöfer P, Brandhorst D, Brandhorst H, Hering B, Bretzel RG, Göke B 1997 Leptin inhibition of insulin secretion from isolated human islets. Acta Diabetol 34:249–252 [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR 1997 Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest 100:2729–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert J, Kieffer TJ, Leech CA, Holz GG, Moritz W, Ricordi C, Habener JF 1999 Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab 84:670–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K, Schulthess FT, Bielman C, Berney T, Bonny C, Prentki M, Donath MY, Roduit R 2008 Glucose and leptin induce apoptosis in human β-cells and impair glucose-stimulated insulin secretion through activation of c-Jun N-terminal kinases. FASEB J 22:1905–1913 [DOI] [PubMed] [Google Scholar]
- Covey SD, Wideman RD, McDonald C, Unniappan S, Huynh F, Asadi A, Speck M, Webber T, Chua SC, Kieffer TJ 2006 The pancreatic β cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab 4:291–302 [DOI] [PubMed] [Google Scholar]
- Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN 2007 Disruption of leptin receptor expression in the pancreas directly affects β cell growth and function in mice. J Clin Invest 117:2860–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller RS, Klein T, Ling Z, Heimberg H, Katoh M, Madsen OD, Serup P 2003 Expression of Wnt, Frizzled, sFRP, and DKK genes in adult human pancreas. Gene Expr 11:141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK 2007 Wnt signaling regulates pancreatic β cell proliferation. Proc Natl Acad Sci USA 104:6247–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinner S, Ulgen F, Papewalis C, Schott M, Woelk A, Vidal-Puig A, Scherbaum WA 2008 Regulation of insulin secretion, glucokinase gene transcription and β cell proliferation by adipocyte-derived Wnt signalling molecules. Diabetologia 51:147–154 [DOI] [PubMed] [Google Scholar]
- Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, et al. 2006 Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38:320–323 [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, et al. 2007 A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P 2007 A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885 [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC 2003 PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273 [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ 2003 Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM 2007 Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic β cell crosstalk. J Clin Invest 117:3463–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Febbraio M, Saltin B 2003 Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil 24:113–119 [DOI] [PubMed] [Google Scholar]
- Ellingsgaard H, Ehses JA, Hammar EB, Van Lommel L, Quintens R, Martens G, Kerr-Conte J, Pattou F, Berney T, Pipeleers D, Halban PA, Schuit FC, Donath MY 2008 Interleukin-6 regulates pancreatic α-cell mass expansion. Proc Natl Acad Sci USA 105:13163–13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G 2007 Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, Hinoi E, Karsenty G, Ducy P 2008 Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 105:5266–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA 2005 Very slow turnover of β-cells in aged adult mice. Diabetes 54:2557–2567 [DOI] [PubMed] [Google Scholar]
- Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, Stewart AF 2006 Molecular control of cell cycle progression in the pancreatic β-cell. Endocr Rev 27:356–370 [DOI] [PubMed] [Google Scholar]
- Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M 1999 Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat Genet 22:44–52 [DOI] [PubMed] [Google Scholar]
- Hino S, Yamaoka T, Yamashita Y, Yamada T, Hata J, Itakura M 2004 In vivo proliferation of differentiated pancreatic islet β cells in transgenic mice expressing mutated cyclin-dependent kinase 4. Diabetologia 47:1819–1830 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Inoue H, Keshavarz P, Mizuta K, Sato A, Sakamoto Y, Moritani M, Kunika K, Tanahashi T, Itakura M 2008 Transgenic expression of a mutated cyclin-dependent kinase 4 (CDK4/R24C) in pancreatic β-cells prevents progression of diabetes in db/db mice. Diabetes Res Clin Pract 82:33–41 [DOI] [PubMed] [Google Scholar]
- Mziaut H, Kersting S, Knoch KP, Fan WH, Trajkovski M, Erdmann K, Bergert H, Ehehalt F, Saeger HD, Solimena M 2008 ICA512 signaling enhances pancreatic β-cell proliferation by regulating cyclins D through STATs. Proc Natl Acad Sci USA 105:674–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF 2005 Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol Cell Biol 25:3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Chen M, Zhang QH, Ma Z, Weinstein LS 2007 β Cell-specific deficiency of the stimulatory G protein α-subunit Gsα leads to reduced β cell mass and insulin-deficient diabetes. Proc Natl Acad Sci USA 104:19601–19606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen BN, Richter HE, Hansen JA, Rhodes CJ, Nielsen JH, Billestrup N, Moldrup A 2003 Signal transducer and activator of transcription 5 activation is sufficient to drive transcriptional induction of cyclin D2 gene and proliferation of rat pancreatic β-cells. Mol Endocrinol 17:945–958 [DOI] [PubMed] [Google Scholar]
- Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KI, White MF, Kasuga M 2005 Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 11:175–182 [DOI] [PubMed] [Google Scholar]
- Rachdi L, Balcazar N, Elghazi L, Barker DJ, Krits I, Kiyokawa H, Bernal-Mizrachi E 2006 Differential effects of p27 in regulation of β-cell mass during development, neonatal period, and adult life. Diabetes 55:3520–3528 [DOI] [PubMed] [Google Scholar]
- Zhong L, Georgia S, Tschen SI, Nakayama K, Bhushan A 2007 Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic β cells. J Clin Invest 117:2869–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK 2007 Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science 318:806–809 [DOI] [PubMed] [Google Scholar]
- Schnepp RW, Chen YX, Wang H, Cash T, Silva A, Diehl JA, Brown E, Hua X 2006 Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Res 66:5707–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozar-Castellano I, Haught M, Stewart AF 2006 The cell cycle inhibitory protein p21cip is not essential for maintaining β-cell cycle arrest or β-cell function in vivo. Diabetes 55:3271–3278 [DOI] [PubMed] [Google Scholar]
- Vasavada RC, Cozar-Castellano I, Sipula D, Stewart AF 2007 Tissue-specific deletion of the retinoblastoma protein in the pancreatic β-cell has limited effects on β-cell replication, mass, and function. Diabetes 56:57–64 [DOI] [PubMed] [Google Scholar]
- Stout LE, Svensson AM, Sorenson RL 1997 Prolactin regulation of islet-derived INS-1 cells: characteristics and immunocytochemical analysis of STAT5 translocation. Endocrinology 138:1592–1603 [DOI] [PubMed] [Google Scholar]
- Friedrichsen BN, Galsgaard ED, Nielsen JH, Moldrup A 2001 Growth hormone- and prolactin-induced proliferation of insulinoma cells, INS-1, depends on activation of STAT5 (signal transducer and activator of transcription 5). Mol Endocrinol 15:136–148 [DOI] [PubMed] [Google Scholar]
- Galsgaard ED, Friedrichsen BN, Nielsen JH, Moldrup A 2001 Expression of dominant-negative STAT5 inhibits growth hormone- and prolactin-induced proliferation of insulin-producing cells. Diabetes 50 (Suppl 1):S40–S41 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs III WH, Wright CV, White MF, Arden KC, Accili D 2002 The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest 110:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Liu Z, Patel S, Doble BW, Li L, Cras-Méneur C, Martinez SC, Welling CM, White MF, Bernal-Mizrachi E, Woodgett JR, Permutt MA 2008 Genetic deficiency of glycogen synthase kinase-3β corrects diabetes in mouse models of insulin resistance. PLoS Biol 6:e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, Burk U, Onichtchouk D, Dohrmann C, Austen M 2007 Inhibition of GSK3 promotes replication and survival of pancreatic β cells. J Biol Chem 282:12030–12037 [DOI] [PubMed] [Google Scholar]
- Rachdi L, Balcazar N, Osorio-Duque F, Elghazi L, Weiss A, Gould A, Chang-Chen KJ, Gambello MJ, Bernal-Mizrachi E 2008 Disruption of Tsc2 in pancreatic β cells induces β cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc Natl Acad Sci USA 105:9250–9255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Gao N, Gorski RK, White P, Hardy OT, Rafiq K, Brestelli JE, Chen G, Stoeckert Jr CJ, Kaestner KH 2007 Expansion of adult β-cell mass in response to increased metabolic demand is dependent on HNF-4α. Genes Dev 21:756–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR 2004 PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest 114:828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Blaha M, Spear C, Nicholson W, Radhika A, Shiota M, Charron MJ, Wright CV, Powers AC 2005 Reduced PDX-1 expression impairs islet response to insulin resistance and worsens glucose homeostasis. Am J Physiol Endocrinol Metab 288:E707–E714 [DOI] [PubMed] [Google Scholar]
- Iguchi H, Ikeda Y, Okamura M, Tanaka T, Urashima Y, Ohguchi H, Takayasu S, Kojima N, Iwasaki S, Ohashi R, Jiang S, Hasegawa G, Ioka RX, Magoori K, Sumi K, Maejima T, Uchida A, Naito M, Osborne TF, Yanagisawa M, Yamamoto TT, Kodama T, Sakai J 2005 SOX6 attenuates glucose-stimulated insulin secretion by repressing PDX1 transcriptional activity and is down-regulated in hyperinsulinemic obese mice. J Biol Chem 280:37669–37680 [DOI] [PubMed] [Google Scholar]
- Iguchi H, Urashima Y, Inagaki Y, Ikeda Y, Okamura M, Tanaka T, Uchida A, Yamamoto TT, Kodama T, Sakai J 2007 SOX6 suppresses cyclin D1 promoter activity by interacting with β-catenin and histone deacetylase 1, and its down-regulation induces pancreatic β-cell proliferation. J Biol Chem 282:19052–19061 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Kulkarni RN, Sarraf P, Ozcan U, Okada T, Hsu CH, Eisenman D, Magnuson MA, Gonzalez FJ, Kahn CR, Spiegelman BM 2003 Targeted elimination of peroxisome proliferator-activated receptor γ in β cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol 23:7222–7229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liadis N, Salmena L, Kwan E, Tajmir P, Schroer SA, Radziszewska A, Li X, Sheu L, Eweida M, Xu S, Gaisano HY, Hakem R, Woo M 2007 Distinct in vivo roles of caspase-8 in β-cells in physiological and diabetes models. Diabetes 56:2302–2311 [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY 2002 Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K 2008 The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology 149:2208–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD, Deeney JT, Robertson K, Morash J, Kulkarni A, Neschen S, Kim S, Greenberg ME, Corkey BE, Shirihai OS, Shulman GI, Lowell BB, Korsmeyer SJ 2008 Dual role of proapoptotic BAD in insulin secretion and β cell survival. Nat Med 14:144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME 1999 Cellular survival: a play in three Akts. Genes Dev 13:2905–2927 [DOI] [PubMed] [Google Scholar]
- Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ 2001 p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci USA 98:9666–9670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC 2007 High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated β-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56:2016–2027 [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ 2007 Endoplasmic reticulum stress contributes to β cell apoptosis in type 2 diabetes. Diabetologia 50:752–763 [DOI] [PubMed] [Google Scholar]
- Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ 2006 GLP-1 receptor activation improves β cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 4:391–406 [DOI] [PubMed] [Google Scholar]
- Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ 2005 Control of mRNA translation preserves endoplasmic reticulum function in β cells and maintains glucose homeostasis. Nat Med 11:757–764 [DOI] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ 2008 Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118:3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki K, Kaufman RJ 2008 Regulation of ER stress-induced macroautophagy by protein kinase C. Autophagy 4:841–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, Kawamori R, Fujitani Y, Watada H 2008 Autophagy is important in islet homeostasis and compensatory increase of β cell mass in response to high-fat diet. Cell Metab 8:325–332 [DOI] [PubMed] [Google Scholar]
- Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, Leibowitz G 2008 mTOR inhibition by rapamycin prevents β-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 57:945–957 [DOI] [PubMed] [Google Scholar]
- Martinez SC, Tanabe K, Cras-Méneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA 2008 Inhibition of Foxo1 protects pancreatic islet β-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes 57:846–859 [DOI] [PubMed] [Google Scholar]
- Lin CY, Gurlo T, Haataja L, Hsueh WA, Butler PC 2005 Activation of peroxisome proliferator-activated receptor-gamma by rosiglitazone protects human islet cells against human islet amyloid polypeptide toxicity by a phosphatidylinositol 3′-kinase-dependent pathway. J Clin Endocrinol Metab 90:6678–6686 [DOI] [PubMed] [Google Scholar]
- Han SJ, Kang ES, Hur KY, Kim HJ, Kim SH, Yun CO, Choi SE, Ahn CW, Cha BS, Kang Y, Lee HC 2008 Rosiglitazone inhibits early stage of glucolipotoxicity-induced β-cell apoptosis. Horm Res 70:165–173 [DOI] [PubMed] [Google Scholar]
- Doria A, Patti ME, Kahn CR 2008 The emerging genetic architecture of type 2 diabetes. Cell Metab 8:186–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Boström K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, et al. 2007 Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336 [DOI] [PubMed] [Google Scholar]
- Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, Styrkarsdottir U, Gretarsdottir S, Emilsson V, Ghosh S, Baker A, Snorradottir S, Bjarnason H, Ng MC, Hansen T, Bagger Y, Wilensky RL, Reilly MP, Adeyemo A, Chen Y, Zhou J, Gudnason V, Chen G, Huang H, Lashley K, et al. 2007 A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 39:770–775 [DOI] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT 2007 Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P, Sjögren M, Ling C, Eriksson KF, Lethagen AL, Mancarella R, Berglund G, Tuomi T, Nilsson P, Del Prato S, Groop L 2007 Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 117:2155–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K 2008 Transcription factor 7-like 2 regulates β-cell survival and function in human pancreatic islets. Diabetes 57:645–653 [DOI] [PubMed] [Google Scholar]
- Liu Z, Habener JF 2008 Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic β cell proliferation. J Biol Chem 283:8723–8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelengaris S, Khan M, Evan GI 2002 Suppression of Myc-induced apoptosis in β cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell 109:321–334 [DOI] [PubMed] [Google Scholar]
- Cano DA, Rulifson IC, Heiser PW, Swigart LB, Pelengaris S, German M, Evan GI, Bluestone JA, Hebrok M 2008 Regulated β-cell regeneration in the adult mouse pancreas. Diabetes 57:958–966 [DOI] [PubMed] [Google Scholar]
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA 2007 Growth and regeneration of adult β cells does not involve specialized progenitors. Dev Cell 12:817–826 [DOI] [PubMed] [Google Scholar]
- Bigatel T.A., Cozar-Castellano I., Velazquez-Garcia S., Harb G., Fiaschi-Taesch N, Selk K, Takane KK, Stewart SF 2007 Comprehensive comparative cell cycle analysis reveals critical differences in human vs. murine β cell regulation: human islets contain Cdk-6, but lack Cdk-4. Diabetes 56:A416 [Google Scholar]
- Cozar-Castellano I, Takane KK, Bottino R, Balamurugan AN, Stewart AF 2004 Induction of β-cell proliferation and retinoblastoma protein phosphorylation in rat and human islets using adenovirus-mediated transfer of cyclin-dependent kinase-4 and cyclin D1. Diabetes 53:149–159 [DOI] [PubMed] [Google Scholar]
- Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R 2002 Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 143:4397–4408 [DOI] [PubMed] [Google Scholar]
- Xu G, Stoffers DA, Habener JF, Bonner-Weir S 1999 Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48:2270–2276 [DOI] [PubMed] [Google Scholar]
- Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M 2004 Glucagon-like peptide-1 prevents β cell glucolipotoxicity. Diabetologia 47:806–815 [DOI] [PubMed] [Google Scholar]
- Lamont BJ, Drucker DJ 2008 Differential antidiabetic efficacy of incretin agonists versus DPP-4 inhibition in high fat fed mice. Diabetes 57:190–198 [DOI] [PubMed] [Google Scholar]
- Wang Q, Brubaker PL 2002 Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia 45:1263–1273 [DOI] [PubMed] [Google Scholar]
- Hansotia T, Maida A, Flock G, Yamada Y, Tsukiyama K, Seino Y, Drucker DJ 2007 Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest 117:143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ, Schreiber WE, Zhong E, Liu FF, Kornfeld BD, Wondisford FE, Hussain MA 2008 Exendin-4 stimulation of cyclin A2 in β-cell proliferation. Diabetes 57:2371–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Butler AE, Galasso R, Rizza RA, Butler PC 2006 Increased islet β cell replication adjacent to intrapancreatic gastrinomas in humans. Diabetologia 49:2689–2696 [DOI] [PubMed] [Google Scholar]
- Boushey RP, Abadir A, Flamez D, Baggio LL, Li Y, Berger V, Marshall BA, Finegood D, Wang TC, Schuit F, Drucker DJ 2003 Hypoglycemia, defective islet glucagon secretion, but normal islet mass in mice with a disruption of the gastrin gene. Gastroenterology 125:1164–1174 [DOI] [PubMed] [Google Scholar]
- Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, Friesen HG, Sorenson RL 1993 Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132:879–887 [DOI] [PubMed] [Google Scholar]
- Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, Kendall W, Oden J, Bridges S, Binart N, Breant B, Kelly PA 2002 Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 143:1378–1385 [DOI] [PubMed] [Google Scholar]
- Robertson K, Kopchick JJ, Liu JL 2006 Growth hormone receptor gene deficiency causes delayed insulin responsiveness in skeletal muscles without affecting compensatory islet cell overgrowth in obese mice. Am J Physiol Endocrinol Metab 291:E491–E498 [DOI] [PubMed] [Google Scholar]
- Liu JL, Coschigano KT, Robertson K, Lipsett M, Guo Y, Kopchick JJ, Kumar U, Liu YL 2004 Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab 287:E405–E413 [DOI] [PubMed] [Google Scholar]
- Vasavada RC, Garcia-Ocaña A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF 2000 Targeted expression of placental lactogen in the β cells of transgenic mice results in β cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 275:15399–15406 [DOI] [PubMed] [Google Scholar]
- Fujinaka Y, Sipula D, Garcia-Ocaña A, Vasavada RC 2004 Characterization of mice doubly transgenic for parathyroid hormone-related protein and murine placental lactogen: a novel role for placental lactogen in pancreatic β-cell survival. Diabetes 53:3120–3130 [DOI] [PubMed] [Google Scholar]
- Vasavada RC, Cavaliere C, D'Ercole AJ, Dann P, Burtis WJ, Madlener AL, Zawalich K, Zawalich W, Philbrick W, Stewart AF 1996 Overexpression of parathyroid hormone-related protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J Biol Chem 271:1200–1208 [DOI] [PubMed] [Google Scholar]
- Cebrian A, García-Ocaña A, Takane KK, Sipula D, Stewart AF, Vasavada RC 2002 Overexpression of parathyroid hormone-related protein inhibits pancreatic β-cell death in vivo and in vitro. Diabetes 51:3003–3013 [DOI] [PubMed] [Google Scholar]
- Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF 2000 Hepatocyte growth factor overexpression in the islet of transgenic mice increases β cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem 275:1226–1232 [DOI] [PubMed] [Google Scholar]
- Roccisana J, Reddy V, Vasavada RC, Gonzalez-Pertusa JA, Magnuson MA, Garcia-Ocaña A 2005 Targeted inactivation of hepatocyte growth factor receptor c-met in β-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of β-cell mass. Diabetes 54:2090–2102 [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E 2001 Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest 107:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissios P, Ozcan U, Kokkotou E, Okada T, Liew CW, Liu S, Peters JN, Dahlgren G, Karamchandani J, Kudva YC, Kurpad AJ, Kennedy RT, Maratos-Flier E, Kulkarni RN 2007 Melanin concentrating hormone is a novel regulator of islet function and growth. Diabetes 56:311–319 [DOI] [PubMed] [Google Scholar]