Abstract
Circulating ghrelin elevates abdominal adiposity by a mechanism independent of its central orexigenic activity. In this study we tested the hypothesis that peripheral ghrelin induces a depot-specific increase in white adipose tissue (WAT) mass in vivo by GH secretagogue receptor (GHS-R1a)-mediated lipolysis. Chronic iv infusion of acylated ghrelin increased retroperitoneal and inguinal WAT volume in rats without elevating superficial sc fat, food intake, or circulating lipids and glucose. Increased retroperitoneal WAT mass resulted from adipocyte enlargement probably due to reduced lipid export (ATP-binding cassette transporter G1 mRNA expression and circulating free fatty acids were halved by ghrelin infusion). In contrast, ghrelin treatment did not up-regulate biomarkers of adipogenesis (peroxisome proliferator-activated receptor-γ2 or CCAAT/enhancer binding protein-α) or substrate uptake (glucose transporter 4, lipoprotein lipase, or CD36) and although ghrelin elevated sterol-regulatory element-binding protein 1c expression, WAT-specific mediators of lipogenesis (liver X receptor-α and fatty acid synthase) were unchanged. Adiposity was unaffected by infusion of unacylated ghrelin, and the effects of acylated ghrelin were abolished by transcriptional blockade of GHS-R1a, but GHS-R1a mRNA expression was similar in responsive and unresponsive WAT. Microarray analysis suggested that depot-specific sensitivity to ghrelin may arise from differential fine tuning of signal transduction and/or lipid-handling mechanisms. Acylated ghrelin also induced hepatic steatosis, increasing lipid droplet number and triacylglycerol content by a GHS-R1a-dependent mechanism. Our data imply that, during periods of energy insufficiency, exposure to acylated ghrelin may limit energy utilization in specific WAT depots by GHS-R1a-dependent lipid retention.
Circulating acylated ghrelin elevates fat mass in specific abdominal depots by a GHSR1a-dependent reduction in lipid export and an increase in adipocyte size.
Given the startling rise in obesity, understanding the mechanisms regulating fat deposition has never been more pressing. At the cellular level, expansion of white adipose tissue (WAT) volume arises from either increased adipocyte number (adipogenesis) and/or increased adipocyte size as a result of enhanced substrate uptake, elevated lipid synthesis (lipogenesis), decreased lipid utilization (lipolysis), and/or reduced lipid export.
The gastric hormone, ghrelin, is now thought to play a significant role in the regulation of lipid storage in WAT. Ghrelin is secreted from the stomach (1) in response to fasting (2) or food restriction (3), and has been identified in a discrete population of hypothalamic interneurons (4). Activating GH secretagogue receptors (GHS-R1a) (1) in the hypothalamus, ghrelin stimulates orexigenic neurons (4,5,6,7) and promotes the preferential ingestion of fat (8). Although acute ghrelin exposure also induces GH secretion (1,9), the net effect of prolonged ghrelin exposure is increased fat mass (2,10).
However, the effect of ghrelin exposure on adipocytes remains controversial. Ghrelin has been reported to inhibit (11) and enhance adipogenesis (2,12,13), augment fat storage enzyme activity (14), to elevate triglyceride content (15), and reduce fat utilization (2)/lipolysis (15,16). These contradictory results may arise from the use of different modes of ghrelin exposure and analysis of adipocytes from different locations. To clarify this situation we have tested the hypothesis that peripheral ghrelin elicits depot-specific increases in WAT mass by inducing GH secretagogue receptor (GHS-R1a)-dependent lipolysis.
In this study, we used magnetic resonance imaging (MRI) to establish the nonuniform effect of chronic acylated ghrelin exposure on abdominal WAT volume in rats. We then determined whether these effects of ghrelin are mimicked by unacylated ghrelin (UAG), which is the most abundant form of ghrelin in the circulation (18). Although UAG does not bind to GHS-R1a (1), it is equipotent with acylated ghrelin in stimulating adipogenesis in bone marrow (12). To elucidate the cellular mechanisms underlying the effects of ghrelin on abdominal adiposity, we quantified circulating energy substrates and the expression of adipogenic, substrate uptake, lipogenic, lipolytic, and lipid export biomarkers in responsive abdominal WAT in rats after treatment with acylated ghrelin. To establish whether the ghrelin-induced elevation in WAT mass is mediated by GHS-R, we measured abdominal adiposity after infusion of acylated ghrelin in loxTB-GHS-R mice, in which transcriptional blockade prevents expression of GHS-R (18). To determine the mechanism by which individual abdominal WAT depots are sensitive to ghrelin exposure, we quantified GHS-R mRNA expression in a range of abdominal fat depots and performed a transcriptome microarray in ghrelin-responsive and ghrelin-unresponsive intraabdominal WAT. Finally, to determine whether the influence of ghrelin on lipid storage is WAT specific, we quantified the effect of acylated ghrelin on hepatic lipid content in rats and loxTB-GHS-R mice.
Results
Study 1: ghrelin induces depot-specific increases in abdominal WAT
A 2-wk continuous iv infusion of acylated ghrelin significantly altered abdominal WAT distribution (Fig. 1, A and B). Although body weight gain (122% of vehicle-treated; P = 0.054; data not shown) and total WAT volume (Fig. 1C; 117%; P = 0.071) were not significantly elevated by ghrelin treatment, inguinal and retroperitoneal WAT volumes were increased by 20% (Fig. 1E; P = 0.030) and 50%, respectively (Fig. 1G; P = 0.025). Mesenteric (Fig. 1F; 117% of vehicle-treated; P = 0.067) and epididymal (Fig. 1H; 122%; P = 0.212) WAT volumes were not significantly increased by ghrelin exposure, and superficial sc WAT volume was unaffected (Fig. 1D).
Figure 1.
Ghrelin induces a depot-specific increase in abdominal WAT. Analysis of fat-only MRI images of rat abdomen after 2-wk iv infusion of vehicle (A; 24 μl/d) or ghrelin (B; 80 μg/d) enabled quantification of total WAT volume (C) and the volumes of superficial sc (D), inguinal (E), mesenteric (F), retroperitoneal (G), and epididymal (H) WAT depots. Values shown are mean ± sem (n = 6) with statistical comparisons made by unpaired Student’s t test (*, P < 0.05).
Study 2: UAG does not increase abdominal adiposity
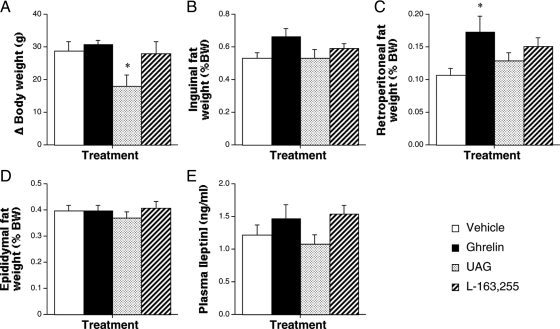
Because we had previously shown that the adipogenic action of ghrelin in bone marrow is mimicked by UAG (12), we investigated whether the effects of ghrelin in abdominal WAT could be seen after UAG treatment. Male rats received 1-wk iv infusions of acylated ghrelin, UAG, or the GHS-R1a-specific ligand, L-163,255 (19,20), and dissected WAT depots were weighed post mortem. Ghrelin increased retroperitoneal (Fig. 2C; 62% higher; P < 0.05) and perirenal (64% higher; P < 0.05; data not shown) WAT mass, without significantly affecting inguinal (Fig. 2D; 124%) or epididymal (Fig. 2D) WAT weights or circulating leptin (Fig. 2E; 120%). In contrast, UAG reduced body weight gain (Fig. 2A; 37% lower; P < 0.05), without affecting any of the parameters of adiposity. None of the parameters measured were significantly affected by L-163,255 infusion, although retroperitoneal fat weight was 141% of that in vehicle-treated rats (P > 0.05). Thus, UAG did not promote abdominal fat deposition.
Figure 2.
UAG does not increase abdominal adiposity. Quantification of body weight gain (A) and the weight of inguinal (B), retroperitoneal (C), and epididymal (D) WAT depots and circulating leptin (E) in male rats after 1-wk iv infusion of vehicle (24 μl/d), ghrelin (80 μg/d), UAG (80 μg/d), or L-163,255 (160 μg/d). Values shown are mean ± sem [n = 6 (ghrelin and UAG), n = 5 (vehicle), n = 4 (L-163,255)] with statistical comparisons made by one-way ANOVA and Bonferroni’s selected pairs post hoc test (*, P < 0.05). BW, Body weight.
Study 3: ghrelin does not elevate circulating substrate
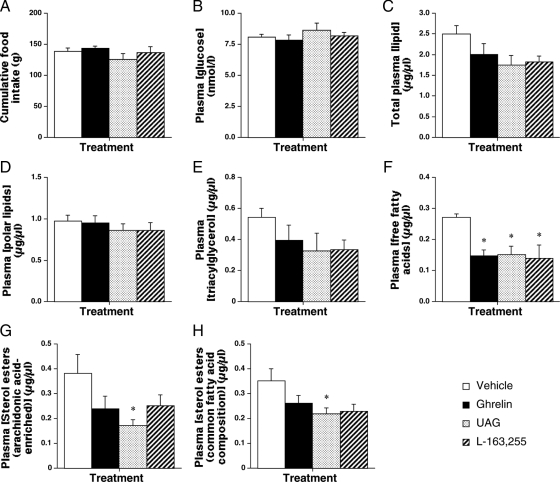
To determine whether the ghrelin-induced elevation in abdominal adiposity is due to an increase in circulating substrate, glucose and lipid profiling was performed on terminal plasma samples from infused rats in study 2. Peptide infusion did not affect cumulative food intake (Fig. 3A) and, instead of increasing circulating substrates (Fig. 3, B–H), plasma free fatty acids (FFAs) were halved by ghrelin, UAG, and L-163,255 treatment (Fig. 3F). In addition, UAG reduced the diet-derived sterol esters (arachidonic acid enriched by 55%; Fig. 3G; esters of common fatty acid composition by 37%; Fig 3H) in the circulation. Thus, ghrelin did not elevate abdominal adiposity by increasing the availability of circulating metabolic substrates but appeared to reduce lipid export from WAT.
Figure 3.
Ghrelin does not elevate circulating substrate. Quantification of cumulative food intake (A) and the concentrations of glucose (B), total lipid (C), polar lipids (D), triacylglycerol (E), free fatty acids (F), and arachidonic acid-enriched (G) or common fatty acid composition (H) sterol esters in terminal plasma from male rats after 1-wk iv infusion of vehicle, ghrelin, UAG, or L-163,255. Values shown are mean ± sem [n = 6 (ghrelin and UAG), n = 5 (vehicle), n = 4 (L-163,255)] with statistical comparisons made by one-way ANOVA and Bonferroni’s selected pairs post hoc test (*, P < 0.05).
Study 4: ghrelin reduces ATP-binding cassette transporter G1 (ABCG1) expression, a lipid export biomarker
To determine the underlying cellular mechanism of the ghrelin-induced abdominal WAT mass, we used PCR to quantify the expression of adipogenic, lipogenic, and lipolytic transcripts in retroperitoneal fat from rats treated with acylated ghrelin. Neither biomarker of adipogenesis, peroxisome proliferator-activated receptor (PPAR)γ2, and C/EBPα, were affected by ghrelin infusion (Table 1), but AP2 mRNA expression (a marker of terminally differentiated adipocytes) was down-regulated (Table 1). Expression of CD36 (which regulates lipid uptake) was significantly reduced, whereas the transcripts associated with glucose (GLUT4) and triglyceride uptake (lipoprotein lipase) were unaffected. Expression of biomarkers of lipid storage (perilipin) and adipocyte growth [liver X receptor (LXR)β] was also significantly reduced. Although sterol-regulatory element-binding protein (SREBP)1c expression was tripled by ghrelin exposure, the WAT-specific mediators of lipogenesis (LXRα and fatty acid synthase) were unaffected. Expression of biomarkers of fat utilization (uncoupling proteins 1 and 2) and lipolysis (hormone-sensitive lipase) was not significantly reduced, but expression of the cholesterol exporter ABCG1 was halved. Thus, ghrelin-induced elevation in WAT mass is not due to elevated substrate uptake or triglyceride synthesis but is likely to be due to reduced lipid export.
Table 1.
The effect of ghrelin infusion on the expression of adipogenic, lipogenic and lipolytic mRNA transcripts in retroperitoneal adipose tissue
| Transcript | Vehicle | Ghrelin | P Value |
|---|---|---|---|
| Adipogenic | |||
| PPARγ2 | 1.00 ± 0.11 | 1.33 ± 0.38 | 0.425 |
| C/EBPα | 1.00 ± 0.15 | 1.26 ± 0.10 | 0.174 |
| Uptake mechanisms | |||
| GLUT4 | 1.00 ± 0.17 | 0.88 ± 0.14 | 0.595 |
| Lipoprotein lipase | 1.00 ± 0.11 | 0.70 ± 0.15 | 0.133 |
| CD36 | 1.00 ± 0.12 | 0.57 ± 0.09 | 0.015 |
| Lipogenic/storage | |||
| AP2 | 1.00 ± 0.08 | 0.67 ± 0.10 | 0.029 |
| SREBP1c | 1.00 ± 0.08 | 2.88 ± 0.53 | 0.006 |
| LXRα | 1.00 ± 0.08 | 0.82 ± 0.08 | 0.129 |
| LXRβ | 1.00 ± 0.05 | 0.43 ± 0.04 | <0.0001 |
| Fatty acid synthase | 1.00 ± 0.19 | 0.75 ± 0.12 | 0.285 |
| Utilization | |||
| UCP1 | 1.00 ± 0.48 | 0.06 ± 0.03 | 0.078 |
| UCP2 | 1.00 ± 0.19 | 0.60 ± 0.08 | 0.076 |
| Lipolytic | |||
| Perilipin | 1.00 ± 0.09 | 0.62 ± 0.08 | 0.013 |
| Hormone-sensitive lipase | 1.00 ± 0.12 | 0.66 ± 0.13 | 0.079 |
| Export mechanisms | |||
| ABCG1 | 1.00 ± 0.19 | 0.50 ± 0.07 | 0.035 |
Values shown are mean ± sem (n = 6 ghrelin-treated; n = 5 vehicle-treated) with statistical comparisons performed by unpaired Student’s t test. UCP, Uncoupling protein.
Study 5: ghrelin increases abdominal adiposity via GHS-R
To establish whether the ghrelin-induced elevation in adiposity is dependent upon GHS-R1a, wild-type (W-T) and loxTB-GHS-R (GHS-R-null) mice received 1-wk iv infusions of either vehicle or acylated ghrelin. In W-T mice ghrelin treatment tripled mean weight gain (Fig. 4A; P > 0.05), increasing inguinal (66%; Fig. 4B; P < 0.05) and epididymal (48%; Fig. 4E; P < 0.05) WAT mass and circulating leptin (2.5-fold; Fig. 4F; P < 0.01), without significantly increasing retroperitoneal WAT (Fig. 4D; 113%). Histological analysis revealed that ghrelin increased inguinal adipocyte size by 40% (Fig. 4C and inset; P < 0.001). In contrast, ghrelin infusion had no effect on weight gain (Fig. 4A) or any of the parameters of adiposity in loxTB-GHS-R mice. Thus, ghrelin-induced elevation of abdominal adiposity is dependent upon GHS-R.
Figure 4.
Ghrelin increases abdominal adiposity via GHS-R. Quantification of body weight gain (A) and inguinal (B), retroperitoneal (D), and epididymal (E) WAT weights, inguinal adipocyte size [C and inset (scale bar, 20 μm)] and circulating leptin (F) in male W-T and loxTB-GHS-R mice after 1-wk iv infusion of vehicle (24 μl/d) or ghrelin (48 μg/d). Values shown are mean ± sem [n = 6 (W-T groups), n = 5 (loxTB-GHS-R groups)] with statistical comparisons performed by one-way ANOVA and Bonferroni’s selected pairs post hoc test (*, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. vehicle-treated W-T; †, P < 0.05; ††, P < 0.01 vs. ghrelin-treated W-T).
Study 6: sensitivity to ghrelin is independent of depot-specific GHS-R1a mRNA expression
Because the ghrelin-induced elevation in adipose tissue mass was dependent upon the presence of GHS-R, we quantified GHS-R mRNA expression in individual WAT depots by PCR. Although significantly lower than in the hypothalamus (<30%; Fig. 5; P < 0.01), GHS-R1a mRNA expression was detectable in all WAT depots, being highest in mesenteric adipose tissue and lowest in tibial marrow adipocytes. Because there were no inter-depot differences in GHS-R1a mRNA expression (Fig. 5) and relative expression did not correspond to ghrelin responsiveness (Figs. 1 and 2), the sensitivity of individual WAT depots appears to be independent of relative GHS-R1a expression. Similarly, GHS-R1b mRNA expression in WAT was less than 40% of that in the hypothalamus, and there were no interdepot differences (data not shown).
Figure 5.
Depot-specific sensitivity to ghrelin is independent of relative GHS-R1a mRNA expression. Semiquantitative PCR analysis of GHS-R1a mRNA expression in hypothalamus (Hyp) and bone marrow (BM), epididymal (Epi), inguinal (Ing), mesenteric (Mes), perirenal (Prr), and retroperitoneal (Rtp) adipose tissue of W-T male rats. GHS-R1a mRNA was detectable in all adipose depots at a lower level than in hypothalamus, but relative expression (% β-actin) was not significantly different between individual depots. A, Values shown are mean ± sem (n = 6 for all tissues) with statistical comparisons performed by one-way ANOVA and Bonferroni’s post hoc test (**, P < 0.01 vs. Hyp). Representative gels for GHS-R1a and β-actin are shown (B and C).
Study 7: depot specificity may be related to differential regulation of signal transduction and lipid handling
Given the relatively uniform expression of GHS-R1a mRNA ghrelin-responsive and ghrelin-unresponsive WAT depots, we used microarray analysis to examine whether depot sensitivity may be due to differential expression of genes associated with the GHS-R1a signal transduction pathway or the mechanisms of lipid handling. The microarray was validated by the expected high levels of expression of genes involved in lipid handling (lipoprotein lipase, hormone-sensitive lipase, GLUT4, fatty acid synthase) or adipogenesis (PPARγ) in both depots (Table 2) and the observation that there was no significant difference in the levels of GHS-R1a mRNA expression (as seen in Fig. 5). Of the genes studied 12% (3917) were differentially expressed between ghrelin-responsive retroperitoneal and ghrelin-unresponsive epididymal adipose tissue (Figs. 1 and 2), with 228 genes showing a greater than 2-fold difference [the complete dataset is lodged with the Minimum Information About a Microarry Experiment (MIAME)-compliant ArrayExpress European Bioinformatics Institute repository (http://www.ebi.ac.uk/microarray-as/ae/; experiment reference: E-MEXP-1993]. Among those genes showing differential expression, guanylate cyclase activator 2a and the regulator of G protein signaling, RGS7, were more highly expressed in ghrelin-responsive retroperitoneal fat, whereas phospholipase C and RGS4 were more highly expressed in epididymal WAT. Given the emerging evidence that heterodimerization of G protein-coupled receptors (21) influences function, we noted that several receptors associated with activating G proteins (CRH-R1, dopamine receptor 1a, and the secretin receptor) were more highly expressed in retroperitoneal WAT, whereas others (Calmodulin receptor 3, GPR88, and VPAC2) were more highly expressed in epididymal fat. In addition, SST-R5, which is associated with an inhibitory G protein, is more highly expressed in retroperitoneal WAT. As expected, the expression of PPARγ2 was not significantly different between the two depots studied, and neither were the majority of genes regulating lipid uptake and storage. However, the glucose uptake transporter, GLUT4, and fatty acid synthase were both more highly expressed in ghrelin-responsive adipose tissue (P < 0.05). Conversely, steroidogenic acute regulatory protein was more highly expressed in epididymal adipose tissue. This analysis suggests that the sensitivity of individual WAT depots to ghrelin exposure may be determined by differential regulation of signal transduction (including receptor oligomerization) and/or lipid handling.
Table 2.
Microarray analysis of mRNA expression in WAT from retroperitoneal and epididymal depots of untreated male rats
| Epididymal WAT | Retroperitoneal WAT | Fold Δ | P Value | |
|---|---|---|---|---|
| Signal transduction | ||||
| Guanylate cyclase activator 2a | 19.29 | 24.19 | 1.3↑ | <0.05 |
| PLC δ1 | 297.2 | 258.3 | 1.2↓ | <0.05 |
| PKC γ | 16.76 | 14.78 | 1.1 | >0.05 |
| Regulators of signal transduction | ||||
| RGS4 | 407.9 | 255.2 | 1.6↓ | <0.05 |
| RGS7 | 212.3 | 684.2 | 3.3↑ | <0.05 |
| G protein-coupled receptors | ||||
| GHS-R | 34.3 | 37.45 | 1.1 | >0.05 |
| Calmodulin-R3 | 497.9 | 374.4 | 1.3↓ | <0.05 |
| CRH-R1 | 12.33 | 15.34 | 1.2↑ | <0.05 |
| Dopamine-R1a | 8.966 | 10.11 | 1.1↑ | <0.05 |
| Secretin-R | 27.62 | 59.69 | 2.2↑ | <0.05 |
| GPR88 | 105.1 | 37.26 | 2.8↓ | <0.05 |
| SST-R5 | 15.46 | 18.16 | 1.2↑ | <0.05 |
| VPAC2 | 712.7 | 127.4 | 5.6↓ | <0.05 |
| Adipogenic | ||||
| PPARγ2 | 1,655 | 2,460 | 1.5 | >0.05 |
| Lipid regulators | ||||
| GLUT4 | 1,298 | 2,410 | 1.9↑ | <0.05 |
| Lipoprotein lipase | 12,929 | 10,556 | 1.2 | >0.05 |
| CD36 | 676.5 | 465.1 | 1.5 | >0.05 |
| Fatty acid synthase | 8,886 | 12,475 | 1.4↑ | <0.05 |
| Perilipin | 321.5 | 310.8 | 1.0 | >0.05 |
| LXRα | 591.9 | 631.0 | 1.1 | >0.05 |
| UCP1 | 15.24 | 166.7 | 11 | >0.05 |
| Lipolytic | ||||
| Hormone-sensitive lipase | 3,599 | 3,258 | 1.1 | >0.05 |
| ABCG1 | 83.93 | 83.91 | 1.0 | >0.05 |
| STAR | 125 | 1,317 | 11↑ | <0.05 |
Values shown are means of raw data obtained from samples from four different rats, with statistical comparisons performed by Student’s t test and Benjamini and Hochberg False Discovery Rate test.
PLC, Phospholipase C; PKC, protein kinase C; RGS, regulator of G protein signaling; CRH-R, CRH receptor; SST-R, somatostatin receptor; VPAC, vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor; UCP, uncoupling protein; STAR, steroidogenic acute regulatory protein.
↑, Higher in retroperitoneal adipose tissue.
↓, Higher in epididymal adipose tissue.
Study 8: ghrelin induces GHS-R-dependent hepatic steatosis
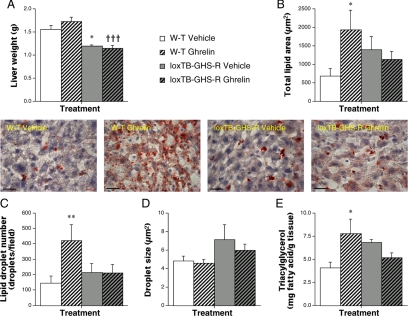
To establish whether ghrelin-induced lipid storage is specific to WAT, we quantified lipid content in sections of liver from ghrelin-treated rats and mice. Total lipid area was elevated by 50% in ghrelin-infused rats but unaffected by UAG treatment (data not shown; P < 0.05). In W-T mice, ghrelin infusion tripled total lipid area (Fig. 6B and inset; P < 0.05) and lipid droplet number (Fig. 5C; P < 0.01) and doubled triacylglycerol content (Fig. 6E; P < 0.05), without significantly affecting liver weight (Fig. 6A) or droplet size (Fig. 6D). None of the parameters of hepatic lipid content were increased in ghrelin-treated loxTB-GHS-R mice (Fig. 6). Thus, ghrelin also induces hepatic steatosis by a GHS-R-dependent mechanism.
Figure 6.
Ghrelin induces GHS-R-dependent hepatic steatosis. Quantification of liver weight (A) and total lipid area (B), droplet number (C), and droplet size (D) in oil red-O-stained sections (inset: scale bar, 20 μm) and triacylglycerol content (E) in livers from male W-T and loxTB-GHS-R mice after 1-wk iv infusion of vehicle (24 μl/d) or ghrelin (48 μg/d). Values shown are mean ± sem [n = 6 (W-T groups), n = 5 (loxTB-GHS-R groups)] with statistical comparisons performed by one-way ANOVA and Bonferroni’s selected pairs post hoc test (*, P < 0.05; **, P < 0.01 vs. vehicle-treated W-T; †††, P < 0.001 vs. ghrelin-treated W-T).
Discussion
Body fat distribution in humans varies with age and gender, and the profile of WAT distribution is predictive of the risk of developing the comorbidities of obesity (22). However, the factors governing the development of particular adiposity profiles remain unclear (23). Despite the differences in lipid metabolism between humans and rodents, the current study provides evidence that peripheral ghrelin regulates expansion of adipocytes in specific abdominal WAT compartments in rats by a GHS-R1a-dependent mechanism.
Using MRI we demonstrated that ghrelin exposure increased the volume of retroperitoneal and inguinal (a deep sc depot) WAT in rats, without influencing epididymal or superficial sc compartments. This finding was replicated in our quantification of WAT mass except that inguinal WAT mass was not significantly elevated, possibly reflecting the shorter treatment time. This depot-specific profile, which is similar to our previous findings in GH-deficient rats (12), differs from the more ubiquitous changes in adiposity induced by manipulating central ghrelin (14,24), which are thought to be mediated by the sympathetic nervous system (14).
At the cellular level, the increase in WAT volume in the current study resulted from adipocyte enlargement (Fig. 4C), without increasing the expression of biomarkers of preadipocyte differentiation, PPARγ2 and C/EBPα (25). Several mechanisms may give rise to this hypertrophy.
First, increased adipocyte volume may arise from increased availability of circulating metabolic substrate. Although central ghrelin injections induce feeding behavior (6,7), chronic peripheral treatment did not affect cumulative food intake in the current study (Fig. 3A) or increase circulating glucose or dietary-derived lipids (Fig. 3, G and H). When combined with our observation that ghrelin did not enhance circulating triacylglycerol, we concluded that adipocyte hypertrophy was not due to elevated availability of circulating substrate.
Alternatively, ghrelin may increase WAT volume by enhancing substrate uptake. Although measurement of mRNA transcripts does not necessarily equate to protein expression, our data do not support this proposal. GLUT4 and lipoprotein lipase expression, the primary regulators of glucose and triacyglycerol uptake, were unaffected by ghrelin treatment, and the expression of CD36, which mediates fatty acid uptake in adipocytes (26), was significantly down-regulated.
Similarly, evidence that ghrelin increases WAT volume by inducing lipogenesis is not convincing. In responsive WAT, expression of SREBP1c, the master regulator of hepatic lipogenesis (27), was tripled by ghrelin exposure. However, SREBP1c does not regulate lipid synthesis in WAT (28) but promotes cholesterol metabolism (27). The WAT-specific mediators of lipogenesis, fatty acid synthase, and LXRα (29) were unaffected by ghrelin exposure. Indeed, the expression of the cytosolic lipid chaperone, aP2, and the nuclear receptor, LXRβ, which is necessary for adipocyte growth (30), was significantly reduced. The difference between this result and a previous report that ghrelin up-regulates expression of lipogenic transcripts (31), may lie in the pattern-dependent and depot-specific nature of this action of ghrelin (12).
In addition, ghrelin may increase WAT volume by inhibiting lipolysis. Although the reduction in perilipin expression indicates that ghrelin may destabilize stored lipid droplets by permitting access of the cytosolic lipases (32), expression of biomarkers of lipolysis or lipid utilization (hormone-sensitive lipase and the uncoupling proteins) was not significantly affected. This differs from previous indirect calorimetric evidence suggesting that ghrelin reduces lipid utilization (2).
Finally, ghrelin may increase adipocyte volume by reducing lipid export. This seems the most plausible option. Ghrelin exposure halved the expression of ABCG1, the primary mediator of cholesterol efflux (33), reduced circulating fatty acids (Fig. 3F), and has previously been reported to suppress glycerol release from cultured epididymal adipocytes (15). Although the precise mechanisms governing the export of FFAs from adipocytes remain to be elucidated (34,35), we conclude that exposure to ghrelin appears to induce adipocyte hypertrophy by enhancing lipid retention in responsive adipocytes.
The effect of peripheral ghrelin on the retroperitoneal WAT transcriptome differs significantly from the increase in lipid storage enzymes and reduction in fatty acid oxidation enzymes induced in epididymal WAT by intracerebroventricular ghrelin treatment (14). Because the effects of central ghrelin are thought to be mediated by the sympathetic nervous system (14), the different molecular profiles suggest that peripheral ghrelin may have a more direct influence.
To investigate this, we quantified GHS-R1a mRNA expression in individual abdominal WAT depots, showing that GHS-R1a and the truncated variant, GHS-R1b, are both expressed in abdominal adipose tissue at much lower levels than in the hypothalamus. The difference between this result and the majority of previous reports (16,36,37,38) may arise from our use of intron-spanning primers. The functional significance of GHS-R in this system is demonstrated by the failure of UAG to elevate WAT mass and the ineffectiveness of ghrelin in elevating adiposity in GHS-R-null mice. In addition, the GHS-R1a-specific ligand, L-163,255, appears to produce a similar profile of responses to ghrelin (Figs. 2 and 3), but the lack of statistical significance (except in the case of circulating FFAs), may indicate that the dose used was insufficient.
Thus, in contrast to the GHS-R1a-independent induction of adipogenesis in bone marrow (12), ghrelin-induced lipid accumulation in intraabdominal WAT is clearly dependent upon the cognate receptor. This corroborates previous reports that GHS-R-null mice are resistant to diet-induced obesity (18,39). However, our data do not establish that the hypertrophic effects of peripheral ghrelin in WAT are mediated directly by GHS-R expression in adipocytes. Although ghrelin regulates leptin secretion directly from retroperitoneal adipocytes in culture (40), our observation, that the relative expression of GHS-R1a does not correspond with the responsiveness of individual WAT depots, implies that this receptor is not the sole determinant of sensitivity to ghrelin.
Our microarray analysis confirmed that GHS-R mRNA expression did not differ between retroperitoneal and epididymal fat but suggested that the sensitivity of specific depots to ghrelin exposure may be determined by several additional factors. First, a number of G protein-coupled receptor species were more highly expressed in ghrelin-responsive WAT. The significance of this observation lies in the fact that there is growing evidence that heterooligomerization of G protein-coupled receptors may regulate the presentation of receptors to the cell surface and their subsequent activation. Of particular interest in this regard was the higher expression of the dopamine receptor 1a in ghrelin-responsive WAT, because ghrelin has been shown to amplify dopamine signaling via agonist-dependent formation of GHS-R/ dopamine receptor 1a heterodimers (41). Second, higher expression of guanylate cyclase activator 2a and lower expression of RGS4 suggest a differential fine tuning of the mechanisms of signal transduction between individual WAT depots. Third, although ghrelin exposure did not elevate the expression of any of the regulators of lipid handling, the constitutive expression of GLUT4 and fatty acid synthase was significantly higher in ghrelin-responsive fat. Further investigation is required to establish the relative importance of these mechanisms.
Although UAG failed to elevate WAT mass, it remains premature to preclude a role for this hormone in regulating adiposity. Unlike acylated ghrelin, UAG significantly reduced body weight gain, a phenomenon repeatedly observed in our laboratory and reflected in the growth retardation of UAG-overexpressing transgenic mice (40). The role of UAG in the regulation of feeding remains controversial (42,43,44) and in the current study, food intake was not significantly affected. However, the reduction in circulating dietary-derived lipids (Fig. 3, G and H) implies that UAG may limit substrate absorption. Because both UAG and ghrelin halved circulating FFAs, it is possible that both forms of the hormone have similar effects on lipid export from WAT, except that any influence of UAG on adipocyte size is masked by the reduction of available dietary-derived substrate. Whereas the inhibition of isoproteronol-induced lipolysis by UAG (16) appears to support this hypothesis, the recent report that adipocyte-specific overexpression of UAG reduced WAT mass (38) suggests that this may not be the case. However, if the 17-fold elevation in circulating UAG in aP2-ghrelin-transgenic mice reduced the absorption of dietary lipids, this could account for the ubiquitous reduction in fat pad weight in the absence of reduced food intake (38). Another possibility, that the observed effects of UAG in the present study are mediated by GHS-R1a, after acylation with ghrelin O-acyltransferase, also seems unlikely, because this rate-limiting enzyme is not expressed in WAT (45). Thus, the possibility that UAG acts via an unidentified receptor remains open.
Finally, we demonstrated that ghrelin-induced lipid accumulation is not specific to WAT. Ghrelin markedly increased the number of lipid droplets in the livers of treated rats and mice, which corresponded with a significant increase in triacylglycerol content. Although this hepatic steatosis was clearly dependent upon the expression of the cognate receptor, previous reports that hepatocytes show no appreciable expression of GHS-R1a (37,46) suggest that, in the liver at least, this effect is indirect.
In conclusion, our data support the hypothesis that ghrelin increases WAT mass in selective abdominal depots by a GHS-R1a-dependent mechanism. However, evidence from the current study suggests that this is due to decreased lipid export rather than a decrease in lipolysis per se. Thus, during periods of energy insufficiency, ghrelin may prevent lipid loss from responsive adipocytes, permitting depot-specific utilization of energy reserves. When combined with our previous evidence that these effects of ghrelin are not seen with intermittent exposure (11), our data imply that interruption of ghrelin signaling may be an essential component in any program of sustainable fat loss from those depots most associated with the metabolic syndrome.
Materials and Methods
Animals
The procedures described conformed to the institutional and national guidelines for animal experimentation, including those involving genetically modified animals, and were specifically approved by local ethical review. Male Sprague Dawley (SD) rats (Harlan UK Ltd., Bicester, Oxon, UK) were housed under conditions of 14-h light, 10-h dark (lights on at 0500 h), with food (Harlan Teklad Rodent Maintenance Diet containing 4.9% oil; 14.2% protein) and water available ad libitum. Homozygous loxTB-GHS-R mice (University of Texas Southwestern Medical Center, Dallas, TX) and W-T (C57Bl6; W-T) controls (Harlan UK Ltd.) were housed in the animal facility at Cardiff University as above. The day before the commencement of study 5, the diet was replaced by an expanded rodent breeding diet (Rat and Mouse No. 3 Breeding Diet, Special Diet Services Ltd., Witham, Essex, UK, containing 4.25% oil; 22.39% protein).
Study 1: the effect of iv ghrelin infusion on fat distribution in male rats
Male SD rats (205–232 g) were housed individually in metabolic cages 3 d before implantation of a single-bore jugular vein cannula connected to an sc osmotic minipump (Alzet model 2001; Alza Corp., Palo Alto, CA) under halothane anesthesia. Minipumps were primed to deliver vehicle (sterile saline containing BSA (1 mg/ml) and heparin (5 U/ml); n = 6) at 1 μl/h, or full-length acylated ghrelin (80 μg/d; Phoenix Pharmaceuticals, Belmont, CA; n = 6). This dose was previously shown to elevate abdominal adiposity in GH-deficient rats (12). After 7 d, minipumps were replaced with similarly primed pumps. Body weight and food intake were monitored throughout the 14-d infusion, at the end of which the rats were weighed, concussed, and killed by cervical dislocation. Abdominal WAT volumes were quantified by MRI in the Experimental MRI Centre (School of Biosciences, Cardiff University) using a Bruker 9.4 Tesla Biospec Scanner (Bruker BioSpin MRI Ltd., Coventry, UK). A two-dimensional multislice spin echo method was used to obtain two sets of 80 × 1-mm-thick axial images of the abdomen, with and without chemical-shift selective fat suppression. Subtraction of fat-suppressed from nonsuppressed images enabled WAT visualization, and depot volumes were quantified using Fat Analysis Tool (FAT) software (Cardiff University).
Study 2: the effect of UAG on abdominal adiposity
After 7 d acclimatization to metabolic cages, male SD rats (182–220 g) were prepared with single-bore jugular vein cannulae as above. Minipumps were primed to deliver either vehicle (n = 5), acylated ghrelin (80 μg/d; n = 6), UAG (80 μg/d; Phoenix Pharmaceuticals; n = 6), or L-163,255 (a potent spiropiperidine GHS-R1a agonist (19,20); 160 μg/d; n = 4), for 7 d. At the end of the infusion period, rats were weighed, concussed, and decapitated. Plasma from trunk blood was stored at −20 C before quantification of circulating leptin. Inguinal, retroperitoneal, perirenal, and epididymal fat pads were dissected and weighed.
Study 3: the effect of ghrelin on circulating metabolic substrates
A full lipid profile and circulating glucose levels were determined (see below) in terminal plasma samples from study 2.
Study 4: the effect of ghrelin on adipogenic and lipogenic markers
Subsamples of retroperitoneal fat from study 2 were snap frozen in TRIzol Reagent (Invitrogen, Paisley, Scotland, UK) and stored at −70 C for subsequent mRNA analysis by quantitative real-time PCR. Total RNA was isolated using the RNeasy kit (QIAGEN AB, Solna, Sweden) and reverse transcribed using the SuperScript II reverse transcriptase kit (Invitrogen). Quantitative real-time PCR was performed using the Power SYBR Green master mix (Applied Biosystems, Stockholm, Sweden) and amplified in an ABI Prism 7500 Sequence detector. Primers were designed using Primer Express software (primer sequences are available on request). Amplification of specific transcripts was confirmed by dissociation curve analysis and electrophoresis. We calculated relative changes by the comparative CT method using 18S as the reference gene and data normalized to values from vehicle-treated animals.
Study 5: the effect of ghrelin on abdominal adiposity in GHS-R-null mice
loxTB-GHS-R mice (21.8–26.2 g; 14 wk of age) and age-matched W-T controls (27.9–34.9 g; P < 0.001; Harlan) were prepared with single-bore jugular vein cannulae and osmotic minipumps (Alzet model 2001) under isofluorane anesthesia. The pumps were primed to deliver either vehicle [1 μl/h; n = 6 (W-T), n = 5 (loxTB-GHS-R), or acylated ghrelin (48 μg/d; n = 6 (W-T), n = 5 (loxTB-GHS-R)] for 7 d. We had previously found that this dose gave a reproducible response in W-T mice (our unpublished data). At term, the mice were weighed, reanesthetized with isofluorane, and decapitated. Plasma from trunk blood was stored at −20 C for subsequent measurement of leptin concentration. Inguinal, retroperitoneal (including perirenal), and epididymal fat depots were dissected and weighed.
Study 6: quantification of GHS-R mRNA expression in abdominal WAT
After being concussed and killed by cervical dislocation, hypothalami and samples of inguinal, mesenteric, epididymal, retroperitoneal, and perirenal fat were excised from six male SD rats (20 wk old) and snap frozen in TRIzol reagent. Marrow fat was obtained by centrifugation of tibial marrow into sterile saline, the floating adipocytes being removed and snap frozen in TRIzol reagent. Samples were stored at −70 C before analysis of expression of GHS-R1a and GHS-R1b mRNAs by RT-PCR. Total RNA was extracted using phenol-chloroform-ethanol, and cDNA generated by reverse transcription using the Affinity Script multiple temperature reverse transcriptase (Stratagene, Amsterdam, The Netherlands). Semiquantitative gene expression analysis PCRs were performed in triplicate for GHS-R1a, GHS-R1b, and β-actin (sequences of intron-spanning primers available on request). Densitometry was performed on each amplimer after 30 cycles, and the results were normalized to β-actin.
Study 7: microarray analysis of retroperitoneal and epididymal fat depots
Microarray analysis was performed on RNA extracted from samples of intraabdominal WAT. Four male SD rats (∼220 g) were concussed and killed by cervical dislocation, and samples of retroperitoneal (most sensitive) and epididymal (least sensitive) adipose tissue were excised and snap frozen in TRIzol reagent. Samples were stored at −70 C before RNA extraction and microarray analysis (see below).
Study 8: the effect of ghrelin on hepatic lipid content
Liver samples excised from rats and mice in studies 2 and 5 were stored at −70 C before determination of lipid and triacylglycerol content.
Tissue analysis
Plasma variables
Plasma glucose and leptin concentrations were determined by spectrophotometric measurement of reduced nicotinamide adenine dinucleotide phosphate using an Abbott Aeroset Spectrophotometer (Abbott Diagnostics, Maidenhead, Berkshire, UK) and RIA (Linco Research, Inc., St. Charles, MO). Lipids were extracted (from plasma and liver) by the Folch method (47). Triacylglycerols and total polar lipids were separated by thin-layer chromatography and quantified by their fatty acid contents. Fatty acid methyl esters were separated using a Clarus 500 gas chromatograph (PerkinElmer, Norwalk, CT), identified by comparing retention times with fatty acid standards (Nu-Chek Prep., Inc., Elysian, MN) and quantified against an internal pentadecanoate standard. One FFA value in vehicle-treated rats (which was >4 sems from the mean) was omitted from the calculations.
Microarray analysis
Total RNA was extracted from retroperitoneal and epididymal fat pads using the TRIzol Reagent and hybridized with the Affymetrix Rat Gene Chip 230 2.0 (n = 4 per depot). Affymetrix microarray chip data were analyzed using Genespring GX version 7.3 (Agilent Technologies, Palo Alto, CA). All data presented underwent Robust Microarray Average preprocessing [including data transformation (values <0.01 to 0.01) and normalization (per chip; normalized to the 50th percentile: per gene; normalized to median value)] before filtering data, based on fold change and expression.
Histology
Sections (5 μm) of inguinal WAT (study 5) were stained with Masson’s trichrome, and 15-μm cryostat sections of liver (study 8) were stained with oil red-O and counterstained with Meyer’s hematoxylin. Digital images (one image per section, three sections per mouse) were obtained with a Leica DFC300FX digital camera (Leica Microsystems Imaging Solutions Ltd., Cambridge, UK) and a Leica DMLB microscope. Adipocyte size was quantified in those cells with transversely sectioned peripheral nuclei (minimum 20 cells per section) using Image J and hepatic lipid droplets were measured using Leica Q-Win.
Statistical analyses
Results are expressed as mean ± sem, and differences between groups were compared using GraphPad Prism (GraphPad Software, Inc., San Diego, CA) by either unpaired Student’s t test, or one-way ANOVA followed either by the Dunnett’s (vs. control) or Bonferroni’s (for selected pairs) post hoc test, as appropriate, with P < 0.05 considered significantly different. For microarray analysis, genes were highlighted that showed more than 2-fold difference in expression between retroperitoneal and epididymal fat, with statistical comparisons performed using Student’s t test and Benjamini and Hochberg false discovery rate test with P < 0.05 considered significantly different.
Acknowledgments
We thank Dr. Stuart Faulkner and the staff of the Experimental MRI facility, Cardiff University, and Derek Scarborough (Cardiff University) for excellent technical support.
Footnotes
This work was funded by the Biotechnology and Biosciences Research Council [BBSRC; Grant Nos. 72/S11914 (to T.W. and J.S.D.), BB/C505032 (to T.W., B.A.J.E. and J.S.D.)], the Swedish Science Council and Karolinska Institutet funds (to P.K. and A.M.), the National Institutes of Health (K08DK068069-01A2), and a University of Texas Southwestern Medical Centers Disease-Orientated Clinical Scholars Award (to J.M.Z.).
Present address for J.S.D.: Institute of Life Sciences, School of Medicine, Swansea University, Swansea SA2 8PP, United Kingdom.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 19, 2009
Abbreviations: ABCG1, ATP-binding cassette transporter G1; FFA, free fatty acid; GHS, GH secretagogue; GHS-R1a, GH secretagogue receptor; LXR, liver X receptor; MRI, magnetic resonance imaging; PPAR, peroxisome proliferator-activated receptor; SD, Sprague Dawley; SREBP, sterol-regulatory element-binding protein; UAG, unacylated ghrelin; WAT, white adipose tissue; W-T, wild type.
References
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Guallilo O, Caminos JE, Nogueiras R, Seoane LM, Arvat E, Ghigo E, Casanueva FF, Diéguez C 2002 Effect of food restriction on ghrelin in normal-cycling female rats and in pregnancy. Obes Res 10:682–687 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Freidman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL 2003 The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37:649–661 [DOI] [PubMed] [Google Scholar]
- Hewson AK, Dickson SL 2000 Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate neurones of fasted and fed rats. J Neuroendocrinol 12:1047–1049 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Snape AC, Baudoin FM, Luckman SM 2002 Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 143:155–162 [DOI] [PubMed] [Google Scholar]
- Shimbara T, Mondal MS, Kawagoe T, Toshinai K, Koda S, Yamaguchi H, Date Y, Nakazato M 2004 Central administration of ghrelin preferentially enhances fat ingestion. Neurosci Lett 369:75–79 [DOI] [PubMed] [Google Scholar]
- Tolle V, Zizzari P, Tomasetto C, Rio MC, Epelbaum J, Bluet-Pajot M-T 2001 In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat. Neuroendocrinology 73:54–61 [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR 2001 Ghrelin causes hyperphagia and obesity in rats. Diabetes 50:2540–2547 [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhao L, Lin TR, Chai B, Fan Y, Gantz I, Mulholland MW 2004 Inhibition of adipogenesis by ghrelin. Mol Biol Cell 15:2484–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, Wells T 2004 Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 145:234–242 [DOI] [PubMed] [Google Scholar]
- Choi K, Roh S-G, Hong Y-H, Shrestha YB, Hishikawa D, Chen C, Kojima M, Kangawa K, Sasaki S-I 2003 The role of ghrelin and growth hormone secreatgogues receptor on rat adipogenesis. Endocrinology 144:654–759 [DOI] [PubMed] [Google Scholar]
- Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schürmann A, Szanto I, Tschöp MH, Rohner-Jeanerenaud F 2006 Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 116:1983–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubone T, Masaki T, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H 2005 Ghrelin regulates adiposity in white adipose tissue and UCP1 mRNA expression in brown adipose tissue in mice. Regul Pept 130:97–103 [DOI] [PubMed] [Google Scholar]
- Muccioli G, Pons N, Ghè C, Catapano F, Granata R, Ghigo E 2004 Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type 1a growth hormone secretagogue receptor. Eur J Pharmacol 498:27–35 [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Matsuo H, Kangawa K 2000 Ghrelin and des-acyl ghrelin: two major forms of ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279:909–913 [DOI] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK 2005 Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115:3564–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Rickes EL, McGuire L, Frazier E, Chen H, Barakat K, Nargund R, Patchett A, Smith RG, Hickey GJ 1996 Growth hormone (GH) and insulin-like growth factor I responses after treatments with an orally active GH secretagogue L-163,255 in swine. Endocrinology 137:4851–4856 [DOI] [PubMed] [Google Scholar]
- Moulas AN, Krieg Jr RJ, Veldhuis JD, Chan JC 2002 Effect of the GH secretagogue L-163,255 and restricted feeding time on GH pulsatility in the rat. Eur J Endocrinol 147:143–148 [DOI] [PubMed] [Google Scholar]
- Prinster SC, Hague C, Hall RA 2005 Heterodimerization of G protein-coupled receptors: specificity and functional significance. Pharmacol Rev 57:289–298 [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL 2000 Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21:697–738 [DOI] [PubMed] [Google Scholar]
- Votruba SB, Jensen MD 2007 Regional fat deposition as a factor in FFA metabolism. Annu Rev Nutr 27:149–163 [DOI] [PubMed] [Google Scholar]
- Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamura H, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I 2002 Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest 109:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire FM, Smas CM, Sul HS 1998 Understanding adipocyte differentiation. Physiol Rev 78:783–809 [DOI] [PubMed] [Google Scholar]
- Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, Silverstein RL 1999 A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 274:19055–19062 [DOI] [PubMed] [Google Scholar]
- Kersten S 2001 Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep 21:282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya M, Yahagi N, Matsuzaka T, Takeuchi Y, Nakagawa Y, Takahashi H, Okazaki H, Iizuka Y, Ohashi K, Gotoda T, Ishibashi S, Nagai R, Yamazaki T, Kadowaki T, Yamada N, Osuga J, Shimano H 2007 SREBP-1-independent regulation of lipogenic gene expression in adipocytes. J Lipid Res 48:1581–1591 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B 2000 Role of LXRs in control of lipogenesis. Genes Dev 14:2831–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin I, Dolinsky VW, Shackman JG, Kennedy RT, Chiang SH, Burant CF, Steffensen KR, Gustafsson JÅ, MacDougald OA 2005 LXRβ is required for adipocyte growth, glucose homeostasis and β cell function. J Biol Chem 280:23024–23031 [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G 2005 Ghrelin regulates mitochondrial lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab 288:E228–E235 [DOI] [PubMed] [Google Scholar]
- Brasaemle DL 2007 The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 48:2547–2559 [DOI] [PubMed] [Google Scholar]
- Vaughan AM, Oram JF 2005 ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not lipid-depleted apolipoproteins. J Biol Chem 280:30150–30157 [DOI] [PubMed] [Google Scholar]
- Kampf JP, Kleinfeld AM 2007 Is membrane transport of FFA mediated by lipid, protein, or both? Physiology 22:7–14 [DOI] [PubMed] [Google Scholar]
- Bonen A, Chabowski A, Luiken JJFP, Glatz JFC 2007 Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein mediated cellular fatty acid uptake: molecular, and physiological evidence. Physiology 22:15–28 [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD 1997 Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Mol Brain Res 48:23–29 [DOI] [PubMed] [Google Scholar]
- Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M 2002 The tissue distribution of the mRNA of ghrelin and the subtypes of its receptor, GHS-R in humans. J Clin Endocrinol Metab 87:2988–2991 [DOI] [PubMed] [Google Scholar]
- Zhang W, Chai B, Li JY, Wang H, Mulholland MW 2008 Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology 149:4710–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo KA, Charoenthongtrakul S, Giuliana DJ, Govek EK, McDonagh T, Qi Y, DiStefano PS, Geddes BJ 2008 Improved insulin sensitivity and metabolic flexibility in ghrelin receptor knockout mice. Regul Pept 150:55–61 [DOI] [PubMed] [Google Scholar]
- Giovambattista A, Piermaría J, Suescun MO, Calandra RS, Gaillard RC, Spinedi E 2006 Direct effect of ghrelin on leptin production by cultured rat white adipocytes. Obesity 14:19–27 [DOI] [PubMed] [Google Scholar]
- Jiang H, Betancourt L, Smith RG 2006 Ghrelin amplifies dopamine signalling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol 20:1772–1785 [DOI] [PubMed] [Google Scholar]
- Ariyasu H, Takaya K, Iwakura H, Hosoda H, Akamizu T, Arai Y, Kangawa K, Nakao K 2005 Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology 146:355–364 [DOI] [PubMed] [Google Scholar]
- Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, Murakami N, Miyazato M, Kangawa K, Nakazato M 2006 Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology 147:2306–2314 [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M 2005 Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 54:18–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL 2008 Identification of the acyltransferase that octanoylates ghrelin, and appetite-stimulating peptide hormone. Cell 132:387–396 [DOI] [PubMed] [Google Scholar]
- Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, Ghigo E, van der Lely AJ 2005 Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab 90:1055–1060 [DOI] [PubMed] [Google Scholar]
- Kates M 1986 Techniques in lipidology. 2nd ed. Amsterdam: Elsevier [Google Scholar]