Abstract
A 246-bp region upstream of placenta-specific exon I.1 of the human aromatase (hCYP19) gene mediates placenta-specific, developmental, and O2 regulation of expression. In this study, trophoblast differentiation and associated induction of CYP19 expression were prevented when cytotrophoblasts were cultured in phenol red-free medium containing charcoal-stripped serum or with the estrogen receptor (ER) antagonist, ICI 182,780, suggesting a stimulatory role of estrogen/ER. ERα protein was expressed in human trophoblasts and increased during syncytiotrophoblast differentiation, whereas ERβ was undetectable. Mutational analysis revealed that an estrogen response element-like sequence (ERE-LS) at −208 bp is required for inductive effects of estradiol/ERα on hCYP19I.1 promoter activity in transfected COS-7 cells. Increased binding of syncytiotrophoblast compared with cytotrophoblast nuclear proteins to the ERE-LS was observed in vitro; however, ERα antibodies failed to supershift the complex and in vitro-transcribed/translated ERα did not bind. Nonetheless, chromatin immunoprecipitation assays in cultured trophoblasts revealed recruitment of endogenous ERα to the −255- to −155-bp region containing the ERE-LS before induction of hCYP19 expression; this was inhibited by ICI 182,780. Chromatin immunoprecipitation also revealed increased acetylated histone H3(K9/14) and decreased methylated histone H3(K9) associated with this region during trophoblast differentiation. These modifications were prevented when trophoblasts were incubated with ICI 182,780, suggesting that ERα recruitment to the −255- to −155-bp region promotes histone modifications leading to increased hCYP19 transcription. Thus, during trophoblast differentiation, estrogen/ERα exerts a positive feedback role, which promotes permissive histone modifications that are associated with induction of hCYP19 gene transcription.
Estrogen/ERα exert a positive feedback role on human trophoblast, which promotes differentiation and permissive histone modifications with induction of hCYP19 expression.
Synthesis of estrogens from C19-steroids is catalyzed by aromatase P450 (P450arom, product of the CYP19 gene) (1). The ability of the human placenta to synthesize estrogens is vastly increased after the ninth week of gestation (2), in association with cytotrophoblast invasion and enlargement of the uterine arterioles, increased blood flow, and O2 availability to the floating chorionic villi (3,4). The trophoblast stem cells of the placenta and the cytotrophoblasts do not express aromatase; however, when cytotrophoblasts fuse to form multinucleated syncytiotrophoblast, aromatase expression is markedly induced (5). Human CYP19 (hCYP19) is a single-copy gene spanning about 130 kb. Expression of the hCYP19 gene in various estrogen-producing tissues is driven by tissue-specific promoters upstream of tissue-specific alternative first exons, which encode the 5′-untranslated regions of hCYP19 mRNA transcripts (6). These alternative first exons, located from about +110 bp to about 100,000 bp upstream of the hCYP19 translation initiation site in exon II, are alternatively spliced onto a common site just upstream of the translation start codon so that the aromatase protein synthesized in each of these tissues is identical (7). In placenta, the majority of the hCYP19 mRNA transcripts contain 5′-untranslated sequences encoded by exon I.1 (7), which lies approximately 95,000 bp upstream of the start site of translation in exon II (6).
In previous studies, transgenic mice carrying hCYP19I.1:hGH fusion genes containing various amounts of DNA flanking the 5′-end of placenta-specific exon I.1 linked to the human GH (hGH) structural gene, as reporter, were used to define the genomic regions that mediate hCYP19 expression in the placenta. It was observed that 501 bp of hCYP19 exon I.1 5′-flanking DNA was sufficient to mediate placenta-specific transgene expression that was developmentally regulated and restricted to the labyrinth of mouse placenta (8), a region that contains syncytial cells and is analogous to human syncytiotrophoblast. In subsequent deletion mapping studies, we observed that a transgene containing only 246 bp of exon I.1 5′-flanking DNA also was expressed in a placenta-specific manner in both labyrinth and giant cells, whereas transgenes containing 201 or fewer base pairs of 5′-flanking sequence were not expressed in placenta or in other tissues (9). Because mouse placenta does not express CYP19, these results also indicate that the transcription factors that mediate placental hCYP19 expression are conserved between mouse and human, whereas the CYP19 genetic response elements that bind these factors and/or the epigenetic mechanisms that control their access are species specific.
Although our findings suggest the importance of response elements within −246 bp upstream of hCYP19 exon I.1 in placenta-specific expression (9), the regulatory hormones, transcription factors, and mechanisms that mediate trophoblast differentiation and induce CYP19 expression in human placenta remain undefined. There is evidence to suggest that placentally derived estrogen may play an autocrine role in trophoblast differentiation. Estrogen was found to stimulate GnRH-induced human chorionic gonadotropin release from cultured human placental cells, suggesting a local modulatory role of estrogen (10). Connexin 43, a gap junction protein expressed during villous trophoblast differentiation, also was observed to be stimulated by estrogen (11). During early pregnancy in the baboon, lower estrogen levels were suggested to promote trophoblast vascular invasion, whereas the increase in estrogen levels during the last trimester of pregnancy was proposed to suppress further trophoblast invasion of the uterine spiral arteries (12). Estrogen receptor (ER)α and -β proteins have been localized in nuclei of cultured human syncytiotrophoblast cells using immunohistochemistry (13).
In the present study, we observed that ERα, but not ERβ, protein is expressed in midgestation human trophoblast cells before and after differentiation in culture and established that estrogen acting through ERα up-regulates hCYP19 gene expression in human placenta. Importantly, we identified an estrogen response element-like sequence (ERE-LS) at −208 bp upstream of the transcription start site in exon I.1, which is required for the inductive effect of estrogen. Although ERα does not appear to bind directly to this site in vitro, using chromatin immunoprecipitation (ChIP), we observed increased recruitment of endogenous ERα to the genomic region from −255 to −155 bp containing the ERE-LS in placental trophoblast cells during differentiation in culture. This was associated with an ER-dependent increase in levels of acetylated and decrease in levels of methylated histone H3(K9) bound to the ERE-LS-containing region. These findings suggest that locally produced estrogen acting through ERα may play a stimulatory role by promoting local histone modifications associated with an opening of chromatin structure leading to activation of hCYP19 gene expression.
Results
Culturing placental trophoblasts in phenol red-free medium containing charcoal-stripped fetal bovine serum (FBS) inhibits differentiation and aromatase induction
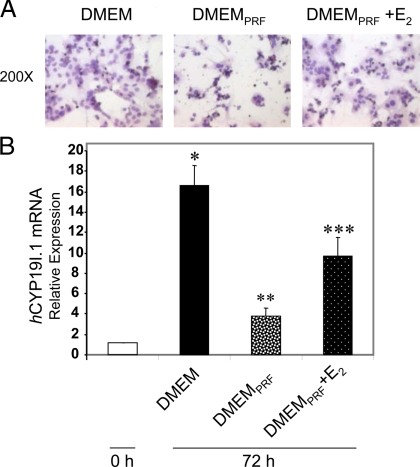
We previously observed that midgestation human placental cytotrophoblasts have low to undetectable levels of aromatase activity and P450arom mRNA. Within 24 h of culture in FBS-containing (2%) medium, the cytotrophoblasts begin to spontaneously fuse to form multinucleated syncytiotrophoblast, and aromatase activity and P450arom mRNA are markedly induced (5). Serum-containing medium is replete with growth factors and hormones, and phenol red binds to ER and has estrogenic properties (14). To investigate trophoblast differentiation and induction of hCYP19I.1 expression in the absence of these factors, freshly isolated trophoblasts were cultured in phenol red-free (PRF) medium containing 2% charcoal-stripped FBS (DMEMPRF). After 72 h of culture in phenol red-containing DMEM with 2% FBS or in DMEMPRF with or without 10 nm E2, the cells were stained with hematoxylin and eosin and viewed by light microscopy. As shown in Fig. 1A (left panel), when trophoblasts were cultured in DMEM containing 2% FBS, there was clear syncytia formation; however, when cells were cultured in DMEMPRF, syncytia formation was markedly reduced (middle panel). By contrast, addition of 10 nm E2 to the DMEMPRF (right panel) reversed this inhibition by DMEMPRF of trophoblast differentiation. Also, induction of placenta-specific aromatase mRNA transcripts (hCYP19I.1 mRNA) was prevented when the trophoblast cells were cultured in DMEMPRF (Fig. 1B). However, when 17β-estradiol (E2) (10 nm) was added to DMEMPRF, placenta-specific aromatase mRNA levels increased significantly. These results clearly demonstrate that estrogens in the culture medium contribute to the spontaneous induction of trophoblast differentiation and hCYP19I.1 gene expression.
Figure 1.
Induction of aromatase expression and syncytiotrophoblast differentiation are inhibited by culture of human trophoblasts in PRF medium containing charcoal-stripped serum. Syncytia formation and CYP19 mRNA are increased by E2 treatment. Freshly isolated human placental cytotrophoblasts were suspended in phenol red-containing DMEM with 2% FBS (DMEM) or in PRF DMEM containing 2% charcoal-stripped FBS (DMEMPRF) with or without E2 and plated at a density of 2 × 106 cells per dish. A, After 72 h of culture in DMEM (left panel), in DMEMPRF (middle panel), or in DMEMPRF plus E2 (right panel), the cells were stained with hematoxylin and eosin and viewed by light microscopy. B, RNA was isolated from cells either before culture (d 0) or after 72 h of culture in DMEM, in DMEMPRF, or in DMEMPRF plus10 nm estradiol, and expression of hCYP19I.1 mRNA transcripts was analyzed by qRT-PCR. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are expressed relative to expression levels in cytotrophoblasts before culture. *, P < 0.05 for 0 h vs. DMEM; **, P < 0.05 for DMEM vs. DMEMPRF; ***, P < 0.05 for DMEMPRF vs. DMEMPRF plus E2.
ER antagonist ICI 182,780 inhibits hCYP19I.1 activity and expression
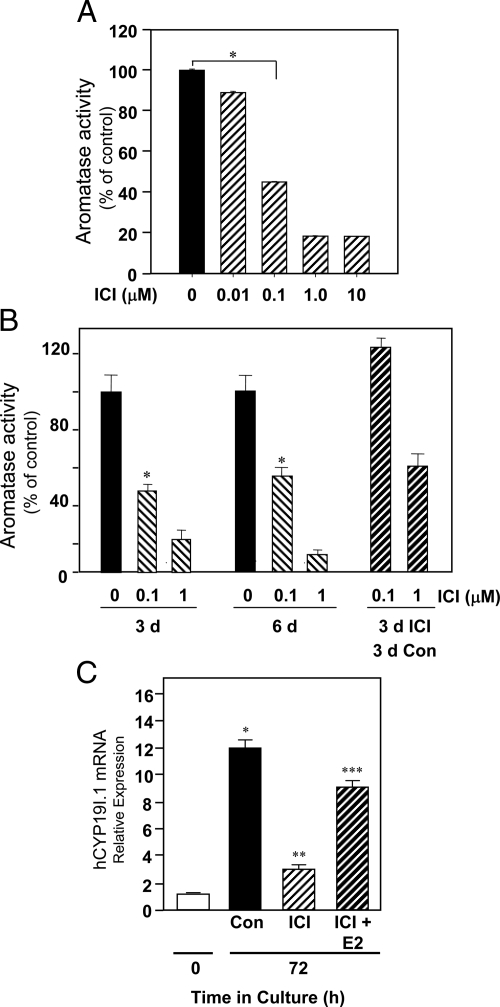
To analyze the role of the ER in aromatase expression in human placenta, freshly isolated cytotrophoblasts were cultured for 72 h in phenol red-containing medium with 2% FBS, either in the absence or presence of various concentrations of ER antagonist ICI 182,780 (0.01–10 μm). As can be seen, aromatase activity was markedly decreased when the cells were cultured in the presence of 0.1 μm ICI, with a further reduction when the ICI concentration was increased to 1.0 μm (Fig. 2A). To verify that ICI 182,780 is not toxic to the cells, freshly isolated cytotrophoblasts were cultured in medium containing 2% FBS either in the absence or presence of ICI 182,780 (0.1 or 1 μm) for up to 6 d. After 3 d, some dishes that had been cultured in the presence of ICI were rinsed thoroughly with fresh medium and incubated for another 72 h in fresh medium without ICI. Aromatase activity was then assayed on d 3 and 6 of culture. We observed that aromatase activity of cells cultured in the presence of 0.1 μm ICI for 72 h and then cultured in its absence for another 72 h was comparable to that of cells cultured in the absence of ICI for the entire 6-d period (Fig. 2B). These findings suggest that ICI 182,780 inhibition of trophoblast hCYP19 expression is specific and not due to a toxic effect on the cells.
Figure 2.
The ER antagonist ICI 182,780 reversibly inhibits spontaneous induction of aromatase expression in cultured human trophoblasts. A, Freshly isolated human cytotrophoblasts were cultured in medium containing 2% FBS either in the absence or presence of the ER antagonist ICI 182,780 (0.01–10 μm). Aromatase activity (expressed as picomoles of androstenedione metabolized to estrogen per milligram per minute) was analyzed after 3 d culture. Shown are the mean ± sem of data (expressed as percentage of activity in untreated/control cells) from three independent experiments, each conducted in triplicate. B, Freshly isolated cytotrophoblasts were cultured in medium containing 2% FBS either in the absence or presence of ICI 182,780 (0.1 or 1 μm) for up to 6 d. After 3 d, some dishes that had been cultured in the presence of ICI were rinsed thoroughly with fresh medium and incubated for another 72 h in fresh medium without ICI. Aromatase activity was then assayed on d 3 and 6 of culture. *, P < 0.05 for 0 vs. 0.1 μm ICI. Con, Control. C, Freshly isolated human placental cytotrophoblasts were cultured in medium containing 2% FBS in the absence or presence of the ER antagonist ICI 182,780 (0.1 μm), either alone or in combination with E2 (10 nm). RNA was isolated from cells before culture or after 72 h culture, and the expression of hCYP19I.1 was analyzed by qRT-PCR. Data are the mean ± sem of mRNA values from three independent experiments each conducted in triplicate and are expressed as a percentage of mRNA levels in cytotrophoblasts before culture. *, P < 0.05 for 0 h vs. Con 72 h; **, P < 0.05 for Con 72 h vs. ICI 72 h; ***, P < 0.05 for ICI vs. ICI+E2.
As can be seen in Fig. 2C, ICI 182,780 (0.1 μm) also inhibited the up-regulation of hCYP19I.1 mRNA during trophoblast differentiation. However, if the trophoblast cells were cocultured with the ER antagonist plus 10 nm E2, hCYP19I.1 mRNA inhibition was largely prevented, suggesting that ICI may exert its effects by antagonizing the action of E2/ERα on hCYP19 gene expression.
ERα protein and mRNA are expressed in human trophoblasts and are induced with differentiation
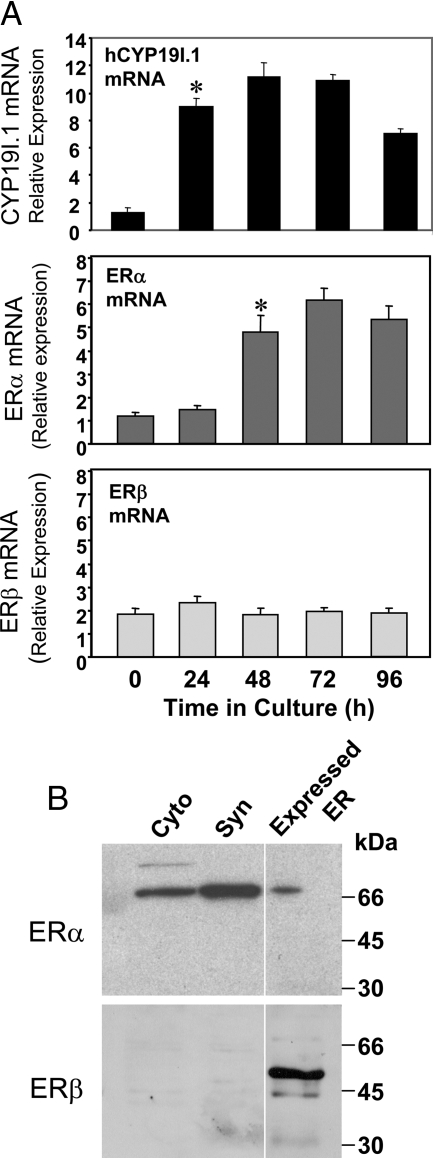
To determine whether ERα and/or ERβ are expressed in midgestation human trophoblasts and whether protein and mRNA expression levels change with differentiation, immunoblotting of nuclear proteins and quantitative RT-PCR (qRT-PCR) of RNA isolated from cells before and after 24–96 h of culture were performed. COS-7 cells overexpressing ERα or ERβ were analyzed as a control. Our findings suggest that ERα mRNA is expressed in cytotrophoblasts and increases with syncytiotrophoblast differentiation and induction of hCYP19I.1 mRNA expression. By contrast, ERβ mRNA was expressed at considerably lower levels in cytotrophoblasts and declined with differentiation (Fig. 3A). As can be seen in Fig. 3B, ERα protein also increased with trophoblast differentiation, whereas ERβ protein was essentially undetectable.
Figure 3.
ERα is selectively expressed in human trophoblasts and increases with trophoblast differentiation. Freshly isolated human placental cytotrophoblasts were suspended in DMEM containing 2% FBS and cultured for up to 96 h. A, RNA was isolated from cells either before culture (day 0) or after 24, 48, 72, or 96 h, and the expression of hCYP191.1, ERα, and ERβ were analyzed by qRT-PCR. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are expressed relative to those of cytotrophoblasts before culture. *, P < 0.05 for 0, 24, and 48 h. B, Nuclear proteins (30 μg) extracted from freshly isolated cytotrophoblasts (Cyto) and from syncytiotrophoblast (Syn) after 72 h of culture were analyzed by immunoblotting using antisera to ERα and ERβ [Ab-7 (AER320) mouse monoclonal; Neomarkers, Fremont, CA] and ERβ (PA 1-310B, rabbit polyclonal; Affinity Bioreagents, Golden, CO). COS-7 cells overexpressing ERα or ERβ were analyzed as a control.
An ERE-like sequence at −208 bp is essential for E2 and ERα stimulation of hCYP19(I.1) promoter activity
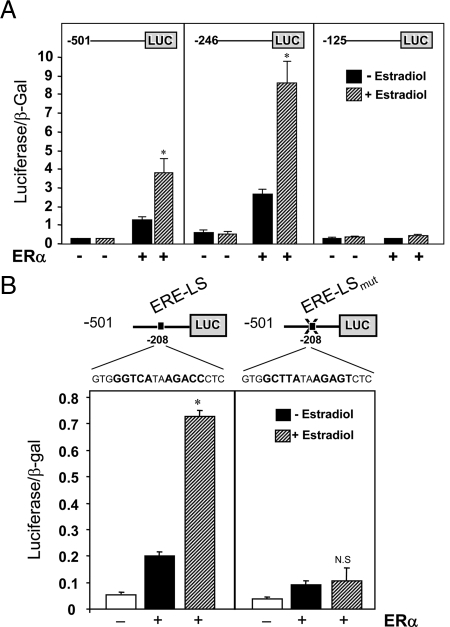
It next was of interest to identify the genomic sequences that mediate the inductive effect of estrogen/ERα on aromatase expression. We chose to use COS-7 cells for these studies because they lack endogenous ERα and manifested relatively low basal activity of the transfected promoter constructs. The cells were cotransfected with hCYP19I.1 reporter constructs containing various amounts of DNA flanking the 5′-end of hCYP19 exon I.1 fused to the luciferase (LUC) gene, as reporter. The cells were cotransfected with an ERα expression vector or a control vector and cultured in the absence or presence of estradiol (10−8 m) for 48 h. Expression of the hCYP19I.1 reporter constructs containing −501 and −246 bp of hCYP19I.1 5′-flanking DNA were increased in cells cotransfected with ERα and further induced by E2 treatment (Fig. 4A). As compared with the −246-bp reporter construct, ERα and E2 had no effect on hCYP19I.1−125: LUC reporter activity (Fig. 4A). These findings suggest that response elements between −246 and −125 bp mediate the stimulatory effects of ERα/E2 on aromatase expression. Within this region, we found an ERE-LS at −208 bp (GGGTCAtaa_GACCC) that differs from the consensus ERE by the lack of a single nucleotide. To determine whether this putative ERE-LS is functional, we performed site-directed mutagenesis of this site within the hCYP19I.1−501:LUC fusion gene to disrupt potential ERα binding and the resulting construct was cotransfected into COS-7 cells with or without ERα, in the absence or presence of E2 (Fig. 4B). As observed above, expression of the wild-type construct was induced by cotransfection of ERα and further enhanced by E2 (Fig. 4B). By contrast, little or no effect of ERα and E2 were observed in cells cotransfected with the reporter construct in which the ERE-LS was mutated. These findings clearly suggest that the ERE-LS at −208 bp is required for E2 and ERα stimulation of hCYP19I.1 expression.
Figure 4.
An ERE-LS at −208 bp is required for estrogen activation of CYP19(I.1) promoter activity in cotransfected COS-7 cells. A, COS-7 cells were cotransfected either with CYP19I.1−501:LUC, CYP19I.1−246:LUC, or CYP19I.1−125:LUC reporter plasmids and an expression vector for β-galactosidase in the absence or presence of an ERα expression plasmid. The transfected cells were cultured in the absence or presence of E2 (10 nm), and 72 h after transfection, the cells were lysed and assayed for luciferase and β-gal activities. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are expressed as luciferase activity corrected for transfection efficiency with β-gal activity. *, P < 0.05 for untreated vs. E2 treated. B, COS-7 cells were transiently cotransfected with CYP19I.1−501:LUC or CYP19I.1−501EREmut:LUC fusion genes (ERE-LS mutated) and expression vector for β-gal in the absence or presence of ERα expression plasmid. The transfected cells were cultured in the absence or presence of E2 (10 nm). At 72 h after transfection, the cells were lysed and assayed for luciferase and β-gal activities. Data are the mean ± sem of values from three independent experiments, each conducted in triplicate, and are expressed as luciferase activity corrected for transfection efficiency with β-gal activity. *, P < 0.05 for untreated vs. E2 treated; N.S., Not significant.
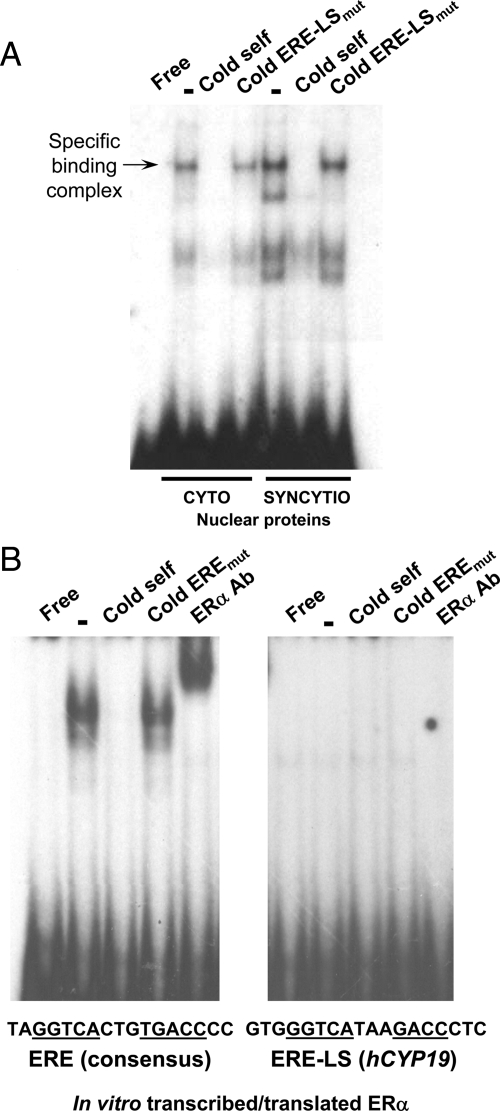
ERα does not bind directly to the ERE-LS
EMSA was performed using the ERE-LS at −208 bp as a probe and nuclear proteins from human trophoblast before and after differentiation in culture for 48 h in phenol red-containing medium with 2% FBS. We observed increased binding to the ERE-LS of syncytiotrophoblast, as compared with cytotrophoblast, nuclear proteins (Fig. 5A). Binding was competed by nonradiolabeled self probe but not by mutated ERE-LS probe; thus trophoblast nuclear proteins specifically bind to the ERE-LS and binding increases with differentiation of the trophoblast cells. However, the finding that ERα antibody did not supershift the complex of syncytiotrophoblast nuclear proteins bound to the ERE-LS (data not shown) suggested that ERα is not in the protein complex. To further determine whether ERα binds directly to the ERE-LS, EMSA was carried out using in vitro-transcribed/translated ERα and either a consensus ERE (known to bind ER) or the ERE-LS from hCYP19 promoter I.1, as probes. Although expressed ERα specifically bound to the ERE consensus sequence, was competed by non-radiolabeled self oligonucleotides but not by mutated oligonucleotides, and was supershifted by ERα antibody, the expressed ERα failed to bind to the ERE-LS (Fig. 5B). These results suggest that ERα may not directly bind the ERE-LS in DNA but either interacts with another protein that binds directly to the ERE-LS or that estrogen/ERα induces or activates a transcription factor that directly binds to this site.
Figure 5.
Syncytiotrophoblast nuclear proteins specifically bind to the ERE-LS at −208 bp. A, Nuclear extracts from human cytotrophoblasts and syncytiotrophoblast were incubated with 32P-labeled oligonucleotide containing the hCYP19I.1 ERE-LS in the absence or presence of 100-fold excess of cold wild-type or mutated oligonucleotides. B, In vitro-transcribed and translated ERα protein was incubated with a radiolabeled probe containing the consensus ERE (left panel) or the mutated ERE-LS (right panel) in the absence or presence of a 100-fold excess of the non-radiolabeled ERE consensus probe, a 100-fold excess of the non-radiolabeled probe containing a mutated ERE-LS or the ERα antibody (2 μl/lane). Protein was omitted from incubations with probe run in the first lane (Free).
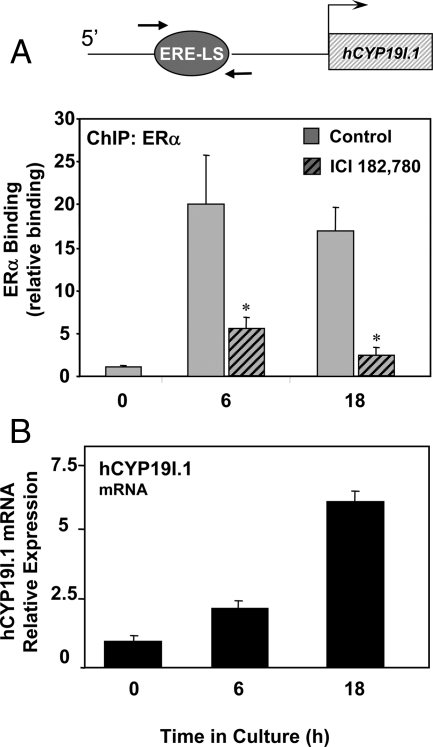
Endogenous ERα is recruited to ERE-LS-containing region of the hCYP19I.1 promoter during differentiation of human trophoblast cells in culture
As shown above (Fig. 2), the induction of hCYP19I.1 gene expression in human trophoblasts during differentiation in culture was inhibited by ICI 182,780, indicating a role of the ER in syncytiotrophoblast differentiation. To analyze the binding of endogenous ERα to the region of the hCYP19I.1 promoter containing the ERE-LS during trophoblast differentiation, we performed ChIP assays using formaldehyde-cross-linked chromatin from freshly isolated human trophoblast cells (0 h) or from cells cultured for either 6 or 18 h in the absence or presence of ICI 182,780. After immunoprecipitation of the chromatin complexes with ERα antibody, quantitative real-time PCR was carried out using primers to amplify an approximately 100-bp region between −255 and −155 bp containing the ERE-LS. We observed that binding of endogenous ERα to the hCYP19I.1 promoter was low to undetectable in freshly isolated cytotrophoblasts. Within 6 h of culture, there was a marked increase in recruitment of ERα to the ERE-LS-containing region, and this was inhibited by incubation with ICI 182,780 (Fig. 6A). A similar up-regulation of ERα binding was observed when the trophoblast cells were cultured for 18 h; this again was blocked by incubation of the cells with ICI 182,780. These profound changes in endogenous ERα binding were associated with the differentiation-associated induction of hCYP19I.1 mRNA expression (Fig. 6B). Thus, although EMSA did not demonstrate direct binding of ERα to the ERE-LS, ChIP findings suggest that ERα may bind indirectly through interaction with other transcription factors and that its interaction increases with syncytiotrophoblast differentiation.
Figure 6.
Endogenous ERα is recruited to the hCYP19I.1 −255- to −155-bp genomic region in human trophoblast cells during culture; ERα binding is inhibited by ICI treatment. Freshly isolated human placental cytotrophoblasts (0 h) were cultured in the absence or presence of ER antagonist ICI 182,780 for 6 or 18 h. A, Freshly isolated cytotrophoblasts and trophoblast cells cultured with or without ICI were treated with 1% formaldehyde and subjected to ChIP analysis for ERα binding to an approximately 100-bp genomic region surrounding the CYP19I.1 ERE-LS (−255 to −155 bp), indicated by the arrows. The ChIP data were normalized to input control data and expressed as arbitrary units relative to binding at the 0-h time point. The results shown are the means ± sem of values from three independent experiments. B, RNA was isolated from cells at the same time points, and expression of hCYP191.1 mRNA was analyzed by qRT-PCR. *, P < 0.05 for untreated vs. ICI-treated cells.
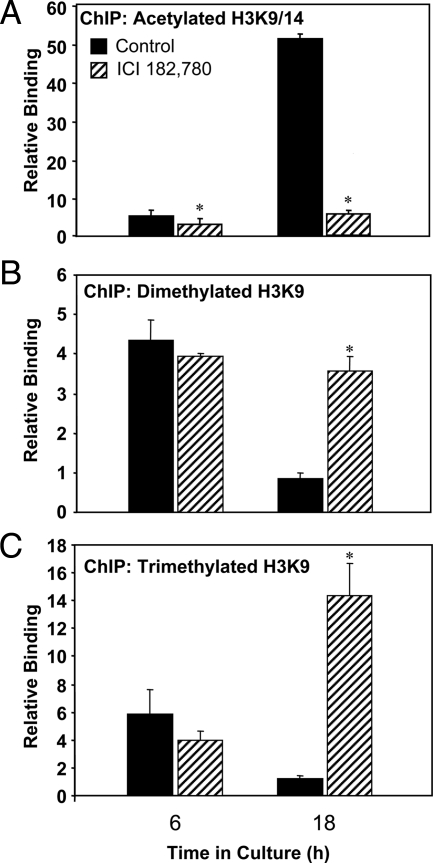
Histone modifications associated with active chromatin within the hCYP19I.1 −255- to −155-bp region increase during syncytiotrophoblast differentiation and are repressed by ICI 182,780 treatment
To assess histone modifications linked to active chromatin within the −255 to −155 region during syncytiotrophoblast differentiation and the role of ERα binding, we used ChIP to assess changes in local levels of histone H3 acetylated on lysines (K) 9 and 14 in cells cultured for 6 h and 18 h with or without ICI 182,780. As shown in Fig. 7A, ChIP assays revealed a pronounced temporal increase in histone H3-K9/14 acetylation at 18 h as compared with 6 h. However, in cells treated with ICI 182,780, the induction of histone acetylation was blocked, suggesting that ERα recruitment plays a role in H3-K9/14 acetylation. We also used ChIP to examine di- and trimethylation of H3-K9 (marks of repressed chromatin) within the ERE-LS-containing region after 6 and 18 h culture as well as the effects of ICI 182,780 treatment. Interestingly, the ChIP assays revealed that dimethylation (Fig. 7B) and trimethylation (Fig. 7C) of H3-K9 were relatively high at 6 h and declined dramatically at 18 h in trophoblasts cultured in the absence of the ER antagonist. However, when the cells were cultured with ICI 182,780, the levels of H3-K9me2 and H3-K9me3 within the −255 to −155 region failed to decline at the 18-h time point and were similar or greater than those of untreated cells at 6 h. These findings indicate that key changes in chromatin modification within the ERE-LS-containing region of the hCYP19I.1 promoter in human trophoblast cells are dependent upon ERα recruitment and associated with the induction of hCYP19I.1 gene expression.
Figure 7.
Histone modifications associated with active chromatin at the hCYP19I.1 −255 to −155 region increase during syncytiotrophoblast differentiation and are prevented by ICI treatment. Freshly isolated human cytotrophoblasts were cultured in the absence or presence of the ER antagonist ICI 182,780 for either 6 or 18 h. The cells were then treated with 1% formaldehyde and subjected to ChIP analysis using antibodies for acetylated histone H3 Lys 9/14 (A), dimethyl histone H3 Lys 9 (B), or trimethyl histone H3 Lys 9 (C). qRT-PCR was performed using primers to an approximately 100-bp region surrounding the CYP19I.1 ERE-LS. The ChIP data were normalized to input control data and expressed as relative binding units. The results shown are the means ± sem of values from three independent experiments. *, P < 0.05 for untreated vs. ICI-treated cells.
Discussion
The syncytiotrophoblast of human placenta represents the major site of endocrine activity, because it secretes hormones, including estrogens, progesterone, chorionic gonadotropin, placental lactogen, and GHs that are necessary for fetal and placental growth and for the maintenance of pregnancy. Trophoblast stem cells and cytotrophoblasts do not express hCYP19; however, when cytotrophoblasts fuse to form multinucleated syncytiotrophoblast, hCYP19 gene expression is markedly induced. The hormones and factors that regulate hCYP19 expression in human placenta have not been defined. In the present study, we discovered that estrogen acting through ERα has an autostimulatory effect on hCYP19I.1 expression in cultured human trophoblast cells. When cytotrophoblasts from midgestation placenta are cultured in DMEM containing FBS, they spontaneously fuse to form syncytiotrophoblast-like cells and hCYP19I.1 expression is induced (5). However, when these cells were cultured in DMEMPRF, syncytia formation was markedly reduced and expression of placenta-specific hCYP19 mRNA transcripts (hCYP19I.1) and aromatase activity were inhibited. The findings that treatment of the trophoblast cells with the pure antiestrogen, ICI 182,780 (fulvestrant) (15), markedly inhibited syncytiotrophoblast differentiation and the associated induction of aromatase expression and activity, whereas cotreatment with estrogen rescued this inhibitory effect, clearly demonstrate that estrogen acting via the ER has a stimulatory effect on differentiation and hCYP19I.1 expression in human placenta. It is likely that these actions of estrogen are mediated by ERα, because ERα protein and mRNA expression increased during syncytiotrophoblast differentiation, whereas ERβ protein was undetectable and ERβ mRNA levels were relatively low and declined with trophoblast differentiation.
Estrogen, acting via ERα or ERβ, modulates gene expression through multiple mechanisms. These include direct binding of ER dimers to specific response elements within the promoters of estrogen-responsive genes (16), indirect binding through interaction/tethering of ER to other DNA-bound transcription factors such as activator protein-1 (AP-1) (17,18,19) or Sp1 (20,21,22), and estrogen/ER-induced expression and/or activation of other transcription factors that in turn bind to their response elements in DNA. E2/ERα was reported to induce hCYP19 expression in MCF-7 human breast cancer cells acting through the placenta-specific hCYP19 promoter I.1 (23). Although a putative ERE was identified, binding of ERα to that element was not detected. Furthermore, the stimulatory effect of E2 on hCYP19 promoter I.1 activity was blocked by a MAPK inhibitor, suggesting that E2/ERα was acting nongenomically through activation of another transcription factor (23). It should be noted that the response element identified in that study differs in sequence and localization from the ERE-LS characterized in the present investigation. Recent studies from our laboratory also indicate that estrogen induces hCYP19 expression in cultured human endometrial cells via a positive feedback loop by enhancing recruitment of the orphan nuclear receptor, steroidogenic factor-1 (SF-1) to its response element in the ovary-specific promoter (hCYP19IIa) (24).
In the present study, we identified a genomic region between −246 and −125 bp relative to the transcription start site in hCYP19 exon I.1 that is required for the inductive effect of estrogen on hCYP19I.1 promoter activity. Mutation of the ERE-LS within this region resulted in the loss of estrogen/ERα-induced hCYP19I.1 promoter activity in transfected COS-7 cells. Although EMSA demonstrated specific binding of trophoblast nuclear proteins to the ERE-LS that increased with syncytiotrophoblast differentiation, direct in vitro binding of ERα to this element was not detected using EMSA. These findings suggest that although the ERE-LS is required for ERα/E2 induction of hCYP19I.1 promoter activity, it may serve a role in basal promoter activity that is requisite for estrogen responsiveness.
On the other hand, ChIP assays revealed enhanced ERα recruitment during trophoblast differentiation to the genomic region between −255 and −155 bp containing the ERE-LS. The finding that ICI 182,780 blocked both ERα recruitment to the −255- to −155-bp region and induction of hCYP19I.1 expression further suggests the importance of ERα interaction with this genomic region in estrogen-mediated up-regulation of hCYP19I.1 gene expression. These collective findings suggest that ERα may enhance hCYP19I.1 promoter activity via interaction with other, as yet unidentified, transcription factors bound to the ERE-LS. Of particular interest are members of the Fos/Jun families, because the ERE-LS also has similarities to an AP-1 response element. Interestingly, JunB-deficient mouse embryos die between 8.5 and 10 d postcoitum due to defective placental vascular development (25). On the other hand, in human placenta, JunD was found to be expressed in nuclei of villous syncytiotrophoblast, whereas expression of AP-1 family members c-Jun, JunB, c-Fos, FosB, Fra-1, and Fra-2 was not detectable (26). However, in COS-7 cell cotransfection studies with hCYP19I.1−501:LUC fusion genes, we were unable to demonstrate effects of JunD to further enhance ERα/E2 induction of hCYP19I.1 promoter activity (Kumar, P., and C. R. Mendelson, unpublished observations).
As mentioned, ERα also has been reported to increase gene expression indirectly via interaction with transcription factor Sp1 bound to DNA (21,22,27). We previously identified a G/C-rich sequence at −233 bp upstream of hCYP19 exon I.1 that binds transcription factor Sp1 and is required for increased promoter I.1 activity in transfected placental cells (5). Because this element lies within the genomic region amplified in our ChIP analysis, it is possible that endogenous ERα may interact with Sp1 proteins bound to the G/C-rich site (20,21,22). Notably, Sp1/Sp3 compound heterozygous mice are not viable and are reported to manifest severe placental defects (28). However, in preliminary studies, we observed that Sp1 cotransfection did not further enhance ERα/E2 induction of hCYP19I.1−501:LUC expression and that mutagenesis of the G/C box did not impair ERα/E2 induction of hCYP19I.1 promoter activity (Kumar, P., A. Kamat, and C. R. Mendelson, unpublished observations). Nonetheless, it is possible that ERα interaction with other transcription factors bound to adjacent sites is required to stabilize direct binding of ERα to the ERE-LS.
Although in the present study, the ERE-LS was found to be relatively close (∼−200 bp) to the transcription start site in hCYP19 exon I.1, recent analysis of ERα binding sites in MCF-7 breast cancer cells using genome-wide ChIP combined with tiling microarrays (ChIP-chip) suggested that most of the ERα binding sites in the human genome were far removed (>100 kb) from the promoters of target genes (29,30,31). Interestingly, the majority of these sites corresponded to binding sites for the pioneer factor FoxA1 and were associated with H3K4 methylation (31), a mark of active chromatin (32). Although some ER binding sites were within 1.0 kb of the transcription start sites of target genes, these in fact were in the minority (30). However, in another ChIP-chip analysis of E2-induced ERα binding to rapidly activated target genes, coupled with enhanced recruitment of steroid receptor coactivators and RNA polymerase II, increased local levels of acetylated histone H3 and enhanced gene transcription, it was found that about 36% of genes manifesting these combined properties exhibited ERα binding within 1 kb of the transcription start site (33). Thus, ERα bound to a distal enhancer element may possibly interact with a transcription factor bound to the estrogen-responsive region of the hCYP19 promoter I.1 by DNA looping.
Numerous studies have demonstrated that binding of activating transcription factors to DNA results in the recruitment of coactivator complexes containing histone-modifying enzymatic activities (e.g. histone acetylases, demethylases, and kinases) that cause dynamic posttranslational modifications of key residues in the N-terminal tails of core histones. The addition of acetyl groups to the N-terminal tails of histones at key lysine residues results in an opening of chromatin structure, which facilitates recruitment of general transcription factors and of RNA polymerase II, leading to activation of transcription initiation (34). By contrast, binding of inhibitory transcription factors and recruitment of corepressors block transcriptional activation by causing deacetylation and methylation of these histone residues to promote formation of heterochromatin. In the present study, we observed recruitment of ERα to the 100-bp genomic region containing the ERE-LS after 6 h culture of freshly isolated cytotrophoblasts; this was sustained through 18 h incubation. Correspondingly, there was a marked increase in histone H3K9/14 acetylation and decreased di- and trimethylation of H3K9 after 18 h, as compared with the 6-h time point. Treatment of the cells with the ER antagonist ICI 182,780 blocked the recruitment of ERα to the ERE-LS and prevented the activating histone modifications in this genomic region at 18 h culture. These findings clearly suggest that ERα recruitment to the ERE-LS-containing region of the hCYP19I.1 promoter promotes local histone modifications resulting in the induction of hCYP19 gene expression in cultured human trophoblasts.
The epigenetic regulation of a number of genes that are expressed during trophoblast differentiation in mammalian placenta has been investigated. For example, increased expression of genes within the human GH cluster during placental cytotrophoblast differentiation is accompanied by sequential local increases in histone H3K4 di- and trimethylation and histone H3 and H4 acetylation (35,36), marks of active chromatin. Furthermore, differentiation of trophoblast stem cells and induced expression of the dimethylarginine dimethylaminohydrolase 2 (Ddah2) gene was associated with activating chromatin modifications, including hyperacetylation of histones H3 and H4, hypermethylation of H3K4, and decreased H3K9 methylation in its 5′-flanking region (37). Human trophoblast fusion is mediated by the fusogenic protein, syncytin (38). Syncytin gene expression is enhanced by promoter binding of the transcription factor glial cells missing 1a (GCM1a) (39) and of the histone acetylase, cAMP response element-binding protein (CREB)-binding protein (CBP), and is repressed by binding of histone deacetylase 3 (HDAC3) (40). Importantly, histone deacetylase 3 was not found to be associated with the syncytin promoter in actively fusing trophoblasts (40). Collectively, these findings, together with those presented herein, suggest the importance of histone modification in the dynamic changes in chromatin reorganization associated with the process of syncytiotrophoblast differentiation.
As mentioned, the cellular mechanisms that promote enhanced placental aromatase expression after the ninth week of human gestation remain obscure. Our previous findings suggest that early in gestation when the placenta is poorly vascularized and relatively hypoxic, increased upstream stimulatory factors 1 and 2 (USF1/2) expression may prevent trophoblast differentiation and block hCYP19 gene expression via binding to two E-boxes surrounding the placenta-specific transcription start site in exon I.1 (41). In the second trimester, increased vascularization and O2 availability to cells of the chorionic villi promote proteasomal degradation of USF1/2 (42), resulting in syncytiotrophoblast differentiation and induction of hCYP19 expression with an associated increased estrogen synthesis. Our current findings suggest that the estrogens produced by the syncytiotrophoblast act via ERα in a stimulatory positive feedback loop to further enhance hCYP19 promoter I.1 activity. ERα expression and binding to hCYP19 promoter I.1 also was found to increase in concert with trophoblast differentiation and hCYP19 gene expression. These changes were associated with posttranslational modifications of histone H3 within the 100-bp genomic region containing the ERE-LS that are indicative of the transition from repressed to active chromatin. The critical role of ERα was underscored by the finding that the ER antagonist ICI 182,780 blocked ERα recruitment to the promoter and maintained repressive marks on H3K9 within the ERE-LS-containing region. Studies are in progress to define the transcription factors and coregulators with which ERα interacts at hCYP19 promoter I.1 to effect these changes in chromatin modification and aromatase expression.
Materials and Methods
Primary culture of human trophoblast cells
Midtrimester human placental tissues were obtained from Advanced Bioscience Resources (Alameda, CA) in accordance with the Donors Anatomical Gift Act of the State of Texas. Protocols were approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. A placental primary culture system (43) was modified (5) for isolation and culture of cytotrophoblasts from midgestation human placenta. Briefly, the placental tissues were washed with Hanks’ balanced salt solution (pH 7.4) (GIBCO, Grand Island, NY) and then finely minced and digested with 0.125% trypsin in Hanks’ balanced salt solution at 37 C for 20 min. This procedure was repeated three times. At the end of each digestion step, the supernatant was collected, layered over 10 ml serum, and then briefly centrifuged. The pellet was suspended in DMEM (GIBCO), filtered, and layered over a Percoll gradient (70 to 5%). The gradients were centrifuged at 1200 × g for 20 min at room temperature, and cells in the middle layer (density, 1.045–1.062 g/ml) were collected, washed, and counted. The cells were then resuspended in DMEM supplemented with 10% FBS and 1.2% antibiotic/antimycotic solution (GIBCO) and plated at a density of 2 × 106 cells per 35-mm culture dish or 15 × 106 cells per 100-mm dish. The cells were cultured overnight, and then the medium was changed to DMEM containing 2% FBS. To determine the effects of estrogen, the cells were suspended and plated in DMEM medium without phenol red, containing 2% charcoal-stripped FBS (DMEMPRF).
Morphological analysis
Cytotrophoblasts were cultured on glass chamber slides in either DMEM containing 2% FBS or DMEMPRF with or without E2 (10 nm) and cultured for 3 d. The cells were rinsed in PBS and fixed in 10% formalin. Hematoxylin and eosin Y were used to stain nuclei and cytoplasm, respectively. Morphology was analyzed by light microscopy.
qRT-PCR
Total RNA from trophoblast cells cultured for 24, 48, or 72 h was extracted by the one-step method (TRIzol; Invitrogen, Carlsbad, CA). RNA was treated with deoxyribonuclease to remove any contaminating DNA, and 2 μg were reverse transcribed using random primers and Superscript II ribonuclease H-reverse transcriptase (Invitrogen). Primer sets directed against human CYP19I.1 along with the constitutively expressed h36B4 were generated utilizing Primer Express software (PE Applied Biosystems, Boston, MA) based on published sequences: hCYP19I.1 forward primer, 5′-ACG GAA GGT CCT GTG CTC G-3′, and reverse primer, 5′-GTA TCG GGT TCA GCA TTT CCA-3′; H36B4 forward primer, 5′-TGC ATC AGT ACC CCA TTC TAT CA-3′, and reverse primer, 5′-AAG GTG TAA TCC GTC TCC ACA GA-3′.
The relative abundance of each transcript was determined by qRT-PCR using a modification of previously published methods (44). For the quantitative analysis of mRNA expression, the ABI Prism 7700 Detection System (Applied Biosystems, Foster City, CA) was employed using the DNA binding dye SYBR Green (PE Applied Biosystems) for the detection of PCR products. The cycling conditions were 50 C for 2 min and 95 C for 10 min, followed by 40 cycles of 95 C for 15 sec and 60 C for 1 min. The cycle threshold was set at a level at which the exponential increase in PCR amplification was approximately parallel between all samples. All primer sets produced amplicons of the expected size and sequence. The relative fold changes were calculated using the comparative cycle times (Ct) method with H36B4 as the reference guide.
Cell culture and transfections
COS-7 cells (American Type Culture Collection, Manassas, VA) grown in DMEM (Life Technologies, Grand Island, NY) supplemented with 10% FBS were transfected with hCYP19I.1:luciferase (LUC) reporter constructs containing various deletions of the placenta-specific promoter, β-gal expression vector, and human ERα expression vector or vector alone as a control, using LipofectAMINE Plus and OPTI-MEM medium (Invitrogen). Twenty-four hours after transfection, fresh DMEMPRF, with or without E2 (10 nm), was added to the cells; 72 h after transfection, the cells were lysed in reporter lysis buffer (Promega, Madison, WI) and assayed for β-gal and luciferase (Promega).
EMSA
Nuclear proteins were purified from freshly isolated cytotrophoblast cells and from cultured trophoblasts as described previously (5,45). Double-stranded oligonucleotides corresponding to the CYP19I.1 ERE-LS are as follows: sense primer: 5′-GTG GGT CAT AAG ACC ATA-3′, and antisense primer, 5′-GAG GGT TTA TGA CCC AC-3′.
For EMSA, the double-stranded oligonucleotides were end labeled with [γ-32P]ATP by using T4 kinase (Invitrogen) and incubated with trophoblast nuclear proteins (3 μg) or in vitro-transcribed/translated ERα (10 μl) for 30 min at room temperature in binding buffer [20 mm HEPES (pH 7.4), 12% glycerol, 84 mm KCl, 1 mm EDTA, and 1 mm dithiothreitol] and 1 μg poly(deoxyinosine-deoxycytosine)-poly(deoxyinosine-deoxycytosine) (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) as nonspecific competitor. The DNA-protein complexes were resolved on 5% nondenaturing polyacrylamide gels and visualized by autoradiography. In vitro-transcribed/translated ERα was synthesized using the TNT Coupled Transcription/Translation System (Promega). For supershift EMSA, the nuclear proteins were incubated for 1 h at 4 C in binding buffer in the absence or presence of IgG (1 μg) for ERα (F-10, sc-2008 X; Santa Cruz Biotechnology, Santa Cruz, CA). Labeled CYP19I.1 ERE-LS oligonucleotide was added to the reaction mixture, and the incubation was continued for another 30 min at room temperature before separation on 5% native polyacrylamide gels and visualization by autoradiography.
ChIP
Human trophoblasts were cultured overnight in DMEM with 2% FBS, in the absence or presence of ICI 182,780 (10−6 m). ChIP was performed using a modification (46) of previously published methods (47). Trophoblast cells were washed once with PBS and incubated with 1% formaldehyde (in control medium) for 10 min at room temperature to cross-link proteins and DNA. Cross-linking was terminated by the addition of glycine (0.125 m, final concentration). The cells were washed twice with cold PBS and placed in 500 μl lysis buffer [50 mm Tris (pH 8.1), 150 mm NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), protease inhibitor cocktail (Roche, Indianapolis, IN), and 5 mm EDTA]. The lysates were sonicated on ice to produce sheared, soluble chromatin. The soluble chromatin was precleared with Protein A/G Plus agarose beads (60 μl) at 4 C for 1 h. The samples were microfuged at 14,000 rpm to pellet the beads, and the supernatant containing the sheared chromatin was placed in new tubes. The precleared chromatin was aliquoted into 300-μl amounts and incubated either with antibodies for ERα (HC-20; Santa Cruz Biotechnology), acetyl histone H3 Lys 9/14 (06-599), dimethyl histone H3 Lys 9 (07–441) from Millipore Biosciences (Danvers, MA) or trimethyl histone H3 Lys 9 (Abcam Inc., Cambridge, MA) at 4 C overnight. An aliquot incubated with nonimmune IgG was reserved as control. Protein A/G Plus agarose beads (60 μl) were added to each tube, the mixtures were incubated for 2 h at 4 C, and the immune complexes were collected by centrifugation. The beads containing the immunoprecipitated complexes were washed sequentially for 5 min in wash buffer I [20 mm Tris-HCl (pH 8.1), 2 mm EDTA, 0.1% SDS, 1% Triton X-100, and 150 mm NaCl], wash buffer II (same as wash buffer I, except containing 500 mm NaCl), wash buffer III [10 mm Tris-HCl (pH 8.1), 1 mm EDTA, 1% Nonidet P-40, 1% deoxycholate, 0.25 m LiCl], and Tris-EDTA buffer. The beads were eluted with 250 μl elution buffer (1% SDS, 0.1 mm NaHCO3, and 20 μg salmon sperm DNA; Sigma Chemical Co., St. Louis, MO) at room temperature. This was repeated once, and eluates were combined. Cross-linking of the immunoprecipitated chromatin complexes and input controls (10% of the total soluble chromatin) was reversed by heating the samples at 65 C for 4 h. Proteinase K (15 μg; Invitrogen) was added to each sample in buffer [50 mm Tris-HCl (pH 8.5), 1% SDS, and 10 mm EDTA] and incubated for 1 h at 45 C. The DNA was purified by phenol-chloroform extraction and precipitated in ethanol overnight at −20 C. Samples and input controls were diluted in 10–100 μl Tris-EDTA buffer just before PCR. Quantitative PCR was employed using forward (5′-TGC CCT CCT TTC ATC CAC C-3′) and reverse (5′-TCC TTC CTC CAG GGT ATG GG-3′) primers that amplify an approximately 100-bp region surrounding the CYP19I.1 ERE-LS.
Statistical analysis
All assays were repeated at least three times. The results of qRT-PCR studies were reported as mean ± sem. Differences were analyzed by Students’s t test. P < 0.05 was regarded as significant, and such differences are indicated in the figures by asterisks.
Acknowledgments
We thank Ms. Jo Smith for her expert assistance in preparing the primary human trophoblast cultures for these studies.
Footnotes
This work was supported by National Institutes of Health Grant 5 R01 DK031206 (C.R.M.).
Disclosure Summary: None of the authors has anything to declare regarding potential conflicts of interest.
First Published Online March 19, 2009
Abbreviations: AP-1, Activator protein-1; ChIP, chromatin immunoprecipitation; E2, 17β-estradiol; ER, estrogen receptor; ERE-LS, estrogen response element-like sequence; FBS, fetal bovine serum; PRF, phenol red-free; qRT-PCR, quantitative RT-PCR; SDS, sodium dodecyl sulfate.
References
- Thompson Jr EA, Siiteri PK 1974 The involvement of human placental microsomal cytochrome P-450 in aromatization. J Biol Chem 249:5373–5378 [PubMed] [Google Scholar]
- Everett RB, MacDonald PC 1979 Endocrinology of the placenta. Annu Rev Med 30:473–488 [DOI] [PubMed] [Google Scholar]
- Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ 1996 Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest 97:540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Kamat A, Mendelson CR 2000 Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2). Mol Endocrinol 14:1661–1673 [DOI] [PubMed] [Google Scholar]
- Kamat A, Alcorn JL, Kunczt C, Mendelson CR 1998 Characterization of the regulatory regions of the human aromatase (P450arom) gene involved in placenta-specific expression. Mol Endocrinol 12:1764–1777 [DOI] [PubMed] [Google Scholar]
- Kamat A, Hinshelwood MM, Murry BA, Mendelson CR 2002 Mechanisms in tissue-specific regulation of estrogen biosynthesis in humans. Trends Endocrinol Metab 13:122–128 [DOI] [PubMed] [Google Scholar]
- Means GD, Mahendroo MS, Corbin CJ, Mathis JM, Powell FE, Mendelson CR, Simpson ER 1989 Structural analysis of the gene encoding human aromatase cytochrome P-450, the enzyme responsible for estrogen biosynthesis. J Biol Chem 264:19385–19391 [PubMed] [Google Scholar]
- Kamat A, Graves KH, Smith ME, Richardson JA, Mendelson CR 1999 A 500-bp region, approximately 40 kb upstream of the human CYP19 (aromatase) gene, mediates placenta-specific expression in transgenic mice. Proc Natl Acad Sci USA 96:4575–4580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A, Smith ME, Shelton JM, Richardson JA, Mendelson CR 2005 Genomic regions that mediate placental cell-specific and developmental regulation of human CYP19 (aromatase) gene expression in transgenic mice. Endocrinology 146:2481–2488 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Florio P, Nappi C, Genazzani AR 1996 Peptide signaling in human placenta and membranes: autocrine, paracrine, and endocrine mechanisms. Endocr Rev 17:156–186 [DOI] [PubMed] [Google Scholar]
- Cronier L, Guibourdenche J, Niger C, Malassiné A 1999 Oestradiol stimulates morphological and functional differentiation of human villous cytotrophoblast. Placenta 20:669–676 [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ 2006 Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta 27:483–490 [DOI] [PubMed] [Google Scholar]
- Schiessl B, Mylonas I, Hantschmann P, Kuhn C, Schulze S, Kunze S, Friese K, Jeschke U 2005 Expression of endothelial NO synthase, inducible NO synthase, and estrogen receptors α and β in placental tissue of normal, preeclamptic, and intrauterine growth-restricted pregnancies. J Histochem Cytochem 53:1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS 1986 Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA 83:2496–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A 2006 Pure oestrogen antagonists for the treatment of advanced breast cancer. Endocr Relat Cancer 13:689–706 [DOI] [PubMed] [Google Scholar]
- Beato M, Herrlich P, Schütz G 1995 Steroid hormone receptors: many actors in search of a plot. Cell 83:851–857 [DOI] [PubMed] [Google Scholar]
- Gaub MP, Bellard M, Scheuer I, Chambon P, Sassone-Corsi P 1990 Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell 63:1267–1276 [DOI] [PubMed] [Google Scholar]
- Weisz A, Rosales R 1990 Identification of an estrogen response element upstream of the human c-fos gene that binds the estrogen receptor and the AP-1 transcription factor. Nucleic Acids Res 18:5097–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umayahara Y, Kawamori R, Watada H, Imano E, Iwama N, Morishima T, Yamasaki Y, Kajimoto Y, Kamada T 1994 Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. J Biol Chem 269:16433–16442 [PubMed] [Google Scholar]
- Petz LN, Ziegler YS, Loven MA, Nardulli AM 2002 Estrogen receptor α and activating protein-1 mediate estrogen responsiveness of the progesterone receptor gene in MCF-7 breast cancer cells. Endocrinology 143:4583–4591 [DOI] [PubMed] [Google Scholar]
- Dong J, Tsai-Morris CH, Dufau ML 2006 A novel estradiol/estrogen receptor α-dependent transcriptional mechanism controls expression of the human prolactin receptor. J Biol Chem 281:18825–18836 [DOI] [PubMed] [Google Scholar]
- Safe S 2001 Transcriptional activation of genes by 17β-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm 62:231–252 [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Chen S 2003 Induction of aromatase (CYP19) expression in breast cancer cells through a nongenomic action of estrogen receptor α. Cancer Res 63:3546–3555 [PubMed] [Google Scholar]
- Bukulmez O, Hardy DB, Carr BR, Auchus RJ, Toloubeydokhti T, Word RA, Mendelson CR 2008 Androstenedione up-regulation of endometrial aromatase expression via local conversion to estrogen: potential relevance to the pathogenesis of endometriosis. J Clin Endocrinol Metab 93:3471–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorpp-Kistner M, Wang ZQ, Angel P, Wagner EF 1999 JunB is essential for mammalian placentation. EMBO J 18:934–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger AM, Bamberger CM, Aupers S, Milde-Langosch K, Löning T, Makrigiannakis A 2004 Expression pattern of the activating protein-1 family of transcription factors in the human placenta. Mol Hum Reprod 10:223–228 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Petz LN, Nardulli AM 2003 Estrogen receptor α and Sp1 regulate progesterone receptor gene expression. Mol Cell Endocrinol 201:165–175 [DOI] [PubMed] [Google Scholar]
- Krüger I, Vollmer M, Simmons DG, Elsässer HP, Philipsen S, Suske G 2007 Sp1/Sp3 compound heterozygous mice are not viable: impaired erythropoiesis and severe placental defects. Dev Dyn 236:2235–2244 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M 2005 Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M 2008 FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132:958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, Ren B 2007 Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39:311–318 [DOI] [PubMed] [Google Scholar]
- Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL 2007 Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27:5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 [DOI] [PubMed] [Google Scholar]
- Kimura AP, Liebhaber SA, Cooke NE 2004 Epigenetic modifications at the human growth hormone locus predict distinct roles for histone acetylation and methylation in placental gene activation. Mol Endocrinol 18:1018–1032 [DOI] [PubMed] [Google Scholar]
- Kimura AP, Sizova D, Handwerger S, Cooke NE, Liebhaber SA 2007 Epigenetic activation of the human growth hormone gene cluster during placental cytotrophoblast differentiation. Mol Cell Biol 27:6555–6568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomikawa J, Fukatsu K, Tanaka S, Shiota K 2006 DNA methylation-dependent epigenetic regulation of dimethylarginine dimethylaminohydrolase 2 gene in trophoblast cell lineage. J Biol Chem 281:12163–12169 [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith Jr JC, McCoy JM 2000 Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403:785–789 [DOI] [PubMed] [Google Scholar]
- Yu C, Shen K, Lin M, Chen P, Lin C, Chang GD, Chen H 2002 GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem 277:50062–50068 [DOI] [PubMed] [Google Scholar]
- Chuang HC, Chang CW, Chang GD, Yao TP, Chen H 2006 Histone deacetylase 3 binds to and regulates the GCMa transcription factor. Nucleic Acids Res 34:1459–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Mendelson CR 2003 USF1 and USF2 mediate inhibition of human trophoblast differentiation and CYP19 gene expression by Mash-2 and hypoxia. Mol Cell Biol 23:6117–6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Mendelson CR 2005 O2 enhancement of human trophoblast differentiation and hCYP19 (aromatase) gene expression are mediated by proteasomal degradation of USF1 and USF2. Mol Cell Biol 25:8824–8833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringler GE, Strauss 3rd JF 1990 In vitro systems for the study of human placental endocrine function. Endocr Rev 11:105–123 [DOI] [PubMed] [Google Scholar]
- Condon JC, Hardy DB, Kovaric K, Mendelson CR 2006 Upregulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of NF-κB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 20:764–775 [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG 1983 Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR 2006 Progesterone receptor (PR) plays a major anti-inflammatory role in human myometrial cells by antagonism of NF-κB activation of cyclooxygenase 2 (COX-2) expression. Mol Endocrinol 20:2724–2733 [DOI] [PubMed] [Google Scholar]
- Chakrabarti SK, James JC, Mirmira RG 2002 Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem 277:13286–13293 [DOI] [PubMed] [Google Scholar]