Abstract
Prosthetic heart valve dysfunction due to thrombus or pannus formation can be a life-threatening complication. The present report describes a 47-year-old woman who developed valvular cardiomyopathy after chorda-sparing mitral valve replacement, and subsequently underwent heart transplantation for progressive heart failure. The explanted mitral valve prosthesis showed significant thrombus and pannus leading to reduced leaflet mobility and valvular stenosis. The present report illustrates the role of the subvalvular apparatus and pannus in prosthesis dysfunction.
Keywords: Mitral valve-sparing surgery, Pannus, Prosthetic heart valve, Synthetic chordae tendinae, Thrombus
Abstract
Le dysfonctionnement d’une valvule cardiaque prothétique causée par la formation d’un thrombus ou d’un pannus est une complication qui peut mettre en jeu le pronostic vital. Le présent rapport décrit le cas d’une femme de 47 ans qui a développé une myocardiopathie valvulaire après un remplacement de la valvule mitrale épargnant la chorda et qui a finalement dû subir une transplantation cardiaque en raison d’une insuffisance cardiaque progressive. La prothèse de valvule mitrale explantée a révélé un important thrombus et un important pannus entraînant une diminution de la mobilité des valves et une sténose valvulaire. Le présent rapport démontre le rôle de l’appareil sous-valvulaire et du pannus dans le dysfonctionnement prothétique.
The incidence of valvular heart disease is increasing worldwide, and for patients with severe disease, replacement with a prosthetic valve is often the only option. Approximately 55% of implanted prostheses are mechanical, while 45% are biological (1). Outcomes of mechanical valve replacement have improved considerably since their introduction in 1960 (1). Nonetheless, mechanical valve obstruction remains a significant and life-threatening complication, with an incidence of 1% to 6% (2). The causes of such dysfunction are thrombosis (common), pannus (increasing awareness) or both (2). We report a patient whose mitral valve was replaced with a bileaflet mechanical prosthesis with mitral chordal preservation and who, seven years later, underwent heart transplantation for valvular cardiomyopathy and progressive heart failure.
CASE PRESENTATION
A 47-year-old woman with progressive symptoms of congestive heart failure was diagnosed with nonischemic dilated cardiomyopathy. Seven years earlier, she had a bileaflet mechanical, mitral site prosthesis inserted for mitral insufficiency. In recent years, she had clinical and echocardiographic findings of moderate mitral regurgitation and stenosis. On admission, echocardiography revealed a severely dilated left atrium and left ventricle, moderate left ventricular hypertrophy, left ventricular hypokinesis and severely decreased global left ventricular systolic function, with an ejection fraction of less than 10%. In the setting of poor left ventricular function and inotrope dependence, redo mitral valve replacement has a higher operative mortality than heart transplantation. Therefore, the patient was assessed for heart transplantation. An appropriate male donor became available and the transplant was performed without complications. Her relevant medical history included hypertension and hyperlipidemia. She was a nonsmoker and was never diagnosed with diabetes or coronary artery disease. In addition, she had a stroke four years before her transplant, possibly resulting from a thromboembolism in the setting of chronic atrial fibrillation and/or noncompliance with her anticoagulation protocol.
Pathology
The explanted heart showed cardiomegaly with biventricular hypertrophy and dilation, interstitial fibrosis and mitral valve replacement with a CarboMedics bileaflet mechanical valve (Sulzer CarboMedics UK Ltd, United Kingdom). There was pannus on the atrial surface, covering the sewing cuff (Figures 1 to 4), and thrombus on one leaflet, which was ‘fixed’ in the nearly closed position (Figure 2A). The other leaflet was freely mobile. On the nonflow surface, the posterior and a part of the native anterior mitral leaflets had been conserved, along with most chordae tendinae (Figure 2B). In addition, two synthetic chordae had been used to resuspend the papillary muscles. These, as well as a knot at the tip of the papillary muscle, were covered with pannus (Figures 3, 4 and 5). The thrombus found on this prosthesis was likely related to the significant degree of pannus seen on the flow and nonflow surfaces. Pannus covered the sewing cuff completely, and overhung the nonflow surface of the orifice by 1.0 mm to 3.0 mm. The overhang was greatest in the region of the thrombus and on the side of the fixed leaflet (Figure 2A). There was no evidence of inflammation other than around the synthetic fabric of the sewing cuff. The aortic valve leaflets were soft and supple, with no evidence of calcification. Finally, the ascending aorta did not show any signs of clinically significant atherosclerosis.
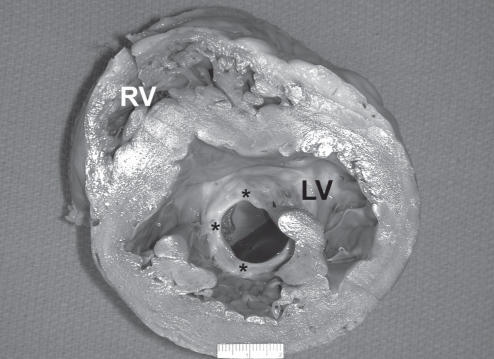
Figure 1).
Cross-section of the left ventricle (LV), showing significant dilation. The mechanical mitral valve prosthesis (ventricular surface) shows significant pannus (asterisks), which covers the entire circumference of the valve and is markedly pronounced in the region of the anterior leaflet. The valve is oriented nearly in the ante-anatomic position. RV Right ventricle
Figure 4).
Histological section of the mechanical valve sewing cuff (SC), atrial endocardium (At) and native mitral (M) leaflet tissue. A significant degree of pannus (P) deposition is seen covering the SC region, and extending into the surrounding atrial and native mitral leaflet tissue. Thrombus (arrow) is also seen close to the mitral leaflet region (original magnification ×1.6; Movat pentachrome stain)
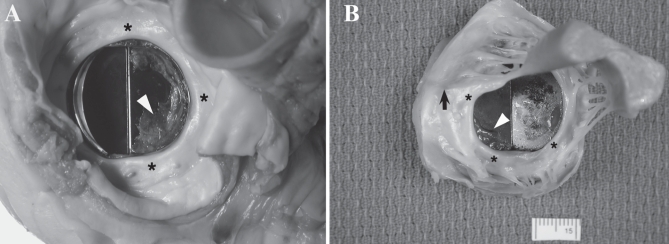
Figure 2).
The mechanical prosthesis in the mitral site in the explanted heart. A The flow surface shows pannus (asterisks) covering the sewing cuff and extending into the surrounding regions. A significant degree of thrombus (arrowhead) is seen covering the right hemi-leaflet, restricting its mobility. B The ventricular surface shows the native mitral apparatus in place. Pannus (asterisks) is seen covering the apparatus and extending onto the base of the mechanical valve prosthesis. Synthetic chordae (arrow) and thrombus (arrowhead) adherent to one leaflet are also seen
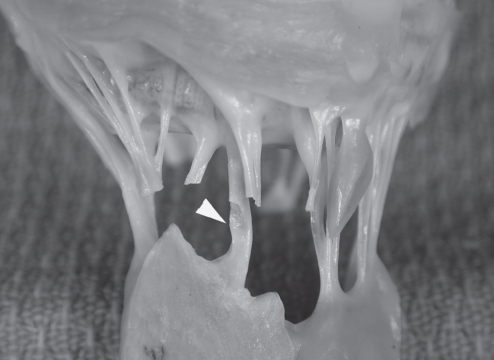
Figure 3).
A magnified view of the explanted prosthesis with the synthetic chordae tendinae (arrowhead). The synthetic chordae show extensive fibrosis and thickening but are otherwise unremarkable (original magnification ×1.6; Movat pentachrome stain)
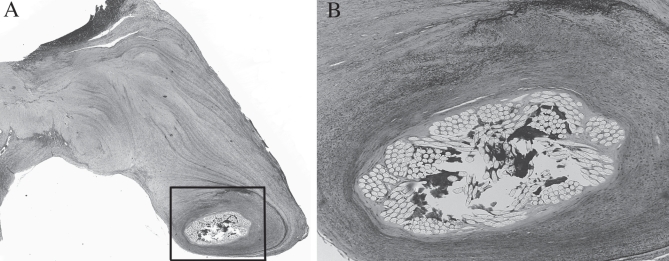
Figure 5).
A Histological section showing the synthetic chorda surrounded by tissue fibrosis (original magnification ×2.5; hematoxylin and eosin stain). B Higher magnification (box from A) showing intact synthetic chorda with collagen deposition in the interstices of the Dacron sutures. There was no evidence of chorda degradation. No inflammatory cells were seen (original magnification ×10; Movat pentachrome stain)
DISCUSSION
We have presented the case of a 47-year-old woman who underwent orthotopic heart transplantation following previous mitral- and chorda-preserving mitral valve replacement with a 29 mm CarboMedics bileaflet prosthesis. The significant findings were the considerable amount of pannus and thrombus on both surfaces of the prosthesis, which likely contributed to the patient’s prosthetic valvular dysfunction and symptoms of progressive congestive heart failure.
Mitral valve replacement with partial or complete chordal sparing offers the advantage of preserving left ventricular geometry and function. Komeda et al (3) showed improved late left ventricle hemodynamic recovery with a mitral valve-sparing procedure compared with conventional mitral valve replacement. While such procedures improve left ventricular function, they can also increase the risk of thrombus and/or pannus formation. Reports suggest that the unresected mitral valve leaflets may contribute to prosthesis dysfunction by exacerbating the host tissue response to the prosthesis (4). We report clinically significant thrombus and pannus growth on a CarboMedics mechanical valve prosthesis after a mitral valve-sparing procedure and papillary muscle resuspension with synthetic chordae tendinae. Pannus also covered the synthetic chordae, giving them the appearance of native chordae.
Pannus is a normal reaction to injury and likely to the insertion of a foreign material. It helps by covering the surface with host tissue and lessens the possibility of further thrombus deposition on the injured surfaces and the foreign materials. However, the accelerated growth of pannus in mitral valve-sparing procedures can impinge on the prosthesis orifice and affect leaflet function. The greater the excursion of the leaflets beyond the lower margin of the valve ring, the greater the likelihood of restriction of leaflet movement by excess pannus and/or thrombus deposition. The CarboMedics prosthesis has its hinge area within the valve ring; as a result, there is a greater movement of leaflets outside the valve ring (1). In comparison, the St Jude Medical valve (St Jude Medical Inc, USA) has its hinge area above the level of the valve ring; therefore, few of its leaflets swing out beyond the lower edge of the prosthesis ring, and this design makes the leaflets less susceptible to dysfunction due to surrounding pannus and/or thrombus (1). In addition, Grunkemeier and Wu (5) showed that the CarboMedics prosthesis has a greater rate of valve thrombosis than the St Jude Medical valve, taking into account 11 published series on the St Jude Medical valve and eight published series on the CarboMedics valve in the mitral position. We believe that this evidence suggests that the St Jude Medical valve is a better option for mitral valve replacement in the setting of subvalvular tissue preservation and papillary muscle resuspension.
We have previously reported the case of a patient who underwent mitral valve repair 13 years earlier with synthetic chordae tendinae (6). Gross examination revealed extensive fibrosis and thickening surrounding the chordae, rendering them indistinguishable from native tissue. In this case, similar growth of fibrous tissue and thickening surrounding the synthetic chordae was seen. Clinically significant pannus can develop more rapidly than previously reported. For example, Rizzoli et al (7) reported that clinically significant pannus formation leading to reoperation required a median interval of 13 years. The unresected mitral valve apparatus, with its intimate contact with the prosthetic valve sewing cuff, accelerates the development of pannus on the mechanical prosthesis. Pannus overgrowth can cause low flow areas and thrombus deposition on the overhanging pannus (8). Hence, along with atrial fibrillation, pannus overgrowth likely contributed to the incidence of stroke and ongoing cerebral ischemic changes in our patient.
CONCLUSION
The present case adds to the growing body of literature documenting accelerated obstruction of mechanical prostheses by pannus overgrowth after chorda-sparing mitral valve replacement. As more surgeons turn to the valve-sparing surgical procedure, complications related to prosthesis dysfunction may occur earlier compared with conventional mitral valve replacement, and the type of prosthesis used should be chosen, keeping the native valve-conserving procedure in mind.
REFERENCES
- 1.Butany J, Ahluwalia MS, Munroe C, et al. Mechanical heart valve prostheses: Identification and evaluation. Cardiovasc Pathol. 2003;12:1–22. doi: 10.1016/s1054-8807(02)00128-x. [DOI] [PubMed] [Google Scholar]
- 2.Aoyagi S, Nishimi M, Tayama E, et al. Obstruction of St Jude medical valves in the aortic position: A consideration for pathogenic mechanism of prosthetic valve obstruction. Cardiovasc Surg. 2002;10:339–44. doi: 10.1016/s0967-2109(02)00021-2. [DOI] [PubMed] [Google Scholar]
- 3.Komeda M, David TE, Rao V, Sun Z, Weisel RD, Burns RJ. Late hemodynamic effects of the preserved papillary muscles during mitral valve replacement. Circulation. 1994;90:II190–4. [PubMed] [Google Scholar]
- 4.Fayet C, Butany J, Leask RL, Ahluwalia MS, Feindel C, Fornasier VM. Host tissue overgrowth in a mitral valve conserving procedure. Cardiovasc Pathol. 2003;12:91–3. doi: 10.1016/s1054-8807(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 5.Grunkemeier GL, Wu Y. “Our complication rates are lower than theirs”: Statistical critique of heart valve comparisons. J Thorac Cardiovasc Surg. 2003;125:290–300. doi: 10.1067/mtc.2003.53. [DOI] [PubMed] [Google Scholar]
- 6.Privitera S, Butany J, Silversides C, Leask RL, David TE. Artificial chordae tendinae: Long-term changes. J Card Surg. 2005;20:90–2. doi: 10.1111/j.0886-0440.2005.200380.x. [DOI] [PubMed] [Google Scholar]
- 7.Rizzoli G, Guglielmi C, Toscano G, et al. Reoperations for acute prosthetic thrombosis and pannus: An assessment of rates, relationship and risk. Eur J Cardiothorac Surg. 1999;16:74–80. doi: 10.1016/s1010-7940(99)00124-4. [DOI] [PubMed] [Google Scholar]
- 8.Tang GH, Rao V, Siu S, Butany J. Thrombosis of mechanical mitral valve prosthesis. J Card Surg. 2005;20:481–6. doi: 10.1111/j.1540-8191.2005.200474.x. [DOI] [PubMed] [Google Scholar]