Abstract
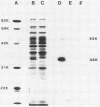
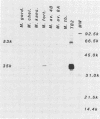
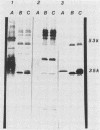
Recombinant plasmids containing DNA from Mycobacterium tuberculosis were transformed into Escherichia coli, and three colonies were selected by their reactivity with polyclonal antisera to M. tuberculosis. The three recombinant vectors contained DNA inserts of different sizes flanking a common 4.7-kilobase (kb) sequence. Each recombinant produced 35- and 53-kilodalton proteins (35K and 53K proteins, respectively) which were absent in the control E. coli. In Western blotting experiments, both proteins bound several antisera to M. tuberculosis but not antisera to other commonly isolated mycobacteria. Rabbits immunized with the recombinant 35K protein produced antisera which bound to both the 35K and 53K protein bands, a single 35K protein band present in a culture filtrate of M. tuberculosis, and single protein bands with differing molecular weights in whole-cell homogenates from other Mycobacterium spp. An additional recombinant vector containing a 2.2-kb subclone of the 4.7-kb sequence was constructed and, when used as a probe, demonstrated homology with various fragments of chromosomal digests of selected mycobacteria. Reactivity of this probe to Mycobacterium bovis and M. bovis BCG was indistinguishable from reactivity to M. tuberculosis. Immunoglobulin G reactivity to the 35K antigen was detected in antisera from 8 of 20 persons with active tuberculosis, 4 of 18 persons with leprosy, and none of 14 healthy controls. In contrast, reactivity to various proteins in M. tuberculosis culture filtrate was present in 18 of 20 patients with tuberculosis, 16 to 18 patients with leprosy, and 5 of 14 controls. The production of M. tuberculosis proteins by E. coli circumvents many difficulties encountered in the growth and manipulation of M. tuberculosis and may facilitate the development of better diagnostic and immunizing reagents.
Full text
PDF

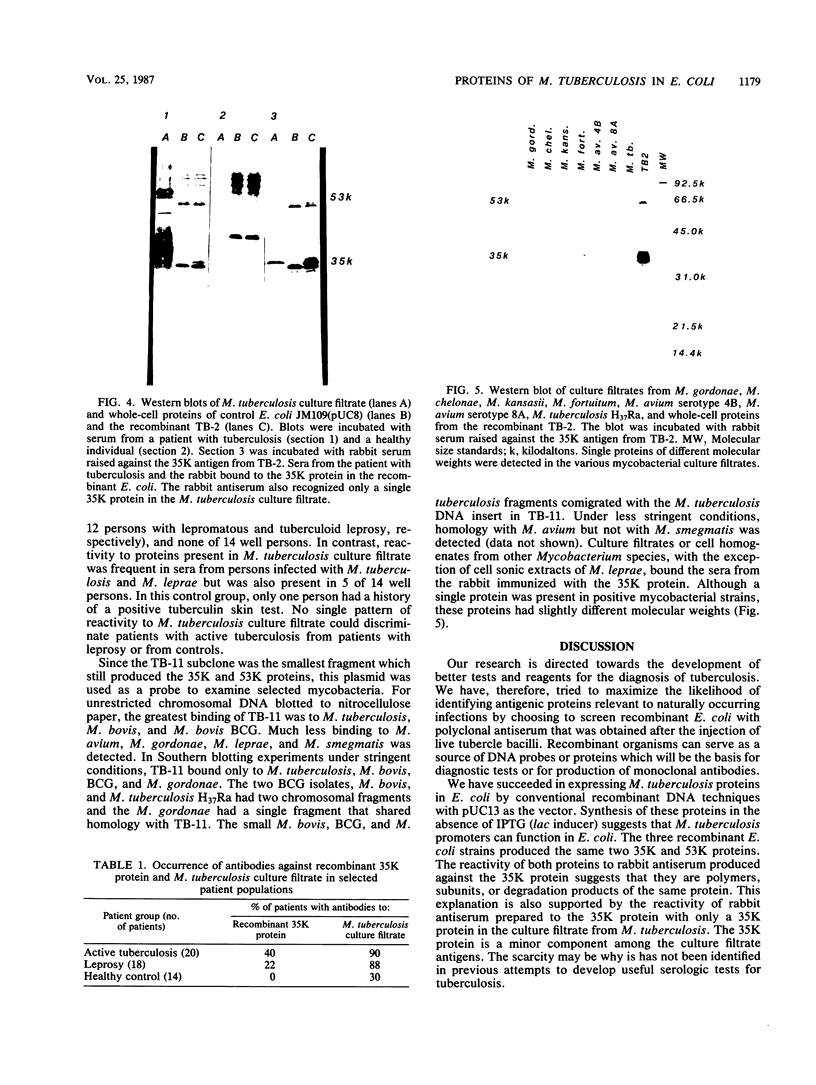

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baess I. Isolation and purification of deoxyribonucleic acid from mycobacteria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):780–784. doi: 10.1111/j.1699-0463.1974.tb02375.x. [DOI] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Cohen M. L., Falkow S. Protein antigens from Staphylococcus aureus strains associated with toxic-shock syndrome. Science. 1981 Feb 20;211(4484):842–844. doi: 10.1126/science.7466361. [DOI] [PubMed] [Google Scholar]
- Crawford J. T., Cave M. D., Bates J. H. Evidence for plasmid-mediated restriction-modification in Mycobacterium avium intracellulare. J Gen Microbiol. 1981 Dec;127(2):333–338. doi: 10.1099/00221287-127-2-333. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kenna J. G., Major G. N., Williams R. S. Methods for reducing non-specific antibody binding in enzyme-linked immunosorbent assays. J Immunol Methods. 1985 Dec 27;85(2):409–419. doi: 10.1016/0022-1759(85)90150-4. [DOI] [PubMed] [Google Scholar]
- Parekh B. S., Mehta H. B., West M. D., Montelaro R. C. Preparative elution of proteins from nitrocellulose membranes after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1985 Jul;148(1):87–92. doi: 10.1016/0003-2697(85)90631-1. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Zaatari F. A., Reiss E., Yakrus M. A., Bragg S. L., Kaufman L. Monoclonal antibodies against isoelectrically focused Nocardia asteroides proteins characterized by the enzyme-linked immunoelectro-transfer blot method. Diagn Immunol. 1986;4(2):97–106. [PubMed] [Google Scholar]