Abstract
The purpose of this study was to confirm the expression of interleukin-1 receptor antagonist (IL-1 Ra) in the human cornea. Four samples of human ex vivo corneal epithelium were obtained from patients undergoing photorefractive keratectomy. RT-PCR was performed using mRNA isolated from the corneal epithelium and oligo-dT primers. PCR was performed on the cDNA products using primers specific for human IL-1Ra. The PCR products were subcloned and sequenced. Human cornea sections were prepared from eyes enucleated for choroidal melanoma. Immunocytochemistry was performed using goat anti-mouse polyclonal IL-1Ra IgG and NL-577 conjugated donkey anti-goat IgG. IL-1 Ra mRNA was expressed in all ex vivo corneal epithelium samples as confirmed by sequencing of the PCR products. Immunofluorescence studies revealed strongest expression of IL-1Ra in the superficial apical layer of corneal epithelium. Expression of IL-1 Ra may represent an endogenous mechanism of down-regulating the effects of epithelial- and tear-derived IL-1α and IL-1β on the intact epithelium in the unwounded cornea and stromal cells after injury.
Introduction
Inflammation is an important component of the corneal response to injury. Interleukin 1 (IL-1) has been shown to be an important cytokine in the initiation of corneal inflammation (Hong, et al., 2001; Stapleton, et al., 2008). Binding of IL-1 to its receptor results in several downstream inflammatory effects, including leukocyte extravasation, protease and prostaglandin production, and T-cell activation (Dinarello, 1988; Le, et al., 1987). In the cornea, IL-1α and IL-1β have been shown to mediate inflammatory cell recruitment from the limbal vessels, cytokine production, and keratocyte apoptosis in the stroma (Hong, et al., 2001; Wilson, et al., 1999). Three members of the IL-1 family, IL-1α, IL-1β, and IL-1 receptor antagonist (IL-1 Ra), are located adjacent to one another on 2q14 (Nicklin, et al., 1994). On the ocular surface, IL-1α and IL-1β have been shown to be constitutively present in the tear film and corneal epithelium (Wilson, et al., 1994; Weng, et al., 1997; Afonso, et al., 1999). Prolonged inflammation is deleterious to the surrounding tissues and must be controlled to reduce collateral tissue injury. IL-1 Ra represents one mechanism through which IL-1 activity can be attenuated. IL-1 Ra is a 22–25 kDa protein that binds to IL-1 receptors with similar affinity as IL-1α and IL-1β, without triggering inflammatory downstream effects (Hannum, et al., 1990). Kennedy and coworkers (1995) previously demonstrated IL-1Ra expression in cultured human corneal epithelial and stromal cells, as well as the epithelium and stroma of corneal sections, using immunocytochemistry. The present study investigated the expression of IL-1 Ra in ex vivo human corneal epithelium removed during PRK and in human corneal tissue sections using immunocytochemistry methods.
Methods
Approval from the Cleveland Clinic Institutional Review Board was obtained for use of human pathology specimens: epithelium removed at the time of PRK and corneo-scleral rims of eyes removed for choroidal melanoma.
RNeasy Mini Kit (Qiagen, Valencia, CA) was used according to the manufacturer’s protocol to isolate mRNA from ex vivo corneal epithelium obtained from photorefractive keratectomy patients. The eyes underwent epithelial scrape with a #64 Beaver blade (Becton Dickinson, Franklin Lakes, NJ) without ethanol denaturation. RT-PCR was performed using oligo-dT primers, as previously described (Weng, et al., 1997). PCR was performed using primers specific for human IL-1Ra. The forward primer sequence was 5′ GCA AGA TGC AAG CCT TCA 3′, and the reverse primer sequence was 5′ CTC CTG GAA GTA GAA TTT GGT 3′. The PCR products were sub-cloned into the pDRIVE vector (Qiagen, Valencia, CA) using the TA cloning kit. The insert was sequenced at the sequencing core at Lerner Research Institute at Cleveland Clinic.
Sections of human cornea were obtained from eyes with normal anterior segments enucleated for choroidal melanoma. Full thickness corneal tissue was trephined from the eye immediately after enucleation and snap frozen in Optimal Cutting Temperature (OCT) compound (Sakura Finetek, Torrance, CA). The frozen block was sectioned (8 μm) with a cryolathe and mounted on glass slides. Immunocytochemistry was performed, as previously described (Netto, et al., 2007), using goat anti-mouse IL-1Ra IgG (R&D Systems, Minneapolis, MN) and donkey anti-goat IgG conjugated with NL-577 (R&D Systems, Minneapolis, MN). IgG isolated from non-immune goat serum was used as the primary antibody in control specimens.
Results
PCR using primers specific for human IL-1Ra was expected to yield a 429 base pair fragment. The product of the anticipated length was amplified from PCR of cDNA synthesized from each human ex-vivo corneal epithelium sample (Figure 1). Sub-cloning and sequence analysis of the fragments revealed a 99.5% match to human IL-1 Ra mRNA (data not shown).
Figure 1.
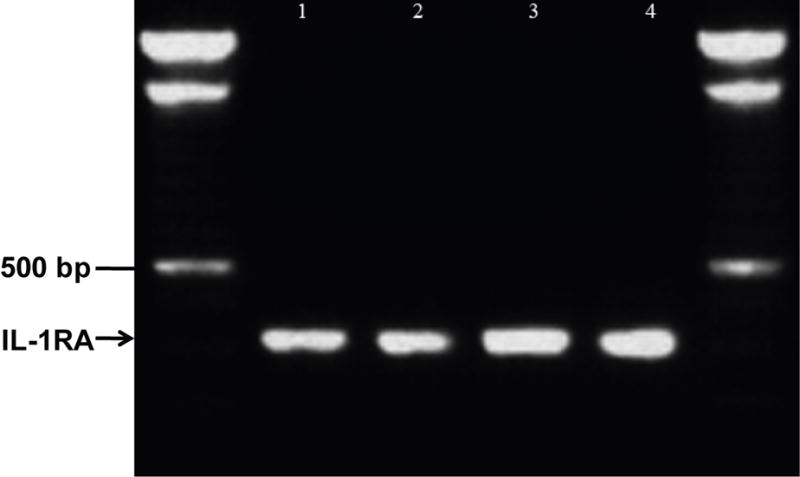
Detection of IL-1 Ra mRNA in ex vivo human corneal epithelium using RT-PCR. cDNA was synthesized from RNA isolated from central corneal epithelium removed at the time of photorefractive keratectomy. The arrowhead highlights 429 bp PCR product amplified from independent epithelial cell cDNA samples (lanes 1–4). Flanking the PCR products is DNA ladder (L). BLAST alignment of PCR product sequence. The alignment showed a 427/429 (99.5%) match to human IL-1 Ra mRNA (data not shown).
Immunocytochemistry was performed on unwounded human cornea sections. The sections were stained using goat anti-mouse IL-1 Ra polyclonal IgG and donkey anti-goat IgG conjugated with NL-577, a red fluorophore. The corneal sections showed intense staining of the outer-most, superficial cells of the corneal epithelium (Figure 2, panels A and C). Control sections that were stained with non-immune goat IgG did not show red fluorescence (Figure 2, panels D and F). Theses results show that IL-1 Ra is expressed constitutively in the corneal epithelium and the expression is the strongest in the outermost cells of the corneal epithelium. Some keratocytes throughout the stroma also expressed IL-1 Ra in the unwounded cornea (not shown).
Figure 2.
IL-1 Ra detected in the central human cornea by immunocytochemistry. Representative corneal sections from multiple experiments are shown. A) Overlay of red and blue fluorescence in a section stained with goat anti-mouse IL-1 Ra and donkey anti-goat IgG conjugated with NL-577 and counterstained with DAPI. IL-1Ra was most highly expressed in the superficial apical cells of the epithelium (arrows). The thickness of the epithelium in this section is an artifact of sectioning. Very low levels of IL-1 Ra were also detected at the microscope in keratocytes throughout the stroma, but are not well seen in photographs. B) Overlay of red and blue fluorescence in a control section stained with non-immune goat IgG and donkey anti-goat IgG conjugated with NL-577 and counterstained with DAPI. e indicates epithelium and s indicates stroma. Magnification 400X.
Discussion
IL-1Ra mRNA is constitutively expressed in exvivo human corneal epithelium and IL-1Ra was most prominently produced in apical corneal epithelial cells of fresh human cornea. The results of this study confirm an earlier finding that showed expression of IL-1 Ra in the corneal epithelium and some stromal cells (Kennedy, et al., 1995). The authors of the previous study hypothesized that corneal IL-1 Ra expression has a role in modulating cytokine-mediated inflammatory responses in the cornea. This hypothesis was supported by recent studies in mice which demonstrate that topical IL-1 Ra profoundly down-regulates inflammatory cell infiltration into the corneal stroma in response to epithelial injury (Stapleton, et al., 2008). In addition, the very high level of expression of IL-1 Ra in the apical surface epithelial cells suggests a role for the receptor antagonist in attenuating the effects of tear IL-1α and IL-1β derived from corneal and conjunctival epithelial cells and, possibly, lacrimal glands (Solomon, et al, 2001). Alternatively, it is possible that the high levels of IL-1 Ra detected in apical corneal epithelial cells is in whole or in part absorbed from tears and, thus, that the messenger RNA detected in the epithelium is not translated in physiologically significant amounts. If this were the case, the tear IL-1 Ra might be derived from lacrimal gland, conjunctiva or other sources.
A remarkable property of the cornea is its capacity to maintain transparency despite exposure to the outside environment. Thus, the cornea is likely exposed to chronic mechanical, viral and other inflammatory stimuli that, left unchecked, could lead to a loss of transparency. Epithelial IL-1 Ra may have a role in homeostatic suppression of the inflammatory response to stimuli and thereby play a role in the maintenance of corneal transparency. For example, it has been shown that overexpression of IL-1 Ra can reduce corneal scarring secondary to HSV infection in a mouse model of HSV keratitis (Biswas, et al., 2004). A polymorphism in intron 2 of IL-1 Ra has been shown to be associated with several diseases of epithelial origin, including ulcerative colitis, alopecia areata and lichen sclerosus (Clay, et al., 1994; Tarlow, et al., 1993; Tarlow, et al., 1994; Tountas, et al., 1999). This association between IL-1 Ra polymorphisms and inflammatory diseases of epithelial origin suggests that IL-1 Ra plays an important role in maintaining tonic suppression of inflammation in the cornea.
Acknowledgments
This study was supported by EY10056, EY015638, and Research to Prevent Blindness. Steven E. Wilson is a recipient of the RPB Physician-Scientist Award. The authors also would like to thank Arun D. Singh for providing the human cornea for the immunofluorescence studies and Vandana Agrawal for cutting and mounting corneal sections.
Supported in part by US Public Health Service grants EY010056 and EY015638 from the National Eye Institute and Research to Prevent Blindness, New York, NY. Dr. Wilson is the recipient of a Research to Prevent Blindness Physician-scientist Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso AA, Sobrin L, Monroy DC, Selzer M, Lokeshwar B, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999;40:2506–2512. [PubMed] [Google Scholar]
- Biswas PS, Banerjee K, Kim B, Rouse BT. Mice transgenic for IL-1 receptor antagonist protein are resistant to herpetic stromal keratitis: possible role for IL-1 in herpetic stromal keratitis pathogenesis. J Immunol. 2004;172:3736–3744. doi: 10.4049/jimmunol.172.6.3736. [DOI] [PubMed] [Google Scholar]
- Clay FE, Cork MJ, Tarlow JK, Blakemore AI, Harrington CI, Lewis F, Duff GW. Interleukin 1 receptor antagonist gene polymorphism association with lichen sclerosus. Hum Genet. 1994;94:407–410. doi: 10.1007/BF00201602. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biology of interleukin 1. Faseb J. 1988;2:108–115. [PubMed] [Google Scholar]
- Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Hong JW, Liu JJ, Lee JS, Mohan RR, Mohan RR, Woods DJ, He YG, Wilson SE. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Invest Ophthalmol Vis Sci. 2001;42:2795–2803. [PubMed] [Google Scholar]
- Kennedy MC, Rosenbaum JT, Brown J, Planck SR, Huang X, Armstrong CA, Ansel JC. Novel production of interleukin-1 receptor antagonist peptides in normal human cornea. J Clin Invest. 1995;95:82–88. doi: 10.1172/JCI117679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987;56:234–248. [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Medeiros FW, Dupps WJ, Jr, Sinha S, Krueger RR, Stapleton WM, Rayborn M, Suto C, Wilson SE. Femtosecond laser and microkeratome corneal flaps: comparison of stromal wound healing and inflammation. J Refract Surg. 2007;23:667–676. doi: 10.3928/1081-597x-20070901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin MJ, Weith A, Duff GW. A physical map of the region encompassing the human interleukin-1 alpha, interleukin-1 beta, and interleukin-1 receptor antagonist genes. Genomics. 1994;19:382–384. doi: 10.1006/geno.1994.1076. [DOI] [PubMed] [Google Scholar]
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–92. [PubMed] [Google Scholar]
- Stapleton WM, Chaurasia SS, Medeiros FW, Mohan RR, Sinha S, Wilson SE. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp Eye Res. 2008;86:753–757. doi: 10.1016/j.exer.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlow JK, Blakemore AI, Lennard A, Solari R, Hughes HN, Steinkasserer A, Duff GW. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Hum Genet. 1993;91:403–404. doi: 10.1007/BF00217368. [DOI] [PubMed] [Google Scholar]
- Tarlow JK, Clay FE, Cork MJ, Blakemore AI, McDonagh AJ, Messenger AG, Duff GW. Severity of alopecia areata is associated with a polymorphism in the interleukin-1 receptor antagonist gene. J Invest Dermatol. 1994;103:387–390. doi: 10.1111/1523-1747.ep12395398. [DOI] [PubMed] [Google Scholar]
- Tountas NA, Casini-Raggi V, Yang H, Di Giovine FS, Vecchi M, Kam L, Melani L, Pizarro TT, Rotter JI, Cominelli F. Functional and ethnic association of allele 2 of the interleukin-1 receptor antagonist gene in ulcerative colitis. Gastroenterology. 1999;117:806–813. doi: 10.1016/s0016-5085(99)70338-0. [DOI] [PubMed] [Google Scholar]
- Weng J, Mohan RR, Li Q, Wilson SE. IL-1 upregulates keratinocyte growth factor and hepatocyte growth factor mRNA and protein production by cultured stromal fibroblast cells: interleukin-1 beta expression in the cornea. Cornea. 1997;16:465–471. [PubMed] [Google Scholar]
- Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999;18:293–309. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Schultz GS, Chegini N, Weng J, He YG. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp Eye Res. 1994;59:63–72. doi: 10.1006/exer.1994.1081. [DOI] [PubMed] [Google Scholar]



