Abstract
In this review the diagnostic potential of evaluating female pelvic floor muscle (PFM)) function using magnetic and ultrasound imaging in the context of urodynamic observations is considered in terms of determining the mechanisms of urinary continence. A new approach is used to consider the dynamics of PFM activity by introducing new parameters derived from imaging. Novel image processing techniques are applied to illustrate the static anatomy and dynamics PFM function of stress incontinent women pre and post operatively as compared to asymptomatic subjects. Function was evaluated from the dynamics of organ displacement produced during voluntary and reflex activation. Technical innovations include the use of ultrasound analysis of movement of structures during maneuvers that are associated with external stimuli. Enabling this approach is the development of criteria and fresh and unique parameters that define the kinematics of PFM function. Principal among these parameters, are displacement, velocity, acceleration and the trajectory of pelvic floor landmarks. To accomplish this objective, movement detection, including motion tracking algorithms and segmentation algorithms were developed to derive new parameters of trajectory, displacement, velocity and acceleration, and strain of pelvic structures during different maneuvers. Results highlight the importance of timing the movement and deformation to fast and stressful maneuvers, which are important for understanding the neuromuscular control and function of PFM. Furthermore, observations suggest that timing of responses is a significant factor separating the continent from the incontinent subjects.
Keywords: Ultrasonography, Urodynamics, Pelvic floor, Continence, Urethra, Levator ani
1. Introduction
Anatomically, the pelvic floor (PF) contains many visceral organs having diverse functions ranging from: urination, defecation, ejaculation, orgasm, conception, labor and parturition. These multiple organ systems interact and coordinate with each other in performing their normal physiological function. Under certain conditions, these functions are subject to disruption, manifesting as incontinence: sexual dysfunction, pain or any of a spectrum of complex symptoms whose origin may or may not be readily identifiable. In this context, pelvic floor dysfunction (PFD) constitutes a global burden affecting the quality of life of the individual, their family and society in general. Clearly within the general categorization of PFD, there is broad spectrum of symptoms having diverse origins. In women, because of the magnitude of the problem, a great deal of attention has been placed on the implication of PF function as it relates to urinary incontinence (UI) and stress urinary incontinence (SUI) involving the involuntary leakage of urine on coughing, sneezing, exertion or effort to the extent that it has been termed by DeLancey to be a hidden epidemic [1]. As a consequence, diagnostic methodologies under the umbrella of Urodynamic testing evolved to address the epidemic. However, as with urodynamic testing there is an analogous endeavor to formalize and systematize the evaluation of the function of PFM. Evidently the technical requirements of PF dynamics technology are complex and challenging requiring a contextual approach and the least possible invasive means. In current practice, imaging and manual muscle testing per vagina or rectum is the technique used by most clinicians to evaluate the PF muscles. Unfortunately due to the location of the PF muscles defining its normal function in a non-invasive way is clinically and technically challenging.
2. Basic Considerations
The PF is a complex 3D arrangement of muscle and connective tissue, attached to the bony pelvis. The PF is a collective name for the levator ani and ischiococcygeus. The levator ani muscle consists of the pubococcygeus, the puborectalis, and the iliococcygeus muscles. The pubococcygeus and the puborectalis muscles form a U-shape as they originate from the pubic bone on either side of the midline and pass behind the rectum to form a sling. The iliococcygeus muscle arises laterally from the arcus tendineus levator ani and forms a horizontal sheet that spans the opening in the posterior region of the pelvis, thereby providing a “shelf” upon which the pelvic organs rest [2]. The muscles and fascias of the pelvic diaphragm are inserted on the ischial spines either directly or indirectly through the sacrospinous ligament and the tendinous arch of the pelvic fascia. The result of a PFM contraction is a medial pull on the ischial spines to produce a more rigid and narrower pelvic floor [3]. Various diagnostic approaches have been applied to evaluate PFM function directly or indirectly and assess their dynamic properties, contractility and tissue quality and strength using palpation, visual observation, electromyography, dynamometers, ultrasound, (MRI). Compared collectively, each tool has its own qualities and limitations [4]. Most recently, using a reliable instrumented speculum, incontinent women demonstrated lower values in passive force, endurance and speed of contraction than continent women, however, differences between the two groups for maximal force reached the statistically significant level only in the endurance parameter [5]. PFM strengthening exercises do diminish the symptoms SUI [6,7]. Little research has focused upon the mechanisms of therapeutic change to help identify the specific critical muscle components of manipulation [8] so it is unknown whether PFM manipulation mimics the normal physiological behavior of the PFM or is an compensation strategy, nor whether program awareness is indeed the most efficient method of conservative rehabilitation. It seems appropriate to determine whether other properties of muscle function generated from imaging are also important in defining PFM function and dysfunction, as well as gaining a greater understanding of why PFM manipulation is effective in some cases and not others. A useful approach to understand the mechanisms involved can be made by considering the relative effect of PFM activation on the urethra under involuntary reflex conditions as well as during volitional or anticipatory contractions. Figure 1 illustrates the anatomical as well as functional changes taking place during PFM contraction.
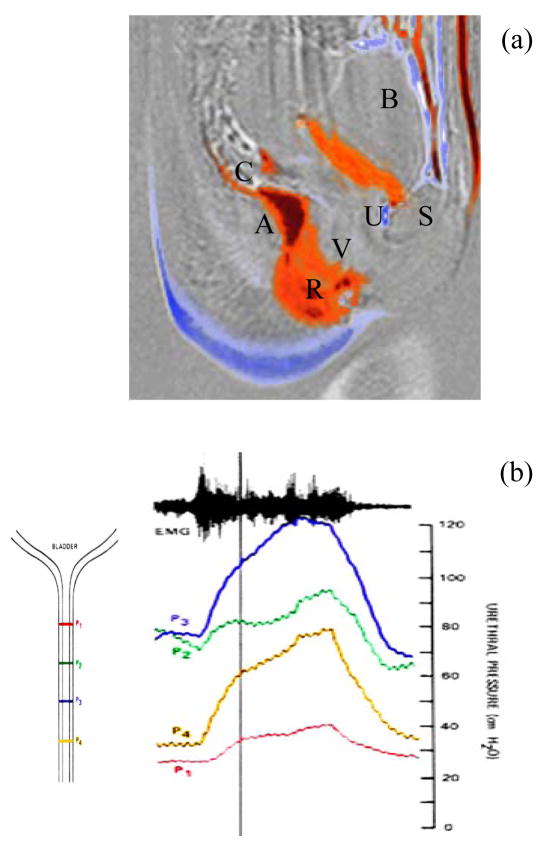
Figure 1.
Subtraction mri image showing (a) the differences between resting and contracting pelvic florr muscles [9,10] where: A is ano-rectal junction, B: bladder, S: Symphysis pubis, R rectum, U urethra and V vagina. Red denotes region where contraction compresses while blue where it is vacated. Figure (b) illustrates the changes in urethra pressures all measured simultaneously at different regions using the approach described in [11]. Urethral pressure rise U is shown by Figure 1(b) to be generated over and above the resting pressures P1–P4. As shown by the schematic diagram associated with Figure 1(b) P1 defines the pressure at the base of the bladder, having the lowest baseline pressure while P2 P3 and P4 are located 7mm apart towards the urethral sphincter and meatus. Figure 1(b) clearly shows that the maximun increase in urethral pressure from baseline can be localized at a region distal sphincter. The electromyogram shown at the top, obtained with co-axial needle electrodes of the anal sphincter, is characteristic of a sustained voluntary contraction.
As indicated by Figure 1, voluntary PFM contraction is elevates the bladder and acts upon the urethra U thereby generating an increase in closure pressures above the resting pressure. The increase in pressure is not uniform and depends on the position along the length of the urethra producing a pressure gradient and closure. Clinically while the increase in the closure pressures produced can be felt during pelvic examination through the vagina Figure 1(b) shows that the urethra is also squeezed synchronously with the PFM contraction. Clearly active PFM contraction pressures are superimposed above the resting vaginal and urethral closure given that neither structures can be considered as passive. Distinction between the relative influence of vagina and urethral can only be made if vaginal closure is measured at rest as well as during PFM contraction. Enabled by a probe system [12], the closure forces at various locations in the vagina were measured. Using this probe, a resting vaginal pressure profile VPP was characterized and Figure 2 illustrates the distribution of closure along the length of the vagina.
Figure 2.
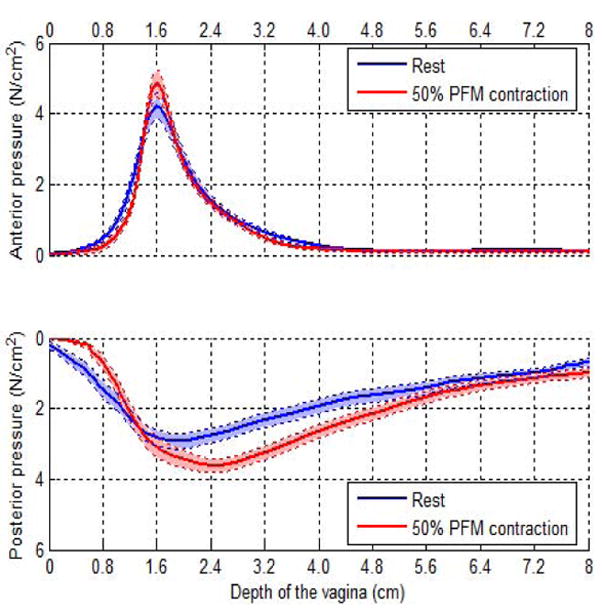
Vaginal pressure profile measured with a vaginal probe at rest (blue) and also during sustained voluntary PFM contraction. As indicated anterior pressures are higher than theposterior values while spread of the pressurized zone in the posterior is higher. [13,14]
In measuring the vaginal pressures with a probe, it is appropriate to consider that there is mechanical deformation of the tissues involved and consequently values obtained in the anterior as compared to the posterior require adjustment [13]. Nonetheless the VPP has provided clinically useful information delineating the distribution of forces along the length of the vagina in continent as well as in SUI subjects [14]. Indeed the VPP, as illustrated by Figure 2, may be considered analogous the urethral pressure profile upp given that the urethra is cradled by the vaginal for a considerable region of its length.
3. Imaging/Urodynamics Studies
As demonstrated by Figure 1, voluntary PFM contractions can be readily viewed using mri primarily because the contraction can be sustained for a sufficiently long time, 10–15 seconds to acquire the image. Thus as indicated, by imaging studies using ultrasound or MRI show that a voluntary contraction of the PFM changes the ano-rectal angle (ARA) [15] and can displace the urethra in a direction towards the pubic symphysis [11,16–19]. Yet PFM contraction in some women increase the intra urethral pressure, but not in others [20]. It is known that in women there is recruitment of PFM motor units [21,22] and an increase in intra-urethral pressure [23] prior to an increase in intra-abdominal pressure during a stress. Two hypothetical questions then arise: Is the contractile force of the PFM as applied to the urethra diminished or the timing of urethra closure in SUI slow in responding. To clarify these questions, it is appropriate to consider the available evidence, in terms of clinical conditions, where urethral response to the cough reflex was evaluated.
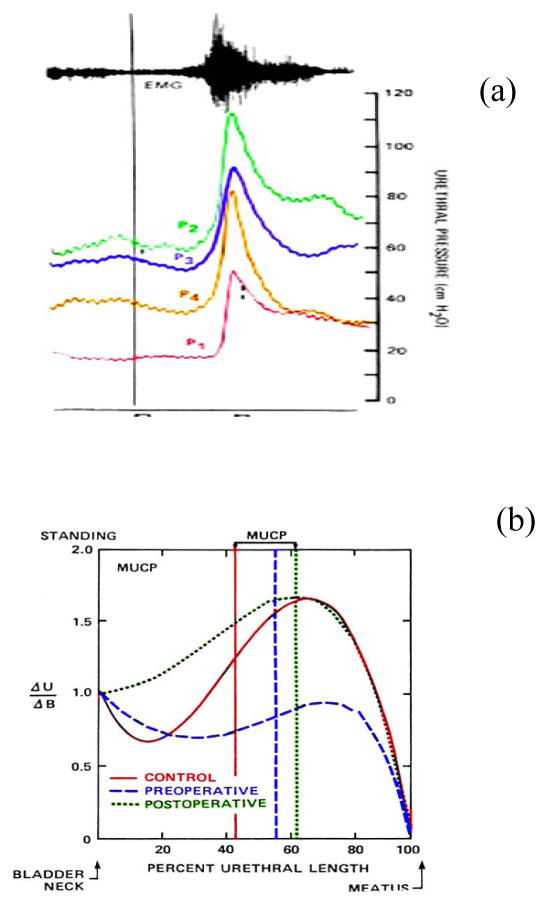
As indicated by Figure 3a the pressure rise in the urethra compared to that of the ratio between the elevation in urethral pressure Δu/and bladder pressure Δu of continent subjects varies by region. The position of maximum transmission is located distal to the external sphincter and is higher than 100% suggesting that an external force elevates the bladder and acts upon the urethral lumen. In SUI subjects, transmission is less than 100% suggesting that the external forces acting on the urethra are attenuated. Such attenuation may be considered to be due to weaker PFM muscles or the anatomical position urethral lumen is such that the PFM are not effective. However, the evidence provided from postoperative urodynamic studies of SUI subjects immediately after endoscopic bladder neck demonstrates that pressure transmission is restored immediately (3–4 days) after surgery. Assuming that there is no significant PFM strengthening during this brief time period, it is evident that increased transmission can be accounted by the anatomical reorganization produced by the surgical procedure. In view of these observations, the question arises as to the importance of timing of the PFM contraction relative to the cough reflex. Altered PFM activation patterns during a cough, enabled by anal electromyography measurements in SUI compared to healthy volunteers have also been reported, [22] as having with shorter activation periods suggesting that the duration of activity may be critical. These observations may be viewed in the context that PFM are involved in generating intra-abdominal pressure given that respiration and incontinence have a strong correlation [24,25]. Furthermore, in studies of continent women, co-activation was observed, suggesting the need to evaluate multiple PFM responses occurring at a relatively fast rate (<0.1 seconds) [26]. In addition to address the need to examine the response of multiple PFM and their influence during activation, it is essential to use the least invasive imaging methods, providing good temporal resolution. Fortunately, developments in two dimensional (2D) ultrasound imaging and three dimensional (3D) real time reconstructions technology, associated with frame by frame software analysis a valuable tool to explore the dynamics of the PFMs to facilitate a better understanding of the mechanisms of continence.
Figure 3.
(a) Clinical data illustrating typical recordings, of urethral pressure distribution along four regions of the urethra associated with a cough using the methodology illustrated by Figure 1b. The electromyogram shown at the top, is characteristic to that of a short duration cough reflex response.
(b) illustrates measurements designed specifically to provide clinical evidence by evaluating the differences between continent subjects and those who present with SUI and are scheduled for surgery. Vertical axis is given as the ratio of the incremental rise of urethral pressures to that of the bladder. This ratio was used in order to normalize the variability between different intensity of coughs and resting pressures. By taking the ratio of bladder pressure rise to urethral pressure rise a more accurate quotient of the relative response can thus be generated. Similarly, the units of the horizontal axis are given as a % of urethral length in order to normalize the individual variations of the urethra. Data to generate these curves were obtained from asymptomatic volunteers and patients presenting with stress urinary incontinence pre-operatively, tested post bladder neck suspension usig the Stamey procedure. Details of the methodology and clinical criteria can be found in [23]
4. Dynamics of 2D and 3D Ultrasound Imaging
In analogy to cardiac imaging, 2D transperineal ultrasound imaging can acquire dynamic information on the morphology of the urogenital organs. In particular, perineal, introital and trans-vaginal ultrasound has become an imaging platform for the evaluation of the PF and for the treatment planning of many uro-gynecological conditions [4, 18, 27]. By its nature, 2D ultrasound imaging provides a very large amount of dynamic data that cannot be visually assimilated by the observer in its totality, particularly during fast occurring reflex events. Such dynamic events contain information relating the integrity of the supporting structures of the bladder neck, the role of the PFM, and the compliance of pelvic floor structures [28] to deform. Furthermore, because the urogenital structures are anatomically interconnected, ultrasound-based dynamic imaging can substantiate the findings of urodynamic observations of the effective spatial and temporal distribution and timing of pressure transmission to the urethra [11,23,28]. State-of-the-art 3D ultrasound imaging techniques provide 3D visualization of the pelvic floor structures with higher resolution. However, current 3D ultrasound technology is not swift enough for the purpose of visualization the movement of tissues in fast and stressful maneuvers like coughing, which may provoke urinary incontinence.
Until recently, real time ultrasound imaging, containing the diagnostically important information of the dynamic response of the PF such as coughing occur at such high speed, that all anatomical movements cannot be assimilated and quantified by the observer during the scanning process. The direction and the timings of the movement of the PF tissues, which may be more important than the amplitudes in the mechanism of female urinary continence, are usually missed and sometimes ignored. Clinical measurements of 2D ultrasound images can only tell us about the resting position of the urethra and the displacement at the end of events such as Valsalva, voluntary PFM contraction and coughing. [18,28,29]. The difficulties with accurately determining the finishing point of any swift maneuver, are numerous and are a potential source of error [30]. The operator has either had to make multiple on-screen measurements, or determine the exact peak moment, or end position of the maneuver, visually freeze it on the screen, and then measure the change in position manually on screen or within in built electronic calipers. Without correcting for probe movement relative to the pubis symphysis the percentage errors range from 18–87% [31]. Clearly in order to define normal PFM function it is essential to capture and visualize the sequence of dynamic changes the PFM produced on the urethra, vagina and rectum using digital image-processing methods. To determine the deviation from normal function it is useful to target the evaluation of asymptomatic volunteers and to develop a number of functional parameters to facilitate comparison with those with SUI.
5. Trans-perineal ultrasound imaging
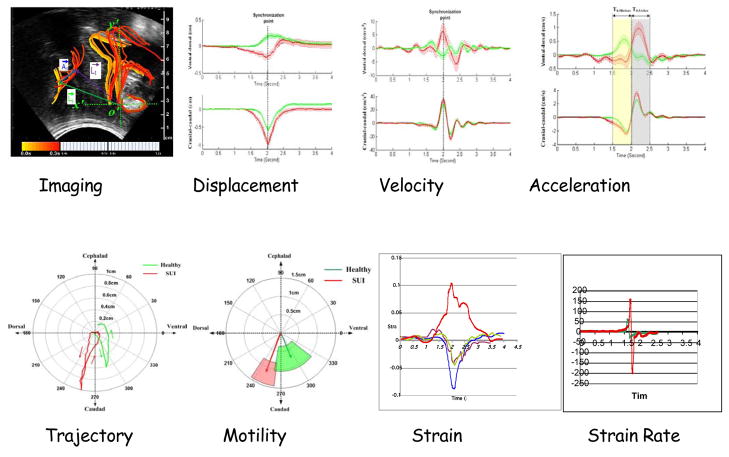
Visual examination of the ultrasound images suggested that the displacement of the PF tissues during maneuvers contains components that can best be defined as a ventral (anterior) component towards or dorsal (posterior) component away from the symphysis pubis and a cephalad (superior) component upwards or caudad (inferior) component downwards. This is supported by other studies that suggest that in a functional PFM contraction, the bladder neck has been shown by Miller et al to move in a ventro-cephlad direction [17] increasing the closure pressure within the urethra as it is displaced towards the symphysis pubis [20] and during Valsalva, as the intra-abdominal pressure increases, the bladder neck moves in an dorsal-caudad direction [29]. Practical details of the ultrasound scanning system and approach are given by Peng et al. [27] Briefly, the approach taken is to outline the symphysis, urethra and rectum interfaces on a frame-by-frame basis for sequences of stress inducing events such a cough, Valsalva and voluntarily induced PFM contractions. During each event, the trajectory of the boundary of each structure was identified to characterize the sequential history of the ensuing movement. The resulting image analysis focused to reveal the anatomical displacement of the urogenital structures and to enable the evaluation of their biomechanical parameters in terms of displacement.
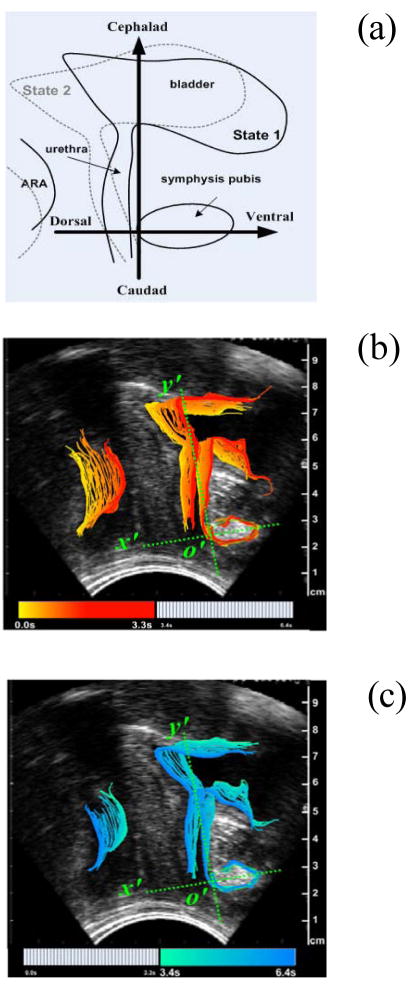
Figure 4(a) shows the two axes of the coordinate system are parallel and vertical to the urethra at rest respectively which is fixed during the maneuver, so when the subject deforms the bladder (From State 1 to State 2), the coordinate system will maintain its original position and the ensuing that measurements can be made relative to this fixed axis. Visual examination of the ultrasound images suggested that the displacement of the PF tissues during maneuvers contains components that can best be defined as a ventral (anterior) component towards or dorsal (posterior) component away from the symphysis pubis and a cephalad (superior) component upwards or caudad (inferior) component downwards. Such movements are supported by other studies that suggest that in a functional PFM contraction. Evidently the transition from State1 to State2, as illustrated by Figure 4a, represents highly simplified characterization of PF dynamics. In our preliminary studies we were able to map the trajectory of the bladder, urethra and ARA and define the physical characteristics associated with the neuromuscular activation of the PF. Thus, the transition from State1 to State2 shown by Figure 1a occurs relatively fast and translate into a sequence of overlapping frames separated in time. A convenient visualization of these frames, is illustrated by Figure 4b and Figure 4c. In these Figures the temporal/spatial transition was color coded to facilitate identification of the path taken by the bladder and anorectal when the PF is activated. Thus the timing of the movement of tissues in pelvic floor during a typical PFM contraction in the same volunteer can be visualized so that when the volunteer contracts her PFM, (Figure 4b) the urogenic structures move in a ventral-cephlad direction (forward and up). As she releases the contraction, or relaxes the PFM (Figure 4c) the tissues return to the original resting position.
Figure 4.
Figure 4(a). Schematic of the localization system fixed on the symphysis pubis illustrating the two axis (ventral-dorsal and cephalad-caudad) of displacements reflect PFM functions of squeezing the urethra and supporting the bladder respectively. (b) Corresponding timing of the movement of tissues in pelvic floor during the onset of a typical PFM contraction of an asymptomatic volunteer. (c) Timing of the relaxation of PFM to original position. Sequence takes approximately 6.4 seconds. Adapted from [33]
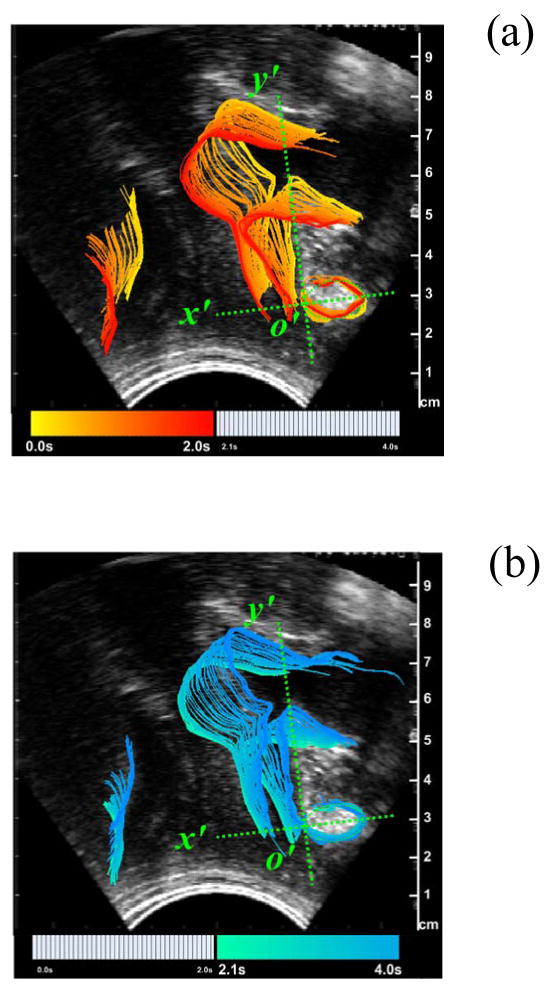
Intuitively the direction of movement in a Valsalva is in the opposite direction compared to the PFM contraction illustrated by Figure 4. Thus, Figure 5a shows the timing of the movement of tissues in pelvic floor during a typical Valsalva. As the volunteer performs this forced expiration technique, the urogenic structures move from their resting position in a dorsal-caudal direction (down and back) before returning to their resting position as the volunteer completes the maneuver.
Figure 5.
Displacement of ARA consequent to a 4 second Valsalva sequence. (a) Onset of Valsalva showing the downward movement for the 2 seconds. (b) Termination of Valsalva showing the restoration of outlined structures to their original state. Adapted from [33]
Motion tracking
Figure 4 demonstrates that the influence of PFM contraction can be identified and from the outlines of structures can are now amenable to analysis that can form the foundation of PFM dynamics. As such, we are enabled to be further parameterize and generate values that potentially can characterize the dynamics of PFM. A readily visible, clearly resolved anatomical structure in perineal ultrasound imaging is the angle the rectal ampulla forms with the anal canal, the ano-rectal angle (ARA). The movement of ARA can be used to analyze PFM function because the sling of the PFM or Levator Ani muscles wrap around the anorectal junction, and its displacement is closely associated with a PFM contraction [7,15]. The utility of motion tracking of the ARA from perineal ultrasonography was demonstrated by Peng et al 2008 using data obtained of 22 asymptomatic females and 9 SUI subjects with a broad age distribution and parity was. Figure 6 illustrates the differences between continent and incontinent women in the magnitude and ventral-dorsal and cranio-caudal displacement during a cough. As clearly indicated by Figure 6, both the direction and temporal sequence of the ARA movement are distinctly different between the continent and incontinent women. During a cough, in continent women the ARA moves ventrally towards the SP. In incontinent women the ARA moves dorsally away from the symphisis. Furthermore, the amplitude of the maximum caudal movement of the incontinent women’ ARA and urethrovesical junction are significantly larger than those of the continent women.
Figure 6.
Characteristic patterns of quantitative data generated by processing and analyzing the response of the pelvic floor. Data generated from the evaluation of assymptomatic and SUI subjects investigated and have been reported by [27, 33]
Figure 6 summarizes the parameters generated from imaging of asymptomatic and SUI subjects On the strength of these studies outlined above, we suggest that by using non invasive ultrasound imaging, we can translate our methodology from the evaluation of the PF of asymptomatic and incontinent subjects to include a generalized assessment of pelvic floor dysfunction. In deriving physiological values from the imaging procedures it is now possible to make comparisons between different dysfunctional conditions using new parameters in a statistically robust way. As Figure 6 shows, the biomechanical and timing parameters generated as a result of reflex contractions can be calculated where the internal displacement produced and associated biomechanical strain in structures contained by PFM can generate the basis of a model a mechanical model. One important parameter of relevance is muscle strain ε and strain rate was evaluated by Rachmanian et al using data from the displacement measurements shown by Figure 6 [33]. The biomechanical results comparing of Maximun strain of the PFM in healthy and SUI women show that strain as a parameter is significantly different between the two groups −0.088 ± 0.007 via 0.041 ± 0.002. Furthermore, consideration of strain rate during reflex contraction shows the maximum strain rate in incontinent women is significantly higher than that of continent subjects, incontinent women having a strain rate that is 716.4 ± 73.3 % higher than controls. Clearly, dynamic visualization of the PFM studies provides an abundance of functional information that occurs so swiftly, it cannot be captured by the observer let alone be quantified. In particular reflex phenomena, the guarding reflex in this case, incorporates multiple physiological processes whose timing and convergence cannot be simultaneously assimilated by the visual observation of images alone. It is therefore essential to process dynamic sequences of image in order to derive obtainable physical parameters. Inevitably in using the term “strain” a number of limitations and assumptions are made that are inherent to the derivation of geometrical parameters from imaging. Principal among these is that the pelvic floor muscle is lined up parallel to the plane of the image, deformation takes place along the defined attachment points, and t reference lengths needs to be determined in a geometrically correct alignment. Practically it is important to be aware of these assumptions are appropriate to keep in mind that these could and could vary. Thus, in considering the analysis and interpretation of our results it would be more accurate to describe the data as relative displacements between two anatomical landmarks rather than specific strain measurements of pelvic floor muscle. It is also important to be vigilant in documenting differences in the “resting position” and consider the magnitude and consistency of these limitations. In our current analysis we are attempting to overcome these limitations by examining the differences in displacements made between maximum contractions which is a forward/upward movement in association with the Valsalva which is a downward/backward movement. Using the fixed reference of the suprapubic line (SP) we are interpolating the “apparent resting position” as the basis in motion tracking.
In summary we quantitatively demonstrated some of the characteristic responses of cough induced contractions on urethral closure using urodynamics and imaging in relation to magnetic resonance and ultrasound imaging. In particular, evidence on of these observations is provided from a more resent analysis using imaging the ARA response to the cough reflex as well as voluntary contractions of asymptomatic women. Evidence was provided to show that the status of continent and asymptomatic women can be clearly identified from those with incontinence on the basis of a number of parameters such as the displacement, velocity and acceleration of the ARA movement. Furthermore because these studies were obtained using the non-invasive nature of trans-perineal ultrasound, the value of the results is enhanced in the study of the population targeted by this application. As a consequence of the degree of the analytical treatment applied to the temporal sequence of the data, important aspects of the role of the PFM in general function gained may prove critical in identifying the physiological impact of the variety of PF dysfunctions. As described, the data obtained by each maneuver will inevitably yield a large number of parameters that represent the neuromuscular characteristics of each individual subject, of a given disposition. It is encouraging that so much useful information can be generated from a test having the minimum of invasion and takes so little time to perform.
While quantitative measures, and analysis of the movement during PFM maneuver yielded new parameters of PF function, confidence is still required to validate the ultrasound approach with subjects having a broader age group and pathology. While visualization and analysis of PF activities using 2D ultrasound imaging is likely to develop further, new measures of the PFM functions that are more sensitive and specific than current methods should be pursued particularly with the availability of 3D ultrasound imaging. In this way more objective ways can be found to categorize different sub groups of patients within a particular pathology and determining the most appropriate treatment intervention and its effects. Ultimately it would be fruitful to translate this approach to become a more widespread, non-invasive, time saving and clinically validated methodology in the study of not only pelvic floor function defined above but also expand our knowledge in terms of parameters such as blood flow. To this end significant progress has already been reported by Noguti et al who have shown using doppler visualization that the vascularization of the levator ani decreases in an age dependent way [34]. Taking these observations into account it may be possible to explain some of the variations in the dynamic parameters observed in these in the context of blood flow.
It is expected that by understanding the processes and, the mechanisms involved in the functioning of the PF we can better identify more sensitive clinical diagnoses and have treatment outcomes in the management of incontinence. In the future, it is anticipated that as the imaging and urodynamic technology develops these techniques will be applicable to study other groups with PFD, such as prolapse, pelvic pain, vulvodynia and sexual dysfunction.
Acknowledgments
This work was funded in part by NIH, grant #1R21 EB001654 and is the result of studies supported with resources and the use of facilities at the Palo Alto VA Medical Center, Palo Alto, California, USA. Author would also like to acknowledge the contributions of, C Payne, Q Peng, R Jones, V Wolfe, and LL Christensen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeLancey JO. The hidden epidemic of pelvic floor dysfunction: achievable goals for improved prevention and treatment. Am J Obstet Gynecol. 2005;192:1488–95. doi: 10.1016/j.ajog.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 2.DeLancey JO, Strohbehn K, Aronson MP. Comparison of ureteral and cervical descents during vaginal hysterectomy for uterine prolapse. Am J Obstet Gynecol. 1998;179:1405–8. doi: 10.1016/s0002-9378(98)70002-x. discussion 1409–10. [DOI] [PubMed] [Google Scholar]
- 3.Abitbol MM. Evolution of the ischial spine and of the pelvic floor in the Hominoidea. Am J Phys Anthropol. 1988;75:53–67. doi: 10.1002/ajpa.1330750107. [DOI] [PubMed] [Google Scholar]
- 4.Bo K, Sherburn M. Evaluation of female pelvic-floor muscle function and strength. Phys Ther. 2005;85:269–82. [PubMed] [Google Scholar]
- 5.Morin M, Bourbonnais D, Dumoulin C, Lemieux M-C. Pelvic floor muscle function in continent and stress urinary incontinent women using dynamometric measurements. Neurourol Urodyn. 2004;23:668–74. doi: 10.1002/nau.20069. [DOI] [PubMed] [Google Scholar]
- 6.Berghmans LC, Hendriks HJ, DeBie RA, vanWaalwijk van Doorn ES. Conservative treatment of stress urinary incontinence in women: a systematic review of randomized clinical trials. Br J Urol. 1998;82:181–91. doi: 10.1046/j.1464-410x.1998.00730.x. [DOI] [PubMed] [Google Scholar]
- 7.Norton C. Chelvanayagam S, Wilson-Barnet J, Redfwrn S, Kamm MA. Randomized controlled trial of biofeedback for fecal incontinence Gastroenterology. 2003;125:1320–9. doi: 10.1016/j.gastro.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 8.Nygaard I. Physiologic outcome measures for urinary incontinence. Gastroenterology. 2004;126:S99–105. doi: 10.1053/j.gastro.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Constantinou CE, Hvistendahl G, Ryhammer A, Nagel LL, Djurhuus JC. Determining the displacement of the pelvic floor and pelvic organs during voluntary contractions using magnetic resonance imaging in younger and older women. BJU Int. 2002;90:408–14. doi: 10.1046/j.1464-410x.2002.02907.x. [DOI] [PubMed] [Google Scholar]
- 10.Christensen LL, Djurhuus JC, Constantinou CE. Imaging of pelvic floor contractions using MRI. Neurourol Urodyn. 1995;14:209–16. doi: 10.1002/nau.1930140302. [DOI] [PubMed] [Google Scholar]
- 11.Constantinou CE. Urethral Pressure in the evaluation of female incontinence. In: O’Donnell, editor. URINARY INCONTINENCE. 1997. pp. 82–93. [Google Scholar]
- 12.Constantinou CE, Omata S. Direction Sensitive Sensor Probe for the Evaluation of Voluntary and Reflex Pelvic Floor Contractions. Neurourol & Urodyn. 2007;26:386–91. doi: 10.1002/nau.20263. [DOI] [PubMed] [Google Scholar]
- 13.Peng Q, Jones R, Shishido K, Constantinou CE. Ultrasound evaluation of dynamic responses of female pelvic floor muscles. Ultrasound Med Biol. 2006;34(3):477–493. doi: 10.1016/j.ultrasmedbio.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shishido K, Peng Q, Jones R, Omata S, Constantinou CE. Influence of Pelvic Floor Muscle Contraction onthe Profile of Vaginal Closure Pressures of Continent and Stress Urinary Incontinent Women. J Urol. 2008;179:1917–22. doi: 10.1016/j.juro.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Costantini S, Esposito F, Nadalini C, Morano S, Lantieri P, Mistrangelo E. Ultrasound imaging of the female perineum: the effect of vaginal delivery on pelvic floor dynamics. Ultrasound Obstet Gynecol. 2006;27(2):183–7. doi: 10.1002/uog.2663. [DOI] [PubMed] [Google Scholar]
- 16.Miller JM, Ashton-Miller JA, DeLancey JO. A pelvic muscle pre-contraction can reduce cough-related urine loss in selected women with mild SUI. J Am Geriatr Soc. 1998;46:870–4. doi: 10.1111/j.1532-5415.1998.tb02721.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller JM, Perucchini D, Carcchidi LT, DeLancey Pelvic floor muscle contraction during a cough and decreased vesical neck mobility. Obstet Gynecol. 2001;97:255–60. doi: 10.1016/s0029-7844(00)01132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietz HP. Ultrasound imaging of the pelvic floor. Part II: three-dimensional or volume imaging. Ultrasound Obstet Gynecol. 2004;23:615–25. doi: 10.1002/uog.1072. [DOI] [PubMed] [Google Scholar]
- 19.Dietz HP, Jarvis SK, Vancaillie TG. The assessment of levator muscle strength: a validation of three ultrasound techniques. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:156–9. doi: 10.1007/s192-002-8346-5. discussion 159. [DOI] [PubMed] [Google Scholar]
- 20.Bump RC, Hurt WG, Fantl JA, Wyman JF. Assessment of Kegel pelvic muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol. 1991;165:322–7. doi: 10.1016/0002-9378(91)90085-6. discussion 327–9. [DOI] [PubMed] [Google Scholar]
- 21.Deindl FM, Vodusek DB, Hesse U, Schussler B. Activity patterns of pubococcygeal muscles in nulliparous continent women. Br J Urol. 1993;72:46–51. doi: 10.1111/j.1464-410x.1993.tb06455.x. [DOI] [PubMed] [Google Scholar]
- 22.Deindl FM, Vodusek DB. Pelvic floor activity patterns: comparison of nulliparous continent and parous urinary stress incontinent women. A kinesiological EMG study. Br J Urol. 1994;73:413–7. doi: 10.1111/j.1464-410x.1994.tb07606.x. [DOI] [PubMed] [Google Scholar]
- 23.Constantinou CE, Govan DE. Spatial distribution and timing of transmitted and reflexly generated urethral pressures in healthy women. J Urol. 1982;127:964–9. doi: 10.1016/s0022-5347(17)54148-8. [DOI] [PubMed] [Google Scholar]
- 24.Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil. 1999;80:1005–12. doi: 10.1016/s0003-9993(99)90052-7. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira PH, Ferreira ML, Hodges PW. Changes in recruitment of the abdominal muscles in people with low back pain: ultrasound measurement of muscle activity. Spine. 2004;29:2560–6. doi: 10.1097/01.brs.0000144410.89182.f9. [DOI] [PubMed] [Google Scholar]
- 26.Sapsford RR, Hodges PW, Richardson CA, Cooper DH, Markwell SJ, Jull GA. Co-activation of the abdominal and pelvic floor muscles during voluntary exercises. Neurourol Urodyn. 2001;20:31–42. doi: 10.1002/1520-6777(2001)20:1<31::aid-nau5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 27.Peng Q, Jones RC, Constantinou CE. 2D Ultrasound image processing in identifying responses of urogenital structures to pelvic floor muscle activity. Ann Biomed Eng. 2006;34:477–93. doi: 10.1007/s10439-005-9059-3. [DOI] [PubMed] [Google Scholar]
- 28.Schaer GN, Perucchini D, Munz E, Koechli OR, DeLancey JO. Sonographic evaluation of the bladder neck in continent and stress-incontinent women. Obstet Gynecol. 1999;93:412–6. doi: 10.1016/s0029-7844(98)00420-7. [DOI] [PubMed] [Google Scholar]
- 29.Howard D, Miller JM, Delancey JO, Ashton-Miller JA. Differential effects of cough, Valsalva, and continence status on vesical neck movement. Obstet Gynecol. 2009;95:535–40. doi: 10.1016/s0029-7844(99)00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pregazzi R, Sartore A, Bortoli P, Grimaldi E, Ricchi G, Guashino S. Perineal ultrasound evaluation of urethral angle and bladder neck mobility in women with stress urinary incontinence. BJOG. 2002;109:821–7. doi: 10.1111/j.1471-0528.2002.01163.x. [DOI] [PubMed] [Google Scholar]
- 31.Reddy AP, DeLancey JO, Zwica LM, Ashton-Miller JA. On-screen vector-based ultrasound assessment of vesical neck movement. Am J Obstet Gynecol. 2001;185:65–70. doi: 10.1067/mob.2001.116373. [DOI] [PubMed] [Google Scholar]
- 32.Omata S, Yoshimura Y, Qiyu P, Constantinou CE. Reproductive Biomechanics. ANNALS OF THE NEW YORK; ACADEMY OF SCIENCES: 2007. Evaluation of the Dynamic Responses of Female Pelvic Floor Using a Novel Vaginal Probe. [DOI] [PubMed] [Google Scholar]
- 33.Rahmanian S, Jones R, Peng Q, Constantinou CE. Visualization of Biomechanical Properties Of Female Pelvic Floor Function Using Video Motion Tracking of Ultrasound Imaging Stud Health. TECHNOLOGY AND INFORMATICS. 2008;132:390–395. [PubMed] [Google Scholar]
- 34.Noguti AS, Jarmy-Di Bella, ZIK Oliviera RA, Castro RA, Lima CB, Sartori MGF, Girao MJBC. Ultrasonographic and dopler velometric evaluation of the levator ani muscle according to the hormonal status. Eur J Obstet & Gynecol. 2008 doi: 10.1016/j.ejogrb.2008.06.008. [DOI] [PubMed] [Google Scholar]