Abstract
In many organisms, including yeasts and humans, meiotic recombination is initiated preferentially at a limited number of sites in the genome referred to as recombination hotspots. Predicting precisely the location of most hotspots has remained elusive. In this study, we tested the hypothesis that hotspots can result from multiple different sequence motifs. We devised a method to rapidly screen many short random oligonucleotide sequences for hotspot activity in the fission yeast Schizosaccharomyces pombe and produced a library of ∼500 unique 15- and 30-bp sequences containing hotspots. The frequency of hotspots found suggests that there may be a relatively large number of different sequence motifs that produce hotspots. Within our sequence library, we found many shorter 6- to 10-bp motifs that occurred multiple times, many of which produced hotspots when reconstructed in vivo. On the basis of sequence similarity, we were able to group those hotspots into five different sequence families. At least one of the novel hotspots we found appears to be a target for a transcription factor, as it requires that factor for its hotspot activity. We propose that many hotspots in S. pombe, and perhaps other organisms, result from simple sequence motifs, some of which are identified here.
MEIOSIS is a form of cell division common to all sexually reproducing organisms. It differs from mitosis in that two cell divisions follow a single round of DNA replication, resulting in four haploid cells known as gametes or spores. The first division of meiosis differs from mitosis in that paternal and maternal homologs segregate to opposite poles, reducing by half the number of chromosomes in the resulting gametes. Prior to the first division, homologous chromosomes recombine with each other at a greatly elevated frequency compared to mitosis (Esposito and Wagstaff 1981). This recombination serves at least two important functions. First, it forms crossovers (chiasmata) between chromosomes, which are required in most organisms for the proper segregation of homologous chromosomes (Baker et al. 1976). Second, the random shuffling of maternal and paternal alleles at each generation increases genetic diversity, which enhances the ability of a species to adapt to its environment through natural selection.
Meiotic recombination events are not distributed evenly throughout the genome of most organisms. Rather, they occur at high frequency at some sites and low frequency at others. Sites that recombine at a frequency significantly higher than the genomic average are known as hotspots. These hotspots coincide with the formation of DNA double-strand breaks (DSBs) in both the fission and budding yeasts and likely many other organisms (Sun et al. 1989; Cao et al. 1990; Fan et al. 1995; Cervantes et al. 2000; Mahadevaiah et al. 2001; Petes 2001; Steiner et al. 2002; Young et al. 2002; Cromie et al. 2007). Formation of DSBs requires a number of different proteins, including Spo11 (Rec12 in Schizosaccharomyces pombe), a widely conserved protein among eukaryotes that has the active site for cleaving the phosphodiester backbone (Keeney et al. 1997; Cervantes et al. 2000; Malik et al. 2007). After the formation of DSBs, the two broken ends of the DNA initiate recombination by invading intact homologous DNA to form joint molecules, which can be resolved to produce both crossover and noncrossover exchanges (Pâques and Haber 1999).
DNA breaks occur at preferred genomic positions during meiosis, but the factors determining the positions of most break sites are not clearly understood. A global analysis of the distribution of DSB sites in the budding yeast Saccharomyces cerevisiae showed that most breaks occur in 5-kb regions where the GC content exceeds the average GC content for the genome (Gerton et al. 2000), but the causal relation, if any, between elevated GC content and DSB formation is unknown. In addition, DSBs occur primarily in intergenic regions (IGRs) (Baudat and Nicolas 1997; Gerton et al. 2000), which supports the possibility that many hotspots are associated with the binding of transcription factors. Indeed, several well-characterized hotspots are known to require transcription factor binding (White et al. 1993; Kon et al. 1997; Petes 2001; Mieczkowski et al. 2006).
Like S. cerevisiae, the fission yeast S. pombe shows a nonrandom distribution of meiotic DSBs (Cervantes et al. 2000; Young et al. 2002). A genomewide analysis of the distribution of DSBs in S. pombe revealed that DSBs occur primarily in intergenic regions, particularly in exceptionally large intergenic regions (Cromie et al. 2007). In fact, it was noted that large IGRs are strongly predictive of DSBs. The median IGR size in S. pombe is 0.7 kb (Wood et al. 2002), but approximately half of all prominent DSBs occur in IGRs >3 kb, and 44% of IGRs >3 kb contain prominent DSBs (Cromie et al. 2007). However, the basis for the association of DSBs and IGRs remains unclear. One possibility is that some IGRs contain specific sequence motifs that create recombination hotspots whereas others do not.
One simple sequence motif, ATGACGTCA, known as M26 or CRE (cyclic-AMP response element), acts as a recombination hotspot at multiple sites in the S. pombe genome. These sites include both IGRs and protein-coding regions, for example, the ade6 gene where this hotspot motif was originally discovered (Gutz 1971; Fox et al. 1997; Steiner and Smith 2005a). The CRE sequence is a binding site for a heterodimeric transcription factor, Atf1-Pcr1, which is required for activity of the hotspot (Kon et al. 1997). Since the CRE hotspot can predict only a small fraction of the DSBs in the S. pombe genome (Cromie et al. 2007 and W. Steiner, unpublished observation), we hypothesized that there may be other simple sequence motifs that contribute to recombination hotspots. The results shown in this study confirm that hypothesis.
MATERIALS AND METHODS
Strains and genetic procedures:
All strains used in this study with assigned ade6 allele numbers are shown in Table 1. The ade6-4001 allele was generated by amplification of the kanMX6-ura4+ construct contained in the plasmid pura4-kanMX6 (Steiner and Smith 2005b). This construct was amplified by PCR using oligonucleotides with 80 base 5′ extensions homologous to the ade6 insertion site (Table 1). The resulting PCR product was used for linear transformation of strain WS121 selecting for uracil prototrophy. Transformants were confirmed by resistance to G418, sequencing, and Southern blot hybridization.
TABLE 1.
Strains
| ade6 allele | Mating type | Description or sequencea | Base pairsb | Motifc | Straind |
|---|---|---|---|---|---|
| + | h90 | NA | WS121 | ||
| 52 | h− | G796Ae | NA | WS3 | |
| M375 | h90 | TGAGGACGTGAGf | 133–144 | NA | WS135 |
| M26 | h90 | GGATGACGTGAGf | 133–144 | CRE | WS136 |
| M26 | h+ | his7-366 | WS322 | ||
| M26 | h+ | his7-366 php2Δ∷kanMX6g | WS310 | ||
| M26 | h+ | his7-366 php3Δ∷kanMX6g | WS324 | ||
| M26 | h+ | php5Δ∷kanMX6g | WS295 | ||
| 469 | h− | C1468Tf | NA | WS315 | |
| 469 | h− | php2Δ∷kanMX6 | WS313 | ||
| 469 | h− | php3Δ∷kanMX6 | WS317 | ||
| 469 | h− | php5Δ∷kanMX6 | WS311 | ||
| 3074 | h90 | GGATGACGTCAGh | 133–144 | CRE | WS137 |
| 4001 | h90 | Δ(-162-451)∷kanMX6-ura4+ | — | NA | WS129 |
| 4002 | h90 | CCAATCAi | 129–134 | 7-7 | WS224 |
| 4002 | h+ | his7-366 | WS326 | ||
| 4002 | h+ | his7-366 php2Δ∷kanMX6 | WS328 | ||
| 4002 | h+ | his7-366 php3Δ∷kanMX6 | WS320 | ||
| 4002 | h+ | his7-366 php5Δ∷kanMX6 | WS293 | ||
| 4003 | h90 | A__CATGACATCAT | 148–160 | 6-6/8-1 | WS330 |
| 4003 | h− | WS182 | |||
| 4005 | h90 | ACGTAA_T | 138–145 | 7-1 | WS149 |
| 4006 | h90 | CGTCATAi | 149–155 | 7-15 | WS150 |
| 4008 | h90 | TATTACGTAAT | 160–168 | 6-1 (Con) | WS331 |
| 4008 | h− | WS184 | |||
| 4009 | h90 | ATAGCGTCATATACT | 152–165 | 7-15 (Con) | WS332 |
| 4009 | h− | WS186 | |||
| 4010 | h90 | GATGACATAA | 151–159 | 6-21 (Con) | WS365 |
| 4010 | h− | WS188 | |||
| 4071 | h90 | ATGATGTCACi | 152–161 | 7-2 (Con) | WS237 |
| 4072 | h90 | ACCCCGCACGCA | 167–177 | 7-4 (Con) | WS240 |
| 4073 | h90 | ACGGCCCCCA_CAATTi | 126–141 | 7-31 (Con) | WS241 |
| 4094 | h90 | GGATGTAAGTj | 130–139 | 10-1 | WS374 |
| 4095 | h90 | GGTCTGGACCj | 130–139 | 10-2 | WS376 |
| 4096 | h90 | GATGACATCAj | 130–139 | 8-1 | WS378 |
| 4099 | h90 | TGAACCCCGCACTGAj | 129–143 | 7-4 (Con) | WS382 |
| 4100 | h90 | GCCCCCACAj | 132–140 | 7-31 (Con) | WS384 |
| 4101 | h90 | ACCCCGCACGTAAT | 167–179 | 7-4 | WS386 |
| 4102 | h90 | ATGGCCCCCA_CTATTi | 126–141 | 7-31 | WS394 |
| 4103 | h90 | TGACCCCGCACGTj | 130–142 | 7-4 | WS409 |
| 4104 | h90 | TGGCCCCCACTATj | 130–142 | 7-31 | WS410 |
Bases are numbered starting at the first nucleotide of the ade6 open reading frame. Bases in regular type indicate wild-type sequence. Boldface type indicates base substitutions. Insertions or deletions are underlined. For any given ade6 allele, the relevant sequence is shown only once. Additional genotypic information for strains containing the same allele of ade6 is also indicated in this column.
The nucleotide positions of the ade6 sequence shown in the third column. Numbering indicates wild-type sequence before insertions or deletions.
Indicates the motif (Table 2, Table S2a, and Table S3) on which the allele is based. NA, not applicable. Con, consensus sequence (Figure S1).
In addition to the ade6 mutations shown in the third column, all strains also contain ura4-D18 and leu1-32, except for WS3 (ura4+ leu1+). All homothallic strains (h90) also contain the plasmid pWS35 (Figure 1).
M. Fox and G. Smith, personal communication.
Complement strand shown.
These strains also contain a closely linked nonsense mutation, A121T.
The plasmid pWS35 was constructed by PCR amplification of a 613-bp fragment of ade6 with primers 5′-NNNNNCTCGAGCTTGGAAATGTAACGATGAC-3′ and 5′-NNNNNCTCGAGTAAGCCAATGTTTTACTTTTCAG-3′, containing XhoI restriction sites near their 5′ ends. (The additional random bases at the 5′ ends were added to permit efficient cleavage by XhoI.) The resulting PCR product was digested with XhoI and ligated into the XhoI site of pSP1 (Cottarel et al. 1993), producing pWS35. This plasmid contains the S. cerevisiae LEU2 gene as a selectable marker. The 613-bp ade6 fragment of pWS35 is the same fragment deleted in the ade6-4001 allele (Table 1).
Growth and sporulation medium have been previously described (Gutz et al. 1974; Steiner and Smith 2005a,b). Heterothallic strains were grown in rich medium supplemented with adenine (A), uracil (U), leucine, histidine, and lysine (YEL-5S). Strains containing the plasmid pWS35 were grown in minimal medium supplemented with adenine and uracil (NBL-AU). Crosses between heterothallic strains were performed on SPA-5S, and homothallic crosses were performed on SPA-AU. Crosses were incubated for 2 days at 25° before harvesting and analysis as described (Steiner and Smith 2005b). Any given cross was performed a minimum of three times for determination of recombination frequencies.
Screen for hotspots:
WS129 was grown overnight in NBL-A to 0.5-1 × 107 cells/ml and transformed using a lithium acetate-mediated transformation procedure (Bähler et al. 1998). Transforming DNA consisted of a 1.6-kb ade6 fragment containing a 15- or 30-bp random sequence substitution from nucleotides 125–139 or 125–154, respectively (nucleotides from start of ade6 open reading frame). Both substitutions also include an A→T substitution at nucleotide 121, which produces a stop codon and insures that all transformants are adenine auxotrophs. The linear DNA used for transformation was generated by overlap extension PCR (Vallejo et al. 1995). The primers used were oWS202: ACGAACATCATTAAGCGCGAAGCG, oWS203: ACGCATGAGTTGTGGAAGTCGAGA, oWS208: GTTAGGCAGGAGAATTTGCTGCA, oWS209: TGCAGCAAATTCTCCTGCCTAACNNNNNNNNNNNNNNNNNNNNNNNNNNNNNNCATCATTTACTGACCCCGATGCAATTG, and oWS241: TGCAGCAAATTCTCCTGCCTAACNNNNNNNNNNNNNNNGTGAGCACATTGATGCATCATTTAC. The product of oWS202 and oWS208 was combined with the product of oWS209 (or 241) and oWS203 for the overlap extension reaction. All PCR products were performed with the high-fidelity polymerases Vent (New England Biolabs) or Pfx (Invitrogen), using conditions recommended by the manufacturers. A plasmid clone of the ade6 gene (pAS1; Szankasi et al. 1988) was used as the starting template for PCR reactions. Approximately 5 μg of linear ade6 DNA was used per 108 cells transformed.
Following transformation, cells were plated on NBA-AU (2–4 × 107 cells/plate) and incubated at 32° for 2 days. Those plates were replica plated to NBA-AU containing 1 mg/ml 5-FOA (Toronto Research Chemicals) to select for cells with ade6 replacements. After 3 days growth, individual colonies became visible. These plates were replica plated a second time to NBA-AU + 5-FOA to reduce background growth. After 4 days growth at 32°, colonies were replica plated to SPA-AU and incubated 2 days at 25° to induce meiosis. Those colonies were then exposed to acetone vapor (Egel 1977) to kill remaining vegetative cells, replica plated to YEA-4S and incubated 3 days at 32°. Red colonies showing many white papillae were picked and streaked for single colonies to NBA containing uracil and limiting adenine (10 μg/ml) to distinguish ade6− colonies from ade6+ recombinants. Red colonies from each streak were patched in a grid pattern to NBA-AU along with 5 control strains: WS129, WS3, WS135, WS136, and WS137 (Table 1). After 2 days growth at 32°, those patches were replica plated to three different media:
SPA-AU to confirm the original meiotic hotspot phenotype as described above and qualitatively estimate the activity of each hotspot relative to ade6-M375, ade6-M26, and ade6-3074 by comparing the density of white papillae. The heterothallic strain, WS3, which cannot sporulate, served as a control for the effectiveness of the subsequent acetone treatment for this step.
YEA-4S to test for a possible mitotic hotspot phenotype. Strains containing a mitotic hotspot should show many white papillae prior to undergoing meiosis (only one strain with a potential mitotic hotspot was found).
YEA-5S containing 100 μg/ml G418 to confirm homologous replacement of the ade6 gene (Storici et al. 2001). Homologous gene replacement results in the simultaneous loss of both the ura4+ and kanMX6 markers found in the ade6-4001 allele, resulting in both resistance to 5-FOA and sensitivity to G418.
Sequence analysis:
G418-sensitive strains that showed an obviously higher density of white papillae than an ade6-M375 control strain were allowed to lose the plasmid, pWS35, by two successive nonselective streaks onto YEA-4S. Plasmid-free derivatives were identified by the inability to grow in the absence of leucine and used for preparation of genomic DNA, which was used as a template for PCR amplification of ade6 using primers oWS202 and oWS203 (above). PCR products were sequenced by the High Throughput Genomics Unit (University of Washington, Seattle).
The sequence substitutions in ade6 were analyzed for common 6- to 10-bp motifs using YMF3.0 (Sinha and Tompa 2002, 2003) (http://wingless.cs.washington.edu/YMF/YMFWeb/YMFInput.pl) and MEME (Bailey et al. 1996) (http://meme.nbcr.net/meme/intro.html).
Sequence randomization was accomplished by using an algorithm (Pearson and Lipman 1988) available through the San Diego Super Computer Center (http://workbench.sdsc.edu/).
Reconstruction of specific motifs:
Specific motifs were reconstructed in the ade6 gene by overlap-extension PCR (Vallejo et al. 1995) using inner primers containing the desired mutations and outside primers oWS202 and oWS203 (above). All reconstructed motifs listed in Table 1 were confirmed by sequencing and Southern blot hybridization.
RESULTS
The screen for recombination hotspots:
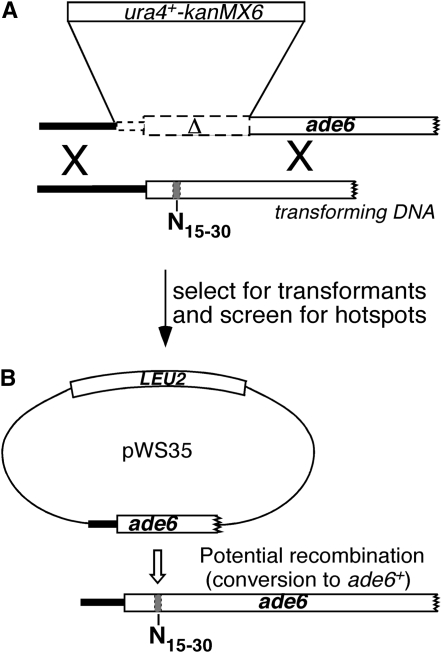
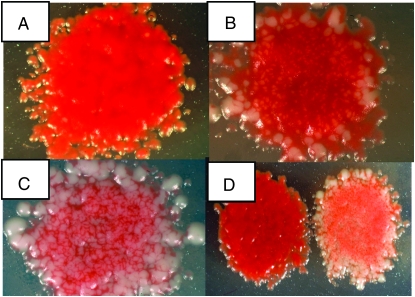
Screening for sequence-dependent recombination hotspots from a large pool of random sequences required a means of rapidly identifying potential candidates. We therefore took advantage of the fact that strains containing mutations in the ade6 gene produce red-colored colonies on medium containing limiting quantities of adenine (Gutz 1971), whereas ade6+ strains form white colonies on the same medium. As shown in Figure 1, strain WS129 was transformed with ade6 DNA containing either a 15- or 30-bp random nucleotide sequence substitution. Transformed cells were selected by loss of the ura4+ gene inserted into ade6 (resistance to 5-FOA) and homologous gene replacement was later confirmed by the simultaneous loss of the kanMX6 gene (G418 sensitivity; Storici et al. 2001). After transformed cells formed colonies on 5-FOA medium, they were replica plated to sporulation medium, producing colonies containing mostly spores. Following treatment with acetone vapor to kill remaining unsporulated cells (Egel 1977), these colonies were replica plated to growth medium containing limiting quantities of adenine. (See materials and methods for full details of the screen.) Since the cells carry a plasmid containing a fragment of the ade6 gene, recombination between chromosome and plasmid can produce ade6+ spores during meiosis. Plasmid × chromosome recombination has previously been shown to accurately reflect chromosome × chromosome recombination in S. pombe (Ponticelli and Smith 1989). These recombination events can be seen at this stage as white papillae in otherwise red colonies. Strains with no recombination hotspot in ade6, for example ade6-M375 (Gutz 1971), produce red colonies with few white papillae, whereas strains containing a recombination hotspot, such as ade6-M26 or ade6-3074 (Steiner and Smith 2005b), produce red colonies with a large number of papillae (Figure 2). Those colonies are easily distinguished from neighboring colonies, most of which resembled ade6-M375 in our screen.
Figure 1.—
Method for screening a large number of unique 15- or 30-bp sequences for hotspot activity. (A) A strain (WS129) with a partial ade6 deletion and ura4+-kanMX6 insertion and plasmid pWS35 (not shown) is transformed with linear ade6 DNA containing a 15- or 30-bp random sequence substitution (shaded bar). (B) Homologous recombinants lose the ura4+-kanMX6 marker and now share homology to a fragment of ade6 carried on the plasmid pWS35. After transfer to sporulation medium, these cells will self-mate and form spores. Strains containing a hotspot in the random sequence region will recombine with the plasmid at high frequency (open arrow) to form ade6+ spores, which can be identified as white papillae on red colonies after replica plating to the appropriate medium (Figure 2). Although this figure implies noncrossover conversion to ade6+, crossover recombinants would also likely produce ade6+ spores, because the plasmid carries 164 bp of the ade6 promoter, which is adequate for ade6 expression (Zahn-Zabal et al. 1995). ade6 genes and fragments are drawn to scale; other genes and constructs are not. Open rectangles represent open reading frames.
Figure 2.—
Visual assay for hotspots. Strains containing known hotspot alleles (ade6-M26 or -3074) and one control allele (ade6-M375) were put through the steps of the screen described in the text. (A) ade6-M375, (B) ade6-M26, (C) ade6-3074, (D) ade6-M375 (left), and ade6-3074 (right). A larger version of this figure is shown in Figure S2.
We screened ∼27,600 colonies each containing a 30-bp random sequence and 18,300 colonies each containing a 15-bp random sequence. Among the 30-bp and 15-bp random sequences, we identified 393 and 102 strains, respectively, showing recombination frequencies obviously higher than ade6-M375. This number includes only strains capable of plasmid loss (see materials and methods), since plasmid integration, particularly if it occurs within the ade6 gene itself, might create a recombination hotspot unrelated to any specific sequence (Virgin et al. 1995). The higher frequency of hotspots among the 30-bp random sequences (1.4%) than the 15-bp sequences (0.6%) is consistent with the expectation that longer sequences have a greater chance of containing any given sequence motif. The complete list of hotspot-containing sequences and their relative activities is shown in supporting information, Table S1.
To confirm that the hyper-rec phenotype observed in our transformed strains was due to the sequence substitution in ade6, we crossed plasmid-free derivatives of the hyper-rec strains with the parent strain WS129. Spores from the cross that were 5-FOA resistant were tested for hotspot activity (papillation) by repeating the steps of the screen described above. In 36 randomly chosen strains, the hyper-rec phenotype showed complete linkage to ade6 among the 50–100 spores tested from each cross. That is, every spore containing the sequence substitution from each of the 36 crosses showed the hyper-rec phenotype. This result is strong evidence that the observed hotspot activity in those strains, and probably all of our hotspot strains, is almost certainly due to the sequence substitution within ade6 and not some other cause.
Common sequence motifs appear frequently:
Since it is unlikely that the entire 15- or 30-bp sequence substitution is required for hotspot activity in any of our hotspot-containing strains, we analyzed those sequences for common 6- to 10-bp motifs as described in materials and methods. The most common motifs found contained the 6-bp sequence TGACGT, referred to here as the CRE-core sequence (Table S2). This result demonstrates that our screen had, in fact, identified sequence-dependent recombination hotspots, as expected. However, the high frequency of the CRE motif was surprising since there was no prior reason to expect that the CRE hotspot would appear more frequently than other potential hotspot motifs. A likely interpretation of this result is that the CRE hotspot has the shortest sequence capable of creating a recombination hotspot, <7 bp in the region of ade6 utilized in our screen (Fox et al. 2000). Hotspots requiring a longer sequence than this would occur at lower frequency.
Since the goal of our screen was to identify novel hotspot motifs, we eliminated 97 sequences from our pool that contained the 6-bp CRE-core sequence, TGACGT, which is required for all known Atf1-Pcr1-dependent hotspots in S. pombe (Fox et al. 2000; Steiner and Smith 2005a). These strains accounted for almost 20% of our total sequence pool. We analyzed the remaining 398 sequences for common motifs as described above (Table 2 and Table S3). To determine the likelihood that any of the observed high-frequency motifs formed hotspots, we compared our results to an analysis of the same set of sequences following sequence randomization. Among random sequences, multiple occurrences of any motif would result from chance and not from any property of the motif, e.g., hotspot activity. For any given motif size, we found a significantly higher number of motifs among the actual sequences, i.e., those containing hotspots, compared to randomized sequences. For example, we found 17 different eight-base motifs that occurred four or more times among our pool of 398 sequences. Among random sequences, there were only four eight-base motifs that occurred at the same frequency and none that occurred more frequently (Table S3b). This result suggests that the majority of those motifs are hotspots, or perhaps form part of a larger hotspot.
TABLE 2.
Common motifs among hotspot sequences lacking CRE hotspot
| 10-base motif number and sequence | Counta | 8-base motif number and sequence | Count | 7-base motif number and sequence | Count | 6-base motif number and sequence | Count | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | GGATGTAAGT | 3 | 1 | TGACATCA | 6 | 1 | ACGTAAT | 14 | 1 | ACGTAA | 22 |
| 2 | GGTCTGGACC | 3 | 4 | CCAATGAG | 4 | 2 | ATGTCAC | 9 | 6 | ACATGA | 16 |
| 5 | AGAGCTCTb | 4 | 4 | CCCCGCA | 8 | 9 | AAAGAT | 15 | |||
| 6 | TCGGCCGAb | 4 | 7 | CCAATCA | 7 | 10 | GTATGA | 15 | |||
| 7 | AGACGCAG | 4 | 8 | CCCACCC | 7 | 45 | CTATTA | 11 | |||
| 8 | GTCTAGACb | 4 | 15 | CGTCATA | 6 | 57 | CATCCC | 10 | |||
| 9 | ATAATTGG | 4 | 31 | CCCCCAC | 5 | 21c | GATGAC | 18 | |||
| 10 | AACAGGCG | 4 | |||||||||
| 11 | ATTGGCGG | 4 | |||||||||
| 12 | AAGCATGA | 4 | |||||||||
| 13 | CGCAGTAA | 4 | |||||||||
| 21 | AATGGATA | 3 | |||||||||
| 22 | CCATTACG | 3 | |||||||||
| 41c | AGGGATGA | 4 | |||||||||
Some of the most common 6- to 10-base motifs among 398 sequences lacking the CRE core sequence, TGACGT. Only two motifs longer than 8-bp that occurred more than twice were found, 10-1 and 10-2. Only sequences tested for hotspot activity (Figure 3 and Table S4) are shown. Motif numbers correspond to those shown in Table S3a.
Number of times that a given motif is found among the 398 sequences lacking the CRE-core sequence, TGACGT.
A palindrome. Palindromes are counted twice, because they occur on both strands.
These motifs are found only among our total pool of sequences and are shown in Table S2a.
Confirmation of hotspot activity by motif reconstruction:
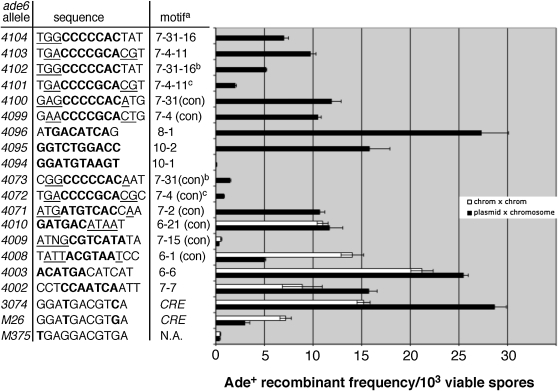
To test whether any of the high-frequency sequence motifs shown in Table 2 were hotspots, we reconstructed some of them in the ade6 gene by specific base-pair changes to the wild-type sequence, for example motifs 6-6, 8-1, 7-1, and 7-15. (The first number of each motif name refers to the length of the motif and the second number refers to the number in that series; Table 2 and Table S3). These experiments generated hotspots in some cases (ade6-4002, -4003, and -4096; Figure 3), but not in others (ade6-4005, 4006; Table 1 and data not shown). We also tested two 10-base motifs that occurred three times each among our pool of sequences (ade6-4094 and -4095; Figure 3), one of which, ade6-4095, produced a hotspot. A hotspot in this case is considered to be any allele that produces a significantly greater frequency of recombinants than ade6-M375 (P < 0.01; Student's t-test), a common control allele for the ade6-M26 hotspot (Gutz 1971).
Figure 3.—
Reconstructed sequence motifs produce hotspots. High-frequency sequence motifs (Table 2 and Figure S1) were reconstructed in the ade6 gene and tested for hotspot activity in homothallic crosses (plasmid pWS35 × chromosome; solid bars) or heterothallic crosses (chromosome × chromosome; open bars). Heterothallic strains were crossed with WS315 (ade6-469; Table 1). Each bar represents the average of at least three crosses ±1 SEM. Sequences show the original motif (Table 2; boldface type) with some flanking nucleotides (regular type). Consensus sequence nucleotides (Figure S1) are underlined when applicable. In this table, only ade6-4009 and ade6-4094 are not significantly more active than ade6-M375 (P > 0.01, Student's t-test). Notes: aThe motif (Table 2) on which the allele is based. Con, consensus sequence. NA, not applicable. Motifs with three numbers indicate reconstruction of a particular allele from a subscreen (Figure S1), e.g., 7-31-16 = sequence number 16 from subscreen of motif 7-31. bThese motifs are inverted relative to ade6-4100 and -4104. cThese motifs are located 36 bp downstream relative to ade6-4099 and -4103.
Motifs that occurred at high frequency but failed to produce hotspots in our reconstruction experiments (ade6-4005, -4006, and -4094) could be explained by either of two possibilities: (1) those motifs are simply not hotspots, that is, they occurred by chance (for example, see Table S3b), or (2) those motifs form only part of a larger sequence necessary for hotspot activity. Reasoning that the second possibility may be true for many motifs, we streamlined our tests for hotspot activity by first testing most of the motifs shown in Table 2 in a “subscreen.” Each subscreen involved repeating the original screen with a given motif flanked by several random nucleotides on one or both sides of the motif (Table S4). Transformants producing hotspots were sequenced and aligned to identify potential consensus sequences (Figure S1). Thus, many of the reconstructed motifs shown in Figure 3 represent consensus sequences identified in those subscreen experiments. Five of the six consensus sequences tested produced hotspots with activity significantly greater than ade6-M375 (P < 0.01, Student's t-test; consensus sequences for motifs 6-1, 6-21, 7-2, 7-4, 7-31; Figure 3), suggesting that the consensus sequence is sufficient for hotspot activity, at least within this narrow region of the genome. However, we cannot infer that the entire consensus sequence is necessary for activity, which can be determined only by systematic mutagenesis.
For some hotspots, we observed an additional level of complexity. For example, the consensus sequence for motif 7-4 found by subscreen was DACCCCGCACD (Figure S1; D = A, G, or T). When the consensus sequence was reconstructed at its original location (bp 131–141), it produced a strong hotspot (ade6-4099, Figure 3). However, when moved only 36 bp away, the same motif produced less than one-tenth as many Ade+ recombinants (ade6-4072). This reduced activity suggests either (1) that additional nucleotides outside of the consensus sequence are required for full activity or (2) that there is a position-dependent effect on hotspot activity. Since each hotspot sequence found by subscreen of motif 7-4 was 13 bp long (Figure S1), we tested whether one of these slightly longer sequences would create a more active hotspot at the new location. One of those motifs (7-4-11, Figure S1) is identical to the relatively weak ade6-4072 hotspot over its 13-bp length except that the last base is a T rather than a C. Making this single base substitution more than doubled activity of the hotspot at that position (ade6-4072 vs. -4101, Figure 3). However, hotspot activity of the ade6-4101 allele still remains significantly lower than the identical 13-bp sequence at its original position (ade6-4103). Thus, neither a position-effect nor an effect of more distant nucleotides can be excluded.
Similar complexity was observed for the 7-31 motif. In that case, the 9-bp consensus sequence, RCCCCCACA, was reconstructed at approximately the same position as in the subscreen, but in the inverted orientation. Activity of that hotspot (ade6-4073) was significantly lower than the same sequence in the forward orientation (ade6-4100). However, two additional base changes (ade6-4102) more than tripled the number of Ade+ recombinants. The ade6-4102 and -4104 alleles have the same 13-bp sequence as motif 7-31-16 found in a subscreen (Figure S1), but are inverted relative to each other. Both alleles also include the additional K (G or T) consensus nucleotide at the first position (Figure S1), which may increase their activities relative to the weaker ade6-4073 hotspot. The similar activities of the ade6-4102 and -4104 alleles suggests that the nucleotides flanking the hotspot motif have a greater influence on hotspot activity than the motif orientation per se.
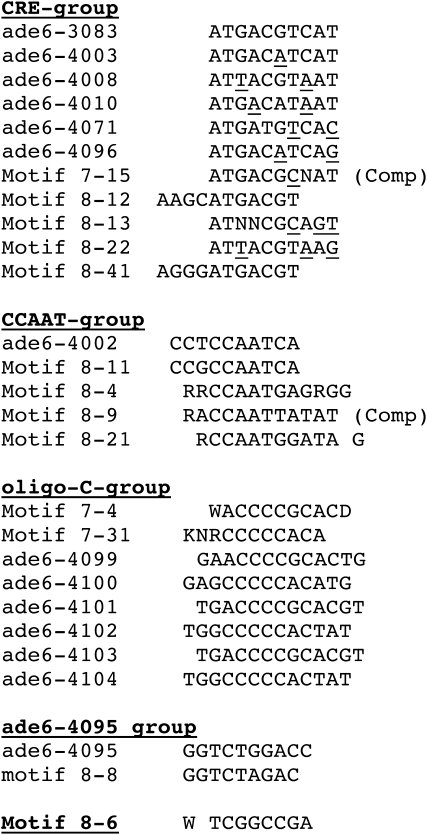
Potential hotspot families:
Upon close inspection of some of the sequences in Figure 3, it became apparent that several of them looked quite similar to the previously characterized CRE hotspot. The most active form of this hotspot is a 10-bp palindrome, ATGACGTCAT (Steiner and Smith 2005b). Figure 3 shows that seven other hotspot motifs differ from the 10-bp CRE palindrome at only one or two positions. Given the previously demonstrated flexibility of the CRE hotspot sequence (Fox et al. 2000), it is conceivable that these related sequences could also be Atf1-Pcr1-dependent hotspots.
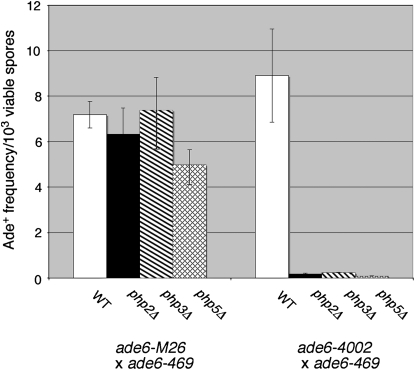
Since several of the hotspots we identified could be grouped into one family (CRE) on the basis of sequence, we also compared other hotspots (Figure 3 or consensus sequence hotspots from Figure S1) to see whether similar groupings were possible. Five motifs were grouped on the basis of their common CCAAT sequence. We speculated that this group of hotspots could be recognized by the CCAAT-binding factor. The CCAAT-binding factor is an evolutionarily conserved heteromeric transcription factor encoded by the php2, php3, and php5 genes and is required for expression of many eukaryotic genes (McNabb et al. 1997). Hotspot activity of one member of this group, ade6-4002, was reduced significantly by deletion of each gene encoding a subunit of the CCAAT-binding factor, while ade6-M26 activity was largely unaffected (Figure 4). In fact, the very low level of recombination observed in these experiments suggests that the ade6-4002 allele is completely dependent on the CCAAT-binding factor for hotspot activity, much as ade6-M26 hotspot activity is completely dependent on the Atf1-Pcr1 transcription factor (Kon et al. 1997).
Figure 4.—
The CCAAT-binding factor is required specifically for activity of the ade6-4002 hotspot, but not ade6-M26. Crosses were performed between heterothallic strains containing the indicated ade6 alleles and mutations in the php2, php3, or php5 genes, which encode subunits of the CCAAT-binding factor. Bars represent the average Ade+ recombinant frequencies ± SEM from a minimum of three experiments for each cross.
A third potential family of hotspots is based on the set of alleles derived from the 7-4 and 7-31 motifs, referred to here as the oligo-C family of hotspots. A search of the TransFac database (http://www.gene-regulation.com/pub/databases.html) suggests that these sequences may be targets for the MIG1 DNA-binding factor in S. cerevisiae, which has several orthologs in S. pombe including Scr1, Rsv1, Hsr1, and Rst2. Yet a fourth family contains two closely related sequences, ade6-4095 and the consensus sequence of motif 8-8. The last potential family has only a single representative, motif 8-6, which shows no obvious similarity to any other identified motif (Figure 5). The very high frequency of hotspots observed in a subscreen of this motif (61%; Table S4), suggests that virtually all of the essential bases are contained within the eight-base motif tested.
Figure 5.—
Potential hotspot families. Hotspot motifs (Figure 3) and hotspot consensus sequences (Figure S1) are aligned to show similarities. In the CRE family of hotspots, bases differing from the 10-bp CRE palindrome (ade6-3083, Steiner and Smith 2005b) are underlined. Comp, complement of consensus sequence (Figure S1).
DISCUSSION
In this study, we devised a method to rapidly screen short nucleotide sequences for hotspot activity and produced a sizable library of 15 and 30 bp sequences containing recombination hotspots. Within those sequences, we identified many shorter motifs ≥6 bp in length that occurred multiple times and hence may form all or part of recombination hotspot sequences. On the basis of our results, we can conclude that the previously characterized CRE hotspot is clearly not the only sequence motif capable of creating a recombination hotspot. However, it was surprising to us how frequently that previously identified hotspot occurred in our library of sequences. The 6-base sequence TGACGT (the CRE core sequence) common to all known Atf1-Pcr1-dependent hotspots (Schuchert et al. 1991; Fox et al. 2000; Steiner and Smith 2005b) was found 75 times, or in 15% of our total pool of hotspot sequences (Table S1 and Table S2a). This frequency increases to almost 20% if one includes sequences from our pool in which the CRE core is formed at the junction between random and nonrandom nucleotides. For example, if the first 4 bases of our 15- or 30-base random region reads GTCA, the 6-base sequence ACGTCA is formed at the junction, which is the complement of the CRE core sequence shown above (see sequence of oligonucleotides oWS209 and oWS241 in materials and methods and Table S1). However, the frequency of CRE-like hotspots may be even greater than the observed frequency of the traditional CRE-core sequence, as several hotspots lacking that core still showed obvious sequence similarity to CRE (Figure 5).
Since our data indicate that other sequence motifs unrelated to CRE also create hotspots, it is possible that the overrepresentation of CRE is due to it having the shortest sequence necessary for observable hotspot activity, which may be fewer than 7 bp in some locations (Fox et al. 2000). Consistent with this, we have observed that the ade6-4002 hotspot requires not only the CCAATCA sequence shown in Figure 3, but also three partially degenerate bases to the left (C. Kalinowski and W. Steiner, unpublished observation). The results from subscreens of other motifs (Table S4) also suggests that hotspots other than CRE may require more than 7 bp for activity. In those experiments, none of the seven or eight base motifs tested produced hotspots in 100% of transformed cells, suggesting that one or more specific nucleotides are required in the random regions flanking each motif.
The number of different hotspot motifs:
What does the frequency of hotspots we observed say about the potential number of different hotspot motifs? We observed that ∼0.6% of random 15mers produced an observable hotspot. In a random 15-bp sequence, there are as many as 9 unique 7-bp sequences when viewed in 7-bp windows moving in steps of 1 bp. Therefore, the probability of finding any unique 7-bp sequence in a random 15mer is (0.25)7 × 9 = 0.055%. The observed frequency of hotspots was ∼10-fold higher than this, suggesting that a minimum of 10 unique 7-bp motifs are required to account for the observed frequency of hotspots. By the same reasoning, ∼50 8-bp motifs, or 225 9-bp motifs would be required. On the basis of the frequency of hotspots among random 30mers, the same calculations produce slightly lower estimates of the number of different hotspots. In either case, however, our data suggest that the number of different hotspot motifs is potentially large.
Without knowing the precise nucleotide sequence required for any given hotspot, it is not possible to determine precisely which of the sequences in our library can have their hotspot activity attributed to a particular motif, with the exception of many of the well-characterized CRE-like hotspots. However, even under generous assumptions about which motifs may create hotspots (for example, that all CRE-core sequences and all CCAAT sequences are hot; see highlighted motifs in Table S1), 197 sequences remain with no identified hotspot motif. Thus, additional hotspot motifs remain to be identified.
The basis of recombination hotspots:
Meiotic recombination hotspots have been most thoroughly analyzed in the budding and fission yeasts S. cerevisiae and S. pombe, respectively. In both organisms, hotspots of recombination are sites of DSBs, which occur preferentially at a limited number of positions throughout their respective genomes. What determines the location of hotspots is incompletely understood, though some correlations have been made. For example, in S. cerevisiae DSBs occur predominantly in intergenic regions where the GC content modestly exceeds the genomic average GC content (Baudat and Nicolas 1997; Gerton et al. 2000; Mieczkowski et al. 2006). In S. pombe DSBs also occur predominantly in intergenic regions, particularly in large intergenic regions, but unlike S. cerevisiae the correlation between GC content and DSBs is very weak (Cromie et al. 2007). In both yeasts, tested DSB hotspots also colocalize to sites of “open” chromatin, i.e., sites that are sensitive to cleavage with S1 or micrococcal nucleases (Wu and Lichten 1994; Mizuno et al. 1997; Hirota et al. 2007).
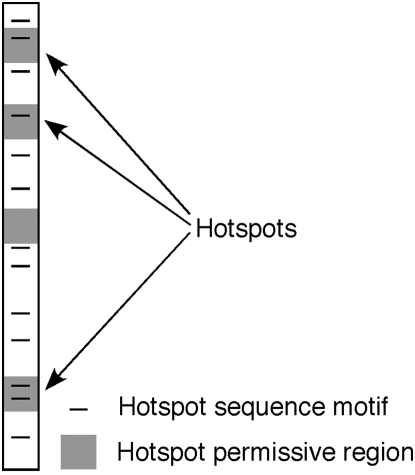
Beyond the aforementioned genomic features, predicting the precise location of recombination hotspots in most cases remains elusive. For example, the well-characterized mbs1 hotspot of S. pombe occurs in an unusually large 7-kb intergenic region (Young et al. 2002; Cromie et al. 2005). Nevertheless, the breaks within that large region are distributed over a much narrower ∼2-kb region. And within that smaller region, the breaks are further focused to four discrete clusters (Cromie et al. 2005). What directs DSBs to these particular sites is unknown. However, a 9-bp CRE motif does predict the location of multiple DSB sites scattered across the S. pombe genome (Steiner and Smith 2005a). This sequence is a binding site for the Atf1-Pcr1 transcription factor, which is required for its hotspot activity (Kon et al. 1997). We propose that there may be many other unrelated short sequence motifs that are responsible for many, and potentially most or all, of the DSB hotspots in S. pombe and perhaps other organisms. However, such hotspot motifs alone are unlikely to be sufficient for hotspot activity at all sites in the genome, since DSBs are not observed at all CRE sites in the genome (Steiner and Smith 2005a). Thus, other factors, such as chromatin structure, also play a role in promoting or permitting hotspots at particular sites. In Figure 6, we propose a model that recombination hotspots are found at positions where these two factors, a hotspot sequence motif and permissive chromatin structure, intersect.
Figure 6.—
Model to explain the location of recombination hotspots. The large rectangle indicates a portion of a chromosome. Shaded regions indicate regions of the genome that are permissive for recombination hotspots. In S. pombe, these regions coincide primarily with large intergenic regions (Cromie et al. 2007). Solid lines indicate hotspot sequence motifs. Hotspots occur where these two chromosomal features coincide.
How do sequence motifs create recombination hotspots? One possibility is that the nucleotide sequence itself possesses some property that makes it unusually susceptible to cleavage during meiosis. For example, tandem repeats of a pentanucleotide sequence reported to inhibit nucleosome formation can create a recombination hotspot in S. cerevisiae (Kirkpatrick et al. 1999). It has also been reported that polypurine/polypyrimidine tracts (PPTs) of ≥12 bp are associated with hotspots in S. cerevisiae (Bagshaw et al. 2006). However, we found no extensive tandem repeats and only a handful of PPTs ≥12 bp among our hotspot sequences (Table S1). Further, a direct test of a 30-bp random PPT in our experimental system did not produce hotspots in any of eight independent transformants (W. Steiner, unpublished observation). Thus, we favor instead the model that most of the sequences in our hotspot library contain target sequences for DNA binding proteins, for example transcription factors, that promote DSBs when bound to DNA. This model is also consistent with current data. First, we are aware of only two previously reported examples of defined sequence motifs creating recombination hotspots, the CRE hotspot of S. pombe (Schuchert et al. 1991) and the Bas1 target sequence, TGACTC, of S. cerevisiae (Mieczkowski et al. 2006). Both of these motifs require the binding of a transcription factor for their hotspot activity (Kon et al. 1997; Mieczkowski et al. 2006). Second, it has been recently observed in S. pombe that hotspots of recombination show significant colocalization to sites expressing noncoding RNAs (Wahls et al. 2008), suggesting that these sites are bound by transcription factors.
How might transcription factors promote recombination when bound to their target sequence? Yamada et al. (2004) showed that the ade6-M26 hotspot was dependent on both a histone acetyl transferase (Gcn5) and an ATP-dependent chromatin remodeling factor (Snf22). These researchers suggested that binding of the Atf1-Pcr1 transcription factor to the M26 motif recruits these chromatin modifying enzymes, resulting in localized chromatin remodeling and making the site accessible to the DSB machinery. It would be interesting to determine whether other hotspot-associated transcription factors operate by a similar mechanism, and what such factors might have in common that results in the recruitment of chromatin modifying enzymes.
It is possible that simple sequence motifs produce hotspots in many different organisms. For example, several sequence motifs have been reported as potential hotspots in humans (Zhang et al. 2004; Myers et al. 2005, 2008). Since recombination hotspots disrupt gene linkages, they complicate efforts to find human disease genes by linkage analysis (Hey 2004; Nishant and Rao 2005). Thus, the ability to identify potential hotspots solely on the basis of sequence is of practical significance. It is possible that some hotspots we have identified in our analysis are also active in other organisms. Two of the motifs reported as potential human hotspots, CGCCCCCGC and CCCCACCCC, show strong similarity to motifs found in our screen, motifs 7-4 and 7-8, respectively (Table 2), at least one of which (7-4) was confirmed to be a hotspot (Figure 3 and Table S4). This result suggests the intriguing possibility that some of the motifs identified here may also be active in other organisms.
Acknowledgments
We thank Simon Labbé for the donation of strains. This work was supported by a National Institutes of Health grant, R15GM078065 (to W.W.S.). W.W.S. was supported by a Leukemia and Lymphoma Society special fellowship, 3230-05, during a portion of this work.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.101253/DC1.
References
- Bagshaw, A. T., J. P. Pitt and N. J. Gemmel, 2006. Association of poly-purine/poly-pyrimidine sequences with meiotic recombination hotspots. BMC Genomics 7 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, T. L., N. Williams, C. Misleh and W. W. Li, 1996. MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 34 W369–W373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, B. S., A. T. C. Carpenter, M. S. Esposito, R. E. Esposito and L. Sandler, 1976. The genetic control of meiosis. Annu. Rev. Genet. 10 53–134. [DOI] [PubMed] [Google Scholar]
- Baudat, F., and A. Nicolas, 1997. Clustering of meiotic double-strand breaks on yeast chromosome III. Proc. Natl. Acad. Sci. USA 94 5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler, J., J.-Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie, III et al., 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14 943–951. [DOI] [PubMed] [Google Scholar]
- Cao, L., E. Alani and N. Kleckner, 1990. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell 61 1089–1101. [DOI] [PubMed] [Google Scholar]
- Cervantes, M. D., J. A. Farah and G. R. Smith, 2000. Meiotic DNA breaks associated with recombination in S. pombe. Mol. Cell 5 883–888. [DOI] [PubMed] [Google Scholar]
- Cottarel, G., D. Beach and U. Deuschle, 1993. Two new multi-purpose multicopy Schizosaccharomyces pombe shuttle vectors, pSP1 and pSP2. Curr. Genet. 23 547–548. [DOI] [PubMed] [Google Scholar]
- Cromie, G. A., R. W. Hyppa, H. P. Cam, J. A. Farah, S. I. S. Grewal et al., 2007. A discrete class of intergenic DNA dictates meiotic DNA break hotspots in fission yeast. PLoS Genet. 3 1496–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromie, G. A., C. A. Rubio, R. W. Hyppa and G. R. Smith, 2005. A natural meiotic DNA break site in Schizosaccharomyces pombe is a hotspot of gene conversion, highly associated with crossing over. Genetics 169 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel, R., 1977. Selective spore survival during replica-plating of fission yeast. Arch. Microbiol. 112 109–110. [DOI] [PubMed] [Google Scholar]
- Esposito, M. S., and J. E. Wagstaff, 1981. Mechanisms of mitotic recombination, pp. 341–370 in The Molecular Biology of the Yeast Saccharomyces, edited by J. N. Strathern, E. W. Jones and J. R. Broach. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Fan, Q., F. Xu and T. D. Petes, 1995. Meiosis-specific double-strand DNA breaks at the HIS4 recombination hot spot in the yeast Saccharomyces cerevisiae: control in cis and trans. Mol. Cell. Biol. 15 1679–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. E., J. B. Virgin, J. Metzger and G. R. Smith, 1997. Position- and orientation-independent activity of the Schizosaccharomyces pombe meiotic recombination hot spot M26. Proc. Natl. Acad. Sci. USA 94 7446–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. F., T. Yamada, K. Ohta and G. R. Smith, 2000. A family of CRE-related DNA sequences with meiotic recombination hotspot activity in Schizosaccharomyces pombe. Genetics 156 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerton, J. L., J. DeRisi, R. Shroff, M. Lichten, P. O. Brown et al., 2000. Global mapping of meiotic recombination hotspots and coldspots in the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 97 11383–11390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz, H., 1971. Site specific induction of gene conversion in Schizosaccharomyces pombe. Genetics 69 317–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz, H., H. Heslot, U. Leupold and N. Loprieno, 1974. Schizosaccharomyces pombe, pp. 395–446 in Handbook of Genetics, edited by R. C. King. Plenum, New York.
- Hey, J., 2004. What's so hot about recombination hotspots? PLoS Biol. 2 0730–0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota, K., W.W. Steiner, T. Shibata and K. Ohta, 2007. Chromatin configuration at natural meiotic recombination hot spots in fission yeast. Eukaryot. Cell 6 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney, S., C. N. Giroux and N. Kleckner, 1997. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88 375–384. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, D. T., Y. H. Wang, M. Dominska, J. D. Griffith and T. D. Petes, 1999. Control of meiotic recombination and gene expression in yeast by a simple repetitive DNA sequence that excludes nucleosomes. Mol. Cell. Biol. 19 7661–7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon, N., M. D. Krawchuk, B. G. Warren, G. R. Smith and W. P. Wahls, 1997. Transcription factor Mts1/Mts2 (Atf1/Pcr1, Gad7/Pcr1) activates the M26 meiotic recombination hotspot in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 94 13756–13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah, S. K., J. M. A. Turner, F. Baudat, E. P. Rogakou, P. deBoer et al., 2001. Recombinational DNA double strand breaks in mice precede synapsis. Nat. Genet. 27 271–276. [DOI] [PubMed] [Google Scholar]
- Malik, S.-B., M. A. Ramesh, A. M. Hulstrand and J. M. Logsdon Jr., 2007. Protist homologs of the meiotic Spo11 gene and topoisomerase VI reveal an evolutionary history of gene duplication and lineage-specific loss. Mol. Biol. Evol. 24 2827–2841. [DOI] [PubMed] [Google Scholar]
- McNabb, D. S., K. A.-S. Tseng and L. Guarente, 1997. The Saccharomyces cerevisiae Hap5p homolog from fission yeast reveals two conserved domains that are essential for assembly of heterotetrameric CCAAT-binding factor. Mol. Cell. Biol. 17 7008–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier, A., B. Pelletier and S. Labbé, 2006. A transcription factor cascade involving Fep1 and the CCAAT-binding factor Php4 regulates gene expression in response to iron deficiency in the fission yeast Schizosaccharomyces pombe. Eukaryotic Cell 5 1866–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieczkowski, P. A., M. Dominska, M. J. Buck, J. L. Gerton, J. D. Lieb et al., 2006. A global analysis of the relationship between the binding of the Bas1p transcription factor and meiosis-specific double-strand DNA breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 26 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno, K.-i., Y. Emura, M. Baur, J. Kohli, K. Ohta et al., 1997. Remodeling of chromatin structure around a single nucleotide mutation in ade6–M26 that creates meiotic recombination hotspot in fission yeast. Genes Dev. 11 876–886. [DOI] [PubMed] [Google Scholar]
- Myers, S., L. Bottolo, C. Freeman, G. McVean and P. Donnelly, 2005. A fine-scale map of recombination rates and hotspots across the human genome. Science 310 321–324. [DOI] [PubMed] [Google Scholar]
- Myers, S., C. Freeman, A. Auton, P. Donnelly and G. McVean, 2008. A common sequence motif associated with recombination hot spots and genome instability in humans. Nat. Genet. 40 1124–1129. [DOI] [PubMed] [Google Scholar]
- Nishant, K. T., and M. R. S. Rao, 2005. Molecular features of meiotic recombination hotspots. Bioessays 28 45–56. [DOI] [PubMed] [Google Scholar]
- Pearson, W., and D. Lipman, 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85 2444–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes, T. D., 2001. Meiotic recombination hot spots and cold spots. Nat. Rev. Genet. 2 360–370. [DOI] [PubMed] [Google Scholar]
- Pâques, F., and J. E. Haber, 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli, A. S., and G. R. Smith, 1989. Meiotic recombination deficient mutants of Schizosaccharomyces pombe. Genetics 123 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchert, P., M. Langsford, E. Käslin and J. Kohli, 1991. A specific DNA sequence is required for high frequency of recombination in the ade6 gene of fission yeast. EMBO J. 10 2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, S., and N. Tompa, 2002. Discovery of novel transcription factor binding sites by statistical overrepresentation. Nucleic Acids Res. 30 5549–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, S., and N. Tompa, 2003. YMF: a program for discovery of novel transcription factor binding sites by statistical overrepresentation. Nucleic Acids Res. 31 3586–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, W. W., R. W. Schreckhise and G. R. Smith, 2002. Meiotic DNA breaks at the S. pombe recombination hotspot M26. Mol. Cell 9 847–855. [DOI] [PubMed] [Google Scholar]
- Steiner, W. W., and G. R. Smith, 2005. a Natural meiotic recombination hotspots in the S. pombe genome successfully predicted from the simple sequence motif M26. Mol. Cell. Biol. 25 9054–9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, W. W., and G. R. Smith, 2005. b Optimizing the nucleotide sequence of a meiotic recombination hotspot in Schizosaccharomyces pombe. Genetics 169 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storici, F., L. K. Lewis and M. A. Resnick, 2001. In vivo site-directed mutagenesis using oligonucleotides. Nat. Biotechnol. 19 773–776. [DOI] [PubMed] [Google Scholar]
- Sun, H., D. Treco, N. P. Schultes and J. W. Szostak, 1989. Double-strand breaks at an initiation site for meiotic gene conversion. Nature 338 87–90. [DOI] [PubMed] [Google Scholar]
- Szankasi, P., W. D. Heyer, P. Schuchert and J. Kohli, 1988. DNA sequence analysis of the ade6 gene of Schizosaccharomyces pombe: wild-type and mutant alleles including the recombination hotspot allele ade6–M26. J. Mol. Biol. 204 917–925. [DOI] [PubMed] [Google Scholar]
- Vallejo, A. N., R. J. Pogulis and L. R. Pease, 1995. Mutagenesis by PCR, pp. 603–612 in PCR Primer: A Laboratory Manual, edited by C. W. Dieffenbach and G. S. Dveksler. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Virgin, J. B., J. Metzger and G. R. Smith, 1995. Active and inactive transplacement of the M26 recombination hotspot in Schizosaccharomyces pombe. Genetics 141 33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahls, W. P., E. R. Siegel and M. K. Davisdson, 2008. Meiotic recombination hotspots of fission yeast are directed to loci that express non-coding RNA. PLoS ONE 3 e2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, M. A., M. Dominska and T. D. Petes, 1993. Transcription factors are required for the meiotic recombination hotspot at the HIS4 locus in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 90 6621–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, V., R. G. William, M.-A. Rajandream, M. Lyne, R. Lyne et al., 2002. The genome sequence of the eukaryote fission yeast Schizosaccharomyces pombe. Nature 415 871–880. [DOI] [PubMed] [Google Scholar]
- Wu, T.-C., and M. Lichten, 1994. Meiosis-induced double-strand break sites determined by yeast chromatin structure. Science 263 515–518. [DOI] [PubMed] [Google Scholar]
- Yamada, T., K. Mizuno, K. Hirota, N. Kon, W. P. Wahls, et al., 2004. Roles of histone acetylation and chromatin remodeling factor in a meiotic recombination hotspot. EMBO J. 23 1792–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, J. A., R. W. Schreckhise, W. W. Steiner and G. R. Smith, 2002. Meiotic recombination remote from prominent DNA break sites in S. pombe. Mol. Cell 9 253–263. [DOI] [PubMed] [Google Scholar]
- Zahn-Zabal, M., E. Lehmann and J. Kohli, 1995. Hot spots of recombination in fission yeast: inactivation of the M26 hot spot by deletion of the ade6 promoter and the novel hotspot ura4-aim. Genetics 140 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., F. Li, J. Li, M. Q. Zhang and X. Zhang, 2004. Evidence and characteristics of putative human alpha recombination hotspots. Hum. Mol. Genet. 13 2823–2828. [DOI] [PubMed] [Google Scholar]