Abstract
Dosage compensation modifies the chromatin of X-linked genes to assure equivalent expression in sexes with unequal X chromosome dosage. In Drosophila dosage compensation is achieved by increasing expression from the male X chromosome. The ribonucleoprotein dosage compensation complex (DCC) binds hundreds of sites along the X chromosome and modifies chromatin to facilitate transcription. Loss of roX RNA, an essential component of the DCC, reduces expression from X-linked genes. Surprisingly, loss of roX RNA also reduces expression from genes situated in proximal heterochromatin and on the small, heterochromatic fourth chromosome. Mutation of some, but not all, of the genes encoding DCC proteins produces a similar effect. Reduction of roX function suppresses position effect variegation (PEV), revealing functional alteration in heterochromatin. The effects of roX mutations on heterochromatic gene expression and PEV are limited to males. A sex-limited role for the roX RNAs in autosomal gene expression was unexpected. We propose that this reflects a difference in the heterochromatin of males and females, which serves to accommodate the heterochromatic Y chromosome present in the male nucleus. roX transcripts may thus participate in two distinct regulatory systems that have evolved in response to highly differentiated sex chromosomes: compensation of X-linked gene dosage and modulation of heterochromatin.
MANY male animals carry a euchromatic, gene-rich X chromosome and a largely heterochromatic, gene-poor Y chromosome (Charlesworth 1991). Highly differentiated sex chromosomes pose several problems for the organisms that carry them, the most obvious being that males have a potentially lethal imbalance in X-linked gene dosage. The process of dosage compensation equalizes X-linked gene expression between XY males and XX females, thereby maintaining a constant ratio of X-linked to autosomal gene products (Gupta et al. 2006; Nguyen and Disteche 2006). While strategies to accomplish this differ between species, a unifying theme is coordinated regulation of a whole chromosome by selective recruitment of chromatin-modifying proteins (Lucchesi et al. 2005). Intriguing links between seemingly unrelated systems that coordinately regulate large groups of genes have been observed. A recent study has shown that the Caenorhabditis elegans dosage compensation protein DPY-28 also limits meiotic crossover, another chromosomewide process (Tsai et al. 2008). In an interesting parallel, we show here that some members of the Drosophila melanogaster dosage compensation complex (DCC) also regulate heterochromatin function by modulating the expression of heterochromatic genes in males.
The Drosophila DCC, also known as the male-specific lethal or MSL complex, mediates dosage compensation by increasing expression from the single X chromosome of males. Two noncoding RNAs, roX1 and roX2 (RNA on the X 1 and 2), are essential components of this complex but are functionally redundant for dosage compensation (Meller and Rattner 2002). The roX transcripts assemble with the MSL proteins, encoded by maleless (mle), the male-specific lethals 1, -2, and -3 (msl1, -2, and -3) and males absent on first (mof). A cotranscriptional targeting mechanism localizes the complex within the body of genes (Kind and Akhtar 2007; Larschan et al. 2007). The complex directs acetylation of histone H4 on lysine 16 (H4Ac16), a modification associated with increased expression (Akhtar and Becker 2000). Mutation of a single roX gene is without phenotype, but simultaneous mutation of both roX genes dramatically lowers male survival (Deng et al. 2005). Females appear unaffected by loss of the roX transcripts. In roX1 roX2 males the MSL proteins bind at ectopic autosomal sites and the chromocenter. Microarray expression analysis of roX1 roX2 (null for roX function) and roX2 (control) male larvae confirmed that roX RNA is required for full expression of X-linked genes in males (Deng and Meller 2006).
The highly preferential binding of the Drosophila DCC to the X chromosome promoted the idea that these molecules contribute solely to X-linked gene expression, but this notion has been challenged by the finding that some of the MSL proteins act outside of the complex as general transcriptional regulators. MLE is enriched on the male X chromosome but also found at sites of active transcription in males and females (Kotlikova et al. 2006). MOF is an integral member of the DCC and is enriched on the male X chromosome. In addition, MOF is more modestly enriched at the 5′ end of transcribed genes throughout the genomes of both sexes (Kind et al. 2008). In spite of the general role of these two factors, mutations in mle and mof are lethal only to males.
We now show that roX RNA influences the expression of heterochromatic genes, including those on the small fourth chromosome and in heterochromatic regions of the second and third chromosomes, in male larvae. The MSL1 and MSL3 proteins are necessary for full expression of these genes, but MSL2, a protein of central importance for X chromosome dosage compensation, is unnecessary. This demonstrates that the intact DCC is not involved in regulation of heterochromatic genes. Consistent with the idea that the roX RNAs affect heterochromatin function in males, we find that a roX1 roX2 chromosome is a potent modifier of position effect variegation (PEV) in males, but not in females. We propose a new role for these molecules in sex-specific regulation of heterochromatin. We further speculate that this serves to accommodate the differences in heterochromatin content in males and females that result from the presence of a large, heterochromatic Y chromosome in the male nucleus.
MATERIALS AND METHODS
Drosophila strains:
Flies were maintained at 25° on standard cornmeal–agar fly food in a humidified incubator. The roX1ex6, roX1SMC17A, roX1mb710, roXex7B, and roX1ex33 mutations have been described (Meller et al. 1997; Deng et al. 2005 ). Elimination of roX2 is accomplished by combining a lethal deletion removing roX2 and essential flanking genes, Df(1)52, with a cosmid insertion carrying essential deleted genes but lacking roX2 [w+4Δ4.3] (Meller and Rattner 2002). For convenience this combination is referred to as roX2. A deleted cosmid lacking w+ was used in studies of PEV [w−4Δ4.3]. Mutations in msl1, msl2, msl3, and pof have been previously described (Lindsley and Zimm 1992; Johansson et al. 2007). Variegating transgene insertions used in this study have been described (Sun et al. 2000; Maggert and Golic 2002; Yan et al. 2002).
Males mutated for msl1 and msl2 generally are not healthy enough to be selected by gonad morphology. To select msl11 males, yw; msl11 females were mated to y+w; msl11/CyOy+ males. msl11 males are y and have brown mouth hooks. To select msl21 males, yw; msl21 females were mated to y+w[w+PD27]; msl21 males. The [w+PD27] insertion carries msl2 and rescues msl21 males (Kelley et al. 1995). All male offspring from this cross are yw; msl21 and all female offspring are y+.
Histology:
Immunhistochemical detection on polytene chromosomes of wild- type and roX1ex6roX2 males was done as previously described (Kelley et al. 1999). POF antibody, a generous gift from J. Larsson, was used as previously described (Larsson et al. 2001).
Quantitative RT–PCR:
Total RNA was made from three groups of at least 50 larvae of each genotype. One microgram of total RNA was reverse transcribed using random hexamers and ImProm-II reverse transcriptase (Promega). Quantitative PCR was performed as previously described (Deng et al. 2005). A total of 34 genes were selected from four different gene groups (2 and 3 euchromatic, 2 and 3 heterochromatic, fourth chromosomal, and X chromosomal). The selected genes were expressed at moderate levels, displayed uniform absorbance in arrays of the same genotype, and reflected the average change in expression for their gene group in roX1 roX2 males. An exception is the X-linked Lsp-1α gene, which is known to escape dosage compensation. Bigmax and Dmn are autosomal genes that proved reliable for normalization of expression (data not shown). The primers used in this study are presented in supporting information, Table S1.
Gene expression microarrays:
Total RNA was prepared from groups of at least 50 third instar larvae using the TRIzol method (Invitrogen) and purified using the RNeasy kit (QIAGEN). Three independent RNA preparations for each genotype served as templates for probe synthesis (see protocol at www.Affymetrix.com). Probes were hybridized to Affymetrix Drosophila Genome 2.0 chips (Santa Clara, CA). Background corrected intensity values were quantile normalized (Irizarry et al. 2003). In brief, all probe intensities from mutant and control arrays were assembled into a single ranking. Probes from individual chips were assigned the value of the corresponding quantile, thus preserving the rank order within a chip and standardizing intensity distribution across all chips. Intensities were summarized into one expression value per sample and probe set using the robust multi-array average (RMA) algorithm. The Affymetrix MAS5.0 Present/Absent calls were used to filter out probe sets not present in at least two out of three replicates of each genotype.
Genes and probe sets (Berkeley Drosophila Genome Project annotation release 5.8) were sorted to enrich for heterochromatic genes on the basis of the boundaries between heterochromatic and euchromatic regions (Smith et al. 2007; Hoskins et al. 2007). The coordinates of these boundaries are: 2R;1-1285689, 2L;22000975-23011544, 3R;1-378656, 3L;22955576-24543557, X;22030326-22422827. The coordinates for heterochromatin that is not contiguous with assembled arm sequences are 2LHet;1-368872, 2RHet;1-3288761, 3LHet;1-2555491, 3RHet;1-2517507, XHet;1-204112, YHet; 1-347038. Only probe sets assigned to a chromosome were used. Genes and probe sets assigned to heterochromatic regions were obtained from FlyBase GBrowse. The corresponding gene and probe set information was obtained from the Affymetrix Drosophila_2 annotation file (Drosophila_2.na25) released on March 17, 2008 (Liu et al. 2007).
Statistical methods and descriptions:
The log2 fold change of each gene was computed as the log2 mean RMA expression of mutant samples minus the log2 mean RMA expression of control samples. The significance of differences between groups was assessed by the Wilcoxon test. Analyses were performed in the R software environment (www.r-project.org) using Bioconductor (www.bioconductor.org) (Gautier et al. 2004; Smyth 2005). The raw data can be downloaded from the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo, GSE3990; GSE12054; GSE12076).
qRT–PCR data were analyzed by the efficiency corrected comparative quantification method (Pfaffl 2001). Ct values for three biological replicates (each containing two technical replicates) per genotype were averaged into one Ct value per gene. The relative quantities (mutant:control) were tested for normality using the Shapiro–Wilk test. As the MSL1 and MSL3 data sets were not normally distributed, the nonparametric Mann–Whitney U test was used to determine significance. Descriptive statistics are in File S1.
RESULTS
Fourth-linked genes are underexpressed in roX1 roX2 male larvae:
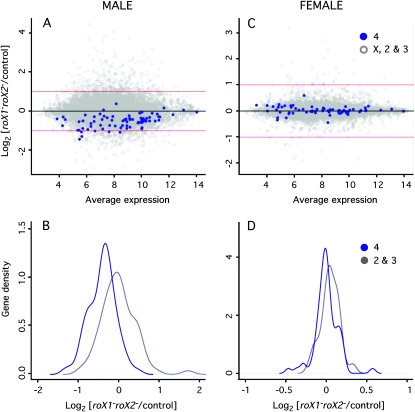
A microarray study was conducted to compare genomewide expression in roX1 roX2 males (null for roX function) and roX2 (control) males. roX1 roX2 males rarely survive past the third larval instar, but males mutated for a single roX gene are developmentally normal with full survival. In support of this, the localization of the MSL proteins on the polytenized X chromosome of roX2 and wild-type males appears identical on chromosome preparations (Deng and Meller 2006). An overall reduction in X-linked gene expression was observed in the roX1 roX2 male larvae (Deng and Meller 2006). However, to our surprise, expression of genes situated on the small fourth chromosome was also reduced by ∼50% in roX1 roX2 males (Figure 1A). qRT–PCR confirmed the reduction in fourth–linked gene expression (Table S2). A plot of the log2 of the expression ratio (mutant: control) of euchromatic genes on the second and third chromosomes has a distribution centered near zero, but the fourth chromosome is shifted left, reflecting this overall decrease (Figure 1B). The change in expression of fourth-linked genes is significantly different from that of any other chromosome (Wilcoxon test, P-value < 10−16).
Figure 1.—
Expression of the fourth chromosome is reduced in roX1 roX2 males. (A) In roX1 roX2 males the expression of fourth-linked genes (blue) decreases in comparison with the rest of the genome (gray). Points represent the log2 of the ratio of gene expression in roX1SMC17AroX2 males to control males (roX2) plotted against expression level (log2 absorbance). Numbers and types of genes plotted are 9880 nonfourth-linked genes and 74 fourth-linked genes. (B) The density distribution of log2 expression (mutant/control) for fourth-linked genes (blue) and second and third chromosome genes (gray) in males. The distribution of fourth-linked genes differs significantly from the remaining autosomal genes (adjusted P-value < 6.6 × 10−16; Wilcoxon test). (C) In roX1 roX2 females the expression of fourth-linked genes (blue) is unchanged. The rest of the genome is shown in gray. Data is presented as the log2 of the ratio of gene expression in roX1SMC17AroX2 females to control females (roX1SMC17AroX2; [w+Hs83- roX1+]) plotted against expression level (log2 absorbance). Genes contributed to this analysis are 8433 nonfourth-linked and 69 fourth-linked genes. (D) The density distribution of log2 expression (mutant/control) for fourth-linked genes (blue) and second and third chromosome genes (gray) in female larvae. The distribution of fourth-linked genes is not significantly different from that of the second and third chromosomes (adjusted P-value 0.92). Only genes present in at least two out of three replicates were included. See File S1 for details of microarray hybridization and analysis.
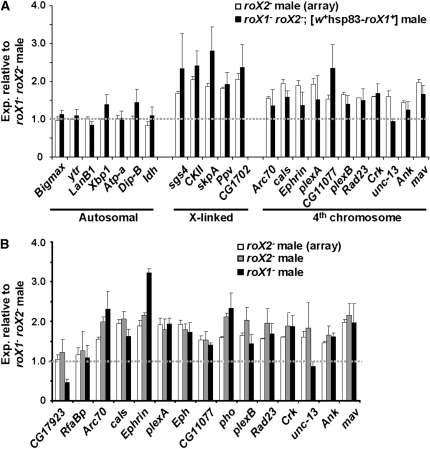
To determine if reduced expression is due to loss of the roX transcripts, the expression of fourth-linked genes was examined in roX1 roX2 males carrying a roX1 transgene, [w+Hs83-roX1+], which rescues both male survival and X localization of the MSL proteins. This transgene fully restores expression of five X-linked genes (Figure 2A). Expression of 10 of 11 fourth chromosome genes is also largely restored. We conclude that the absence of roX RNA reduces expression of fourth–linked genes.
Figure 2.—
roX1 and roX2 are necessary but redundant for full expression of fourth-linked genes. (A) Expression of fourth-linked genes is restored by a roX1 transgene. Quantitative RT–PCR was used to compare the expression of individual genes in male roX1SMC17AroX2 larvae (set to 1) and roX1SMC17AroX2; [w+hsp83-roX1+] larvae (solid bars). The expression in roX2 larvae as determined by microarray (Figure 1A) is shown for comparison (open bars). (B) roX1 and roX2 are redundant for expression of fourth-linked genes in males. Expression of individual genes was measured by qRT–PCR in male roX1SMC17AroX2 larvae (set to 1), roX2 larvae (shaded bars) and roX1SMC17A larvae (solid bars). The expression of each gene in male roX2 larvae by microarray analysis is presented for comparison (open bars).
The roX RNAs appear completely redundant for dosage compensation of the X chromosome (Meller and Rattner 2002). To determine if the roX genes are also redundant for regulation of the fourth chromosome, expression of individual genes was compared in roX1 roX2 males (value set to 1, Figure 2B), roX2 males (shaded bars) and roX1 males (solid bars). Almost all of the fourth-linked genes display similar expression in single mutants but have considerably lower expression in the roX1 roX2 double mutant. Only two tested genes, CG17923 and unc-13, behave differently in roX2 and roX1 male larvae. We speculate that these genes are influenced by genetic factors on the roX1 mutant chromosome used in these studies. This is consistent with the inability of a roX1 transgene to restore unc-13 expression (Figure 2A). We conclude that the roX genes are redundant for expression of fourth-linked genes.
The idea that an autosome is differentially regulated in males and females seemed highly unlikely. However, roX1 is abundant in early embryos of both sexes, and thus might contribute to the expression of fourth-linked genes in both sexes (Meller 2003). To determine if roX also influences fourth-linked gene expression in females, microarrays were hybridized to probes generated from roX1 roX2 and roX1 roX2; [w+Hs83-roX1+] female larvae. Females are not developmentally disrupted by elimination of roX1 and roX2, which may account for the narrow range of expression ratios (compare Figure 1, A and C). The presence of the constitutively expressed roX1 transgene had no influence on fourth-linked genes in roX1 roX2 females (Figure 1, C and D). The roX RNA requirement for full expression of fourth-linked genes, therefore, appears limited to males. Several fourth-linked genes were measured in wild-type male and female larvae to determine whether their expression is normally higher in males. With the exception of the male-preferential CG17923, the fourth-linked genes examined are expressed at similar levels in males and females (Figure S1). This conclusion is supported by published microarray studies of D. melanogaster male and female larvae and adults (Parisi et al. 2003; Liu et al. 2005).
Ectopic localization of the MSL proteins does not repress fourth-linked genes:
There are several potential mechanisms by which loss of the roX transcripts might reduce expression from the fourth chromosome in males. The fourth chromosome shows particularly strong ectopic binding of the MSL proteins in roX1 roX2 males (Meller and Rattner 2002; Deng et al. 2005 ). It is possible that the abnormal binding of these proteins to the fourth chromosome represses expression. To test this idea, we examined expression of fourth-linked genes in female larvae that display an identical pattern of MSL localization. MSL2 is normally present only in males. When MSL2 is expressed in females from the [w+Hs83-M2] transgene, intact MSL complexes form and bind to both X chromosomes, resulting in high female mortality (Kelley et al. 1995). Females are rescued by mutation of both roX genes. These females have normal survival but display ectopic MSL localization indistinguishable from that observed in roX1 roX2 males (Deng et al. 2005). We reasoned that if mislocalized MSL proteins repress expression, this repression will be evident in roX1 roX2; [w+Hs83-M2] females, but not in roX1 roX2 females. Expression of a panel of genes was measured in female larvae of these genotypes by qRT–PCR. Autosomal genes on the second and third chromosomes remain unchanged, but expression from the fourth chromosome actually increases when MSL2 is expressed in roX1 roX2 females (Figure S2). Although unexpected, this increase is consistent with the accumulation of low levels of H4Ac16 at sites of mislocalized MSL proteins (Deng and Meller 2006). We conclude that ectopic binding of the MSL proteins to the fourth chromosome does not cause repression of fourth-linked genes in roX1 roX2 males.
The possibility that mislocalized MSL proteins disrupt centromere function, leading to frequent loss of the fourth chromosome, was also considered. Examination of mitotic neuroblast preparations revealed that the number of visible fourth chromosomes was identical in nuclei from roX1 roX2 and control males (Figure S3). Elevated loss of the fourth chromosome in somatic tissues is thus unlikely to be the source of reduced fourth-linked gene expression.
roX does not interact genetically with painting of fourth:
Several lines of evidence suggest a close relationship between the fourth and the X chromosomes of D. melanogaster (Larsson and Meller 2006). Most suggestive is the chromosomewide targeting mechanism revealed by painting of fourth (POF), which binds along the banded portion of the fourth chromosome in both sexes. In the related D. ananassae and D. malerkotliana, POF binds to the fourth chromosome of both sexes but colocalizes with MSL3 on the male X chromosome, suggesting a role in X chromosome compensation in these species (Larsson et al. 2004). While POF is nonessential in D. melanogaster with two fourth chromosomes, it is necessary for the survival of flies with a single fourth chromosome, suggesting that POF functions in dosage compensation of the D. melanogaster fourth chromosome (Johansson et al. 2007). POF is a putative RNA-binding protein. This raised the suspicion that, like the MSL proteins, POF might require roX RNA for correct localization. We examined the localization of POF in roX1 roX2 and control males, but no differences were discernible by immunostaining of polytene chromosomes (Figure S4). While the roX RNAs are easily detected over the polytenized X chromosome of males, they are not detected binding to the fourth chromosomes (data not shown). The roX RNAs are therefore unnecessary for POF binding and do not colocalize with POF on the fourth chromosome in the larval salivary gland.
No evidence of genetic interactions between roX1 roX2 chromosomes and pof mutations could be detected. roX1 roX2 chromosomes carrying partial loss- of-function roX1 mutations that allow the recovery of escaper males were used for these analyses. Mutation of one copy of pof does not reduce the recovery of males carrying the severely affected roX1mb710roX2 chromosome (Table S3). Elimination of POF in males carrying the partial loss-of-function roX1ex7BroX2 chromosome resulted in a modest decrease in survival consistent with differences in genetic background (22% roX1ex7BroX2 escapers and 18% roX1ex7BroX2; pofΔ119 escapers; see Table S4). Unlike pof mutants, flies carrying a single fourth chromosome and the partial loss-of-function roX1ex7BroX2 chromosome were recovered (Table S5). This suggests that the roX transcripts do not participate in compensation of fourth-linked genes. While POF is proposed to function in dosage compensation of the fourth chromosome in both sexes, roX RNA has a male-limited effect on expression of fourth-linked genes. These studies indicate that the roX and pof genes do not participate in the same process.
Autosomal genes in heterochromatic regions require roX for full expression:
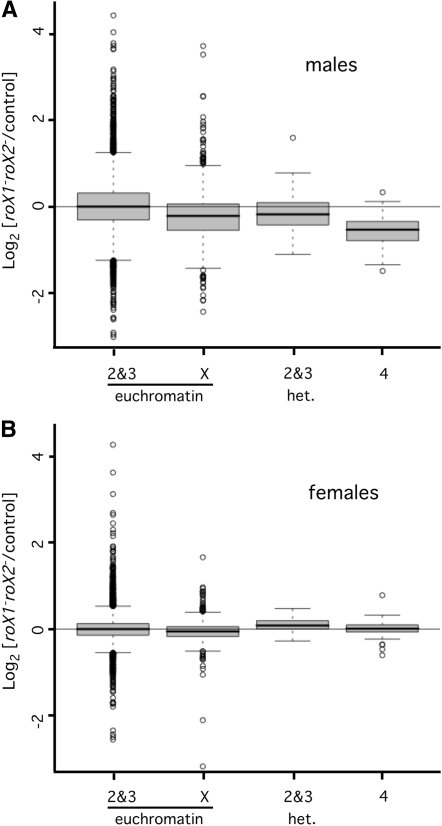
The fourth chromosome has additional unusual features, including enrichment for heterochromatin (reviewed in Riddle and Elgin 2006). Genes embedded in heterochromatin are presumed to have specialized regulatory features that enable expression in spite of their repressive heterochromatic environment (Yasuhara and Wakimoto 2006). If the proximity of fourth-linked genes to heterochromatin makes them dependent upon roX RNA, expression of genes in pericentric heterochromatin on the second and third chromosomes may also depend on roX RNA. To enrich for genes in or near heterochromatin, microarray probe sets were sorted using the heterochromatin/euchromatin boundaries from the Drosophila genome annotation (see materials and methods). Expression of heterochromatin-enriched genes on the second and third chromosomes decreased by 17% in roX1 roX2 males, but remained unchanged in roX1 roX2 females (Figure 3). This suggests that proximity to heterochromatin could account for the dependence of fourth-linked genes on roX RNA.
Figure 3.—
Genes situated in proximal heterochromatin require roX RNA for full expression in males. (A) Genes in proximal heterochromatin have reduced expression in roX1SMC17AroX2 male larvae. Box plots were generated using the log2 expression ratios (mutant/control) presented in Figure 1A. The mean expression of genes in proximal heterochromatin on the second and third chromosomes decreases by 0.17 in roX1SMC17AroX2 males (adjusted P-value of 0.003). The mean expression of X-linked genes decreases by 0.24, and expression of fourth-linked genes decreases by 0.58. Changes of the X and fourth chromosome have an adjusted P-value of <6.6 × 10−16. Only genes present in at least 2 out of 3 arrays contributed to this analysis (8347 in second and third euchromatin; 1533 in X euchromatin, 73 in second and third heterochromatin, and 74 on the fourth chromosome). (B) Fourth-linked and heterochromatic genes do not require roX RNA for full expression in females. Box plots were generated using the log2 expression ratios (mutant/control) presented in Figure 1C. The mean change in expression of X-linked genes in roX1SMC17AroX2 females is −0.04. Second and third chromosome heterochromatic genes and fourth- linked genes have a slight average increase (0.06 and 0.01, respectively) that is not statistically significant. Only genes present in at least 2 out of 3 arrays contributed to this analysis (7097 in second and third euchromatin, 1336 in X euchromatin, 57 in second and third heterochromatin, and 69 on the fourth chromosome). Enrichment for heterochromatic genes is described in File S1.
MSL1 and MSL3, but not MSL2, contribute to expression of heterochromatic genes:
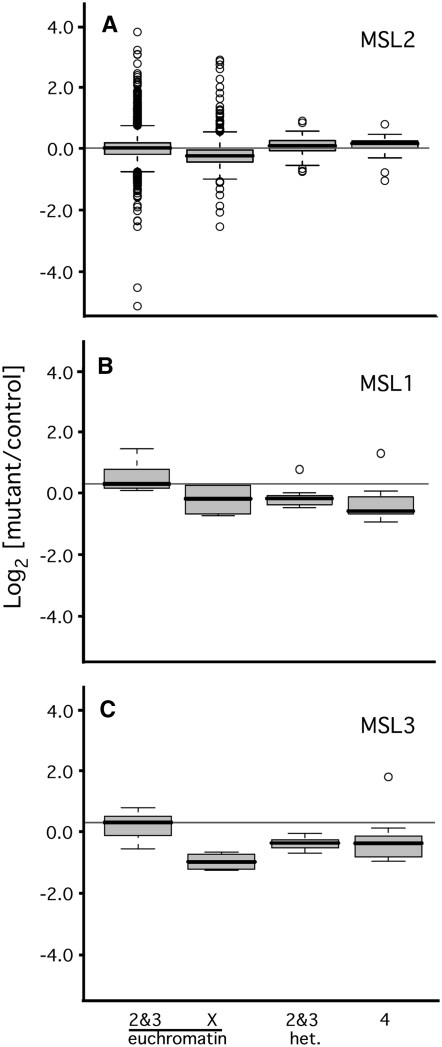
It is possible that the failure of dosage compensation affects fourth-linked and heterochromatic genes indirectly. This might occur by underexpression of critical X-linked factors, or by the global redistribution of chromatin proteins upon disruption of the male X chromosome. To determine if other mutations preventing dosage compensation also reduce expression of fourth-linked and heterochromatic genes, expression was measured in animals lacking different components of the DCC. MSL1 and MSL2 are of central importance to dosage compensation (Copps et al. 1998; Li et al. 2005). All chromatin binding by the remaining DCC proteins is absent in males lacking either MSL1 or MSL2 (Baker et al. 1994; Palmer et al. 1994). Reduced X chromosome expression has been demonstrated in male S2 cells following RNAi knock down of msl2 (Hamada et al. 2005). Examination of data from this study detected no change in fourth-linked or heterochromatic genes (Figure S5). While this is suggestive, it is possible that RNAi knock down is incomplete, or that MSL2 acts transiently to establish a male-specific configuration of heterochromatin. To address these concerns, microarrays were hybridized to probes from msl21 male larvae and msl21/+ controls. MSL2 is not maternally deposited, therefore msl21 larvae lack this protein entirely (Rastelli et al. 1995). Expression from the X chromosome was reduced by 21% in male msl21 larvae (Bonferroni corrected P-value < 2.2 × 10−16; Figure 4A). While this is less than the 50% reduction expected for dosage compensation failure, it compares well with the 22% decrease upon MSL2 knock down in S2 cells (Hamada et al. 2005). By contrast, expression of heterochromatic and fourth-linked genes appears unchanged or slightly increased in msl21 males (0.07- and 0.11-fold, respectively). We conclude that the intact DCC is not necessary for full expression of fourth-linked and heterochromatic genes.
Figure 4.—
MSL1 and MSL3, but not MSL2, are required for full expression of autosomal genes. (A) Fourth-linked and heterochromatic genes are not misregulated in msl21 male larvae. Box plots represent the log2 (mutant:control) expression of the indicated groups of genes. Expression was measured by hybridizing microarrays with probes generated from msl21 and msl21/+ (control) male larvae. Enrichment for heterochromatic genes is described in materials and methods. (B) Fourth-linked and heterochromatic genes require MSL1 for full expression. Expression of a panel of genes was measured in msl11 males and their heterozygous brothers (controls). Seven euchromatic genes on the second and third chromosomes; 4 euchromatic X-linked genes, 12 fourth-linked genes, and 10 heterochromatic genes on the second and third chromosome were assayed (see Table S1). Expression of heterochromatic and fourth-linked genes differs from euchromatic genes on the second and third chromosomes at the 0.003 and 0.002 confidence level, respectively. (C) Fourth-linked and heterochromatic genes require MSL3 for full expression. Expression of a panel of genes was measured in msl32 males and their heterozygous brothers (controls). Seven euchromatic genes on the second and third chromosomes, 4 euchromatic X-linked genes, 13 fourth-linked genes, and 10 heterochromatic genes were measured. Expression of heterochromatic and fourth-linked genes differs significantly from euchromatic genes on the second and third chromosomes at the 0.032 and 0.036 confidence levels. Dmn was used to normalize amplification. Box plots were generated using Bioconductor R. See materials and methods for details of statistical analyses.
To determine the role of other key MSL proteins, we examined the expression of a panel of autosomal and fourth-linked genes in msl11 and msl11/+ male larvae. Expression of fourth-linked genes decreased by 38% in comparison with euchromatic genes on the second and third chromosomes, and genes situated in heterochromatic regions were reduced by 33% (Figure 4B). These changes are significant at the 0.003 and 0.002 level when evaluated using a two-tailed Mann–Whitney U test (see materials and methods for details). Expression was also measured in msl32 males and their msl32/+ brothers (Figure 4C). Expression from fourth-linked genes decreased by 24%, and expression from heterochromatic genes decreased by 36% in msl32 males. These changes are statistically significant at the 0.032 and 0.036 level. Taken together, these findings indicate that some, but not all, of the MSL proteins are necessary for full expression of fourth-linked and heterochromatic genes in male flies.
roX RNA contributes to normal heterochromatin function in males:
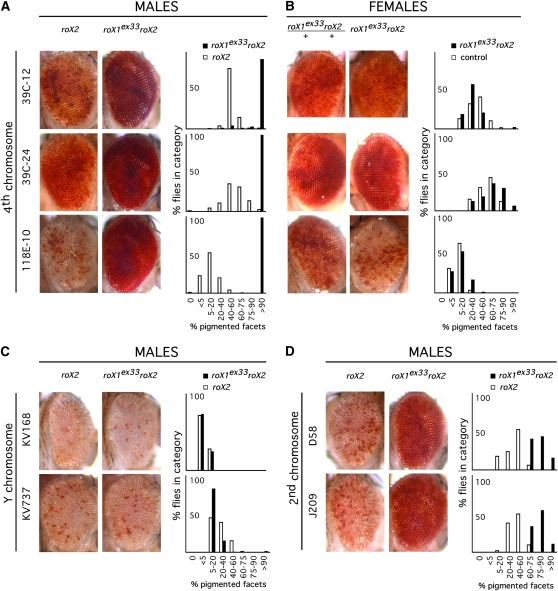
Transgenes inserted in heterochromatin display a variegated silencing (PEV) (Wallrath and Elgin 1995). Modulation of this silencing is a sensitive reporter for local chromatin architecture and has been used to identify mutations that affect heterochromatin function. As loss of roX influences the expression of genes situated in heterochromatic regions, we suspected that roX1 roX2 chromosomes might also affect PEV. Adult male roX1ex33AroX2 and roX2 (control) flies that carry transgenes displaying PEV were generated. roX1ex33A is a partial loss-of-function mutation that supports ∼50% survival of males lacking roX2 (Deng et al. 2005). PEV was detected by expression of the white (w) eye color marker. Increased silencing, or enhancement of PEV, results in fewer red facets in the eye, and decreased silencing, or suppression of PEV, results in more red pigmentation. The expression of w from two different insertions on the Y chromosome is similar in roX1ex33AroX2 and control roX2 males (Figure 5C). In contrast, expression from three insertions on the fourth chromosome is dramatically increased in roX1ex33AroX2 males, but not in females (Figure 5, A and B). The modifying effect of roX1 roX2 on PEV is therefore limited to males. As was observed for other roX phenotypes, suppression of PEV requires simultaneous mutation of both roX genes (data not shown). Expression from insertions on the second chromosome is modestly increased in roX1ex33AroX2 males (Figure 5D). The roX1ex33AroX2 chromosome is therefore a potent suppressor of PEV, but suppression depends on the sex of the fly as well as the position of the variegating transgene.
Figure 5.—
A roX1 roX2 chromosome modifies position effect variegation (PEV) in males. (A) roX1ex33AroX2 suppresses variegation of a w+ marker inserted on the fourth chromosome of males. Control males carry a wild-type roX1 gene. (B) roX1ex33AroX2 females and control females with variegating fourth-linked insertions. Control females are roX1ex33AroX2/++. (C) roX1ex33AroX2 does not modify PEV of insertions on the Y chromosome. (D) roX1ex33AroX2 suppresses variegation of insertions in proximal heterochromatin of the second chromosome in males. Representative eyes are presented beside a histogram illustrating the range of pigmentation in roX1ex33AroX2 (black) and control (white). Details of fly genotypes are presented in materials and methods.
DISCUSSION
Our findings suggest that roX RNA participates in two distinct biological processes that coordinate regulation of large regions; X chromosome dosage compensation and normal heterochromatin function. One of the most striking and unexpected features of our study is the male specificity of roX involvement at heterochromatin. In light of this, it is interesting that mutations in heterochromatin proteins do present sex-biased phenotypes. Depletion of HP1, a major component of heterochromatin, causes higher male lethality and considerably more gene misregulation in males (Liu et al. 2005). The same study identified differences in HP1 distribution in males and females. Our findings suggest that these differences may arise from the fact that heterochromatin itself is different in males and females. The genetic control of heterochromatin has been the subject of many screens for modifiers of PEV. Neither msl nor roX genes have been identified by this method. Simultaneous mutation of both roX genes is required to suppress PEV, making their identification through random mutagenesis highly unlikely (data not shown). In addition, loss of any MSL protein is lethal in males, the sex in which modification of PEV would be expected. It is thus unsurprising that the roX and msl genes have not appeared in screens for modifiers of PEV.
The observation that roX RNA is required for normal heterochromatin function in males is particularly intriguing in light of previous studies suggesting links between dosage compensation and heterochromatin. HP1 is modestly enriched on the male X chromosome and mutation of HP1 or Su(var)3-7, an HP1 binding partner, disrupts the structure of the polytenized male X chromosome (de Wit et al. 2005; Spierer et al. 2005, 2008). The JIL-1 kinase is genetically linked to dosage compensation and also enriched on the male X chromosome (Lerach et al. 2005). JIL-1 mutations suppress PEV in pericentromeric regions and permit proximal heterochromatin to spread into euchromatic regions (Ebert et al. 2004; Lerach et al. 2006; Zhang et al. 2006). While JIL-1 is a plausible link between dosage compensation and heterochromatin, the effect of JIL-1 mutation on heterochromatin and PEV is observed in both sexes.
Although the molecular basis of roX regulation of autosomal genes is currently speculative, we have eliminated the most plausible sources of an indirect effect. MSL1 and MSL2 directly interact and both are thought central to chromatin recognition by the DCC. However, MSL1 is necessary for full expression of heterochromatic genes in males but MSL2 is not. This is inconsistent with the idea that heterochromatic genes are misregulated by a redistribution of chromatin proteins following the failure of dosage compensation. Our studies reinforce the notion that the intact DCC, containing MSL2, is dedicated to recognition of the X chromosome. It is interesting that a short sequence motif recognized by the DCC is enriched on the X chromosome but depleted from the fourth chromosome (Alekseyenko et al. 2008). This suggests selective pressure to prevent inappropriate binding of the DCC to the fourth chromosome and supports our conclusion that the intact DCC does not regulate fourth-linked genes.
It is tempting to speculate that roX RNA, MSL1, and MSL3 associate, as they do in the MSL complex. Although efforts to detect roX1 and MSL proteins on the fourth chromosome of polytene preparations have not been successful, it is possible that these molecules have a transient role in heterochromatic regions. While members of the DCC are interdependent in larvae, in early embryos maternally deposited MSL1 and MSL3 are present and stable prior to the zygotic expression of MSL2 at 3 h after egg laying. Similarly, roX transcripts are unstable in larvae lacking any MSL protein, but roX1 produced in early embryos is stable for several hours, even in the absence of MSL2 (Meller et al. 1997; Meller 2003; Rattner and Meller 2004). roX1 is first transcribed >1 hr before dosage compensation is initiated, but just before heterochromatin becomes visible (Vlassova et al. 1991; Lu et al. 1998 ). MSL1, MSL3, and roX1 are therefore present during the initial formation of heterochromatin, making it plausible that they serve a transient role at this time. Expression of MSL2 in males at 3 hr triggers formation of the intact DCC and sequestration of MSL proteins and roX1 RNA to the X chromosome. The window between 1.2 and 3 hr may thus be a critical time during which roX influences heterochromatin structure. This idea is currently under investigation.
The response of variegating insertions to loss of roX RNA depends on the position of the insertion, raising the possibility of chromosome-specific factors that modulate sensitivity. Y-linked insertions are unaffected by loss of roX RNA, consistent with adaptation of the Y chromosome for expression in male germ cells lacking MSL1 and MSL3 (Rastelli and Kuroda 1998). In contrast, suppression of PEV by loss of roX is strongest for insertions on the fourth chromosome. The fourth chromosome has several unusual features. It is composed of interspersed euchromatin and heterochromatin and is thus enriched for the boundaries between these chromatin states (Sun et al. 2000; Yasuhara and Wakimoto 2008). This organization may influence the sensitivity of fourth-linked genes to loss of the roX transcripts. The DNA sequence elements that underlie heterochromatin formation on the fourth chromosome also appear unusual (Riddle et al. 2008). All heterochromatic regions are marked by H3K9me, which creates a binding site for HP1. While Su(var)3-9 is responsible for the majority of H3K9me deposition throughout the rest of the genome, the dSETDB1 methyltransferase localizes to the fourth chromosome and is responsible for H3K9me accumulation there (Seum et al. 2007; Tzeng et al. 2007). Knock down of ISWI in S2 cells leads to a modest decrease in expression of fourth-linked genes (Bonaldi et al. 2008). This is interesting as the dosage-compensated male X chromosome is particularly sensitive to loss of ISWI (Corona et al. 2002). Finally, PEV of insertions on the distal fourth chromosome are modified by the dose of the fourth chromosome, while insertions on the second chromosome are not (Haynes et al. 2007). This reinforces the idea that factors required for fourth chromosome heterochromatin differ from other heterochromatic regions. At present no evidence suggests that these biochemical features are male limited, but they do support the notion that the structure and regulation of the fourth chromosome is unusual. These differences may render the fourth chromosome particularly sensitive to loss of the roX RNAs.
The observation that heterochromatic genes with similar expression in males and females are differentially regulated raises the question of why this difference exists. A clue may lie in the Y chromosome. The Y chromosome represents 12% of the male genome and is entirely heterochromatic. It has far-reaching affects on other heterochromatic regions because it absorbs a large portion of the proteins that assemble into heterochromatin. Loss of the Y chromosome frees these proteins and enables them to bind elsewhere, thus promoting heterochromatin formation and enhancing PEV throughout the nucleus (Weiler and Wakimoto 1995). As a result, loss of the Y chromosome silences transgenes in proximal heterochromatin and on the fourth chromosome. Loss of roX RNA has the opposite effect, increasing expression from these transgenes. Indeed, the partial loss of function roX1ex33AroX2 chromosome largely restores expression from a variegating fourth chromosome insertion in males lacking a Y chromosome (data not shown). roX and the Y chromosome thus exert opposing influences on heterochromatic silencing.
Dosage compensation is essential in animals with highly differentiated X and Y chromosomes. During the evolution of sex chromosome pairs, the Y chromosome irreversibly loses coding potential and accumulates repetitive sequences, which, in turn, promotes the formation of heterochromatin (Rice 1996). Although the precise origin of the D. melanogaster Y chromosome is debatable, it is similar to mammalian Y chromosomes in being gene poor and heterochromatin rich (Carvalho 2002). In spite of the evidence that the Drosophila Y chromosome exerts a far-reaching influence throughout the nucleus, a mechanism that counteracts the effects of the Y chromosome has never been identified. The roX-dependent modulation of heterochromatin that we have observed is male limited and it influences PEV in a manner opposite to that of the Y chromosome. It thus displays two key features expected for a system that accommodates Y heterochromatin. This model places the roX RNAs in two different domainwide regulatory systems: dosage compensation of the X chromosome and modulation of heterochromatin in males. It is intriguing that both processes serve to accommodate different problems resulting from sex chromosome differentiation.
Acknowledgments
We are grateful to A. Tarca and C. Freeman for statistical analysis, advice, and patience, and we thank Dan Lott of the Applied Genomics and Technology Center at Wayne State University for expert assistance with microarray studies. We thank S. Elgin, J. Larsson, K. Maggert, G. Karpen, and the Bloomington Stock Center for Drosophila strains. Antibodies were gifts from J. Larsson and M. I. Kuroda. This study was supported by start-up funds from Wayne State University, a Graduate Enhancement Research Assistantship to Y.K., graduate enhancement research support to S.K.K., and National Science Foundation award 0641121.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.102087/DC1.
References
- Akhtar, A., and P. B. Becker, 2000. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell 5 367–375. [DOI] [PubMed] [Google Scholar]
- Alekseyenko, A. A., S. Peng, E. Larschan, A. A. Gorchakov, O.-K. Lee et al., 2008. A sequence motif within chromatin entry sites directs MSL establishment on the Drosophila X chromosome. Cell 134 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, B. S., M. Gorman and I. Marin, 1994. Dosage compensation in Drosophila. Annu. Rev. Genet. 28 491–521. [DOI] [PubMed] [Google Scholar]
- Bonaldi, T., T. Straub, J. Cox, C. Kumar, P. B. Becker et al., 2008. Combined use of RNAi and quantitative proteomics to study gene function in Drosophila. Mol. Cell 31 762–772. [DOI] [PubMed] [Google Scholar]
- Carvalho, A. B., 2002. Origin and evolution of the Drosophila Y chromosome. Curr. Opin. Genet. Dev. 12 664–668. [DOI] [PubMed] [Google Scholar]
- Charlesworth, B., 1991. The evolution of sex chromosomes. Science 251 1030–1033. [DOI] [PubMed] [Google Scholar]
- Copps, K., R. Richman, L. M. Lyman, K. A. Chang, J. Rampersad-Ammons et al., 1998. Complex formation by the Drosophila MSL proteins: role of the MSL2 RING finger in protein complex assembly. EMBO J. 17 5409–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona, D. F., C. R. Clapier, P. B. Becker and J. W. Tamkun, 2002. Modulation of ISWI function by site-specific histone acetylation. EMBO Rep. 3 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit, E., F. Greil and B. van Steensel, 2005. Genome-wide HP1 binding in Drosophila: developmental plasticity and genomic targeting signals. Genome Res. 15 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X., and V. H. Meller, 2006. roX RNAs are required for increased expression of X-linked genes in Drosophila melanogaster males. Genetics 174 1859–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X., B. P. Rattner, S. Souter and V. H. Meller, 2005. The severity of roX1 mutations are predicted by MSL localization on the X chromosome. Mech. Dev. 122 1094–1105. [DOI] [PubMed] [Google Scholar]
- Ebert, A., G. Schotta, S. Lein, S. Kubicek, V. Krauss et al., 2004. Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev. 18 2973–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier, L., L. Cope, B. M. Bolstad and R. A. Irizarry, 2004. affy: Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20 307–315. [DOI] [PubMed] [Google Scholar]
- Gupta, V., M. Parisi, D. Sturgill, R. Nuttall, M. Doctolero et al., 2006. Global analysis of X Chromosome compensation. J. Biol. 5 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada, F. N., P. J. Park, P. R. Gordadze and M. I. Kuroda, 2005. Global regulation of X chromosomal genes by the MSL complex in Drosophila melanogaster. Genes Dev. 19 2289–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, K. A., E. Gracheva and S. C. R. Elgin, 2007. A distinct type of heterochromatin within Drosophila melanogaster chromosome 4. Genetics 175 1539–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins, R. A., J. W. Carlson, C. Kennedy, D. Acevedo, M. Evans-Holm et al., 2007. Sequence finishing and mapping of Drosophila melanogaster heterochromatin. Science 316 1625–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry, R. A., B. Hobbs, F. Collin, Y. D. Beazer-Barclay, K. J. Antonellis et al., 2003. Exploration, normalization and summaries of high density oligonucleotide array probe level data. Biostatistics 4 249–264. [DOI] [PubMed] [Google Scholar]
- Johansson, A.-M., P. Stenberg, C. Bernhardsson and J. Larsson, 2007. Painting of fourth and chromosome-wide regulation of the 4th chromosome in Drosophila melanogaster. EMBO J. 26 2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, R. L., V. H. Meller, P. R. Gordadze, G. Roman, R. L. Davis et al., 1999. Epigenetic spreading of the Drosophila dosage compensation complex from roX RNA genes into flanking chromatin. Cell 98 513–522. [DOI] [PubMed] [Google Scholar]
- Kelley, R. L., I. Solovyeva, L. M. Lyman, R. Richman, V. Solovyev et al., 1995. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell 81 867–877. [DOI] [PubMed] [Google Scholar]
- Kind, J., and A. Akhtar, 2007. Cotranscriptional recruitment of the dosage compensation complex to X-linked target genes. Genes Dev. 21 2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind, J., J. M. Vaquerizas, P. Gebhardt, M. Gentzel, N. M. Luscombe et al., 2008. Genome-wide analysis reveals MOF as a key regulator of dosage compensation and gene expression in Drosophila. Cell 133 813–828. [DOI] [PubMed] [Google Scholar]
- Kotlikova, I. V., O. V. Demakova, V. F. Semeshin, V. V. Shloma, L. V. Boldyreva et al., 2006. The Drosophila dosage compensation complex binds to polytene chromosomes independently of developmental changes in transcription. Genetics 172 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larschan, E., A. A. Alekseyenko, A. A. Gortchakov, S. Peng, B. Li et al., 2007. MSL complex is attracted to genes marked by H3K36 trimethylation using a sequence-independent mechanism. Mol. Cell 28 121–133. [DOI] [PubMed] [Google Scholar]
- Larsson, J., J. D. Chen, V. Rasheva, A. Rasmuson-Lestander and V. Pirrotta, 2001. Painting of fourth, a chromosome-specific protein in Drosophila. Proc. Natl. Acad. Sci. USA 98 6273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, J., and V. H. Meller, 2006. Dosage compensation, the origin and afterlife of sex chromosomes. Chromosome Res. 14 417–431. [DOI] [PubMed] [Google Scholar]
- Larsson, J., M. J. Svensson, P. Stenberg and M. Makitalo, 2004. Painting of fourth in genus Drosophila suggests autosome-specific gene regulation. Proc. Natl. Acad. Sci. USA 101 9728–9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerach, S., W. Zhang, X. Bao, H. Deng, J. Girton et al., 2006. Loss of function alleles of the JIL-1 kinase are strong suppressors of position effect variegation of the wm4 allele in Drosophila. Genetics 173 2403–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerach, S., W. Zhang, H. Deng, X. Bao, J. Girton et al., 2005. JIL-1 kinase, a member of the Male-specific lethal (MSL) complex, is necessary for proper dosage compensation of eye pigmentation in Drosophila. Genesis 43 213–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., D. A. Parry and M. J. Scott, 2005. The amino-terminal region of Drosphila MSL1 contains basic, glycine-rich and leucine zipper-like motifs that promote X chromosome binding, self-association and MSL2 binding, respectively. Mol. Cell. Biol. 25 8913–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, D. L., and G. G. Zimm, 1992. The Genome of Drosophila melanogaster. Academic Press, San Diego.
- Liu, L.-P., J.-Q. Ni, Y.-D. Shi, E. J. Oakeley and F.-L. Sun, 2005. Sex-specific role of Drosophila melanogaster HP1 in regulating chromatin structure and gene transcription. Nat. Genet. 37 1361–1366. [DOI] [PubMed] [Google Scholar]
- Liu, T.-Y., C. W. Lin, S. Falcon, J. Zhang and J. W. MacDonald, 2007. drosophila2: Affymetrix Drosophila Genome 2.0 Array Annotation Data (drosophila2). R packager version 2.2.0.
- Lu, B. Y., J. Ma and J. C. Eissenberg, 1998. Developmental regulation of heterochromatin-mediated gene silencing in Drosophila. Development 125 2223–2234. [DOI] [PubMed] [Google Scholar]
- Lucchesi, J. C., W. G. Kelly and B. Panning, 2005. Chromatin remodeling in dosage compensation. Annu. Rev. Genet. 39 615–651. [DOI] [PubMed] [Google Scholar]
- Maggert, K. A., and K. G. Golic, 2002. The Y chromosome of Drosophila melanogaster exhibits chromosome-wide imprinting. Genetics 162 1245–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller, V. H., 2003. Initiation of dosage compensation in Drosophila embryos depends on expression of the roX RNAs. Mech. Dev. 120 759–767. [DOI] [PubMed] [Google Scholar]
- Meller, V. H., and B. P. Rattner, 2002. The roX genes encode redundant male-specific lethal transcripts required for targeting of the MSL complex. EMBO J. 21 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller, V. H., K. H. Wu, G. Roman, M. I. Kuroda and R. L. Davis, 1997. roX1 RNA paints the X chromosome of male Drosophila and is regulated by the dosage compensation system. Cell 88 445–457. [DOI] [PubMed] [Google Scholar]
- Nguyen, D. K., and C. M. Disteche, 2006. Dosage compensation of the active X chromosome in mammals. Nat. Genet. 38 47–53. [DOI] [PubMed] [Google Scholar]
- Palmer, M. J., R. Richman, L. Richter and M. I. Kuroda, 1994. Sex-specific regulation of the male-specific lethal-1 dosage compensation gene in Drosophila. Genes Dev. 8 698–706. [DOI] [PubMed] [Google Scholar]
- Parisi, M., R. Nuttall, D. Naiman, G. Bouffard, J. Malley et al., 2003. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science 299 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl, M. W., 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29 e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastelli, L., and M. I. Kuroda, 1998. An analysis of maleless and histone H4 acetylation in Drosophila melanogaster spermatogenesis. Mech. Dev. 71 107–117. [DOI] [PubMed] [Google Scholar]
- Rastelli, L., R. Richman and M. I. Kuroda, 1995. The dosage compensation regulators MLE, MSL-1 and MSL-2 are interdependent since early embryogenesis in Drosophila. Mech. Dev. 53 223–233. [DOI] [PubMed] [Google Scholar]
- Rattner, B. P., and V. H. Meller, 2004. Drosophila Male Specific Lethal 2 protein controls male-specific expression of the roX genes. Genetics 166 1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, W. R., 1996. Evolution of the Y sex chromosome in animals. Bioscience 46 331–343. [Google Scholar]
- Riddle, N. C., and S. C. R. Elgin, 2006. The dot chromosome of Drosophila: insights into chromatin states and their change over evolutionary time. Chromosome Res. 14 405–416. [DOI] [PubMed] [Google Scholar]
- Riddle, N. C., W. Leung, K. A. Haynes, H. Granok, J. Wuller et al., 2008. An investigation of heterochromatin domains on the fourth chromosome of Drosophila melanogaster. Genetics 178 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seum, C., E. Reo, H. Peng, F. J. Rauscher, P. Spierer et al., 2007. Drosophila SETDB1 is required for chromosome 4 silencing. PLoS Genet. 3 e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. D., S. Shu, C. J. Mungall and G. H. Karpen, 2007. The release 5.1 annotation of Drosophila melanogaster heterochromatin. Science 316 1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, G. K., 2005. Limma: linear models for microarray data, pp. 397–420 in Bioinformatics and Computational Biology Solutions Using R and ‘Bioconductor,’ edited by R. Gentleman, V. Carey, S. Dudoit, R. Irizarry and W. Huber. Springer, New York.
- Spierer, A., F. Begot, P. Spierer and M. Delattre, 2008. Su(var)3–7 links heterochromatin and dosage compensation in Drosophila. PLoS Genet. 4 e1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierer, A., C. Seum, M. Delattre and P. Spierer, 2005. Loss of the modifiers of variegation Su(var)3–7 or HP1 impacts male X polytene chromosome morphology and dosage compensation. J. Cell Sci. 118 5047–5057. [DOI] [PubMed] [Google Scholar]
- Sun, F. L., M. H. Cuaycong, C. A. Craig, L. L. Wallrath, J. Locke et al., 2000. The fourth chromosome of Drosophila melanogaster: interspersed euchromatic and heterochromatic domains. Proc. Natl. Acad. Sci. USA 97 5340–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, C. J., D. G. Mets, M. R. Albrecht, P. Nix, A. Chan et al., 2008. Meiotic crossover number and distribution are regulated by a dosage compensation protein that resembles a condensin subunit. Genes Dev. 22 194–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng, T.-Y., C.-H. Lee, L.-W. Chan and J. Shen, 2007. Epigenetic regulation of the Drosophila chromosome 4 by the histone H3K9 methyltransferase dSETDB1. Proc. Natl. Acad. Sci. USA 104 12691–12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassova, I. E., A. S. Graphodatsky, E. S. Belyaeva and I. F. Zhimulev, 1991. Constitutive heterochromatin in early embryogenesis of Drosophila melanogaster. Mol. Gen. Genet. 229 316–318. [DOI] [PubMed] [Google Scholar]
- Wallrath, L. L., and S. C. R. Elgin, 1995. Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev. 9 1263–1277. [DOI] [PubMed] [Google Scholar]
- Weiler, K. S., and B. T. Wakimoto, 1995. Heterochromatin and gene expression in Drosophila. Annu. Rev. Genet. 29 577–605. [DOI] [PubMed] [Google Scholar]
- Yan, C. M., K. W. Dobie, H. D. Le, A. Y. Konev and G. H. Karpen, 2002. Efficient recovery of centric heterochromatin P-element insertions in Drosophila melanogaster. Genetics 161 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara, J. C., and B. T. Wakimoto, 2006. Oxymoron no more: the expanding world of heterochromatic genes. Trends Genet. 22 330–338. [DOI] [PubMed] [Google Scholar]
- Yasuhara, J. C., and B. T. Wakimoto, 2008. Molecular landscape of modified histones in Drosophila heterochromatic genes and euchromatin-heterochromatin transition zones. PLoS Genet. 4 159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., H. Deng, X. Bao, S. Lerach, J. Girton et al., 2006. The JIL-1 histone H3S10 kinase regulates dimethyl H3K9 modifications and heterochromatic spreading in Drosophila. Development 133 229–235. [DOI] [PubMed] [Google Scholar]