Abstract
Modulating gene dose is an effective way to alter protein levels and modify phenotypes to understand gene function. In addition, combining gene-dose alleles with chemical perturbation can provide insight into drug–gene interactions. Here, we present a strategy that combines diverse loss-of-function alleles to systematically modulate gene dose in Saccharomyces cerevisiae. The generated gene dosage allele set expands the genetic toolkit for uncovering novel phenotypes.
ALTERING gene dose is a well-established means to perturb and thereby infer gene function. In yeast, different alleles that manifest different gene doses such as homozygous deletions (Lee et al. 2005), heterozygous deletions (Giaever et al. 2002), overexpression alleles (Sopko et al. 2006), and other conditional alleles (Mnaimneh et al. 2004; Ben-Aroya et al. 2008) have been used toward this end. Altering gene dose alone is not, however, always sufficient to induce a detectable phenotypic change. For example, ∼64% of homozygous yeast deletion strains and 97% of heterozygote yeast deletion strains manifest wild-type fitness in optimal growth conditions (Winzeler et al. 1999; Giaever et al. 2002; Deutschbauer et al. 2005), suggesting that additional perturbations may be required to uncover a phenotype. This limitation can be circumvented by combining gene-dose alleles with chemical perturbation. A recent study reported that nearly all gene deletions can exhibit a condition-dependent growth phenotype (Hillenmeyer et al. 2008), supporting the hypothesis that chemical perturbation combined with genetic lesions can be a generally useful approach.
To combine gene dose and compound dose to identify gene function one requires a set of well-annotated compounds with known mechanisms of action. Unfortunately, the protein targets of many compounds are still, in large part, unknown and compound selection remains a challenge. Although several yeast genomics-based strategies have been developed to identify drug targets (Sturgeon et al. 2006), each has certain limitations. For example, haploinsufficiency profiling (HIP) represents an assay with the potential to reveal drug targets (Giaever et al. 1999, 2002, 2004; Baetz et al. 2004; Lum et al. 2004; Doostzadeh et al. 2007) but it can fail to identify drug targets if the reduction in gene dose is insufficient. A new pooled assay uses a collection of Decreased Abundance by mRNA Perturbation (DAmP) loss-of-function alleles to identify drug targets (Yan et al. 2008). “DAmPing” involves disrupting the 3′-untranslated region immediately downstream of a gene's stop codon, resulting in the destabilization of the gene's mRNA and decreasing the amount of protein. In practice, protein abundance is decreased to ∼5–50% of wild-type levels (Schuldiner et al. 2005). The DAmP assay can be more sensitive than the HIP assay and has identified several drug targets that were missed by the HIP assay. The DAmP assay is not without limitations. First, only 87.1% of yeast essential genes are represented as DAmP alleles, and the remaining 13% of essential genes are probably inviable as DAmP alleles. Second, of the 958 viable DAmP alleles, 17% manifest fitness defects in rich media, potentially complicating competitive growth assays. Interestingly, when we analyzed DAmP-inviable and DAmP-sick strains and compared them to haploinsufficient heterozygous strains, we found that 47.6% (59/124) of slow-growing heterozygous strains were also slow growing as DAmP strains, suggesting that haploinsufficiency and “DAmP insufficiency” share mechanistic similarities.
We reasoned that, by combining heterozygous alleles with DAmP alleles, we could produce cells or “allele sets” that span a wide range of gene doses and thereby improve the sensitivity of genome-based assays. Toward this end, we developed a strategy to systematically adjust gene dose in yeast. As a proof-of-principle, we created four alleles for several well-characterized essential genes in which gene dose was modulated from (on average) 5 to 100% that of wild type. These strains showed gene-dose-dependent, drug-sensitive phenotypes and experiments with these allele sets using reference compounds show that modulating gene dose, when combined with compound titrations, provides a highly sensitive means to dissect drug–gene interactions.
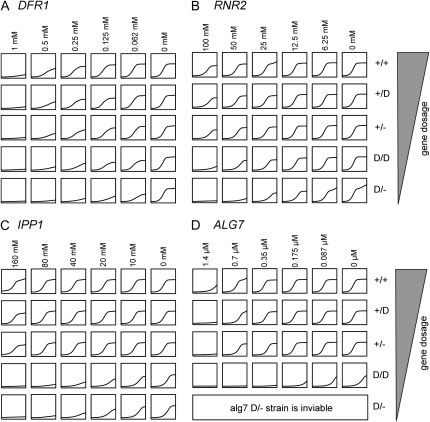
These gene-dose allele sets comprise five diploid strains (wild type and four mutants), each with a different gene dose: (1) a wild-type diploid strain (WT), (2) a diploid DAmP strain (D/+) that contains one wild-type allele and one DAmP allele, (3) a heterozygous diploid strain (−/+) that contains only one wild-type allele, (4) a diploid double DAmP strain (D/D) that contains two DAmP alleles, and (5) a hemizygous DAmP strain (−/D) that only contains a single DAmP allele. The gene doses of these five strains are predicted to be 1, 0.525–0.75, 0.5, 0.05–0.5, and 0.025–0.25, respectively. These gene doses are based on the following assumptions: (1) heterozygous alleles will be, on average, 50% of the WT gene dose, and a typical DAmP allele will produce 5–50% of wild-type protein levels (Schuldiner et al. 2005). The actual gene dose for any one strain will, of course, deviate from these ideal expectations. We created four gene-dose alleles for the following genes: DFR1, ALG7, RNR2, and IPP1. Of 20 possible strains (Table S1), we were able to create 19 and failed to create the alg7 −/D strain, most likely due to the fact that this allele produces too little protein for viability. Indeed the alg7 D/D strain is quite sick, suggesting that this strain produces the minimum amount of gene product required to sustain viability (Figure 1D). All strains were confirmed by PCR with primers flanking the stop codon of each gene and primers flanking the antibiotic gene junction region (Figure S1).
Figure 1.—
Drug sensitivity of gene-dose strains. Each column represents a different drug concentration with concentration indicated at the top. Each row represents a different gene-dose strain with the strain genotype indicated at the right. (A) Growth curve of different DFR1 gene-dose strains. Each strain was grown in YPD medium with or without methotrexate. (B) Growth curve of different RNR2 gene-dose strains. Each strain was grown in YPD medium with or without hydroxyurea. (C) Growth curve of different IPP1 gene-dose strains. Each strain was grown in YPD medium with or without NaF. (D) Growth curve of different ALG7 gene-dose strains. Each strain was grown in YPD medium with or without tunicamycin. Strains and genotypes are listed in Table S1. The diploid parental wild-type strain is BY4743. All DAmP and heterozygous strains were created as described (Giaever et al. 2002; Schuldiner et al. 2005). Primers used to create gene-dose strains are listed in Table S2. Each primer contains 40 bases of homology to its target gene and 18 bases of homology to the KanMX4 or NatMX4 cassette. To create double DAmP strains, two haploid DAmP strains were created, one with the 3′-UTR disrupted using a KanMX4 cassette in strain BY4741 and the other with a NatMX4 cassette in strain BY4742. Haploid DAmP strains were mated and diploids selected on YPD medium containing 250 mg/liter of G418 (AgriBio, no. 3000) and 100 mg/liter of nourseothricin (Werner Bioagents). The hemizygous DAmP strains (D/− allele) were constructed by inserting the NatMX4 cassette immediately after the stop codon of the gene of interest in a heterozygous strain and were selected on YPD medium containing 250 mg/liter of G418 and 100 mg/liter of nourseothricin.
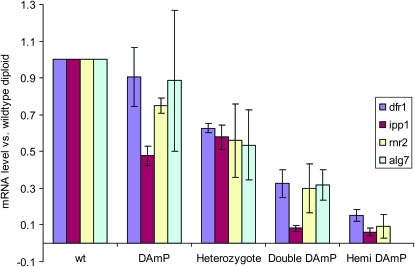
To verify the level of gene dose in each strain, we measured the mRNA levels for each strain using quantitative reverse transcriptase PCR (qRT–PCR). As shown in Figure 2, the wild-type strain had a full complement of RNA per locus (by definition WT = 100% of WT levels) and each allele in the gene-dose allele set had a decreasing amount of mRNA. Levels follow this trend: the WT strain > D/+ strain > the heterozygous −/+ strain > the D/D strain > −/D strain. To test if these gene-dose sets can be useful for assessing drug sensitivity, we treated each allele set strain with a compound known to inhibit the protein product encoded by the altered locus (Table 1). In each case, strains were more sensitive to drug as a function of gene dose (Figure 1).
Figure 2.—
mRNA expression level in gene-dose strains. The mRNA expression level of each gene was determined by quantitative RT–PCR. Histograms represent the expression level of each gene in the gene-dosage strain relative to that of the wild-type strain. Each sample was assayed in triplicate. The bar in each histogram represents the standard deviation. The ALG7 D/– strain was not viable; therefore only four strains are shown in the ALG7 gene-dose allele set. To measure RNA level, exponentially growing yeast cells were collected and RNA was extracted using the RNeasy mini kit (QIAGEN, Valencia, CA; no. 74104). Ten micrograms of total RNA from each strain were treated with turbo DNase (Ambion, no. AM1907) to remove residual genomic DNA. cDNA was reverse transcribed according to manufacturer's instructions (Invitrogen, Carlsbad, CA). Quantitative real-time PCR was performed using SYBR green chemistry (Applied Biosystems, Foster City, CA; no. 4367660) for 40 cycles at an annealing temperature of 60°. The primers used are listed in Table S2. Each sample was assayed in triplicate. mRNA expression levels were normalized relative to the endogenous gene ACT1. PCR efficiency was corrected using the LinRegPCR protocol (Ramakers et al. 2003). The expression level of each strain relative to the wild-type control was then calculated according to the 2 − ΔΔCt method (Livak and Schmittgen 2001).
TABLE 1.
Compounds and their putative protein targets
| Drugs/compounds | Target |
|---|---|
| Methotrexate | DFR1 |
| NaF | IPP1 |
| Hydroxyurea | RNR2 |
| Tunicamycin | ALG7 |
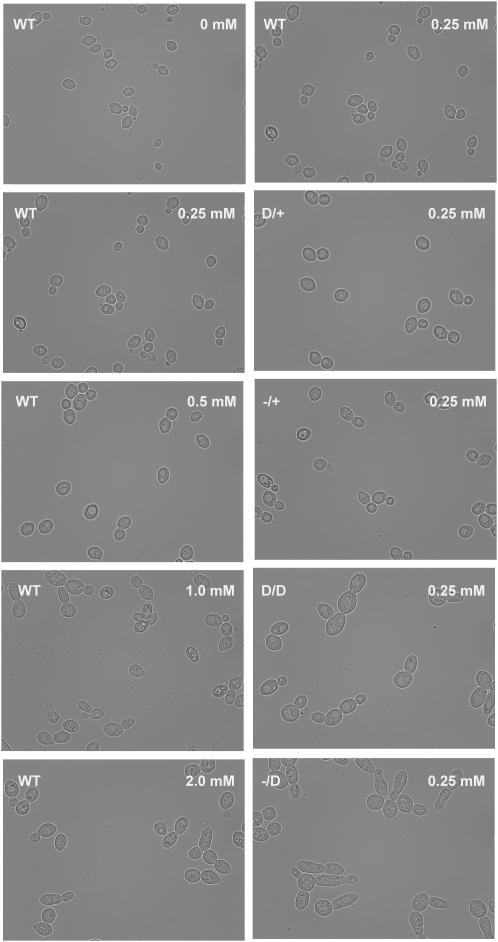
A significant limitation of the current chemical genetics approach is that compounds are often expensive and can be limited in availability. In addition, small molecules can interact with multiple protein targets, which can confound determination of the protein function. To ask if our gene-dose alleles could address some of these limitations, we combined different DFR1 gene-dose alleles with 250 μm methotrexate, a drug that targets DFR1. Methotrexate treatment resulted in a phenotype characterized by enlarged and elongated cells, apparently a result of a defect in cytokinesis. Interestingly, as the gene dose of DFR1 was lowered, this phenomenon became more pronounced (Figure 3). Although similar morphological changes were observed when wild-type cells were treated with methotrexate, they required very high doses (>500 μm) of compound. In addition, the elongated cells in the drug-treated DFR1 −/D strain are more pronounced than those seen in methotrexate-treated wild-type cells, even for WT cells treated by 2 mm of methotrexate. Higher methotrexate doses were lethal for WT yeast cells due to nonspecific toxicity. These results suggest that (1) lowering gene dose either through genetic or chemical perturbation can cause a similar phenotype, (2) gene-dose alleles permit a greater dynamic range within which one can observe a morphological defect, and (3) gene-dose alleles can complement gene–drug interaction studies by amplifying drug-induced phenotypes and can simplify the determination of the appropriate gene dose and drug concentration to amplify the phenotype of interest.
Figure 3.—
Morphological comparison of gene-dosage response to drug-dosage response: morphology of the wild-type strain treated with different concentrations of methotrexate in YPD medium (left column) and morphology of different DFR1 gene-dose strains grown in 250 μm of methotrexate (right column). Cells were grown in YPD with or without methotrexate at 30° for 2 days. Cells were then harvested and observed live with a Zeiss Axiovert 200 microscope using a 63× objective. Images were collected using bright field illumination, using an Axiocam HR.
Acknowledgments
We thank Peter Zandstra for the use of the ABI 7700 RT–PCR machine. This work was supported by grants from the National Genome Research Institute to G.G. and C.N. and from the Canadian Institutes of Health Research to G.G. (MOPS no. 81340) and C.N. (MOPS no. 84305).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.103036/DC1.
References
- Baetz, K., L. McHardy, K. Gable, T. Tarling, D. Reberioux et al., 2004. Yeast genome-wide drug-induced haploinsufficiency screen to determine drug mode of action. Proc. Natl. Acad. Sci. USA 101 4525–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Aroya, S., C. Coombes, T. Kwok, K. A. O'Donnell, J. D. Boeke et al., 2008. Toward a comprehensive temperature-sensitive mutant repository of the essential genes of Saccharomyces cerevisiae. Mol. Cell 30 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschbauer, A. M., D. F. Jaramillo, M. Proctor, J. Kumm, M. E. Hillenmeyer et al., 2005. Mechanisms of haploinsufficiency revealed by genome-wide profiling in yeast. Genetics 169 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doostzadeh, J., R. W. Davis, G. N. Giaever, C. Nislow and J. W. Langston, 2007. Chemical genomic profiling for identifying intracellular targets of toxicants producing Parkinson's disease. Toxicol. Sci. 95 182–187. [DOI] [PubMed] [Google Scholar]
- Giaever, G., D. D. Shoemaker, T. W. Jones, H. Liang, E. A. Winzeler et al., 1999. Genomic profiling of drug sensitivities via induced haploinsufficiency. Nat. Genet. 21 278–283. [DOI] [PubMed] [Google Scholar]
- Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles et al., 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418 387–391. [DOI] [PubMed] [Google Scholar]
- Giaever, G., P. Flaherty, J. Kumm, M. Proctor, C. Nislow et al., 2004. Chemogenomic profiling: identifying the functional interactions of small molecules in yeast. Proc. Natl. Acad. Sci. USA 101 793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenmeyer, M. E., E. Fung, J. Wildenhain, S. E. Pierce, S. Hoon et al., 2008. The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science 320 362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W., R. P. St. Onge, M. Proctor, P. Flaherty, M. I. Jordan et al., 2005. Genome-wide requirements for resistance to functionally distinct DNA-damaging agents. PLoS Genet. 1 e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K. J., and T. D. Schmittgen, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 25 402–408. [DOI] [PubMed] [Google Scholar]
- Lum, P. Y., C. D. Armour, S. B. Stepaniants, G. Cavet, M. K. Wolf et al., 2004. Discovering modes of action for therapeutic compounds using a genome-wide screen of yeast heterozygotes. Cell 116 121–137. [DOI] [PubMed] [Google Scholar]
- Mnaimneh, S., A. P. Davierwala, J. Haynes, J. Moffat, W. T. Peng et al., 2004. Exploration of essential gene functions via titratable promoter alleles. Cell 118 31–44. [DOI] [PubMed] [Google Scholar]
- Ramakers, C., J. M. Ruijter, R. H. Deprez and A. F. Moorman, 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339 62–66. [DOI] [PubMed] [Google Scholar]
- Schuldiner, M., S. R. Collins, N. J. Thompson, V. Denic, A. Bhamidipati et al., 2005. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell 123 507–519. [DOI] [PubMed] [Google Scholar]
- Sopko, R., D. Huang, N. Preston, G. Chua, B. Papp et al., 2006. Mapping pathways and phenotypes by systematic gene overexpression. Mol. Cell 21 319–330. [DOI] [PubMed] [Google Scholar]
- Sturgeon, C. M., D. Kemmer, H. J. Anderson and M. Roberge, 2006. Yeast as a tool to uncover the cellular targets of drugs. Biotechnol. J. 1 289–298. [DOI] [PubMed] [Google Scholar]
- Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson et al., 1999. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285 901–906. [DOI] [PubMed] [Google Scholar]
- Yan, Z., M. Costanzo, L. E. Heisler, J. Paw, F. Kaper et al., 2008. Yeast barcoders: a chemogenomic application of a universal donor-strain collection carrying bar-code identifiers. Nat. Methods 5 719–725. [DOI] [PubMed] [Google Scholar]