Abstract
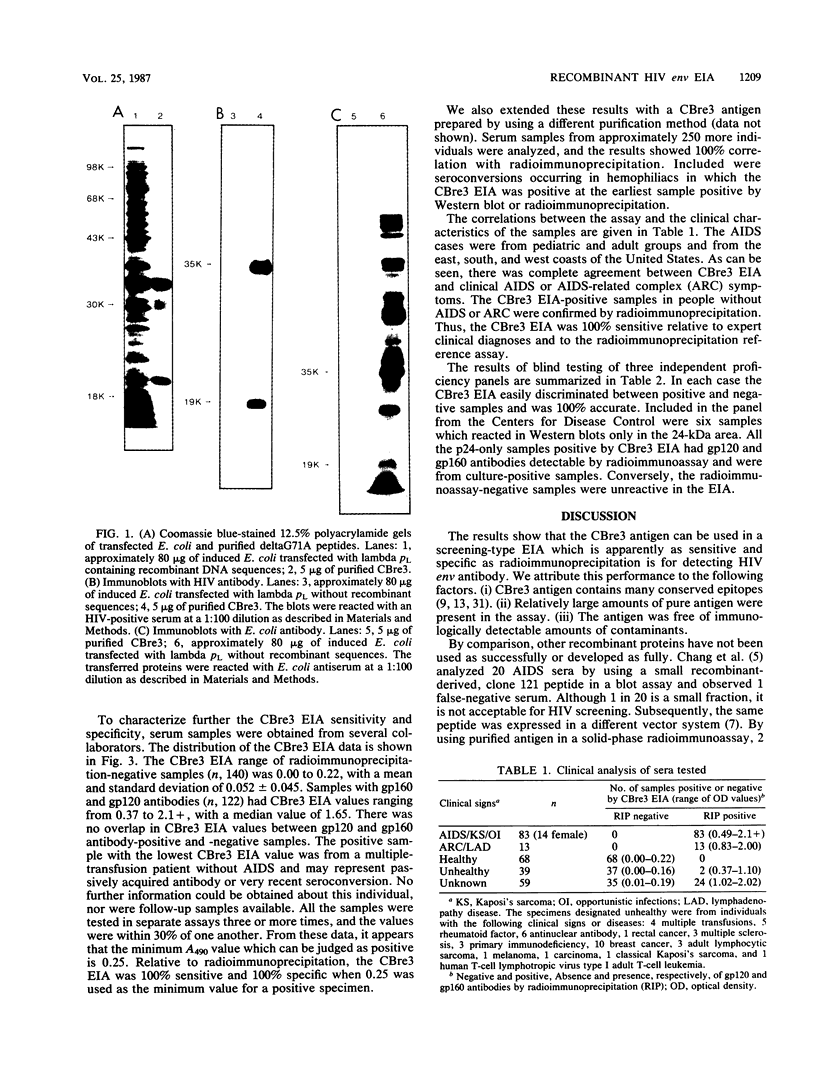
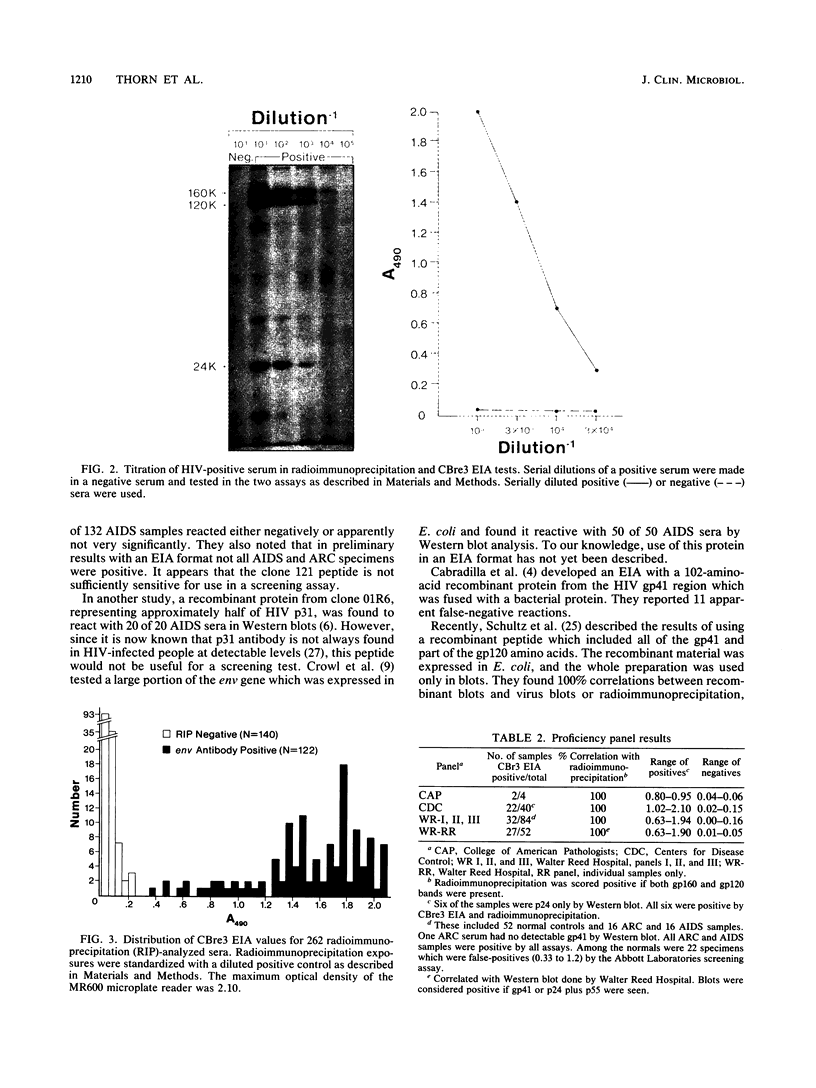
A unique antigen, CBre3, has been synthesized from a genetically engineered clone to detect human immunodeficiency virus (HIV) env antibodies with high sensitivity and specificity. The antigen contains sequences derived from both envelope proteins of HIV, i.e., gp120 and gp41, and was purified free of Escherichia coli proteins detectable by Coomassie stain or immunoblotting with E. coli antiserum. The purified recombinant polypeptides were used as antigen in an enzyme immunoassay (EIA) to screen serum samples from healthy and HIV-infected individuals. The same samples were also tested by radioimmunoprecipitation (RIP) for gp120 and gp160 HIV antibodies. All samples containing gp120 and gp160 antibodies by RIP had CBre3 EIA values greater than 0.35 (n, 122; range, 0.37 to 2.1+; median, 1.65). All RIP HIV antibody-negative samples had CBre3 EIA values less than 0.25 (n, 140; mean, 0.052; standard deviation, 0.045; range, 0.00 to 0.22). The endpoint titer of a standard positive control serum was 1:10,000 by RIP and by CBre3 EIA. The assay was 100% accurate in three proficiency panels. It easily detected six samples from individuals whose infections were confirmed by culture; these samples were reactive only with p24 by Western blot. The samples also were positive for gp120 and gp160 antibodies by RIP. These data suggest that the CBre3 EIA can detect env antibodies as sensitively and specifically as RIP and with more sensitivity than Western blot.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. M. Keeping the AIDS virus out of blood supply. Science. 1986 Aug 1;233(4763):514–515. doi: 10.1126/science.3460177. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Burke D. S., Redfield R. R. False-positive Western blot tests for antibodies to HTLV-III. JAMA. 1986 Jul 18;256(3):347–347. [PubMed] [Google Scholar]
- Chang N. T., Chanda P. K., Barone A. D., McKinney S., Rhodes D. P., Tam S. H., Shearman C. W., Huang J., Chang T. W., Gallo R. C. Expression in Escherichia coli of open reading frame gene segments of HTLV-III. Science. 1985 Apr 5;228(4695):93–96. doi: 10.1126/science.2983429. [DOI] [PubMed] [Google Scholar]
- Chang N. T., Huang J., Ghrayeb J., McKinney S., Chanda P. K., Chang T. W., Putney S., Sarngadharan M. G., Wong-Staal F., Gallo R. C. An HTLV-III peptide produced by recombinant DNA is immunoreactive with sera from patients with AIDS. Nature. 1985 May 9;315(6015):151–154. doi: 10.1038/315151a0. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Crowl R., Ganguly K., Gordon M., Conroy R., Schaber M., Kramer R., Shaw G., Wong-Staal F., Reddy E. P. HTLV-III env gene products synthesized in E. coli are recognized by antibodies present in the sera of AIDS patients. Cell. 1985 Jul;41(3):979–986. doi: 10.1016/s0092-8674(85)80078-7. [DOI] [PubMed] [Google Scholar]
- Esteban J. I., Shih J. W., Tai C. C., Bodner A. J., Kay J. W., Alter H. J. Importance of western blot analysis in predicting infectivity of anti-HTLV-III/LAV positive blood. Lancet. 1985 Nov 16;2(8464):1083–1086. doi: 10.1016/s0140-6736(85)90683-x. [DOI] [PubMed] [Google Scholar]
- Feorino P. M., Kalyanaraman V. S., Haverkos H. W., Cabradilla C. D., Warfield D. T., Jaffe H. W., Harrison A. K., Gottlieb M. S., Goldfinger D., Chermann J. C. Lymphadenopathy associated virus infection of a blood donor--recipient pair with acquired immunodeficiency syndrome. Science. 1984 Jul 6;225(4657):69–72. doi: 10.1126/science.6328663. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., Barin F., M'Boup S., Allan J. S., Romet-Lemonne J. L., Marlink R., McLane M. F., Lee T. H., Arbeille B., Denis F. New human T-lymphotropic retrovirus related to simian T-lymphotropic virus type III (STLV-IIIAGM). Science. 1986 Apr 11;232(4747):238–243. doi: 10.1126/science.3006256. [DOI] [PubMed] [Google Scholar]
- Kanki P. J., McLane M. F., King N. W., Jr, Letvin N. L., Hunt R. D., Sehgal P., Daniel M. D., Desrosiers R. C., Essex M. Serologic identification and characterization of a macaque T-lymphotropic retrovirus closely related to HTLV-III. Science. 1985 Jun 7;228(4704):1199–1201. doi: 10.1126/science.3873705. [DOI] [PubMed] [Google Scholar]
- King N. W. Simian models of acquired immunodeficiency syndrome (AIDS): a review. Vet Pathol. 1986 Jul;23(4):345–353. doi: 10.1177/030098588602300401. [DOI] [PubMed] [Google Scholar]
- Kitchen L. W., Barin F., Sullivan J. L., McLane M. F., Brettler D. B., Levine P. H., Essex M. Aetiology of AIDS--antibodies to human T-cell leukaemia virus (type III) in haemophiliacs. Nature. 1984 Nov 22;312(5992):367–369. doi: 10.1038/312367a0. [DOI] [PubMed] [Google Scholar]
- Kitchen L., Malone G., Orgad S., Barin F., Zaizov R., Ramot B., Gazit E., Kreiss J., Leal M., Wichmann I. Viral envelope protein of HTLV-III is the major target antigen for antibodies in hemophiliac patients. J Infect Dis. 1986 Apr;153(4):788–790. doi: 10.1093/infdis/153.4.788. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange J. M., Coutinho R. A., Krone W. J., Verdonck L. F., Danner S. A., van der Noordaa J., Goudsmit J. Distinct IgG recognition patterns during progression of subclinical and clinical infection with lymphadenopathy associated virus/human T lymphotropic virus. Br Med J (Clin Res Ed) 1986 Jan 25;292(6515):228–230. doi: 10.1136/bmj.292.6515.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Popovic M., Bruch L., Schüpbach J., Gallo R. C. Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science. 1984 May 4;224(4648):506–508. doi: 10.1126/science.6324345. [DOI] [PubMed] [Google Scholar]
- Schulz T. F., Aschauer J. M., Hengster P., Larcher C., Wachter H., Fleckenstein B., Dierich M. P. Envelope gene-derived recombinant peptide in the serodiagnosis of human immunodeficiency virus infection. Lancet. 1986 Jul 12;2(8498):111–112. doi: 10.1016/s0140-6736(86)91648-x. [DOI] [PubMed] [Google Scholar]
- Schüpbach J., Popovic M., Gilden R. V., Gonda M. A., Sarngadharan M. G., Gallo R. C. Serological analysis of a subgroup of human T-lymphotropic retroviruses (HTLV-III) associated with AIDS. Science. 1984 May 4;224(4648):503–505. doi: 10.1126/science.6200937. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Broder S., Essex M., Gallo R. C. Human T-cell leukemia virus: its discovery and role in leukemogenesis and immunosuppression. Adv Intern Med. 1984;30:1–27. [PubMed] [Google Scholar]
- Steimer K. S., Higgins K. W., Powers M. A., Stephans J. C., Gyenes A., George-Nascimento C., Luciw P. A., Barr P. J., Hallewell R. A., Sanchez-Pescador R. Recombinant polypeptide from the endonuclease region of the acquired immune deficiency syndrome retrovirus polymerase (pol) gene detects serum antibodies in most infected individuals. J Virol. 1986 Apr;58(1):9–16. doi: 10.1128/jvi.58.1.9-16.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulstrup J. C., Skaug K., Figenschau K. J., Orstavik I., Bruun J. N., Petersen G. Sensitivity of western blotting (compared with ELISA and immunofluorescence) during seroconversion after HTLV-III infection. Lancet. 1986 May 17;1(8490):1151–1152. doi: 10.1016/s0140-6736(86)91862-3. [DOI] [PubMed] [Google Scholar]
- Wang J. J., Steel S., Wisniewolski R., Wang C. Y. Detection of antibodies to human T-lymphotropic virus type III by using a synthetic peptide of 21 amino acid residues corresponding to a highly antigenic segment of gp41 envelope protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6159–6163. doi: 10.1073/pnas.83.16.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Rutledge R. A., Dias S., Folks T., Theodore T., Buckler C. E., Martin M. A. Identification of conserved and divergent domains within the envelope gene of the acquired immunodeficiency syndrome retrovirus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5038–5042. doi: 10.1073/pnas.83.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]