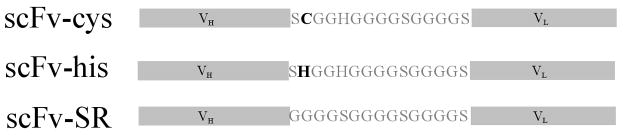Abstract
In this report, the peptide linker connecting scFv VH and VL domains were genetically modified to contain different amino acids (i.e. cysteine (scFv-cys) or histidines ( scFv-his)) to enable the scFv to adsorb or self-assemble onto the gold nanoparticles (NPs). The scFv-cys stabilized gold NPs were used to develop a highly sensitive colorimetric immunosensor. The scFv-cys stabilized gold NPs were characterized by UV-vis spectra, transmission electron microscope (TEM) and FT-IR. After adding the antigen rabbit IgG, the solution of scFv-cys stabilized gold NPs shows obvious visible color change from deep red to light purple due to the aggregation of the gold nanoparticles. Based on the colorimetric aggregation of scFv-cys stabilized gold NPs, the immunosensor exhibits high sensitivity with detection limit of 1.7 nM and good specificity. The good properties of the colorimetric aggregation immunosensor would be attributed to the small size of scFv and the covalent link between the scFv and gold NPs that improve the better orientation and enhance the probe density. With the advantages of speed, simplicity and specificity, the colorimetric immunoassay based on the functionalized scFv stabilized gold NPs represents a promising approach for protein analysis and clinical diagnostics.
Keywords: gold nanoparticle, scFv, colorimetric immunoassay
1. Introduction
Aggregation-based immunoassays were first introduced in 1956 in which antibody molecules, immobilized onto latex microparticles, were used to bind antigens. Upon antigen binding, the antibody-coated particles aggregate to produce an visual or measureable result.(Singer and Plotz 1956). In comparison to traditional immunoassays, nanoparticle aggregation-based immunoassays offer several advantages(Du et al. 2008; Thanh and Rosenzweig 2002) such as simple sample preparation, enhanced assay stability, resistance to photobleaching and a reduction in nonspecific aggregation and false positive assay results. Colorimetric immunoassays have also been developed based on the unique phenomenon that different aggregation states of the gold NP can result in distinctive color changes, in which gold NPs functionalized with antigens aggregate in the presence of complementary antibodies. However, the main disadvantage of the approach is its low sensitivity.(Du et al. 2008) A critical factor in low assay sensitivity may lie in the orientation of antibodies on the gold NP surface. If antibodies are incorrectly oriented, the antibody binding sites would not be available to bind antigen.(Backmann et al. 2005; Peluso et al. 2003) The sensitivity of the immunosensors can be enhanced by maximizing the functional orientation of the antibody binding sites and minimizing the size of antigen-binding molecules.(Backmann et al. 2005; Shen et al. 2005b).
Nanoparticle aggregation-based immunoassays require the conjugation of biological recognition elements (e.g. antibody) with the nanomaterials. The complexity and diversity of biological compounds make the synthesis of stoichiometrically defined nanoparticle–biomolecule complexes a great challenge. Physical adsorption of biomolecules on nanomaterials will generate a random orientated biorecognition elements with poor sensitivity and not rigid. Thus, various chemical means for the directly coupling of inorganic and biological materials were explored. For example, biological molecules (e.g. proteins, DNA) can be conjugated to nanoparticles directly by ligand exchange reactions or a covalent bond. Recently, biotechnological methods was applied to generate de novo protein linker units that can directly recognize distinct surfaces of semiconductor and metal nanomaterials (Christof 2001). In this report, phage display techniques were used to develop engineered single chain fragment variable recombinant antibodies (scFv) containing either a cysteine or histidine in its linker region, its direct coupling with the gold nanoparticles was accomplished by the molecular self-assemble process. The engineered scFv nanoparticle conjugates was used to develop a colorimetric immunoassay with improved sensitivity and specificity.
scFv are small heterodimers comprising the antibody heavy-chain and light-chain variable domains that are connected by a peptide linker to stabilize the molecule. Recombinant scFv antibodies contain no antibody constant regions, typical of traditional antibodies, and represent the smallest functional domains of an antibody necessary for the high-affinity binding of antigen. Due to small size and homogeneity, scFv offer significant advantages over polyclonal and monoclonal antibodies. Moreover, it can be engineered to display unique amino acids (e.g. cysteines or histidines) to immobilize on metallic support (e.g. gold sensor surfaces) and is used as a rigid linker for protein immobilization.(Ackerson et al. 2006; Qian et al. 2008; Shen et al. 2005a; Shen et al. 2005b; Shen et al. 2008). The advantages of scFvs were explored in several earlier studies. For example, scFv and their derivatives containing metal binding domains (scFv: MBD) was demonstrated to significantly improve the labeling fidelity over that obtained with Fab or IgG derivatives for molecular immunolabeling technology (Malecki et al. 2002). A method of conjugation of a glutathione monolayer – protected gold cluster (MPC) with a single chain Fv antibody fragment (scFv), mutated to present an exposed cysteine residue was shown to be able to form a gold –thiolate bond between the MPC and scFv upon activation of the gold cluster by chemical oxidation (Ackerson et al. 2006). The design and synthesis of the appropriate scFv conjugate are key steps in our methods. It is worth stressing here that the location of the cysteine in the scFvs were essential for retaining the paratope affinity. Reports show that a cysteine residue at the end of a C- terminal affinity tag was much more reactive toward a gold cluster than a cysteine residue introduced in the scFv frame work region. Our early studies show, the mutant produced by the phage – display techniques can effectively adsorbed on gold surface of a QCM biosensor and form a perfect order monolayer on Au surface while keeps right orientation for the binding of antigens. In addition, it is also reported that small (<60 nm) gold NPs have extremely high extinction coefficients, and the aggregation colorimetric assays based on gold NPs would provide sensitive detection comparable to or even higher than conventional analytical methods (e.g. fluorescence assays)(Liu and Lu 2003). In this work, the A10B scFv recombinant antibody containing a cysteine (A10B-scFv-cys) or histidines (A10B scFv-his) in the linker peptide (Shen et al. 2005a; Shen et al. 2005b; Shen et al. 2008) was conjugated onto gold NPs. Both A10B scFv binds specifically to rabbit IgG. Upon adding rabbit IgG, the scFv-cys stabilized gold NPs shows obviously visible color change due to the aggregation of gold NPs by the interaction of scFv and rabbit IgG. This phenomena was used to develop and validate an improved colorimetric immunoassays compared to those early reported (Elghanian et al. 1997; He et al. 2005; Hone et al. 2003; Huang et al. 2005; Kim et al. 2001; Li et al. 2005; Liu and Lu 2003, 2004; Sato et al. 2003; Tsai et al. 2005; Viswanadham et al. 1999; Zhao et al. 2007). The colorimetric immunosensor shows much higher sensitivity than that of conventional aggregation immunosensor and a low detection limit of 1.7 nM was achieved. We demonstrate that our simple, one step method allows the formation of a stable rigid antibody-nanoparticle complex without the necessity of pre- chemical functionalization of the nanoparticles. The highly sensitive colorimetric aggregation immunosensor would be promising in the application of simple, fast and high throughput clinical diagnostic.
2. Experimental
Reagents and Materials
Sodium citrate dihydrate was purchased from Aldrich-Sigma Co. (St. Louis, MO, USA). Hydrogen tetrachloroaurate trihydrate was purchased from Acros Organics. Bovine serum albumin (BSA; Catalog no. A-4503), rabbit IgG (Catalog no. I-5006) and anti-mouse IgG (peroxides conjugate, Cot. no. 072k9150) were purchased from Sigma, Co. (St. Louis, MO, USA). All aqueous solutions were prepared using bioanalytical reagent grade water.
The cysteine functionalized scFv (scFv-cys) and non-cysteine functionalized scFv (scFv-SR) in our work are functionalized anti-rabbit IgG scFv from clone A10B. The preparation, purification and characterization of all the functionalized scFv are the same as those reported earlier.(Shen et al. 2005a; Shen et al. 2005b; Shen et al. 2008) The linker sequences of scFv-cys, scFv-his and scFv-SR are shown in figure 1.
Figure 1.
The scheme of the scFv used with their linker sequence shown.
Preparation of Gold Nanoparticles
The gold NPs were prepared via citrate reduction of hydrogen tetrachloroaurate according to a literature method.(Frens 1973) Briefly, a sodium citrate solution (1.75 mL, 38.8 mM) was rapidly added to a boiled HAuCl4 solution (50 mL, 0.01% HAuCl4) under vigorous stirring. The mixed solution was boiled for 15 min. The resulting wine-red solution was cooled to room temperature and was filtered with 0.2 μm Micropore filter. Then, the colloidal solution was stored in the 4 °C refrigerator before use. All glass containers were washed with aqua regia and distill water. The size of the nanoparticles was about 15 nm and the particle concentration of the gold NPs was ca. 3 nM, which was determined according to the beer’s law by using the extinction coefficient of ~2.7×108 M−1cm−1 for 15 nm gold NPs in diameter at 520 nm.
Preparation of scFv stabilized gold nanoparticles
To obtain scFv stabilized gold nanoparticles, the as-synthesized gold nanoparticles were centrifuged for 25 min at 15000g and were redispersed in a PBS solution (5 mM, pH 7.2) with the same volume. After that, 50 μL of scFv solution (0.31 mg/mL) was added into 1 mL above gold NPs solution, and the solution was stand for 24 h after well mixing. Non-bound scFv was removed by centrifugation of the nanoparticle solution for 20 min at 8000 rpm. The scFv stabilized gold nanoparticles were resuspended in PBS solution (5 mM, pH 7.2).
Aggregation assays
A range of concentrations of Rabbit IgG were prepared and each solution was added to scFv stabilized gold nanoparticles with stirring. After 30 min, the reaction was recorded by UV-visible spectrophotometer. The aggregation of gold nanoparticles induce surface plasmon resonance band of gold nanoparticles at about 520 nm red-shift. In addition, the extinction intensity at 520 nm decreases, while extinction intensity at 620 nm increases. As a result, the absorbance ratio of A620/A520 was used to describe the degree of aggregation. The experiments for all scFvs were carried out at the same condition.
Instrumentation
UV-visible absorption measurements were performed using a Cary 100 Bio UV-visible spectrophotometer at room temperature. The light path for UV-vis spectra measurements is 1 cm if it is noted. The concentration of the gold NP was ca. 3 nM, and its diameter was ca. 15 nm. The centrifugations were carried out in a SORVALL RC 5C Plus centrifuge (Sorvall Instrument) at 10 °C. Transmission Electron Microscope (TEM) observations were performed on Jeol-2010 FasTEM operating at 200kV. Samples were prepared by dropping 5 μL of the gold colloidal solution onto holey copper grids. After 5 min, the solution was wicked by filter paper. The grid was subsequently dried and imaged. Attenuated total reflection (ATR)-FTIR spectra were recorded with a Bio-Rad 175c FTIR spectrometer mounted with a Specac ZnSe crystal ATR kit. The system was purged with a N2 stream to remove CO2 and water moisture prior to the collection of IR spectra. The colloidal solution was cast onto the ZnSe and was characterized subsequently.
3. Results and Discussion
A uniform size of gold NPs is required to develop a high intensity surface plasmon absorption band to enhance assay sensitivity. Fifteen nm uniform colloidal gold NPs, for use in bioassays, were prepared by the reduction of gold salt with sodium citrate. The gold colloidal solution obtained by citrate reduction was bright red in color. Figure 2 (dash line) shows the UV-vis absorbance spectra of the gold NPs. A strong plasmon absorption peak at 520 nm attributed to gold NP collective electron oscillations or localized surface plasma resonance (SPR) is presented and is a feature of 15 nm gold NPs. The solid line in Figure 2 shows the UV-vis absorbance spectra of scFv stabilized gold NPs. It is clear that the plasmon absorbance peak of gold NPs shifts from 520 nm to 525 nm after the modification of scFv-cys on their surface. The resonance wavelength and bandwidth of gold NPs are dependent on the particle size and shape, the refractive index of the surrounding medium, and the temperature. This shift after modification of gold NPs with scFv is attributed to the changes in the dielectric nature surrounding the gold NPs due to scFv presence. The inset in figure 2 shows the TEM image of the scFv-cys stabilized gold NPs. Compared to bare gold NPs, a shadow around the gold NPs is clearly observed in the scFv-cys stabilized nanoparticles and represents the scFv protein layer on the surface of gold NPs. The right-up inset represents the high resolution TEM image of the scFv-cys stabilized gold NPs. The thickness of shadow surrounding the gold nanoparticles is about 2.5 nm and is quite close in size to that of the scFv which has a molecular weight of 27k Da.(De Roe et al. 1987) The FT-IR spectra of the gold NPs without (dash line) and with (solid line) modification of scFv-cys molecules are shown in figure S1. Besides the main peak at 3305 cm−1 and 1635 cm−1 due to the hydroxyl stretch and C=O stretching of citrate ligand respectively, several other peaks also appear and the peaks at 3305 cm−1 and 1635 cm−1 become broader and stronger after modification of scFv-cys. The main peak at 2990 cm−1 and 2900 cm−1 are associated with the asymmetric and symmetric CH2 stretching modes, respectively. The peak at 1390 cm−1 is assigned to the action of amide β (C-N). The band at 1240 cm−1 would be ascribed to the wagging and twisting vibration of the CH2 group or C-S band. The peak at 1065 cm−1 can be associated to the C-O stretching vibration. These results verify the conjugation of scFv-cys and gold NPs.
Figure 2.
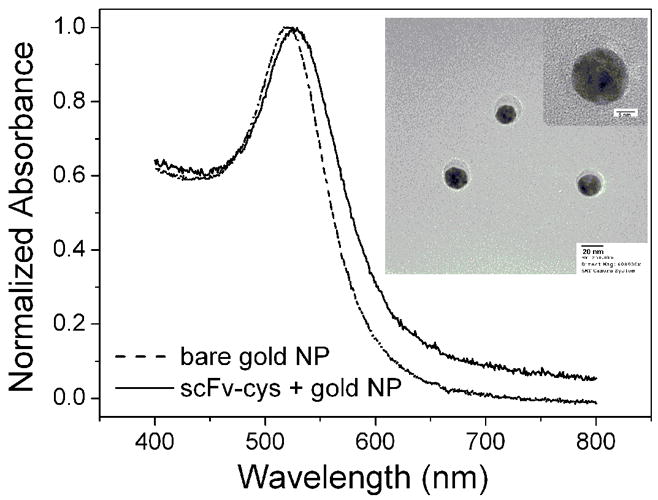
The UV-vis spectra of gold nanoparticles with (solid line) and without (dash line) modification of scFv-cys. The inset is the TEM pattern of the scFv-cys stabilized gold nanoparticles and a high resolution image (up-right).
The solution of scFv-cys stabilized gold NPs retains the bright red coloration and is stable for no less than two weeks in the PBS (pH 7.2, 5mM) at 4 °C. Less aggregation can be observed with addition of NaCl till a concentration of 20 mM, showing the good stability of the scFv stabilized gold NPs. In addition, the gold NPs with size of 30 nm and 50 nm were also used in our experiments. It was found that the scFv-cys stabilized gold NPs of 30 nm and 50 nm were less stable compared to the gold NPs of 15 nm. The larger gold NPs, the worse the stability. Precipitations will be observed after a week in the solution scFv-cys stabilized gold NPs of 30 nm. Moreover, the stability of scFv-his modified gold NPs is also worse than that of scFv-cys modified gold NPs and it shows aggregation in a week of time.
Besides their reduced size that fascinates the direct and dense immobilization on interface (Backmann et al. 2005; Shen et al. 2005b), the most important feature is that scFv molecules are still capable of binding antigens with the same affinity as that of the whole antibody. The scFv molecules can specifically bind to rabbit IgG molecule. Figure S2 shows the photographic patterns of the scFv-cys stabilized gold NPs in the absence (right) and presence (left) of rabbit IgG with a concentration of 85 nM. After addition of rabbit IgG, a visible color change of the scFv-cys stabilized gold NPs solution from bright red to light purple is induced. The phenomena are typical features of the aggregation of gold NPs. Figure 3 shows the UV-vis spectra of the scFv stabilized gold NPs with (solid line) and without (dash line) addition of rabbit IgG. As it is shown, the SPR peak has an obvious red shift from 520 nm to 542 nm and is broaden after the addition of rabbit IgG. Particle aggregation typically results in a massive redshift of the peak position as well as a peak broadening, caused by the near-field coupling between adjacent particles. Moreover, the magnitude of the redshift is highly dependent on the interpartical distance and size of the aggregations. Figure S3a and 3b show the TEM images of scFv-cys stabilized gold NPs (a) and that with addition of rabbit IgG (b), respectively. The gold NPs are well dispersed before adding rabbit IgG. After the addition of rabbit IgG, the gold NPs are brought together to form networks at micrometer scale, and large island like aggregation consisting of hundreds of gold NPs forms. As it is well known, each scFv molecule will capture one “arm” of the rabbit IgG molecule, sharing the antigen with a neighboring scFv molecule (two binding sites per rabbit antibody). Consequently, when rabbit IgG was added into the solution of scFv stabilized gold NPs, aggregations will occur. The aggregation procedure of the scFv stabilized gold NPs are summarized as shown in figure 4.
Figure 3.
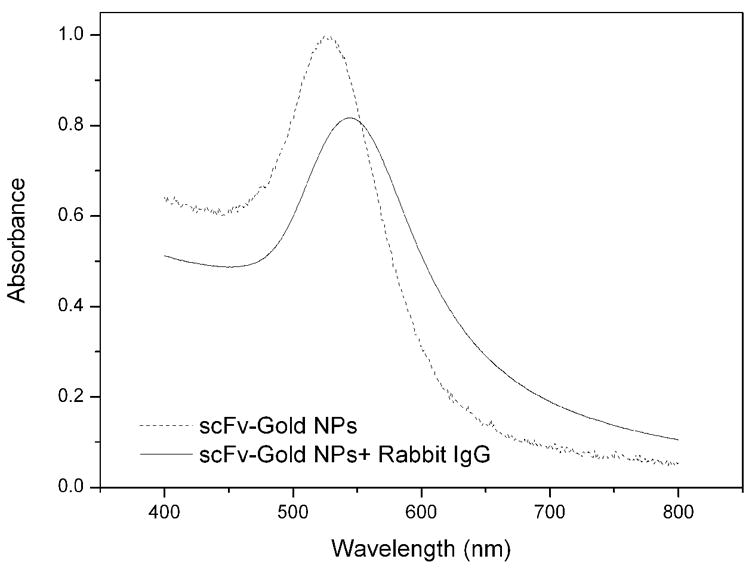
UV-vis spectra of scFv-cys stabilized gold NPs solution before (dash line) and after (solid line) addition of 85 nM rabbit IgG.
Figure 4.
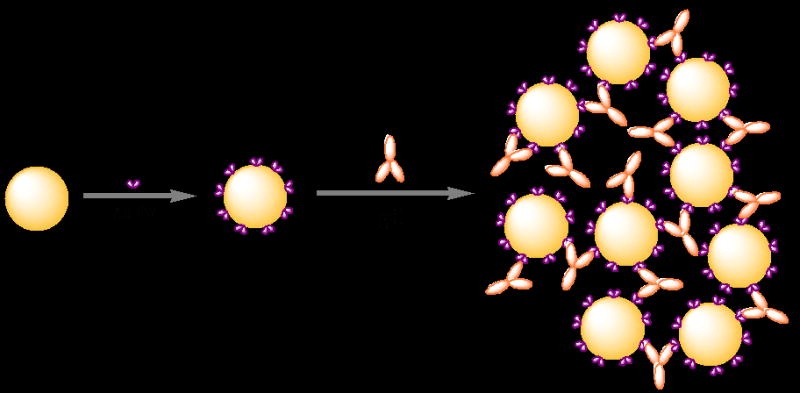
The scheme of the aggregation process of scFv-cys stabilized gold NPs after addition of rabbit IgG.
The kinetic process of the aggregation of scFv stabilized gold nanoparticles is presented in figure S4, in which the position of the maximal SPR band is recorded as a function of time in the presence of various concentrations of rabbit IgG. Upon adding rabbit IgG, the SPR band of the scFv-cys stabilized gold NPs shows obviously red shift. The rate of aggregation, as determined from the change in the redshift of the SPR peak with time is largest in the initial 5 min, and then it slows down to nearly zero after 30 min by adding rabbit IgG. Obviously, both the change of red shift and the rate of aggregation are clearly dependant on the concentration of rabbit IgG. The higher concentration of rabbit IgG gave the larger change of redshift and a faster redshift at initial stage. Figure 5 presents the absorbance ratio A620/A520 of scFv-cys stabilized gold NPs solution after addition of various concentrations of rabbit IgG, which is recorded 30 min after adding rabbit IgG. As the concentrations of rabbit IgG was increased, the A620/A520 value increased. A linear range from 1.7 nM to 170 nM was obtained (Inset in figure 5b) with a relative coefficient of 0.98. The detection limit (1.7 nM, 0.25 μg/mL) at the signal-to-noise ratio of 3 was much lower than those reported aggregation colorimetric immunoassay based antigen conjugated nanoparticles (10 μg/mL for human IgG (Zhang et al. 2003), 1 μg/mL for protein A (Thanh and Rosenzweig 2002)) and a dynamic range of 2 orders of magnitude in concentration was obtained. The linear relationship between A620/A520 and concentrations were also observed after adding rabbit IgG in the range of 1.7 – 170 nM into the scFv-his stabilized gold NPs solution as shown in figure S5. Its relative coefficient is 0.9897. The high sensitivity of the scFv stabilized gold NPs would be attributed to the small size of the scFv and the covalent link between the scFv and gold NPs that allow the better orientation of the binding domain and increase the probe density. At higher concentration, a plateau was reached because the aggregation process is inhibited, which may be due to the blocking of active sites. In addition, the measurement can be carried out 30 min after the addition of analytical sample though precipitation will be observed over several hours. The facts indicated that the method can be used for fast diagnostic.
Figure 5.
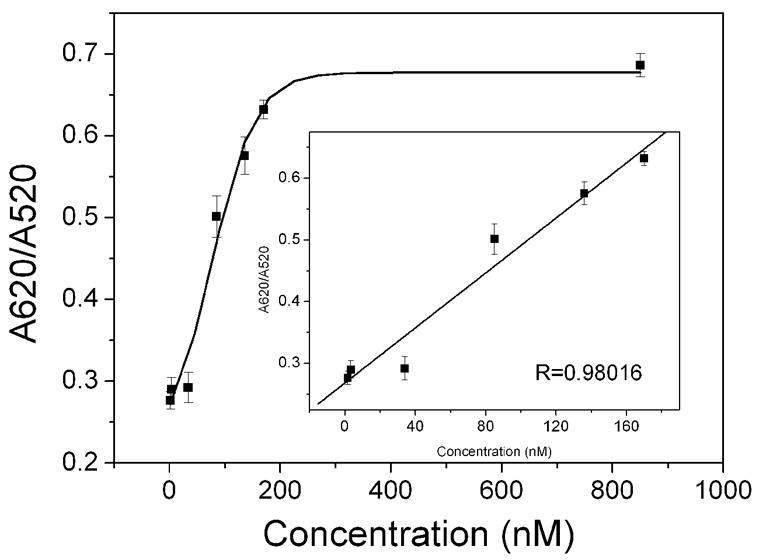
Plot of the absorbance ratio (A620/A520) for the scFv-cys stabilized gold NPs solution at 30 min as a function of the concentration of rabbit IgG. Inset is the linear curve at low concentration of rabbit IgG. (n = 3)
To confirm the specific recognition of A10B scFv conjugated Au NPs by rabbit IgG, control experiments were carried out using BSA, anti-mouse IgG and human serum. When BSA replaces rabbit IgG, the solution of scFv stabilized gold NPs doesn’t show any coloration change and less changes on the absorbance are observed from the UV-vis spectra (figure 6, ii), even though the concentration of BSA is much higher than that of rabbit IgG. The addition of human serum (figure 6, iii) and anti-mouse IgG (figure 6, iv) don’t change the coloration of the gold NPs solution either. The fact suggests that the scFv-cys stabilized gold NPs has good specificity. In addition, we also mixed the gold NPs with unfunctionalized scFv (scFv-SR that has no cysteine or histine in the linker region) and handled at the same experiment condition. The UV-vis spectra of gold NPs is nearly same before and after the addition of scFv-SR, which may be attributed to poor immobilization of scFv-RS on AuNP due to the weak interaction between unfunctionalized scFv and gold NPs. Rabbit IgG was also added into the fresh prepared gold NPs solution. However, no significant response was observed under the same conditions (figure 6, v). The facts indicated a good specificity of the functionalized scFv stabilized gold NPs based immunosensor.
Figure 6.
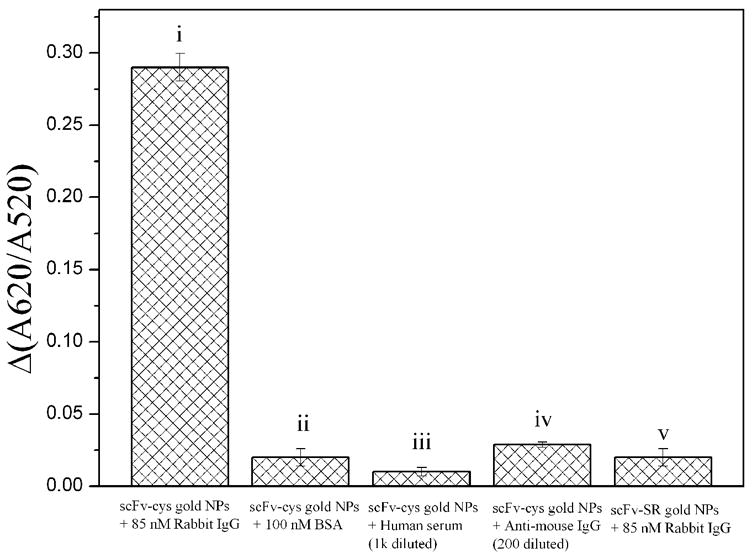
Changes of A620/520 of the scFv-cys stabilized gold NPs solution after addition of after addition of 85 nM rabbit IgG (i), 100 nM BSA (ii), 5k diluted human serum (iii), 200 diluted anti-mouse IgG (iv), and the gold nanoparticles treated with scFv-SR after addition of 85 nM rabbit IgG (v). (n = 3)
4. Conclusion
In conclusion, functionalized scFv stabilized gold nanoparticles were prepared and a simple, fast and sensitive colorimetric immunoassay based on the aggregation of the scFv stabilized gold nanoparticles was developed. Due to the small size of scFv and the covalent conjugation between the scFv and gold NPs, the detection limit of 1.7 nM based on the aggregation immunoassay is achieved. Moreover, the immunosensor has high specificity. With the advantages of speediness, simplicity and specificity, the colorimetric immunoassay based on the functionalized scFv stabilized gold NPs is promising in the protein analysis and clinical diagnostics.
Supplementary Material
Acknowledgments
This work was partly supported by NIH R33EB000672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerson CJ, Jadzinsky PD, Jensen GJ, Kornberg RD. Rigid, Specific, and Discrete Gold Nanoparticle/Antibody Conjugates. J Am Chem Soc. 2006;128(8):2635–2640. doi: 10.1021/ja0555668. [DOI] [PubMed] [Google Scholar]
- Backmann N, Zahnd C, Huber F, Bietsch A, Pluckthun A, Lang HP, Guntherodt HJ, Hegner M, Gerber C. A label-free immunosensor array using single-chain antibody fragments. Proc Nat Aca Sci. 2005;102(41):14587–14592. doi: 10.1073/pnas.0504917102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christof MN. Nanoparticles, Proteins, and Nucleic Acids: Biotechnology Meets Materials Science. Angew Chem Inter Ed. 2001;40(22):4128–4158. doi: 10.1002/1521-3773(20011119)40:22<4128::AID-ANIE4128>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- De Roe C, Courtoy PJ, Baudhuin P. J Histochem Cytochem. 1987;35:1191–1198. doi: 10.1177/35.11.3655323. [DOI] [PubMed] [Google Scholar]
- Du BA, Li ZP, Cheng YQ. Homogeneous immunoassay based on aggregation of antibody-functionalized gold nanoparticles coupled with light scattering detection. Talanta. 2008;75(4):959–964. doi: 10.1016/j.talanta.2007.12.048. [DOI] [PubMed] [Google Scholar]
- Elghanian R, Storhoff JJ, Mucic RC, letsinger RL, Mirkin CA. Science. 1997;277:1078. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- Frens G. Nat. Phys Sci. 1973;241:20–22. [Google Scholar]
- He XR, Liu HB, Li YL, Wang S, Li YJ, Wang N, Xiao JC, Xu XH, Zhu DB. Gold nanoparticle-based fluorometric and colorimetric sensing of copper(II) ions. Adv Mater. 2005;17(23):2811–2815. [Google Scholar]
- Hone DC, Haines AH, Russell DA. Rapid, Quantitative Colorimetric Detection of a Lectin Using Mannose-Stabilized Gold Nanoparticles. Langmuir. 2003;19(17):7141–7144. [Google Scholar]
- Huang CC, Huang YF, Cao Z, Tan W, Chang HT. Aptamer-Modified Gold Nanoparticles for Colorimetric Determination of Platelet-Derived Growth Factors and Their Receptors. Anal Chem. 2005;77(17):5735–5741. doi: 10.1021/ac050957q. [DOI] [PubMed] [Google Scholar]
- Kim Y, Johnson RC, Hupp JT. Gold Nanoparticle-Based Sensing of “Spectroscopically Silent” Heavy Metal Ions. Nano Lett. 2001;1(4):165–167. [Google Scholar]
- Li J, Chu X, Liu Y, Jiang JH, He Z, Zhang Z, Shen G, Yu RQ. A colorimetric method for point mutation detection using high-fidelity DNA ligase. Nucl Acids Res. 2005;33(19):e168. doi: 10.1093/nar/gni163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lu Y. A Colorimetric Lead Biosensor Using DNAzyme-Directed Assembly of Gold Nanoparticles. J Am Chem Soc. 2003;125(22):6642–6643. doi: 10.1021/ja034775u. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu Y. Accelerated Color Change of Gold Nanoparticles Assembled by DNAzymes for Simple and Fast Colorimetric Pb2+ Detection. J Am Chem Soc. 2004;126(39):12298–12305. doi: 10.1021/ja046628h. [DOI] [PubMed] [Google Scholar]
- Malecki M, Hsu A, Truong L, Sanchez S. Molecular immunolabeling with recombinant single-chain variable fragment (scFv) antibodies designed with metal-binding domains. Proc Nat Aca Sci. 2002;99(1):213–218. doi: 10.1073/pnas.261567298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso P, Wilson DS, Do D, Tran H, Venkatasubbaiah M, Quincy D, Heidecker B, Poindexter K, Tolani N, Phelan M, Witte K, Jung LS, Wagner P, Nock S. Optimizing antibody immobilization strategies for the construction of protein microarrays. Anal Biochem. 2003;312(2):113–124. doi: 10.1016/s0003-2697(02)00442-6. [DOI] [PubMed] [Google Scholar]
- Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotech. 2008;26(1):83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- Sato K, Hosokawa K, Maeda M. Rapid Aggregation of Gold Nanoparticles Induced by Non-Cross-Linking DNA Hybridization. J Am Chem Soc. 2003;125(27):8102–8103. doi: 10.1021/ja034876s. [DOI] [PubMed] [Google Scholar]
- Shen Z, Mernaugh RL, Yan H, Yu L, Zhang Y, Zeng X. Engineered Recombinant Single-Chain Fragment Variable Antibody for Immunosensors. Anal Chem. 2005a;77(21):6834–6842. doi: 10.1021/ac0507690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Stryker GA, Mernaugh RL, Yu L, Yan H, Zeng X. Single-Chain Fragment Variable Antibody Piezoimmunosensors. Anal Chem. 2005b;77(3):797–805. doi: 10.1021/ac048655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Yan H, Zhang Y, Mernaugh RL, Zeng X. Engineering Peptide Linkers for scFv Immunosensors. Anal Chem. 2008a;80(6):1910–1917. doi: 10.1021/ac7018624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen ZH, Yan HP, Zhang Y, Mernaugh RL, Zeng XQ. Engineering peptide linkers for scFv immunosensors. Anal Chem. 2008b;80(6):1910–1917. doi: 10.1021/ac7018624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JM, Plotz CM. Am. J Med. 1956;21:888–896. [PubMed] [Google Scholar]
- Thanh NTK, Rosenzweig Z. Development of an Aggregation-Based Immunoassay for Anti-Protein A Using Gold Nanoparticles. Anal Chem. 2002;74(7):1624–1628. doi: 10.1021/ac011127p. [DOI] [PubMed] [Google Scholar]
- Tsai CS, Yu TB, Chen CT. Gold nanoparticle-based competitive colorimetric assay for detection of protein-protein interactions. Chem Commun. 2005;34:4273–4275. doi: 10.1039/b507237a. [DOI] [PubMed] [Google Scholar]
- Viswanadham G, Elghanian R, Mirkin CA, Letsinger RL. Colorimetric detection of pcr amplicons using gold nanoparticle oligonucleotide probes. Abstracts of Papers of the American Chemical Society. 1999;218:U123–U123. [Google Scholar]
- Zhang CX, Wang X, Tang ZM, Lu ZH. Anal. Biochem. 2003;320:136–140. doi: 10.1016/s0003-2697(03)00353-1. [DOI] [PubMed] [Google Scholar]
- Zhao WA, Chiuman W, Lam JCF, Brook MA, Li YF. Simple and rapid colorimetric enzyme sensing assays using non-crosslinking gold nanoparticle aggregation. Chem Commun. 2007:3729–3731. doi: 10.1039/b705335e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.